Characterisation of Olive Oils from the Douro Valley, Portugal: Study of the Volatile Fraction and Its Relationship with Sensory Characteristics
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sensory Analysis
2.3. Volatile Compounds
2.4. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compounds
3.2. Sensory Analysis
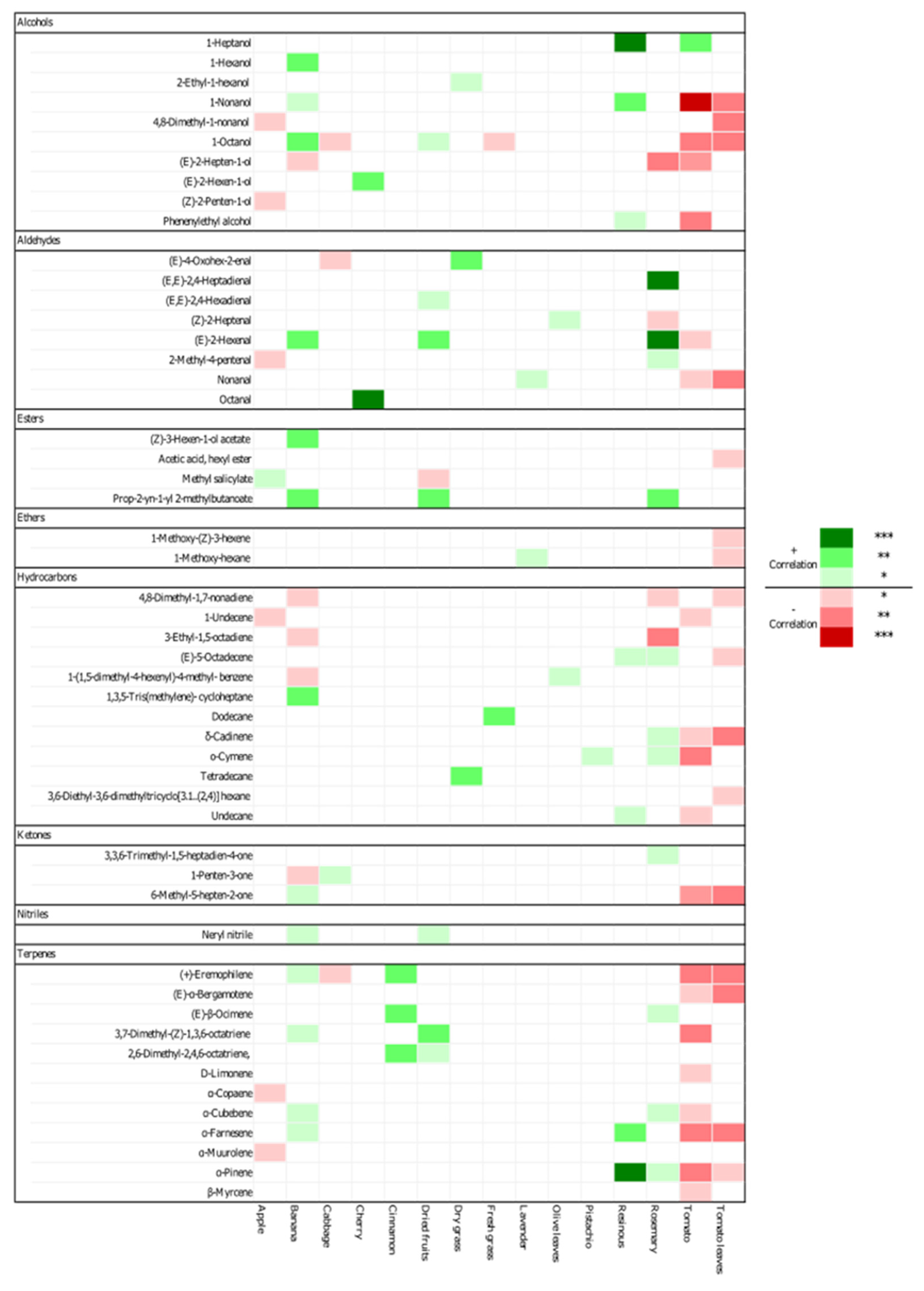
3.3. Relationship between Volatile Compounds and Sensory Attributes
3.4. Assessment of the Possible Separation of Olive Oils into Sub-Regions, Considering Their Volatile Profile and Sensory Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, C.; Belaj, A.; Sánchez-Ortiz, A. Natural Variation of Volatile Compounds in Virgin Olive Oil Analysed by HS-SPME/GC-MS-FID. Separations 2018, 5, 24. [Google Scholar] [CrossRef]
- Aprea, E.; Gasperi, F.; Betta, E.; Sani, G.; Cantini, C. Variability in Volatile Compounds from Lipoxygenase Pathway in Extra Virgin Olive Oils from Tuscan Olive Germoplasm by Quantitative SPME/GC-MS. J. Mass Spectrom. 2018, 53, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Kotti, F.; Jaziri, K.; Arab, F.; Mater, Y.; Sifi, S.; Fares, N.; Hammami, M.; Gargouri, M. Lipoxygenase: Optimisation of Extraction and Evaluation of Its Contribution to Virgin Olive Oil Aroma. Food Biotechnol. 2010, 24, 95–105. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile Compounds in Virgin Olive Oil: Occurrence and Their Relationship with the Quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Arafat, S.; Azza, A.A. Relationship between Volatile Compounds of Olive Oil and Sensory Attributes. Int. Food Res. J. 2013, 20, 197–204. [Google Scholar]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 Varietal Virgin Olive Oils by Their Volatile Compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of Agroclimatic Parameters on Phenolic and Volatile Compounds of Chilean Virgin Olive Oils and Characterization Based on Geographical Origin, Cultivar and Ripening Stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef]
- Chiappetta, A.; Benincasa, C.; Muzzalupo, I. Transcript Levels of LOX Gene and Volatile Compounds Content in Olive (Olea europea L.) Pericarps and Olive Oils: A Comparative Study on Twenty-Five Olive Cultivars Harvested at Two Ripening Stages. Acta Hortic. 2015, 1099, 577–586. