Free and Glycosidically Bound Volatile Compounds in Okinawan Pineapple (Ananas comosus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sample Preparation
2.3. Volatile Component Analysis
2.4. Glycosides Extraction and Hydrolysis
2.5. Sugar Moiety Analysis
2.6. Glycoside Composition Analysis
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Okinawan Pineapple
3.2. Free Volatile Components of Okinawan Pineapple
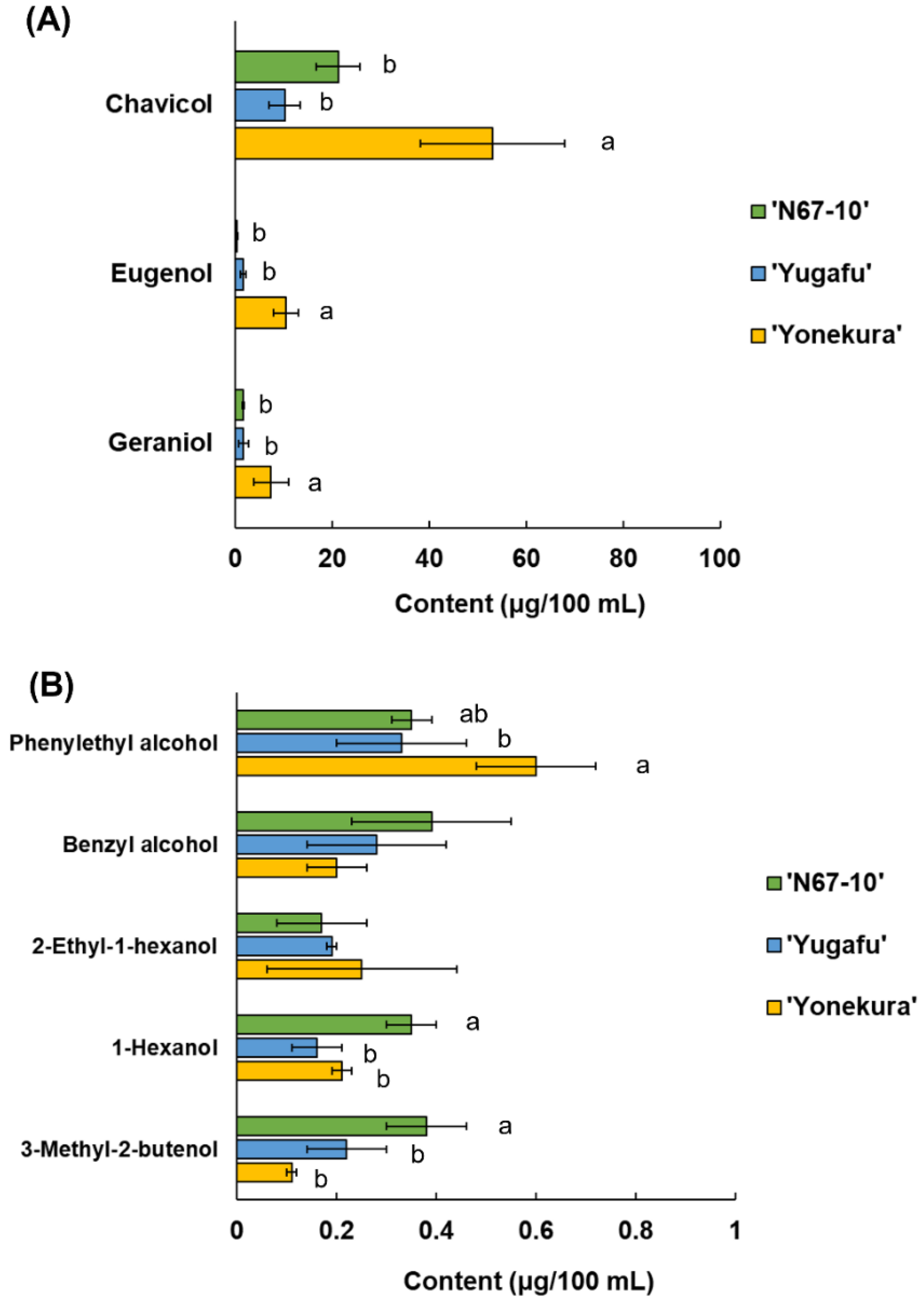
3.3. Released Volatile Components from the Okinawan Pineapple Glycosides
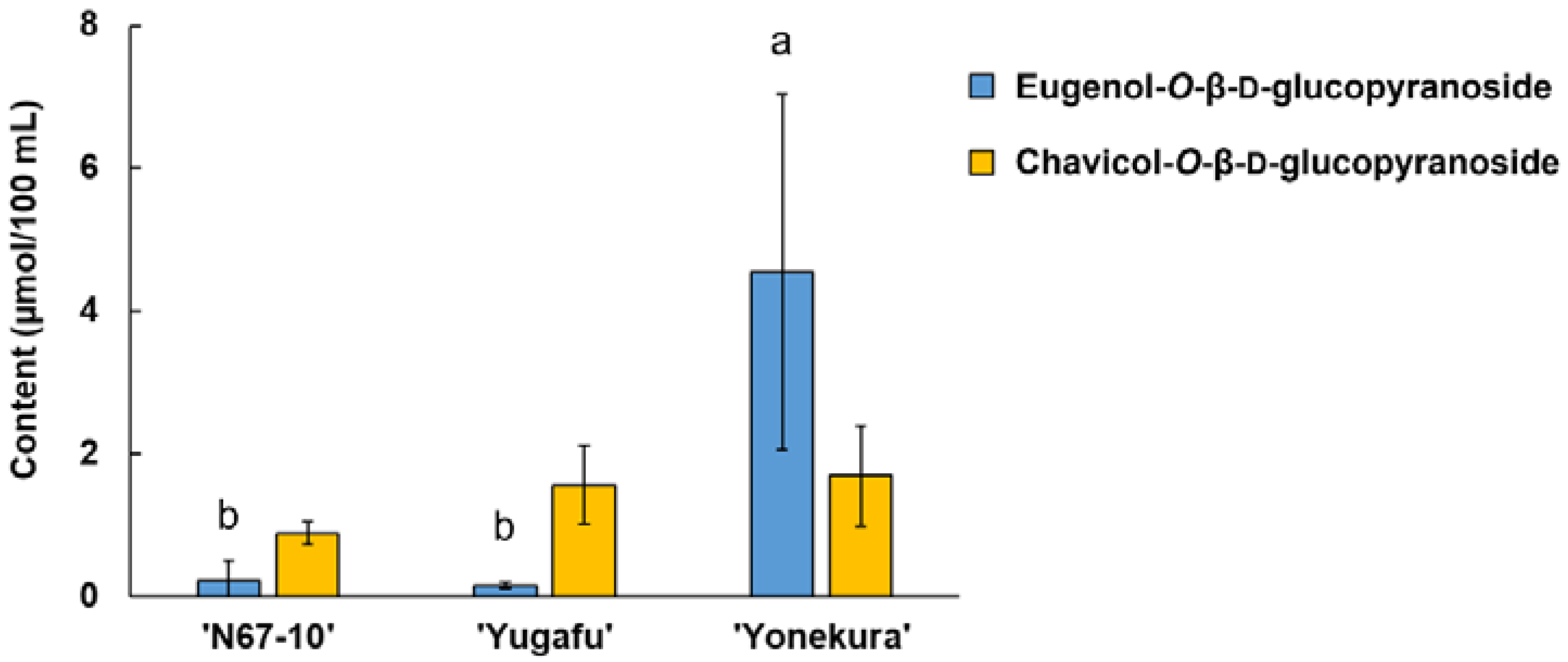
3.4. Glycosidically Bound Volatile Components of Okinawan Pineapple
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaewtathip, T.; Charoenrein, S. Changes in volatile aroma compounds of pineapple (Ananas comosus) during freezing and thawing. Int. J. Food Sci. Technol. 2012, 47, 985–990. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Sun, G.-M.; Liu, Y.-G.; Lv, L.-L.; Yang, W.-X.; Zhao, W.-F.; Wei, C.-B. Aroma volatile compounds from two fresh pineapple varieties in China. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A. Odour-active compounds in pineapple (Ananas comosus [L.] Merril cv. Red Spanish). Int. J. Food Sci. Technol. 2013, 48, 564–570. [Google Scholar] [CrossRef]
- Shoda, M.; Urasaki, N.; Sakiyama, S.; Terakami, S.; Hosaka, F.; Shigeta, N.; Nishitani, C.; Yamamoto, T. DNA profiling of pineapple cultivars in Japan discriminated by SSR markers. Breed. Sci. 2012, 62, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Nishiba, Y.; Takeuchi, M.; Moromizato, C. Carotenoid content in different varieties of pineapple (Ananas comosus L.) cultivated in Okinawa Prefecture. Nippon Shokuhin Kagaku Kogaku Kaishi 2019, 66, 100–107. [Google Scholar] [CrossRef]
- Yao, S. On Asia-Pacific pineapple industry transfer beyond Japanese Empire: Focusing on Hawaii, Taiwan and Okinawa. Hakusan Rev. Anthropol. 2018, 21, 81–104. [Google Scholar]
- Tokitomo, Y.; Steinhaus, M.; Büttner, A.; Schieberle, P. Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Biosci. Biotechnol. Biochem. 2005, 69, 1323–1330. [Google Scholar] [CrossRef]
