Comparative Evaluation of the Antioxidative and Antimicrobial Nutritive Properties and Potential Bioaccessibility of Plant Seeds and Algae Rich in Protein and Polyphenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Polyphenolic Compounds and Antioxidant Properties
2.2.1. Extraction Procedure
2.2.2. Total Polyphenolic Compounds
2.2.3. Antioxidant Activity
2.3. Potential Bioaccessibility
2.4. Microorganism and Culture Conditions
2.4.1. Preparation
2.4.2. Extraction Procedure
2.4.3. Determination of Antimicrobial Properties with Agar Diffusion Assay
2.5. Chemical Composition
2.6. Amino Acid Composition
2.7. Scoring of Amino Acids
2.8. Statistics
3. Results and Discussion
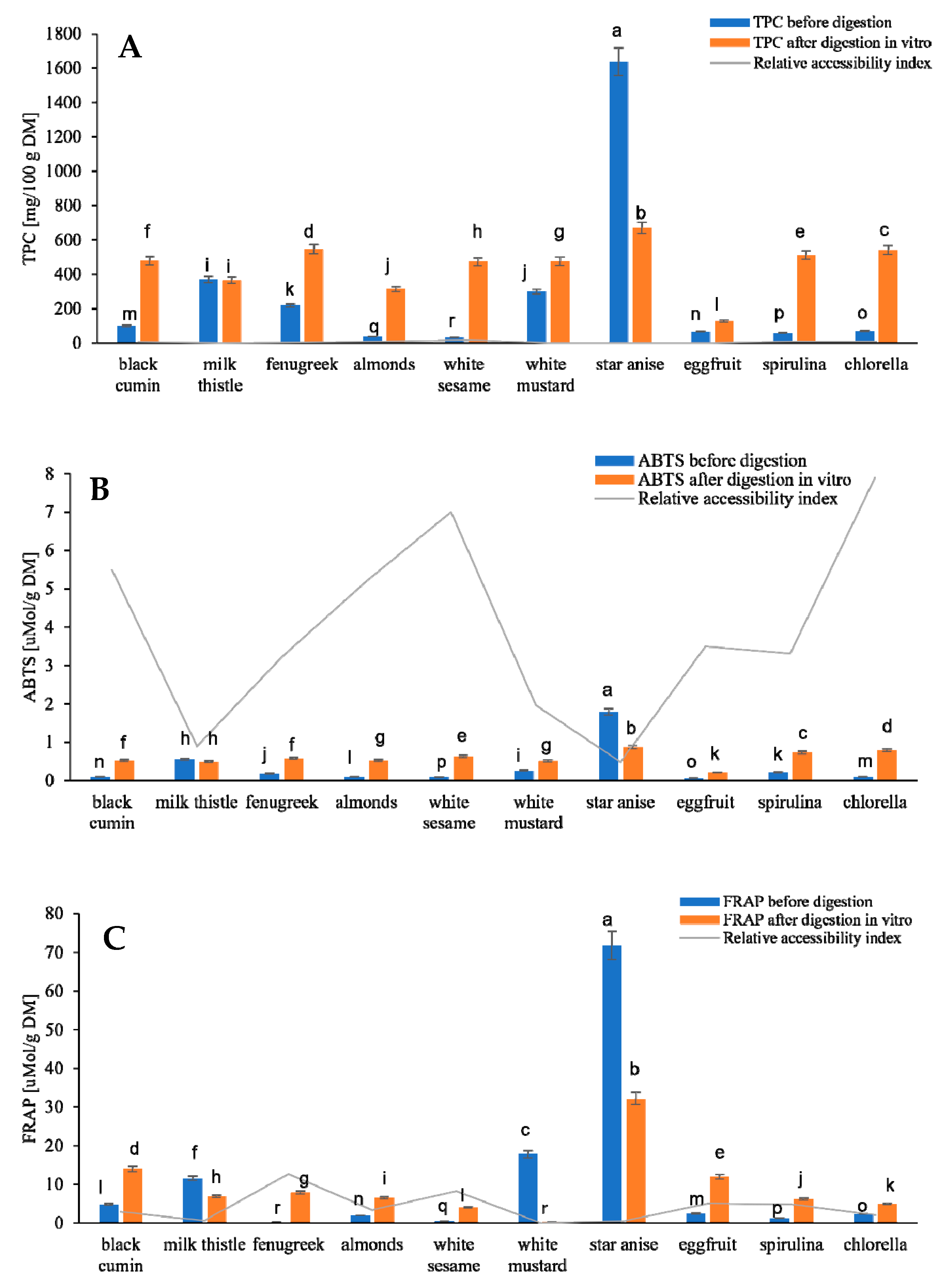
3.1. Polyphenolic Compounds, Antioxidant Activity and Potential Bioaccessibility
3.2. Antimicrobial Activity
3.3. Proximate Composition
3.4. Amino Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO 927:2009; Spices, Culinary Herbs and Condiments. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ceylan, E.; Fung, D.Y.C. Antimicrobial activity of spices. J. Rapid Methods Autom. Microbiol. 2004, 12, 1–55. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; Abd ElRazik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Edelman, M.; Colt, M. Nutrient Value of Leaf vs. Seed. Front. Chem. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Bernal-Castillo, J.; Rozo, C.; Rodríguez, I. Spirulina (Arthrospira): An edible microorganism. A review. Univ. Sci. 2003, 8, 7–24. [Google Scholar]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of Spirulina (Arthrospira platensis) Supplementation on Physical and Chemical Properties of Semolina (Triticum durum) Based Fresh Pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef] [PubMed]
- Całyniuk, B.; Grochowska-Niedworok, E.; Białek, A.; Czech, N.; Kukielczak, A. Food guide pyramid—its past and present. Probl. Hig. I Epidemiol. 2011, 92, 20–24. [Google Scholar]
- Peter, K.V. (Ed.) Handbook of Herbs and Spices; Woodhead Publishing: Sawston, UK, 2012; ISBN 978-0-85709-039-3. [Google Scholar]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Addition of milk to tea infusions: Helpful or harmful? Evidence from in vitro and in vivo studies on antioxidant properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 3188–3196. [Google Scholar] [CrossRef]