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; la Greca, M.; Fregapane, G. Phenolic and Volatile Compounds of Extra Virgin Olive Oil (Olea europaea L. Cv. Cornicabra) with Regard to Fruit Ripening and Irrigation Management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex Interactive Effects of Ripening Degree, Malaxation Duration and Temperature on Oblica Cv. Virgin Olive Oil Phenols, Volatiles and Sensory Quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Servili, M. Characterization of Phenolic and Volatile Composition of Extra Virgin Olive Oil Extracted from Six Italian Cultivars Using a Cooling Treatment of Olive Paste. LWT 2018, 87, 523–528. [Google Scholar] [CrossRef]
- Veillet, S.; Tomao, V.; Bornard, I.; Ruiz, K.; Chemat, F. Chemical Changes in Virgin Olive Oils as a Function of Crushing Systems: Stone Mill and Hammer Crusher. Comptes Rendus Chim. 2009, 12, 895–904. [Google Scholar] [CrossRef]
- Caponio, F.; Summo, C.; Paradiso, V.M.; Pasqualone, A. Influence of Decanter Working Parameters on the Extra Virgin Olive Oil Quality. Eur. J. Lipid Sci. Technol. 2014, 116, 1626–1633. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; de Torres, A.; Moya, M.; Espínola, F. Centrifugation, Storage, and Filtration of Olive Oil in an Oil Mill: Effect on the Quality and Content of Minority Compounds. J. Food Qual. 2019, 2019, 7381761. [Google Scholar] [CrossRef]
- Masella, P.; Parenti, A.; Spugnoli, P.; Calamai, L. Influence of Vertical Centrifugation on Extra Virgin Olive Oil Quality. J. Am. Oil Chem. Soc. 2009, 86, 1137. [Google Scholar] [CrossRef]
- Sacchi, R.; Caporaso, N.; Paduano, A.; Genovese, A. Industrial-Scale Filtration Affects Volatile Compounds in Extra Virgin Olive Oil Cv. Ravece. Eur. J. Lipid Sci. Technol. 2015, 117, 2007–2014. [Google Scholar] [CrossRef]
- Tsartsou, E.; Proutsos, N.; Castanas, E.; Kampa, M. Network Meta-Analysis of Metabolic Effects of Olive-Oil in Humans Shows the Importance of Olive Oil Consumption With Moderate Polyphenol Levels as Part of the Mediterranean Diet. Front. Nutr. 2019, 6, 6. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, S.C.; Shoemaker, C.F. Volatile Constituents in Sensory Defective Virgin Olive Oils. Flavour Fragr. J. 2016, 31, 22–30. [Google Scholar] [CrossRef]
- COI/T.20/Doc. No.22; Method for the Organoleptic Assessment of Extra Virgin Olive Oil Applying to Use a Designation of Origin. 2005. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 4 August 2022).
