Abstract
Hyperthermia is the use of heat to control Varroa destructor. Various apparatuses have been proposed to effectively apply heat and recently the Bee Ethic system was developed in Italy. The Bee Ethic system is a technological hive consisting of a set of heated frames and an electronic control unit. Trials were carried out in the years 2018, 2020 and 2021 to compare colony strength and mite infestation in heat-treated and untreated control bee-hives. In addition, the influence of repeated heat treatments on the development of bee colonies and mite populations was verified by means of a mathematical model. Both in apiary and in silica results show that hyperthermia can be effectively used for V. destructor control within an IPM approach, even in the presence of substantial re-infestation phenomena.
1. Introduction
Varroa destructor can be considered the major pest of honey bees worldwide [1,2]; in addition to the direct damage it causes to adult bees and brood, it is responsible for the spread of numerous viruses and is one of the main causes of colony loss [3]. A correlation has been observed between the presence of V. destructor and infections with deformed wing virus and acute paralysis virus, which are often the cause of colony collapse [4]. Moreover, colony losses, directly or indirectly due to V. destructor infestation, are generally more severe in autumn and winter [5].
Despite the fact that many control methods have been proposed over the years, varroa control is particularly difficult and costly for beekeepers. The use of chemical acaricides has been supplemented by the use of products with a low environmental impact, in particular essential oils and organic acids. Oxalic acid and thymol have demonstrated good efficacy, although they show a strong variability of action, suggesting the use of an integrated pest management (IPM) approach. It is now generally accepted that in order to limit the damage caused by varroa, such an approach should be adopted to minimize the use of acaricides [6,7]; despite this, in current practice, beekeepers still tend to rely mainly on chemical control measures.
Several studies have shown that heat can damage or kill V. destructor [8]. The practice of using heat for varroa control is called thermotherapy or hyperthermia and involves heating the whole bee-hive, the bees or the sealed brood alone, depending on how the hyperthermia is applied, to a temperature that is harmless to the bees but fatal to varroa. This is possible because honey bees and varroa have different sensitivities to heat. Juvenile forms of honey bee, such as larvae, can tolerate up to 42–43 °C while adults can tolerate up to 48 °C; therefore, if they are exposed for a sufficiently long time to temperatures above 40 °C, they are not harmed, unlike the mites, which are killed. The time required to kill or seriously damage most of the mites is inversely proportional to temperature; times up to eight hours were applied in various experiments without hampering adult bee and brood survival [8,9], even extending worker lifespan [10].
In the 1980s, the first devices capable of applying hyperthermia were developed. These were thermal boxes (Hanko thermocube, thermo bell and thermobox) that utilized different methods of heating adult bees to temperatures above 45 °C in order to kill varroa mites in the phoretic phase. These devices could only be used for diagnostic purposes and also had obvious limitations in their use since the entire hive had to be moved inside the heat boxes. Subsequently, devices able to apply heat to the brood were designed, thus succeeding in targeting the parasite during the reproductive phase. During the brood rearing season, most varroa mites are in the brood cells to reproduce, so heat treatment of the brood keeps infestation levels low [11]. Adult bees in the combs react to heat with intense ventilation in order to cool the nest when brood temperature exceeds 36 °C. This suggested the removal of adult bees from the combs to avoid significant bee losses. In 1991, the first thermo-solar hive in history was developed, which was followed by the recent Rašnov thermo-solar hive [11]; the results obtained with this solution have shown high effectiveness, though it has a major limitation as it can only be used in the hottest period of the year, with temperatures above 20 °C. In order to apply thermotherapy under cooler conditions, the Varroa Terminator was developed; it is an electronic device consisting of two electrically heated boards that are placed above and below the hive. Once the device is switched on, the temperature inside the hive rises rapidly and the mites, as a result of falling off the bees, die on contact with the hot surface of the lower panel [12]. In 2006, the Thermovar was developed in Greece; this device performs heat treatment by gradually heating the air inside the system and distributing it among the combs of the hive. However, long treatment times are required to effectively affect varroa in both the reproductive and phoretic phases [8]. The Thermovar, like the other devices described, heats the colony by feeding heat from outside into the hive, failing to consistently heat the brood due to the ventilation carried out by adult bees.