- Romli, S.R.; Easa, A.M.; Murad, M. Influence of post-harvest physiology on sensory perception, physical properties, and chemical compositions of Moris pineapples (Ananas comosus L.). J. Food Sci. 2021, 86, 4159–4171. [Google Scholar] [CrossRef]
- Jana, S.; Mukherjee, S.; Ali, I.; Ray, B.; Ray, S. Isolation, structural features, in vitro antioxidant activity and assessment of complexation ability with β-lactoglobulin of a polysaccharide from Borassus flabellifer fruit. Heliyon 2020, 6, e05499. [Google Scholar] [CrossRef]
- Yauk, Y.K.; Ged, C.; Wang, M.Y.; Matich, A.J.; Tessarotto, L.; Cooney, J.M.; Chervin, C.; Atkinson, R.G. Manipulation of flavour and aroma compound sequestration and release using a glycosyltransferase with specificity for terpene alcohols. Plant J. 2014, 80, 317–330. [Google Scholar] [CrossRef]
- Ghaste, M.; Narduzzi, L.; Carlin, S.; Vrhovsek, U.; Shulaev, V.; Mattivi, F. Chemical composition of volatile aroma metabolites and their glycosylated precursors that can uniquely differentiate individual grape cultivars. Food Chem. 2015, 188, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, S.; Ono, E.; Horikawa, M.; Murata, J.; Totsuka, K.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Matsui, K.; et al. Volatile glycosylation in tea plants: Sequential glycosylations for the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Pankoke, H.; Buschmann, T.; Müller, C. Role of plant β-glucosidases in the dual defense system of iridoid glycosides and their hydrolyzing enzymes in Plantago lanceolata and Plantago major. Phytochemistry 2013, 94, 99–107. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma glycosides in grapes and wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Kamiya, A.; Mizu, M.; Takara, K.; Tamaki, H.; Wada, K. Changes in the physicochemical characteristics, including flavour components and Maillard reaction products, of non-centrifugal cane brown sugar during storage. Food Chem. 2014, 149, 170–177. [Google Scholar] [CrossRef]
- Kamiyoshihara, Y.; Tieman, D.M.; Klee, H.J. Analyses of plant UDP-dependent glycosyltransferases to identify their volatile substrates using recombinant proteins. Methods Mol. Biol. 2016, 1363, 199–207. [Google Scholar] [CrossRef]