- Rodrigues, N.; Casal, S.; Peres, A.M.; Baptista, P.; Pereira, J.A. Seeking for Sensory Differentiated Olive Oils? The Urge to Preserve Old Autochthonous Olive Cultivars. Food Res. Int. 2020, 128, 108759. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Rodrigues, N.; Renard, C.M.G.C.; Pereira, J.A. Volatile Changes in Cv. Verdeal Transmontana Olive Oil: From the Drupe to the Table, Including Storage. Food Res. Int. 2018, 106, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Garcia, B.; Magalhães, J.; Fregapane, G.; Salvador, M.D.; Paiva-Martins, F. Potential of Selected Portuguese Cultivars for the Production of High Quality Monovarietal Virgin Olive Oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1070–1082. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of Malaxation Conditions on Phenol and Volatile Profiles in Olive Paste and the Corresponding Virgin Olive Oils (Olea europaea L. Cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef]
- Blekas, G.; Guth, H. Evaluation and Quantification of Potent Odorants of Greek Virgin Olive Oils. Dev. Food Sci. 1995, 37, 419–427. [Google Scholar]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative Study of Virgin Olive Oil Sensory Defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin Olive Oil Odour Notes: Their Relationships with Volatile Compounds from the Lipoxygenase Pathway and Secoiridoid Compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Study of Volatile Compounds of Virgin Olive Oils with ‘Frostbitten Olives’ Sensory Defect. J. Agric. Food Chem. 2017, 65, 4314–4320. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Morales, M.T. Characterization of Olive Ripeness by Green Aroma Compounds of Virgin Olive Oil. J. Agric. Food Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Cultivar Influence on Virgin Olive (Olea europea L.) Oil Flavor Based on Aromatic Compounds and Sensorial Profile. Sci. Hortic. 2008, 118, 139–148. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, M.V. Relationship between Volatile Compounds and Sensory Attributes of Olive Oils by the Sensory Wheel. J. Am. Oil Chem. Soc. 1996, 73, 1253–1264. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of Volatile Compounds on Virgin Olive Oil Quality Evaluated by Analytical Approaches and Sensor Panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Cerretani, L.; Salvador, M.D.; Bendini, A.; Fregapane, G. Relationship between Sensory Evaluation Performed by Italian and Spanish Official Panels and Volatile and Phenolic Profiles of Virgin Olive Oils. Chemosens. Percept. 2008, 1, 258–267. [Google Scholar] [CrossRef]
- Temime, S.B.; Campeol, E.; Cioni, P.L.; Daoud, D.; Zarrouk, M. Volatile Compounds from Chétoui Olive Oil and Variations Induced by Growing Area. Food Chem. 2006, 99, 315–325. [Google Scholar] [CrossRef]
- Issaoui, M.; Hassine, K.B.; Flamini, G.; Brahmi, F.; Chehab, H.; Aouni, Y.; Mechri, B.; Zarrouk, M.; Hammami, M. Discrimination of Some Tunisian Olive Oil Varieties Compounds and Chemometric Analysis. J. Food Lipids 2009, 16, 164–186. [Google Scholar] [CrossRef]
- Cecchi, T.; Alfei, B. Volatile Profiles of Italian Monovarietal Extra Virgin Olive Oils via HS-SPME–GC–MS: Newly Identified Compounds, Flavors Molecular Markers, and Terpenic Profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Luna, G. Characterisation of Monovarietal Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Casadei, E.; Valli, E.; Aparicio-Ruiz, R.; Ortiz-Romero, C.; García-González, D.L.; Vichi, S.; Quintanilla-Casas, B.; Tres, A.; Bendini, A.; Toschi, T.G. Peer Inter-Laboratory Validation Study of a Harmonized SPME-GC-FID Method for the Analysis of Selected Volatile Compounds in Virgin Olive Oils. Food Control 2021, 123, 107823. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and Off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- COI Analyse Sensorielle de L’huile D’olive: Méthode D’évaluation Organoleptique de L’huile D’olive. 2018. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/05/COI-T20-Doc.-15-REV-10-2018-Fr.pdf (accessed on 4 August 2022).