The next step in the evolution of thermotherapy was the development of devices capable of heating hives directly from the inside; in the United States, the Mite Zapper was developed, the first device able to do so. It is made up of a male brood comb with heating elements incorporated in the wax that generate heat when connected to a source of electricity [13]. The device has shown high efficacy, but treating only male brood does not allow a low level of parasite infestation to be maintained throughout the season, as bees no longer raise male brood towards the end of the summer.
In order to maintain a low infestation as long as possible throughout the season with thermotherapy, heat must be applied to worker brood also. In Switzerland, starting in 2015, a technique was developed to heat the hive from the inside by treating only the female brood using an electrically powered device called the Vatorex, which consists of a thermal comb with an electrical heating element built into the wax. The electrical resistance is connected to a distributor (control unit) that regulates the flow of current to the connected combs. The method involves heating one comb at a time at a temperature of 42 °C for 3 h [14]. The limitations of this device are the heating element, which covers only the central area of the comb, as well as the treatment time, as only one comb is heated at a time.
In 2015, independently from Swiss developments, a device called Bee Ethic was marketed in Italy, equipped, like the Vatorex system, with a thermal comb. This solution differs from the previous ones in terms of its electrical resistance, which is incorporated in the foundation; the electrical resistance is distributed over the entire surface of the comb and not only in its central part, and all the brood combs are equipped with resistance and heated simultaneously.
The efficacy and functionality of the Bee Ethic system were preliminarily tested in 2014, 2015 and 2016 in Umbria (central Italy) on a hundred colonies to define the best ways of using this technology (unpublished results). Subsequently, in the years 2018, 2020 and 2021, further research was carried out in Piedmont (north-western Italy) to further investigate the effects of hyperthermia on bees and varroa. Based on the information collected in the apiary, simulations were then carried out with a well-known mathematical model of the hive that was suitably modified for the specific conditions of hyperthermia. The present work reports the experimental results obtained in Piedmont and what emerged from the simulations developed using the mathematical model.
2. Materials and Methods
2.1. The Bee Ethic System
The Bee Ethic system is a technological hive consisting of a set of heated frames and an electronic control unit integrated in the frame. Bee Ethic has been recognized and financed through Reg 1308/2013-OCM Miele as a valid biotechnology against Varroa by several Italian regions.
Each Bee Ethic hive kit consists of two stainless steel spacers and eight frames with a thermal foundation (Figure 1a). One of the frames (probe frame) includes a microchip (Figure 1b) and must be placed in the hive in a central position (Figure 1c). The foundations are supplied with wax or are to be waxed using a specially designed and manufactured mold (Figure 1d). Brood combs are readily built by honey bees on such foundations (Figure 1e).

Figure 1.
The Bee Ethic system: (a) hive kit: two stainless steel spacers and eight thermal frames; (b) the microchip inserted in the probe frame; (c) the thermal frames in the hive with the one with the microchip in central position; (d) mold for waxing thermal foundations; (e) the microchip and sensors integrated in the probe frame; (f) the main parameters of a hive as shown by BEE-SMART control unit; (g) 24-volt AC/DC transformer to connect the Bee Ethic system to a socket; (h) photovoltaic panel; (i) apiary not served by the electric network.
Thanks to the microchip integrated in the probe frame, the Bee Ethic hive automatically carries out heat treatment every 25 days, bringing the brood temperature to 42 °C for 90 min. Moreover, various sensors and the BEE-SMART control unit allow the beekeeper to remotely check the main parameters of each hive—brood and air temperature inside the hive, as well as humidity and sound frequency—on top of ambient temperature and humidity (Figure 1f).
2.2. Apiary Trials
All experiments were carried out in 2018, 2020 and 2021 at the DISAFA (University of Turin) apiary located in Grugliasco (Turin District) (45°03′59′′ N 7°35′33′′ E). Dadant–Blatt hives with ten frames, eight Bee Ethic frames and two standard frames placed on the sides of the hive were used to carry out the heat treatments; honey supers were added when necessary to store surplus honey. The results obtained were compared with those obtained in identical Dadant–Blatt hives, equipped with standard combs, used as controls; these bee-hives were subjected to standard varroa control practices using trickled oxalic acid and amitraz strips.