- Al-Jasass, F.M.; Al-Jasser, M.S. Chemical Composition and Fatty Acid Content of Some Spices and Herbs under Saudi Arabia Conditions. Sci. World J. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Ncube, N.; Afolayan, A.; Okoh, A. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current Methods and Future Trends. AFRICAN J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Kondo, Y.; Nishida, T.; Hamada, H.; Nakajima, N.; Hamada, H. Biotransformation of thymol, carvacrol, and eugenol by cultured cells of Eucalyptus perriniana. Phytochemistry 2006, 67, 2256–2261. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Pete, U.D.; Zade, C.M.; Padhye, S.B.; Bendre, R.S. Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol. Divers. 2018, 22, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Draijer, R.; van Dorsten, F.; Zebregs, Y.; Hollebrands, B.; Peters, S.; Duchateau, G.; Grün, C. Impact of Proteins on the Uptake, Distribution, and Excretion of Phenolics in the Human Body. Nutrients 2016, 8, 814. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Świeca, M.; Pejcz, E. Biological activity, phytochemical parameters, and potential bioaccessibility of wheat bread enriched with powder and microcapsules made from Saskatoon berry. Food Chem. 2021, 338, 128026. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pęksa, A.; Miedzianka, J.; Nemś, A. Amino acid composition of flesh-coloured potatoes as affected by storage conditions. Food Chem. 2018, 266, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic Recording Apparatus for Use in Chromatography of Amino Acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; Report of an FAO Expert Consultation; FAO Food and Nutrition Paper 92; FAO: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the chemical and nutritional characteristics of almonds (Prunus dulcis (Mill). D.A. Webb) as influenced by harvest time and cultivar. J. Sci. Food Agric. 2018, 98, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Padmashree, A.; Roopa, N.; Semwal, A.D.; Sharma, G.K.; Agathian, G.; Bawa, A.S. Star-anise (Illicium verum) and black caraway (Carum nigrum) as natural antioxidants. Food Chem. 2007, 104, 59–66. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Mashkor, I.M. Phenolic content and antioxidant activity of fenugreek seeds extract. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 841–844. [Google Scholar]
- Lin, X.; Zhou, L.; Li, T.; Brennan, C.; Fu, X.; Liu, R.H. Phenolic content, antioxidant and antiproliferative activities of six varieties of white sesame seeds (Sesamum indicum L.). RSC Adv. 2017, 7, 5751–5758. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Vitaglione, P.; Barone Lumaga, R.; Ferracane, R.; Radetsky, I.; Mennella, I.; Schettino, R.; Koder, S.; Shimoni, E.; Fogliano, V. Curcumin Bioavailability from Enriched Bread: The Effect of Microencapsulated Ingredients. J. Agric. Food Chem. 2012, 60, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Rybicka, I.; Zielińska-Dawidziak, M.; Białas, W.; Lewandowicz, G. Membrane Filtration-Assisted Enzymatic Hydrolysis Affects the Biological Activity of Potato Juice. Molecules 2021, 26, 852. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Miedzianka, J.; Drzymała, K.; Nemś, A.; Kita, A. Comparative evaluation of the antioxidant, antimicrobial and nutritive properties of gluten-free flours. Sci. Rep. 2021, 11, 10385. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Maier, J.; Follo, M.; Spitzmüller, B.; Wittmer, A.; Hellwig, E.; Hübner, J.; Jonas, D. Food-borne Enterococci Integrate Into Oral Biofilm: An In Vivo Study. J. Endod. 2010, 36, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Apetroaie-Constantin, C.; Mikkola, R.; Andersson, M.A.; Teplova, V.; Suominen, I.; Johansson, T.; Salkinoja-Salonen, M. Bacillus subtilis and B. mojavensis strains connected to food poisoning produce the heat stable toxin amylosin. J. Appl. Microbiol. 2009, 106, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Hervio-Heath, D.; Colwell, R.R.; Derrien, A.; Robert-Pillot, A.; Fournier, J.M.; Pommepuy, M. Occurrence of pathogenic vibrios in coastal areas of France. J. Appl. Microbiol. 2002, 92, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ali, Z.; Zou, J.; Jin, G.; Zhu, J.; Yang, J.; Dai, J. Detection methods for Pseudomonas aeruginosa: History and future perspective. RSC Adv. 2017, 7, 51789–51800. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Yu, J.; Zhang, H.; Yuan, Z.; Sun, Y.; Zhang, L.; Zhu, Y.; Song, H. An outbreak of Proteus mirabilis food poisoning associated with eating stewed pork balls in brown sauce, Beijing. Food Control 2010, 21, 302–305. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents 1999, 12, 257–262. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, T.; Filbido, G.S.; de Oliveira Pinheiro, A.P.; Pinto Piereti, P.D.; Dalla Villa, R.; de Oliveira, A.P. In vitro bioaccessibility of the Cu, Fe, Mn and Zn in the baru almond and bocaiúva pulp and, macronutrients characterization. J. Food Compos. Anal. 2020, 86, 103356. [Google Scholar] [CrossRef]
- Ezeagu, I.; Petzke, J.; Metges, C.; Akinsoyinu, A.; Ologhobo, A. Seed protein contents and nitrogen-to-protein conversion factors for some uncultivated tropical plant seeds. Food Chem. 2002, 78, 105–109. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- El-haak, M.A.; Atta, B.M.; Abd Rabo, F.F. Seed yield and important seed constituents for naturally and cultivated milk thistle plants. Egypt. J. Exp. Biol. 2015, 11, 141–146. [Google Scholar]
- Kochhar, A.; Nagi, M.; Sachdeva, R. Proximate Composition, Available Carbohydrates, Dietary Fibre and Anti Nutritional Factors of Selected Traditional Medicinal Plants. J. Hum. Ecol. 2006, 19, 195–199. [Google Scholar] [CrossRef]
- Lunn, J.; Buttriss, J.L. Carbohydrates and dietary fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef]