- Rodrigues, N.; Peres, A.M.; Baptista, P.; Pereira, J.A. Olive Oil Sensory Analysis as a Tool to Preserve and Valorise the Heritage of Centenarian Olive Trees. Plants 2022, 11, 257. [Google Scholar] [CrossRef]
- Malheiro, R.; Rodrigues, N.; Bissaro, C.; Leimann, F.; Casal, S.; Ramalhosa, E.; Pereira, J.A. Improvement of Sensorial and Volatile Profiles of Olive Oil by Addition of Olive Leaves. Eur. J. Lipid Sci. Technol. 2017, 119, 1700177. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Jimenez, A.; Sanchez-Villasclaras, S.; Uceda, M.; Beltran, G. Modulation of Bitterness and Pungency in Virgin Olive Oil from Unripe “Picual” Fruits. Eur. J. Lipid Sci. Technol. 2015, 117, 1463–1472. [Google Scholar] [CrossRef]
- Herrera, B.J.; Velasco, A.R.; Sánchez-Ortiz, A.; Tovar, M.L.L.; Muñoz, M.Ú.; Callejón, R.M.; De Quirós, E.O.B. Influencia del Proceso de Maduración del Fruto en La Calidad Sensorial de Aceites de Oliva Virgen de las Variedades Picual, Hojiblanca y Picudo. Grasas Aceites 2012, 63, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Ruiz, R.; García-González, D.L.; Morales, M.T.; Lobo-Prieto, A.; Romero, I. Comparison of Two Analytical Methods Validated for the Determination of Volatile Compounds in Virgin Olive Oil: GC-FID vs GC-MS. Talanta 2018, 187, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Dorota, D.; Rupert, M.; Wołosiak, R.; Bzducha-Wróbel, A.; Ścibisz, I.; Matuszewska-Janica, A. Volatiles as Markers of Bioactive Components Found in Croatian Extra Virgin Olive Oils. LWT 2021, 139, 110532. [Google Scholar] [CrossRef]
- Sacchi, R.; Caporaso, N.; Squadrilli, G.A.; Paduano, A.; Ambrosino, M.L.; Cavella, S.; Genovese, A. Sensory Profile, Biophenolic and Volatile Compounds of an Artisanal Ice Cream (‘Gelato’) Functionalised Using Extra Virgin Olive Oil. Int. J. Gastron. Food Sci. 2019, 18, 100173. [Google Scholar] [CrossRef]