All hives were standardized during spring in terms of colony strength and level of varroa infestation by intermixing brood frames and workers between hives so that they were as comparable as possible at the start of hyperthermia treatments. The first treatments were applied on 23 and 18 May in 2018 and 2021, respectively; in 2020 it was delayed to 25 June due to lockdown measures introduced in Italy to counteract the spread of COVID-19. Heat treatments were repeated every three weeks until mid-October.
Varroa infestation trends were assessed in 21 hives equipped with the Bee Ethic device (6 hives in 2018, 10 in 2020 and 5 in 2021) and the same number of control hives.
For all hives, both those heat-treated with the Bee Ethic system and the untreated control hives, colony strength was assessed periodically (at one- or two-week intervals in 2018, every three weeks in 2020, and weekly in 2021) using a modified Liebefeld method to evaluate worker and brood cell numbers [15]. Moreover, the varroa mites that fell on the bottom boards were counted weekly all three years [16,17].
To assess the effectiveness of hyperthermia on the reproductive phase of varroa, two brood combs from each of the heat-treated colonies were brought to the laboratory 48 h after every treatment. Here, 300 cells per hive (150 per comb) were uncapped along the diagonals of both sides of the two combs, using the sampling method developed by Floris [18] (Figure 2). The number of live and dead individuals of V. destructor present in the cells was counted, recording the number of adult females, males and juveniles separately.

Figure 2.
Evaluation of effectiveness of hyperthermia by counting the number of live and dead individuals of Varrosa destructor present in the cells.
2.3. Mathematical Model Simulations
In addition to the results of the apiary trials, the influence of repeated heat treatments on the development of bee colonies and varroa populations was verified by means of a mathematical model. To this end, the well-known mathematical model BEEHAVE [19] was modified in 2019 to include the effect of thermotherapy. In the software simulation we carried out, we added a value indicating the number of mites present at the beginning of the season, a parameter indicating daily re-infestation, and a parameter indicating the number of treatments carried out with efficacy both within the cell and on the phoretic mites (see Supplementary Materials S1 for details).
We adopted the weather conditions of the city of Turin during 2018 and rather conservative values for adult and juvenile V. destructor mortality parameters, in line with the experimental data obtained during the apiary trials (see Supplementary Materials S2 for details). Moreover, the presence of 40 V. destructor adult females (20 healthy and 20 infected by deformed wing virus) at the beginning of the year was included. Apart from the above-mentioned settings, BEEHAVE was run under default conditions in order to simulate the trends of colony and parasite population dynamics over the season.
3. Results
3.1. Apiary Trials
The colonies used in the three years of trials developed normally, as can be seen from the graphs shown in Figure 3 that refer to the number of worker cells, without any noticeable differences between the heat-treated hives and the control hives. The number of adult workers followed similar trends.
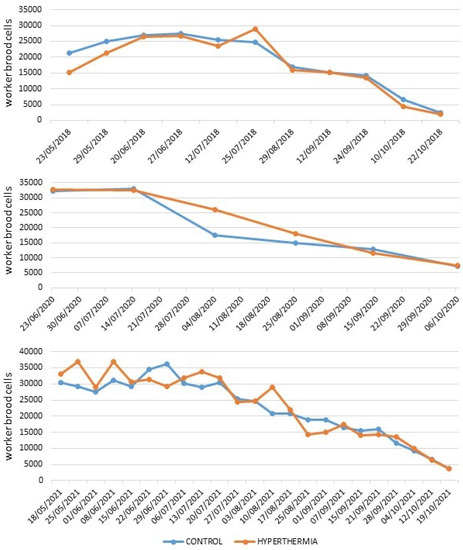
Figure 3.
Average number of worker brood cells in the beehives used in 2018 (above), 2020 (middle), and 2021 (below) tests.
With regard to the presence of varroa, the surveys carried out in 2018 (Figure 4) showed that until the end of July, infestation levels were rather low in the heat-treated bee-hives, since no juvenile forms of the parasite were found in sealed brood-cells and few adults fell on the bottom boards. A similar low degree of infestation was also found in the control colonies. Between the end of July and the beginning of August, the degree of infestation increased in the control colonies and consequently it was necessary to treat them with trickled oxalic acid thrice in August and with the insertion of amitraz strips starting from 12 September. On the contrary, hyperthermia allowed the development of the parasite to be kept under control until the end of September, as the average daily falls recorded meant that no intervention with acaricides was necessary.