- Makinde, F.M.; Akinoso, R. Comparison between the nutritional quality of flour obtained from raw, roasted and fermented sesame (Sesamum indicum L.) seed grown in Nigeria. Acta Sci. Pol. Technol. Aliment. 2014, 13, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Ahsan, M.; Habib, B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals; FAO Fisheries and Aquaculture Circular No. 1034; FAO: Rome, Italy, 2008. [Google Scholar]
- Koru, E. Earth Food Spirulina (Arthrospira): Production and Quality Standarts. In Food Additive; InTech: London, UK, 2012. [Google Scholar]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef]
- Feyzi, S.; Varidi, M.; Zare, F.; Varidi, M.J. Fenugreek (Trigonella foenum graecum) seed protein isolate: Extraction optimization, amino acid composition, thermo and functional properties. J. Sci. Food Agric. 2015, 95, 3165–3176. [Google Scholar] [CrossRef]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- Sadowska, K.; Andrzejewska, J.; Woropaj-Janczak, M. Others Effect of weather and agrotechnical conditions on the content of nutrients in the fruits of milk thistle (Silybum marianum L. Gaertn.). Acta Sci. Pol. Hortorum Cultus 2011, 10, 197–207. [Google Scholar]
- Viegas, C.V.; Hachemi, I.; Mäki-Arvela, P.; Smeds, A.; Aho, A.; Freitas, S.P.; da Silva Gorgônio, C.M.; Carbonetti, G.; Peurla, M.; Paranko, J.; et al. Algal products beyond lipids: Comprehensive characterization of different products in direct saponification of green alga Chlorella sp. Algal Res. 2015, 11, 156–164. [Google Scholar] [CrossRef]
| Seeds and Algae | Gram-Positive Bacteria (G+) | Gram-Negative Bacteria (G−) | Yeast | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. hirae | E. faecalis | P. aeruginosa | E. coli | P. mirabilis | V. harveyi | C. albicans | |
| Black cumin | - | - | - | - | - | - | - | - | - |
| Milk thistle | 1.56 ± 0.02 | - | - | 6.83 ± 0.63 | - | - | 3.85 ± 0.76 | - | - |
| Fenugreek | 3.05 ± 0.64 | - | - | - | - | - | 3.75 ± 0.68 | 3.73 ± 0.53 | 0.28 ± 0.06 |
| Almond | 3.42 ± 0.77 | - | - | - | - | - | - | 2.89 ± 0.49 | - |
| White sesame | 2.98 ± 0.01 | - | - | - | - | - | - | 0.53 ± 0.01 | 1.25 ± 0.01 |
| White mustard | - | - | - | - | 6.95 ± 1.38 | - | 3.09 ± 0.01 | - | - |
| Star anise | 8.89 ± 0.03 | - | - | - | - | - | 2.71 ± 0.01 | - | - |
| Eggfruit | - | - | - | - | - | - | 4.18 ± 0.26 | 3.05 ± 0.50 | - |
| Spirulina | - | - | - | - | - | - | - | - | - |
| Chlorella | - | - | - | - | - | - | - | - | - |
| Raw Material | Dry Matter | Total Protein | Fat | Ash | Carbohydrates |
|---|---|---|---|---|---|
| Black cumin | 94.43 ± 0.08 d | 20.39 ± 1.22 d | 36.85 ± 1.14 c | 4.54 ± 0.02 e | 32.65 ± 3.11 d |
| Milk thistle | 92.83 ± 0.02 f | 16.33 ± 0.08 e | 23.47 ± 0.87 d | 6.51 ± 0.01 c | 46.53 ± 4.52 c |