- Jones, G.V.; Alves, F. Impact of Climate Change on Wine Production: A Global Overview and Regional Assessment in the Douro Valley of Portugal. Int. J. Glob. Warm. 2012, 4, 383–406. [Google Scholar] [CrossRef]
- Martins, J.; Fraga, H.; Fonseca, A.; Santos, J.A. Climate Projections for Precipitation and Temperature Indicators in the Douro Wine Region: The Importance of Bias Correction. Agronomy 2021, 11, 990. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Sacchi, R. Flavor Chemistry of Virgin Olive Oil: An Overview. Appl. Sci. 2021, 11, 1639. [Google Scholar] [CrossRef]
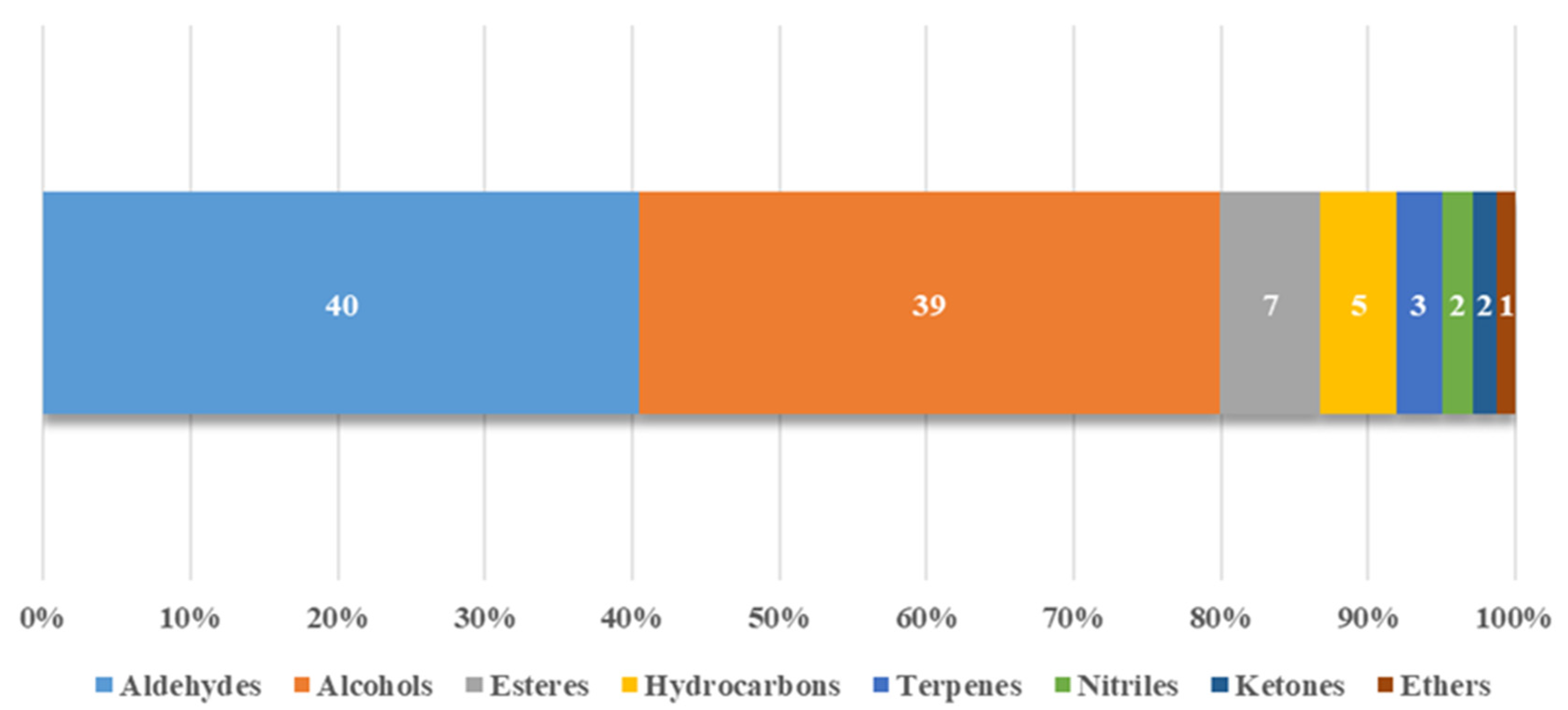

| Volatile Compounds | % of Samples | Concentration (µg/g) | Identification a |
|---|---|---|---|
| Alcohols | |||
| 1-Heptanol | 7 | 0.3 ± 0.2 (0.2–0.7) | MS/IK.DB |
| 1-Hexanol | 89 | 44.4 ± 71.8 (1.4–516.9) | MS/IK.DB |
| 2-Ethyl-1-hexanol | 7 | 0.7 ± 0.3 (0.4–1.2) | MS/IK.DB |
| 1-Nonanol | 91 | 0.7 ± 0.5 (0.1–2.5) | MS/IK.DB |
| 4,8-Dimethyl-1-nonanol | 30 | 0.4 ± 0.2 (0.2–0.8) | MS/IK.DB |
| 1-Octanol | 96 | 0.8 ± 0.5 (0.3–2.9) | MS/IK.DB |
| (E)-2-Hepten-1-ol | 28 | 1.3 ± 0.5 (0.6–2.3) | MS/IK.DB |
| (E)-2-Hexen-1-ol | 19 | 105.4 ± 148.4 (1.9–532.5) | MS/IK.DB |
| (Z)-2-Penten-1-ol | 100 | 4.6 ± 3.8 (1.3–27.0) | MS/IK.DB |
| (Z)-3-Hexen-1-ol | 16 | 156.8 ± 108.6 (62.9–439.9) | MS/IK.DB |
| Phenylethyl Alcohol | 95 | 0.6 ± 0.3 (0.3–1.7) | MS/IK.DB |
| Aldehydes | |||
| (E)-4-Oxohex-2-enal | 96 | 6.0 ± 4.9 (0.6–22.9) | MS/IK.DB |
| (E,E)-2,4-Heptadienal | 32 | 1.2 ± 0.3 (0.7–1.8) | MS/IK.DB |
| (E,E)-2,4-Hexadienal | 96 | 4.3 ± 2.0 (1.3–8.9) | MS/IK.DB |
| (Z)-2-Heptenal | 30 | 0.9 ± 0.6 (0.3–2.6) | MS/IK.DB |
| (E)-2-Hexenal | 100 | 258.9 ± 187.4 (5.3–781.1) | MS/IK.DB |
| (E)-2-Tridecenal | 53 | 0.3 ± 0.2 (0.1–1.0) | MS/IK.DB |
| 2-Methyl-4-pentenal | 100 | 37.7 ± 21.7 (2.8–94.4) | MS/IK.DB |
| Nonanal | 100 | 11.5 ± 6.9 (1.2–32.8) | MS/IK.DB |
| Octanal | 5 | 2.8 ± 0.3 (2.5–3.2) | MS/IK.DB |