Figure 4.
Average daily Varroa destructor mortality in 2018.
In 2020 (Figure 5), the level of varroa infestation was initially quite high in both the control and hyperthermia hives. Heat treatments reduced varroa fall on the bottom boards to around 4 mites/day for the entire season. On the contrary, the mite number in the control hives would have continued to increase exponentially had four treatments with trickled oxalic acid not been carried out in July. On 7 August, amitraz strips were introduced in the control hives and the infestation remained low in both the control and hyperthermia hives until the end of the season.
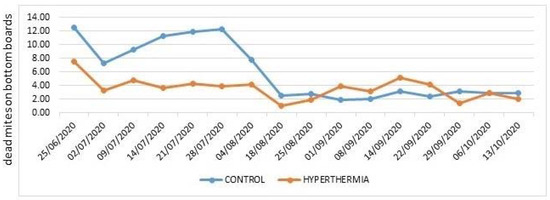
Figure 5.
Average daily Varroa destructor mortality in 2020.
In 2021 (Figure 6), trials began earlier than in the previous year, and infestation levels in the hives subjected to hyperthermia remained at modest levels until September, while in control hives, despite three treatments with trickled oxalic acid carried out between mid-July and early August, natural mite drop on the bottom boards increased alarmingly. No other acaricide treatment was carried out until late October in both the test and control hives.
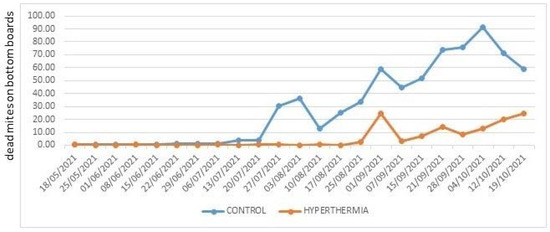
Figure 6.
Average daily Varroa destructor mortality in 2021.
In the brood cells examined after each heat treatment, a low mortality of adult mites was observed, whereas a large proportion of protonymphs and deutonymphs were killed by the adopted combination of time and temperature (Table 1).

Table 1.
Mortality of adult and juvenile forms of Varroa destructor within brood cells due to heat treatments.
3.2. Mathematical Model Simulations
The development of the colony during of the year was simulated using the BEEHAVE model with environmental parameters similar to those present in the apiary where the experiment was carried out. The output of a single run is shown in Figure 7.
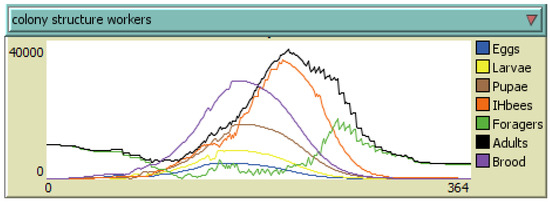
Figure 7.
Yearly development of worker population of a colony according to the BEEHAVE software.
The population dynamics of V. destructor were simulated under different control approaches and various re-infestation hypotheses.
The simulations for the control colonies not subjected to hyperthermia, under different hypotheses of re-infestation, showed high mite levels in the hives at the end of the year even in the case of summer treatment with oxalic acid (Figure 8).
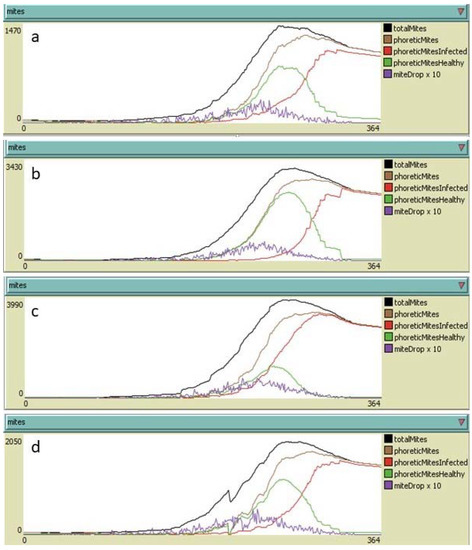
Figure 8.