- Tamura, Y.; Iwatoh, S.; Miyaura, K.; Asikin, Y.; Kusano, M. Metabolomic profiling reveals the relationship between taste-related metabolites and roasted aroma in aged pork. LWT 2022, 155, 112928. [Google Scholar] [CrossRef]
- Steingass, C.B.; Vollmer, K.; Lux, P.E.; Dell, C.; Carle, R.; Schweiggert, R.M. HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Res. Int. 2020, 127, 108709. [Google Scholar] [CrossRef]
- Montero-Calderón, M.; Rojas-Graü, M.A.; Martín-Belloso, O. Aroma profile and volatiles odor activity along gold cultivar pineapple flesh. J. Food Sci. 2010, 75, S506–S512. [Google Scholar] [CrossRef]
- Zhu, G.; Yu, G. A pineapple flavor imitation by the note method. Food Sci. Technol. 2019, 40, 924–928. [Google Scholar] [CrossRef]
- Yauk, Y.K.; Souleyre, E.J.F.; Matich, A.J.; Chen, X.; Wang, M.Y.; Plunkett, B.; Dare, A.P.; Espley, R.V.; Tomes, S.; Chagné, D.; et al. Alcohol acyl transferase 1 links two distinct volatile pathways that produce esters and phenylpropenes in apple fruit. Plant J. 2017, 91, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kong, W.; Yang, C.; Feng, R.; Xi, W. Alcohol acyltransferase is involved in the biosynthesis of C6 esters in apricot (Prunus armeniaca L.) fruit. Front Plant Sci. 2021, 12, 763139. [Google Scholar] [CrossRef] [PubMed]
- Gonda, I.; Lev, S.; Bar, E.; Sikron, N.; Portnoy, V.; Davidovich-Rikanati, R.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Giovannonni, J.J.; et al. Catabolism of L-methionine in the formation of sulfur and other volatiles in melon (Cucumis melo L.) fruit. Plant J. 2013, 74, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Hirose, N.; Tamaki, H.; Ito, S.; Oku, H.; Wada, K. Effects of different drying-solidification processes on physical properties, volatile fraction, and antioxidant activity of non-centrifugal cane brown sugar. LWT-Food Sci Technol. 2016, 66, 340–347. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Lin, L.-Y.; Yang, K.-M.; Chiang, Y.-C.; Chen, M.-H.; Chiang, P.-Y. Effects of roasting sweet potato (Ipomoea batatas L. Lam.): Quality, volatile compound composition and sensory evaluation. Foods 2021, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Nakashima, Y.; Nakahashi, H.; Hara, N.; Nakagawa, H.; Usami, A.; Chavasiri, W. Volatile compounds with characteristic odor of essential oil from Magnolia obovata leaves by hydrodistillation and solvent-assisted flavor evaporation. J. Oleo Sci. 2015, 64, 999–1007. [Google Scholar] [CrossRef]