| Esters | |||
| (Z)-3-Hexen-1-ol acetate | 100 | 45.0 ± 78.2 (2.3–582.5) | MS/IK.DB |
| Acetic acid, hexyl ester | 96 | 5.6 ± 4.9 (0.6–26.5) | MS/IK.DB |
| (E)-Hex-3-enyl butyrate | 7 | 0.3 ± 0.1 (0.1–0.4) | MS/IK.DB |
| Methyl salicylate | 60 | 0.3 ± 0.2 (0.1–0.9) | MS/IK.DB |
| Prop-2-yn-1-yl 2-methylbutanoate | 91 | 3.3 ± 1.7 (0.9–9.6) | MS |
| Ethers | |||
| (Z)-1-Methoxy-3-hexene | 91 | 7.0 ± 4.4 (1.4–18.6) | MS |
| 1-Methoxy-hexane | 63 | 3.4 ± 1.4 (1.5–6.5) | MS/IK.BD |
| Hydrocarbons | |||
| 1,2,4-Metheno-1H-indene, octahydro-1,7a-dimethyl-5-(1-methylethyl)-, [1S-(1.alpha.,2.alpha.,3a.beta.,4.alpha.,5.alpha.,7a.beta.,8s*)] | 70 | 0.8 ± 0.7 (0.1–3.3) | MS/IK.DB |
| 4,8-Dimethyl-1,7-nonadiene | 30 | 4.1 ± 1.6 (1.6–8.7) | MS |
| 1-Undecene | 5 | 1.3 ± 0.4 (0.7–1.7) | MS/IK.DB |
| 3-Ethyl-1,5-octadiene | 100 | 16.0 ± 14.3 (1.5–56.5) | MS/IK.DB |
| (E)-5-Octadecene | 100 | 11.8 ± 10.8 (1.4–43.0) | MS |
| 1-(1,5-Dimethyl-4-hexenyl)-4-methyl-benzene | 25 | 0.4 ± 0.3 (0.1–1.0) | MS/IK.DB |
| 1,3,5-Tris(methylene)-cycloheptane | 95 | 0.8 ± 0.5 (0.2–2.4) | MS |
| 1,1-Dimethyl-2-(1-methyl-2-propenyl)-cyclopropane, | 11 | 2.9 ± 1.6 (0.3–5.3) | MS/IK.DB |
| Dodecane | 67 | 0.4 ± 0.5 (0.1–3.2) | MS/IK.DB |
| (1S-Z)-Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl) | 67 | 0.6 ± 0.3 (0.2–1.6) | MS/IK.DB |
| 2,2,6-Trimethyl-octane | 5 | 0.5 ± 0.3 (0.2–0.9) | MS/IK.DB |
| o-Cymene | 9 | 1.0 ± 0.6 (0.4–1.9) | MS/IK.DB |
| Tetradecane | 5 | 0.3 ± 0.1 (0.2–0.5) | MS/IK.DB |
| 3,6-Diethyl-3,6-dimethyltricyclo[3.1.0.02,4]hexane | 21 | 0.2 ± 0.1 (0.1–0.4) | MS |
| Undecane | 39 | 0.5 ± 0.1 (0.3–0.8) | MS/IK.DB |
| Ketones | |||
| 3,3,6-Trimethyl-1,5-heptadien-4-one | 72 | 1.3 ± 0.6 (0.3–3.5) | MS/IK.DB |
| 1-Penten-3-one | 68 | 3.9 ± 3.3 (0.9–16.8) | MS/IK.DB |
| 2-Heptanone | 5 | 0.6 ± 0.1 (0.4–0.8) | MS/IK.DB |
| 3-Pentanone | 56 | 5.5 ± 2.7 (1.8–17.0) | MS/IK.DB |