Yearly development of Varroa destructor infestation in colonies not subjected to hyperthermia under different hypotheses of re-infestation: (a) no re-infestation; (b) 30 mites per month from June to September (120 mites in total); (c) 150 mites per month from June to September (600 mites in total); (d) 30 mites per month from June to September (120 mites in total) with a dripped oxalic acid treatment at the end of July with 95% efficacy on phoretic mites and efficacy duration of 1 day. Adult mites alive on 31 December: (a) 0; (b) 2283; (c) 2821; (d) 1422.
Simulating the use of hyperthermia leaded to the total disappearance of the infestation before the end of the year even if re-infestation occurred. In this case, the simulations showed infestation levels at the end of the year that were much lower than those of the control colonies without the need of summer treatments for the same level of re-infestations and even in more critical situations (Figure 9).
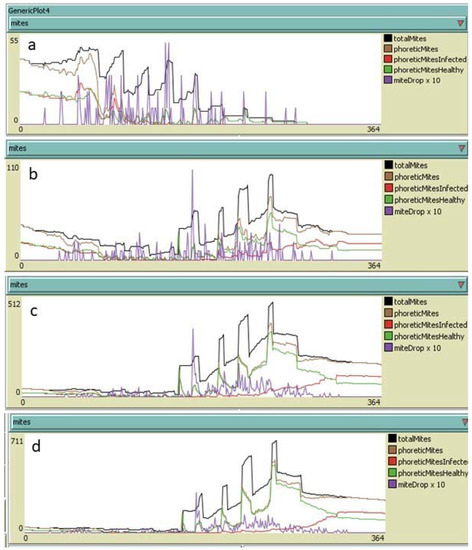
Figure 9.
Yearly development of Varroa destructor infestation in colonies subjected to nine heat treatments under different hypotheses of re-infestation: (a) no re-infestation; (b) 30 mites per month from June to September (120 mites in total); (c) 150 mites per month from June to September (600 mites in total); (d) 150 mites per month in June and July and 250 mites per month in August and September (800 mites in total). Adult mites alive on 31 December: (a) 0; (b) 120; (c) 158; (d) 242.
By adopting an integrated control approach (hyperthermia and summer treatments with oxalic acid), the simulations predicted very low levels of infestation at the end of the year, even in the presence of heavy re-infestation phenomena (Figure 10).
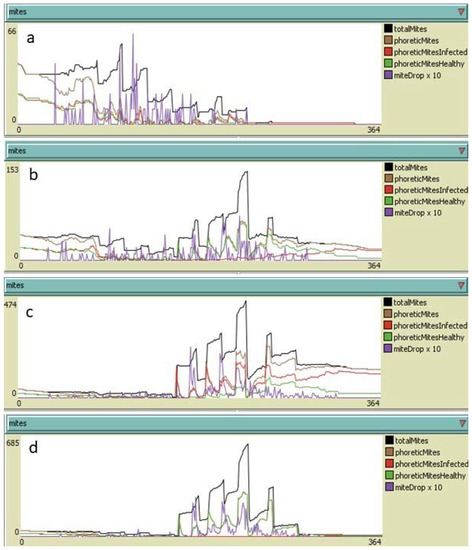
Figure 10.
Yearly development of Varroa destructor infestation in colonies subjected to nine heat treatments under different hypotheses of re-infestation and dripped oxalic acid treatment: (a) no re-infestation and a single acid oxalic treatment at the end of July; (b) 30 mites per month from June to September (120 mites in total) and a single oxalic acid treatment at the end of July; (c) 150 mites per month from June to September (600 mites in total) and a single oxalic acid treatment at the end of July; (d) 150 mites per month from June to September (600 mites in total) and one oxalic acid treatment per month from the end of July to the end of October (4 treatments). Adult mites alive on 31 December: (a) 0; (b) 18; (c) 133; (d) 12.