| Fenugreek | 91.17 ± 0.03 g | 23.70 ± 1.05 c | 5.13 ± 0.22 f | 3.47 ± 0.03 f | 58.87 ± 3.85 b |
| Almonds | 95.58 ± 1.02 b | 24.58 ± 0.07 c | 52.25± 4.27 b | 4.81 ± 0.02 e | 13.94 ± 1.75 f |
| White sesame | 96.38 a ± 0.07 a | 24.06 ± 1.01 c | 51.02 ± 1.89 b | 5.12 ± 0.02 d | 16.18 ± 2.13 e |
| White mustard | 95.08 ± 0.02 c | 20.89 ± 1.52 d | 36.24 ± 0.96 b | 3.72 ± 0.01 f | 34.23 ± 4.74 d |
| Star anise | 89.19 ± 1.58 h | 5.92 ± 0.18 f | 68.75 ± 3.69 a | 3.11 ± 0.03 f | 11.41 ± 1.85 f |
| Eggfruit | 92.75 ± 2.35 f | 1.72 ± 0.98 g | 2.23 ± 0.87 g | 0.52 ± 0.01 g | 88.28 ± 9.55 a |
| Spirulina | 94.37 ± 3.12 d | 69.35 ± 4.05 a | 7.52 ± 1.45 e | 11.47 ± 0.05 b | 6.03 ± 0.15 g |
| Chlorella | 94.07 ± 2.01 e | 55.40 ± 3.21 b | 8.15 ± 1.74 e | 14.13 ± 0.07 a | 16.39 ± 1.45 e |
| Amino Acid | Black Cumin | Milk Thistle | Fenugreek | Almond | White Sesame | White Mustard | Star Anise | Eggfruit | Spirulina | Chlorella | Standard |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential | |||||||||||
| His | 31.70 ± 0.23 d | 51.81 ± 0.13 b | 31.35 ± 0.11 d | 33.80 ± 0.12 d | 35.73 ± 0.03 d | 43.62 ± 0.11 c | 26.12 ± 0.12 e | 100.90 ± 1.98 a | 15.20 ± 0.02 f | 18.66 ± 0.08 f | 16 |
| Ile | 39.64 ± 0.11 e | 79.12 ± 0.14 b | 53.37 ± 0.23 c | 44.64 ± 0.15 d | 47.45 ± 0.04 d | 53.21 ± 0.15 c | 28.60 ± 0.14 f | 95.46 ± 1.65 a | 44.94 ± 0.54 d | 45.61 ± 0.07 d | 30 |
| Leu | 65.68 ± 0.14 f | 119.91 ± 0.15 b | 75.08 ± 0.14 e | 82.50 ± 0.08 d | 84.17 ± 1.11 d | 97.48 ± 1.17 c | 45.05 ± 0.17 g | 132.41 ± 1.77 a | 73.28 ± 0.78 e | 82.61 ± 0.15 d | 61 |
| Lys | 48.64 ± 1.18 d | 92.52 ± 0.09 a | 75.67 ± 0.11 c | 38.33 ± 0.03 e | 35.33 ± 0.03 e | 88.62 ± 0.18 b | 30.34 ± 0.09 f | 83.21 ± 0.88 b | 42.31 ± 0.17 e | 50.40 ± 0.26 d | 48 |
| Met + Cys | 26.19 ± 2.01 d | 42.80 ± 0.07 b | 19.87 ± 0.05 e | 16.67 ± 0.01 f | 35.83 ± 0.03 c | 38.39 ± 0.02 b | 16.69 ± 0.08 f | 65.15 ± 0.77 a | 20.46 ± 0.08 e | 18.45 ± 0.01 e | 23 |
| Phe + Tyr | 82.30 ± 0.22 e | 154.02 ± 1.12 b | 79.91 ± 1.11 e | 106.68 ± 2.04 c | 102.77 ± 1.56 c | 96.98 ± 0.09 d | 59.85 ± 1.15 g | 224.11 ± 2.12 a | 69.62 ± 0.88 f | 75.43 ± 0.58 f | 41 |
| Thr | 46.99 ± 0.13 d | 78.82 ± 1.13 b | 41.88 ± 1.12 d | 37.96 ± 1.04 e | 49.76 ± 0.42 d | 65.54 ± 1.02 c | 25.77 ± 0.05 f | 92.48 ± 0.88 a | 43.45 ± 0.54 d | 46.62 ± 0.23 d | 25 |
| Val | 53.08 ± 1.15 c | 100.78 ± 0.08 a | 44.35 ± 0.08 d | 53.92 ± 1.20 c | 61.09 ± 0.91 c | 71.37 ± 0.94 b | 32.80 ± 0.19 e | 112.81 ± 1.55 a | 56.71 ± 0.74 c | 58.74 ± 0.45 c | 40 |
| AAS (%) | 101.3 ± 1.17 c | 178.3 ± 0.05 a | 82.8 ± 0.06 d | 69.5 ± 1.17 e | 73.6 ± 0.08 e | 159.8 ± 2.01 b | 63.3 ± 1.19 f | 173.5 ± 1.44 a | 88.1 ± 1.15 d | 105.0 ± 1.25 c | 100 |
| Non-essential | |||||||||||