- Yuan, F.; Qian, M.C. Aroma potential in early- and late-maturity Pinot noir grapes evaluated by aroma extract dilution analysis. J. Agric. Food Chem. 2016, 64, 443–450. [Google Scholar] [CrossRef]
- Niu, Y.; Kong, J.; Xiao, Z.; Chen, F.; Ma, N.; Zhu, J. Characterization of odor-active compounds of various Chinese “Wuliangye” liquors by gas chromatography–olfactometry, gas chromatography–mass spectrometry and sensory evaluation. Int. J. Food Prop. 2017, 20, S735–S745. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, C.; Yan, H.; Peng, Y.; Hu, E.; Qi, J.; Lin, Q. Characteristic aroma compound in cinnamon bark extract using soybean oil and/or water. Appl. Sci. 2022, 12, 1284. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drug Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Compositional analysis and aroma evaluation of feijoa essential oils from New Zealand grown cultivars. Molecules 2019, 24, 2053. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Koeduka, T.; Aida, M.; Suzuki, H.; Iijima, Y.; Matsui, K. Biosynthesis of volatile terpenes that accumulate in the secretory cavities of young leaves of Japanese pepper (Zanthoxylum piperitum): Isolation and functional characterization of monoterpene and sesquiterpene synthase genes. Plant Biotechnol. 2017, 34, 17–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwab, W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules 2013, 18, 6936–6951. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Honda, T.; Fujita, A.; Kurobayashi, Y.; Kitahara, T. “Aqua-space®”, a new headspace method for isolation of natural floral aromas using humidified air as a carrier gas. Biosci. Biotechnol. Biochem. 2004, 68, 454–457. [Google Scholar] [CrossRef]
- Caffrey, A.; Ebeler, S.E. The occurrence of glycosylated aroma precursors in Vitis vinifera fruit and Humulus lupulus hop cones and their roles in wine and beer volatile aroma production. Foods 2021, 10, 935. [Google Scholar] [CrossRef]