4. Discussion
Hyperthermia applied using the Bee Ethic system at three-week intervals from late spring to autumn kept V. destructor population at tolerable levels, whereas alarming increases in mite number were observed in untreated controls. Even if hyperthermia is a rather old approach to varroa control [9,11], most of the tests were carried out in the laboratory [9,10,20] and several reports of apiary trials are of anecdotal nature [11,13,14]. The experimental conditions adopted in our trials (42 °C for 90 min) were intermediate among those adopted in other studies and proved to be effective in varroa control for most of the season. In late summer and early autumn, sudden increases in V. destructor infestation rate were often observed and hyperthermia alone was no longer able to control mites to tolerable levels. It is difficult to decide if such increases were due to a lesser effectiveness of thermic treatments or to re-infestation, but the latter appears as the more sensible explanation since re-infestation is widespread and of relevant occurrence [21]. In any case, to turn to an IPM approach coupling hyperthermia with rather soft chemical treatments would be sufficient to keep mite infestation under control. It is worth mentioning that throughout the entire three-year testing period, average daily mite falls were lower in heat-treated colonies.
The results obtained from sealed brood inspections demonstrate that the main effect of heat treatments applied with the Bee Ethic system is on the reproductive phase of V. destructor, with the mortality of the juvenile forms ranging from 76% to 98%. On the contrary, the impact on adult mites was less positive as the maximum mortality recorded was only 45%. Adult and juvenile mite mortality in sealed brood cells has been reported as rather low [22] and some preliminary observations confirmed that this was also the case in our experimental hives. Such results are in accordance with the lethal temperatures verified in laboratory tests for V. destructor adults and juveniles [20]. Since the aim of our investigations was to ascertain the fate of adult and juvenile mites within sealed brood cells, a random sampling method was adopted and therefore our data do not permit for verification of whether hyperthermia has any effect on mite reproduction. Such an effect appears rather likely and further investigations should be carried out to clarify it.
The simulations with BEEHAVE produced trends in colony development that closely resembled those experimentally observed in apiary trials. As far as mite population dynamics are concerned, apiary data were subject to the obvious limitations that derive from the difficulty in quantifying re-infestation and the necessity of acaricide treatments in summer to prevent colony collapse before winter. Therefore, the mathematical model proved to be quite useful in comparing different re-infestation phenomena and alternative chemical control strategies. Since viral infections are often associated with high levels of V. destructor and can be even more dangerous for honey bees than mites [3], model simulations were also useful for generating trends of healthy and virus-infected mites during the year.
Viruses are quite sensitive to temperature and therefore it has been hypothesized that hyperthermia could be at least partly effective against the spread of viruses in the apiary. Some preliminary tests were carried out in 2018 (unpublished results). Samples of 40 worker bees were taken from the brood combs of each test hive with the intention of quantifying via qPCR the viral load of five pathogenic viruses commonly found in bees: deformed wing virus, acute bee paralysis virus complex, black queen cell virus, sackbrood bee virus and chronic bee paralysis virus. In spite of the small number of repeats, there was a promising tendency for DWV and ABPV viruses to increase their viral load less in the colonies subjected to thermotherapy than in the control colonies. It remains unclear whether this trend is directly attributable to the effect of heat or rather to the lower number of mites in the treated hives. In any case, further research work in this direction would be useful.
5. Conclusions
The Bee Ethic system helps in controlling varroa mite infestation in an IPM approach.
The Bee Ethic system appears feasible and applicable in practice by beekeepers, who already use it extensively in Italy.
However, further research is needed to investigate if hyperthermia as applied by means of the Bee Ethic system takes direct action on viruses and is sufficient to combat re-infestation without the use of acaricides.
6. Patents
The Bee Ethic system is protected by two patents (ITRN20130051A1 and ITRN20130052A1), with PCT extension.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12168138/s1.