| 6-Methyl-5-hepten-2-one | 95 | 1.6 ± 0.8 (0.2–4.2) | MS/IK.DB |
| Nitriles | |||
| Neryl nitrile | 100 | 16.7 ± 9.9 (1.2–48.2) | MS |
| Terpenes | |||
| (+)-Eremophilene | 93 | 0.8 ± 0.6 (0.1–2.6) | MS/IK.DB |
| (E)-α-Bergamotene | 32 | 0.4 ± 0.4 (0.1–1.9) | MS/IK.DB |
| (E)-β-Ocimene | 51 | 0.3 ± 0.1 (0.1–0.7) | MS/IK.DB |
| (Z)-3,7-Dimethyl-1,3,6-octatriene | 100 | 6.7 ± 3.7 (1.2–18.1) | MS/IK.DB |
| 2,6-Dimethyl-2,4,6-octatriene | 98 | 0.5 ± 0.3 (0.1–1.5) | MS/IK.DB |
| α-Bergamotene | 47 | 0.6 ± 0.4 (0.2–1.8) | MS/IK.DB |
| α-Copaene | 100 | 4.5 ± 3.5 (0.8–19.7) | MS/IK.DB |
| α-Cubebene | 75 | 0.7 ± 0.4 (0.2–1.8) | MS/IK.DB |
| α-Farnesene | 100 | 3.0 ± 2.2 (0.4–11.3) | MS/IK.DB |
| α-Muurolene | 70 | 0.6 ± 0.6 (0.1–2.7) | MS/IK.DB |
| α-Pinene | 26 | 3.2 ± 4.1 (0.3–15.2) | MS/IK.DB |
| Caryophyllene | 60 | 0.3 ± 0.2 (0.1–1.0) | MS/IK.DB |
| D-Limonene | 39 | 1.8 ± 2.8 (0.3–11.9) | MS/IK.DB |
| β-Myrcene | 14 | 1.3 ± 1.3 (0.4–4.5) | MS/IK.DB |
| Attributes | % Incidence of Attribute | Median | Min–Max |
|---|---|---|---|
| Olfactory | |||
| Fruity | |||
| Ripe Fruity | 65 | 5.1 | 2.6–8.0 |
| Green Fruity | 35 | 4.4 | 1.8–7.1 |
| Positive attributes | |||
| Lavender | 16 | 2.5 | 1.1–3.4 |
| Apricot | 4 | 3.9 | 3.2–4.6 |
| Banana | 54 | 2.6 | 1.4–5.1 |
| Cinnamon | 2 | 2.9 | 2.9–2.9 |
| Cherry | 9 | 2.9 | 2.2–3.3 |
| Cabbage | 30 | 3.7 | 1.1–6.2 |
| Dry grass | 33 | 3.2 | 0.6–4.8 |
| Olive leaves | 4 | 2.8 | 2.7–2.9 |
| Dried fruits | 98 | 3.0 | 1.1–5.6 |
| Kiwi | 5 | 3.5 | 0.8–4.3 |
| Apple | 77 | 3.5 | 1.3–6.5 |
| Pistachio | 4 | 2.5 | 1.7–3.2 |
| Tomato leaves | 56 | 3.7 | 1.2–5.6 |
| Fresh grass | 37 | 3.7 | 0.9–5.4 |
| Resinous | 2 | 2.5 | 2.5–2.5 |
| Rosemary | 32 | 2.8 | 1.5–5.3 |
| Tomato | 84 | 3.7 | 1.2–6.0 |
| Harmony | 7.0 | 4.6–8.9 | |
| Gustatory | |||
| Fruity | |||
| Ripe Fruity | 63 | 5.3 | 1.1–7.8 |
| Green Fruity | 39 | 4.2 | 1.9–7.5 |
| Positive attributes | |||