| Asp | 102.78 ± 2.22 e | 190.59 ± 0.04 b | 120.31 ± 2.06 d | 145.02 ± 2.33 c | 107.76 ± 1.17 e | 96.57 ± 0.15 f | 55.44 ± 1.23 g | 296.71 ± 2.12 a | 85.85 ± 1.19 f | 88.98 ± 0.45 f | - |
| Glu | 264.27 ± 0.11 c | 417.83 ± 1.10 a | 197.64 ± 2.11 d | 371.79 ± 3.01 b | 257.21 ± 2.21 c | 260.87 ± 2.15 c | 86.14 ± 1.78 f | 204.03 ± 2.03 d | 122.40 ± 1.42 e | 126.93 ± 1.29 e | - |
| Ala | 48.34 ± 0.08 e | 85.58 ± 0.88 b | 44.02 ± 1.14 f | 56.80 ± 2.12 d | 60.12 ± 1.15 d | 62.27 ± 0.13 d | 33.29 ± 0.33 g | 150.37 ± 1.98 a | 70.90 ± 0.58 c | 70.20 ± 0.57 c | - |
| Arg | 95.30 ± 1.16 d | 208.01 ± 0.51 a | 116.47 ± 0.14 c | 161.82 ± 0.15 b | 167.48 ± 2.03 b | 85.90 ± 0.87 e | 47.71 ± 0.77 g | 86.75 ± 0.19 e | 72.00 ± 0.95 f | 66.05 ± 0.56 f | - |
| Gly | 70.60 ± 0.09 c | 109.81 ± 1.56 a | 54.88 ± 0.13 e | 84.67 ± 0.09 b | 65.74 ± 0.08 d | 71.99 ± 0.22 c | 36.60 ± 0.55 g | 109.68 ± 1.11 a | 43.94 ± 0.49 f | 49.51 ± 0.96 f | - |
| Pro | 41.21 ± 1.13 e | 79.94 ± 0.34 c | 48.63 ± 0.01 d | 54.96 ± 0.07 d | 34.56 ± 0.17 f | 86.85 ± 0.23 b | 33.51 ± 0.21 f | 118.33 ± 1.16 a | 29.22 ± 0.09 g | 30.07 ± 0.03 f | - |
| Ser | 52.45 ± 2.02 c | 102.55 ± 2.13 a | 55.04 ± 0.14 b | 50.75 ± 0.07 c | 59.38 ± 0.26 b | 59.50 ± 0.15 b | 32.63 ± 0.11 e | 109.29 ± 1.84 a | 41.67 ± 0.23 d | 41.93 ± 0.05 d | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedzianka, J.; Lachowicz-Wiśniewska, S.; Nemś, A.; Kowalczewski, P.Ł.; Kita, A. Comparative Evaluation of the Antioxidative and Antimicrobial Nutritive Properties and Potential Bioaccessibility of Plant Seeds and Algae Rich in Protein and Polyphenolic Compounds. Appl. Sci. 2022, 12, 8136. https://doi.org/10.3390/app12168136
Miedzianka J, Lachowicz-Wiśniewska S, Nemś A, Kowalczewski PŁ, Kita A. Comparative Evaluation of the Antioxidative and Antimicrobial Nutritive Properties and Potential Bioaccessibility of Plant Seeds and Algae Rich in Protein and Polyphenolic Compounds. Applied Sciences. 2022; 12(16):8136. https://doi.org/10.3390/app12168136
Chicago/Turabian StyleMiedzianka, Joanna, Sabina Lachowicz-Wiśniewska, Agnieszka Nemś, Przemysław Łukasz Kowalczewski, and Agnieszka Kita. 2022. "Comparative Evaluation of the Antioxidative and Antimicrobial Nutritive Properties and Potential Bioaccessibility of Plant Seeds and Algae Rich in Protein and Polyphenolic Compounds" Applied Sciences 12, no. 16: 8136. https://doi.org/10.3390/app12168136
APA StyleMiedzianka, J., Lachowicz-Wiśniewska, S., Nemś, A., Kowalczewski, P. Ł., & Kita, A. (2022). Comparative Evaluation of the Antioxidative and Antimicrobial Nutritive Properties and Potential Bioaccessibility of Plant Seeds and Algae Rich in Protein and Polyphenolic Compounds. Applied Sciences, 12(16), 8136. https://doi.org/10.3390/app12168136