| Properties | ‘N67-10’ | ‘Yugafu’ | ‘Yonekura’ |
|---|---|---|---|
| Fruit weight | 1321.8 ± 169.1 | 1172.8 ± 162.5 | 1351.0 ± 170.2 |
| Color space L* | 66.84 ± 2.02 | 63.95 ± 0.91 | 65.95 ± 2.76 |
| Color space a* | −3.42 ± 0.48 ab | −4.11 ± 0.65 a | −3.19 ± 0.40 b |
| Color space b* | 19.10 ± 2.97 b | 10.62 ± 1.23 c | 26.34 ± 2.57 a |
| Total soluble solid (°Brix) | 14.66 ± 0.55 ab | 15.70 ± 0.86 a | 13.60 ± 0.65 b |
| Titratable acidity (%) | 0.62 ± 0.09 b | 0.76 ± 0.06 a | 0.58 ± 0.04 b |
| Total soluble solid/titratable acidity ratio | 23.80 | 20.77 | 23.29 |
| Peak No. | RI | Compound | ‘N67-10′ | ‘Yugafu’ | ‘Yonekura’ |
|---|---|---|---|---|---|
| 1 | 829 | Methyl acetate | 0.81 ± 0.06 b | 0.07 ± 0.01 c | 1.96 ± 0.37 a |
| 2 | 892 | Ethyl acetate | 1.82 ± 0.12 b | 12.58 ± 0.15 a | 2.40 ± 0.44 b |
| 3 | 909 | Methyl propanoate | 0.42 ± 0.02 b | 0.53 ± 0.01 b | 4.80 ± 0.80 a |
| 4 | 923 | Methyl 2-methylpropanoate | nd | 0.07 ± 0.01 a | 0.60 ± 0.14 b |
| 5 | 963 | Ethyl propanoate | nd | 0.88 ± 0.12 | nd |
| 6 | 972 | Ethyl 2-methylpropanoate | nd | 0.22 ± 0.05 | nd |
| 7 | 983 | Methyl butanoate | 4.42 ± 0.10 b | 1.46 ± 0.14 b | 23.46 ± 4.10 a |
| 8 | 1012 | 2-Methylpropyl acetate | nd | 0.15 ± 0.04 | 0.16 ± 0.03 |
| 9 | 1017 | Methyl 3-methylbutanoate | 0.29 ± 0.02 b | 0.25 ± 0.06 b | 3.27 ± 0.64 a |
| 10 | 1042 | Ethyl butanoate | 0.40 ± 0.03 a | 0.27 ± 0.03 b | 0.36 ± 0.07 ab |
| 11 | 1051 | Ethyl 2-methylbutanoate | nd | 11.48 ± 1.98 a | 0.28 ± 0.05 b |
| 12 | 1086 | Methyl pentanoate | 0.60 ± 0.04 b | 0.17 ± 0.04 b | 3.28 ± 0.57 a |
| 13 | 1120 | 2-Methylbutyl acetate | nd | 0.21 ± 0.00 b | 2.70 ± 0.43 a |
| 14 | 1122 | 3-Methylbutyl acetate | nd | 0.53 ± 0.28 | nd |
| 15 | 1134 | Ethyl pentanoate | nd | 0.24 ± 0.03 | nd |
| 16 | 1187 | Methyl hexanoate | 18.84 ± 1.57 b | 11.40 ± 2.22 b | 263.91 ± 48.16 a |
| 17 | 1234 | Ethyl hexanoate | 0.15 ± 0.00 b | 10.13 ± 1.71 a | 1.50 ± 0.29 b |
| 18 | 1255 | 3-Methyl-2-butenyl acetate | 0.65 ± 0.08 b | 1.24 ± 0.49 b | 2.47 ± 0.26 a |
| 19 | 1259 | Methyl (Z)-3-hexenoate | 0.22 ± 0.02 b | nd | 1.71 ± 0.24 a |
| 20 | 1265 | Methyl (E)-3-hexenoate | 0.46 ± 0.03 b | nd | 2.49 ± 0.47 a |