| Lavender | 12 | 2.7 | 1.6–3.4 |
| Apricot | 4 | 2.5 | 1.9–3.0 |
| Banana | 81 | 2.7 | 0.7–6.0 |
| Vanilla | 2 | 4.2 | 4.2–4.2 |
| Cherry | 16 | 2.8 | 1.6–4.7 |
| Cabbage | 30 | 3.7 | 1.4–7.3 |
| Dry grass | 33 | 3.5 | 0.7–6.1 |
| Wildflowers | 2 | 1.8 | 1.8–1.8 |
| Fig leaves | 2 | 4.2 | 4.2–4.2 |
| Olive leaves | 9 | 2.6 | 1.2–4.4 |
| Dried fruits | 100 | 3.0 | 0.8–5.6 |
| Kiwi | 5 | 2.0 | 0.6–3.7 |
| Apple | 74 | 3.5 | 1.1–6.3 |
| Pistachio | 4 | 2.1 | 1.8–2.4 |
| Tomato leaves | 58 | 3.5 | 1.1–4.8 |
| Fresh grass | 42 | 2.8 | 0.6–5.7 |
| Resinous | 2 | 3.1 | 3.1–3.1 |
| Rosemary | 37 | 2.8 | 1.3–5.1 |
| Tomato | 82 | 4.0 | 1.2–5.8 |
| Sweet | 4.2 | 1.3–6.4 | |
| Bitter | 1.9 | 0.9–5.0 | |
| Pungent | 2.4 | 0.7–5.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.; Rodrigues, N.; Pereira, J.A.; Ramalhosa, E. Characterisation of Olive Oils from the Douro Valley, Portugal: Study of the Volatile Fraction and Its Relationship with Sensory Characteristics. Appl. Sci. 2022, 12, 9246. https://doi.org/10.3390/app12189246
Silva K, Rodrigues N, Pereira JA, Ramalhosa E. Characterisation of Olive Oils from the Douro Valley, Portugal: Study of the Volatile Fraction and Its Relationship with Sensory Characteristics. Applied Sciences. 2022; 12(18):9246. https://doi.org/10.3390/app12189246
Chicago/Turabian StyleSilva, Kevin, Nuno Rodrigues, José Alberto Pereira, and Elsa Ramalhosa. 2022. "Characterisation of Olive Oils from the Douro Valley, Portugal: Study of the Volatile Fraction and Its Relationship with Sensory Characteristics" Applied Sciences 12, no. 18: 9246. https://doi.org/10.3390/app12189246
APA StyleSilva, K., Rodrigues, N., Pereira, J. A., & Ramalhosa, E. (2022). Characterisation of Olive Oils from the Douro Valley, Portugal: Study of the Volatile Fraction and Its Relationship with Sensory Characteristics. Applied Sciences, 12(18), 9246. https://doi.org/10.3390/app12189246