Author Contributions
Conceptualization, M.P. and A.M.; methodology, M.P. and D.C.; software, V.P.; investigation, D.C, S.L., S.C. and V.P.; writing, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The 2020 trials and the preliminary virus analyses were carried out under the EU Interreg-Alcotra program Innov’Api.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Giulia Molinatto for virus analyses and to Tiziano Gardi, University of Perugia (Italy) and co-workers (including the beekeepers of “Gubbio and Gualdo Tadino Cooperative”) for making available the unpublished results of investigations carried out on the Bee Ethic system in the years 2014–2016 under a grant of the Italian Ministry for Agricultural, Food and Forestry Politics within Reg. CE 12347/07.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Spivak, M. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 1996, 27, 245–260. [Google Scholar] [CrossRef]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Flenniken, M.-L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.M.; Gámiz, V.; Jiménez-Marín, A.; Flores-Cortés, A.; Gil-Lebrero, S.; Garrido, J.J.; Hernando, M.D. Impact of Varroa destructor and associated pathologies on the colony collapse disorder affecting honey bees. Res. Vet. Sci. 2021, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Imdorf, A.; Charriere, J.D.; Kilchenmann, V.; Bogdanov, S.; Fluri, P. Alternative strategy in central Europe for the control of Varroa destructor in honey bee colonies. Apiacta 2003, 38, 258–278. [Google Scholar]
- Jack, C.J.; Ellis, J.D. Integrated Pest Management Control of Varroa destructor (Acari: Varroidae), the Most Damaging Pest of Apis mellifera L. (Hymenoptera: Apidae) Colonies. J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Goras, G.; Tananaki, C.; Gounari, S.; Dimou, M.; Lazaridou, E.; Karazafiris, E.; Kanelis, D.; Liolios, V.; El Taj, H.; Thrasyvoulou, A. Hyperthermia—A non-chemical control strategy against varroa. J. Hell. Vet. Med. Soc. 2018, 66, 249–256. [Google Scholar] [CrossRef]
- Engels, W. Efficiency of biotechnical control of varroasis, by means of hyperthermia. Apiacta 1998, 33, 49–55. [Google Scholar]
- Kablau, A.; Berg, S.; Rutschmann, B.; Scheiner, R. Short-term hyperthermia at larval age reduces sucrose responsiveness of adult honeybees and can increase life span. Apidologie 2020, 51, 570–582. [Google Scholar] [CrossRef]
- Tihelka, E. History of Varroa Heat Treatment in Central Europe (1981–2013). Bee World 2016, 93, 4–6. [Google Scholar] [CrossRef]
- Bičík, V.; Vagera, J.; Sádovská, H. The effectiveness of thermotherapy in the elimination of Varroa destructor. Acta Mus. Sil. Sci. Nat. 2016, 65, 263–269. [Google Scholar] [CrossRef]
- Huang, Z. Mite Zapper—A New and Effective Method for Varroa Mite Control. Am. Bee J. 2001, 140, 730–732. [Google Scholar]
- Vatorex. Available online: watorex.com (accessed on 9 June 2022).
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Amsler, T.; Schmid, L. Controllo Della Varroa in Apicoltura Biologica; Forschungsinstitut für Biologischen Landbau (FiBL): Frick, Switzerland, 2010; 4p, Available online: https://orgprints.org/id/eprint/18018/1/amsler-schmid-2010-varroase.pdf (accessed on 9 June 2022).
- Rinderer, T.E.; De Guzman, L.I.; Frake, A.M.; Tarver, M.R.; Khongphinitbunjong, K. An Evaluation of the Associations of Parameters Related to the Fall of Varroa destructor (Acari: Varroidae) From Commercial Honey Bee (Hymenoptera: Apidae) Colonies as Tools for Selective Breeding for Mite Resistance. J. Econ. Entomol. 2014, 107, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Floris, I. Dispersion indices and sampling plans for the honeybee (Apis mellifera ligustica Spin.) mite Varroa jacobsoni Oud. Apicoltura 1991, 7, 161–170. [Google Scholar]
- Becher, M.A.; Grimm, V.; Thorbek, P.; Horn, J.; Kennedy, P.J.; Osborne, J.L. BEEHAVE: A systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 2013, 51, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Kablau, A.; Berg, S.; Härtel, S.; Scheiner, R. Hyperthermia treatment can kill immature and adult Varroa destructor mites without reducing drone fertility. Apidologie 2020, 51, 307–315. [Google Scholar] [CrossRef]
- Peck, D.T.; Seeley, T.D. Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS ONE 2019, 14, e0218392. [Google Scholar] [CrossRef] [PubMed]
- Marti, S.J. Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 1994, 18, 87–100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).