| 21 | 1288 | Methyl heptanoate | 0.16 ± 0.01 b | nd | 1.71 ± 0.27 a |
| 22 | 1290 | Methyl 2-hydroxy-2-methylbutanoate | nd | nd | 0.61 ± 0.05 |
| 23 | 1391 | Methyl octanoate | 0.84 ± 0.16 b | 0.19 ± 0.02 b | 10.28 ± 2.74 a |
| 24 | 1437 | Ethyl octanoate | 0.22 ± 0.02 | 0.27 ± 0.11 | nd |
| 25 | 1514 | Dimethyl propanedioate | 0.80 ± 0.07 b | nd | 1.98 ± 0.31 a |
| 26 | 1525 | Methyl 3-(methylthio)propanoate | 18.67 ± 0.74 b | 2.20 ± 0.13 b | 128.49 ± 20.15 a |
| 27 | 1530 | 2,3-Butanediyl diacetate | 0.18 ± 0.01 b | 0.13 ± 0.02 b | 0.41 ± 0.04 a |
| 28 | 1569 | Ethyl 3-(methylthio)propanoate | 0.23 ± 0.01 b | 9.45 ± 0.96 a | 0.90 ± 0.16 b |
| 29 | 1730 | Benzyl acetate | 0.71 ± 0.02 a | 0.48 ± 0.04 b | 0.59 ± 0.10 b |
| Total esters | 50.89 | 64.60 | 460.32 | ||
| 30 | 955 | Ethanol | nd | 1.31 ± 0.13 | nd |
| 31 | 1360 | 1-Hexanol | 0.47 ± 0.02 a | 0.47 ± 0.03 a | 0.33 ± 0.04 b |
| 32 | 1463 | 1-Heptanol | 0.22 ± 0.02 | 0.23 ± 0.04 | 0.31 ± 0.10 |
| 33 | 1496 | 2-Ethyl-1-hexanol | 0.95 ± 0.04 a | 0.66 ± 0.13 b | 1.14 ± 0.03 a |
| 34 | 1565 | 1-Octanol | 0.27 ± 0.01 b | 0.33 ± 0.04 b | 0.89 ± 0.10 a |
| 35 | 1646 | Menthol | 0.49 ± 0.01 a | 0.20 ± 0.04 c | 0.31 ± 0.01 b |
| 36 | 1662 | 2-Furanmethanol | 1.17 ± 0.21 b | 0.99 ± 0.11 b | 2.10 ± 0.46 a |
| Total alcohols | 3.57 | 4.19 | 5.08 | ||
| 37 | 1207 | Eucalyptol | 0.38 ± 0.03 b | 0.67 ± 0.15 a | 0.29 ± 0.03 b |
| 38 | 1232 | (E)-β-Ocimene | nd | nd | 1.99 ± 1.79 |
| 39 | 1488 | α-Copaene | 0.75 ± 0.11 b | 0.18 ± 0.00 b | 1.50 ± 0.32 a |
| 40 | 1588 | β-Elemene | 0.54 ± 0.13 | nd | nd |
| 41 | 1606 | Terpinen-4-ol | 0.26 ± 0.02 b | 0.28 ± 0.04 b | 0.83 ± 0.16 a |
| 42 | 1686 | γ-Muurolene | 0.28 ± 0.10 | nd | nd |
| 43 | 1722 | α-Muurolene | 0.47 ± 0.10 ab | 0.20 ± 0.03 b | 0.81 ± 0.21 a |
| 44 | 1756 | δ-Cadinene | nd | nd | 0.34 ± 0.08 |
| Total terpenes | 2.68 | 1.33 | 5.76 | ||
| 45 | 1338 | Methyl heptenone | nd | nd | 0.27 ± 0.01 |
| 46 | 1596 | 4-Methoxy-2,5-dimethyl-3(2H)-furanone | 0.46 ± 0.05 b | nd | 13.72 ± 2.32 a |
| 47 | 1700 | γ-Hexalactone | 0.19 ± 0.02 b | 0.28 ± 0.03 b | 4.84 ± 0.69 a |
| 48 | 1916 | γ-Octalactone | 0.44 ± 0.04 b | 0.66 ± 0.01 b | 3.06 ± 0.45 a |
| 49 | 1969 | δ-Octalactone | nd | nd | 0.71 ± 0.13 |
| 50 | 2043 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | 0.96 ± 0.31 b | 0.84 ± 0.18 b | 1.86 ± 0.61 a |
| Total ketones | 2.05 | 1.78 | 24.46 | ||
| 51 | 1856 | Hexanoic acid | 1.10 ± 0.22 b | 0.80 ± 0.59 b | 3.97 ± 0.64 a |
| 52 | 2073 | Octanoic acid | 0.43 ± 0.19 | 0.32 ± 0.19 | 0.64 ± 0.10 |
| Total carboxylic acids | 1.53 | 1.12 | 4.61 | ||
| 53 | 1081 | Hexanal | 0.18 ± 0.02 b | 0.13 ± 0.02 b | 0.54 ± 0.09 a |
| 54 | 1393 | Nonanal | 0.17 ± 0.02 b | 0.19 ± 0.03 b | 2.05 ± 0.35 a |
| 55 | 1644 | 2-Decenal | 0.36 ± 0.04 b | 0.43 ± 0.12 b | 1.49 ± 0.11 a |
| Total aldehydes | 0.71 | 0.75 | 4.08 | ||
| 56 | 1333 | tert-Pentylbenzene | 0.96 ± 0.11 a | 0.49 ± 0.04 b | nd |
| 57 | 1401 | Tetradecane | 0.31 ± 0.17 | 0.25 ± 0.03 | 0.43 ± 0.12 |
| 58 | 1451 | 1,3,5,8-Undecatetraene | nd | 0.19 ± 0.06 b | 1.19 ± 0.46 a |
| Total hydrocarbons | 1.27 | 0.93 | 1.62 | ||
| Total identified (content, μg/100 mL) | 62.70 | 74.70 | 505.93 | ||
| Total identified (relative concentration, %) | 78.08 | 88.55 | 84.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asikin, Y.; Shimoda, K.; Takeuchi, M.; Maekawa, R.; Kamiyoshihara, Y.; Takara, K.; Wada, K. Free and Glycosidically Bound Volatile Compounds in Okinawan Pineapple (Ananas comosus). Appl. Sci. 2022, 12, 9522. https://doi.org/10.3390/app12199522
Asikin Y, Shimoda K, Takeuchi M, Maekawa R, Kamiyoshihara Y, Takara K, Wada K. Free and Glycosidically Bound Volatile Compounds in Okinawan Pineapple (Ananas comosus). Applied Sciences. 2022; 12(19):9522. https://doi.org/10.3390/app12199522
Chicago/Turabian StyleAsikin, Yonathan, Kazuki Shimoda, Makoto Takeuchi, Ryota Maekawa, Yusuke Kamiyoshihara, Kensaku Takara, and Koji Wada. 2022. "Free and Glycosidically Bound Volatile Compounds in Okinawan Pineapple (Ananas comosus)" Applied Sciences 12, no. 19: 9522. https://doi.org/10.3390/app12199522
APA StyleAsikin, Y., Shimoda, K., Takeuchi, M., Maekawa, R., Kamiyoshihara, Y., Takara, K., & Wada, K. (2022). Free and Glycosidically Bound Volatile Compounds in Okinawan Pineapple (Ananas comosus). Applied Sciences, 12(19), 9522. https://doi.org/10.3390/app12199522








