Valorization of Pineapple Residues from the Colombian Agroindustry to Produce Cellulose Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pineapple Waste
2.3. Obtention of Cellulose Nanofibers
2.3.1. Native Nanofibers
2.3.2. Obtention of Nanofibers by Chemical Methods Combined with Ultrasound
2.4. Characterization of the Nanofibers
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Infrared Analysis (FITR)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Transmission Electron Microscopy (TEM)
3. Results and Discussion
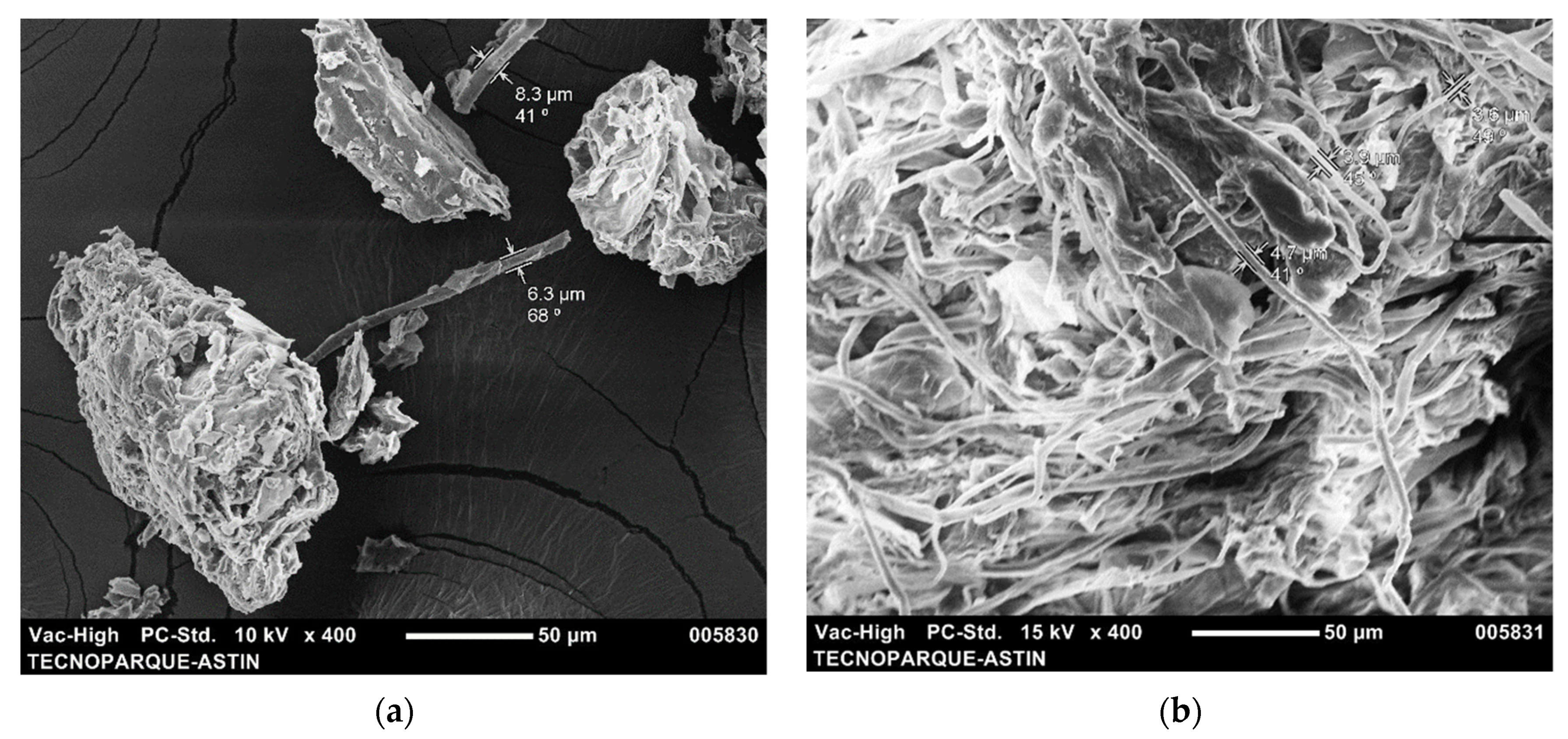
3.1. Morphology Analysis
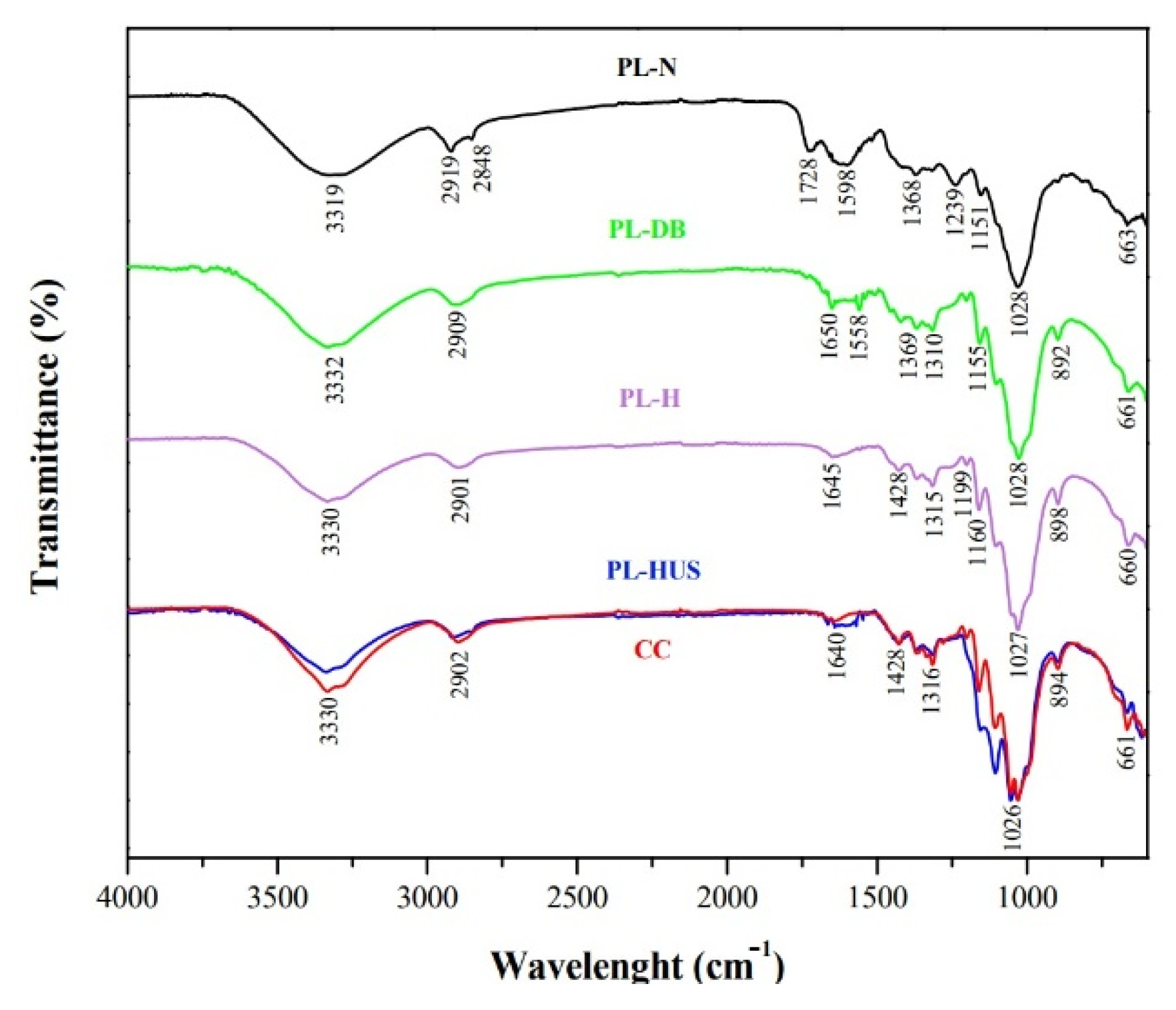
3.2. Functional Groups Analysis
3.3. Thermal Stability Analysis
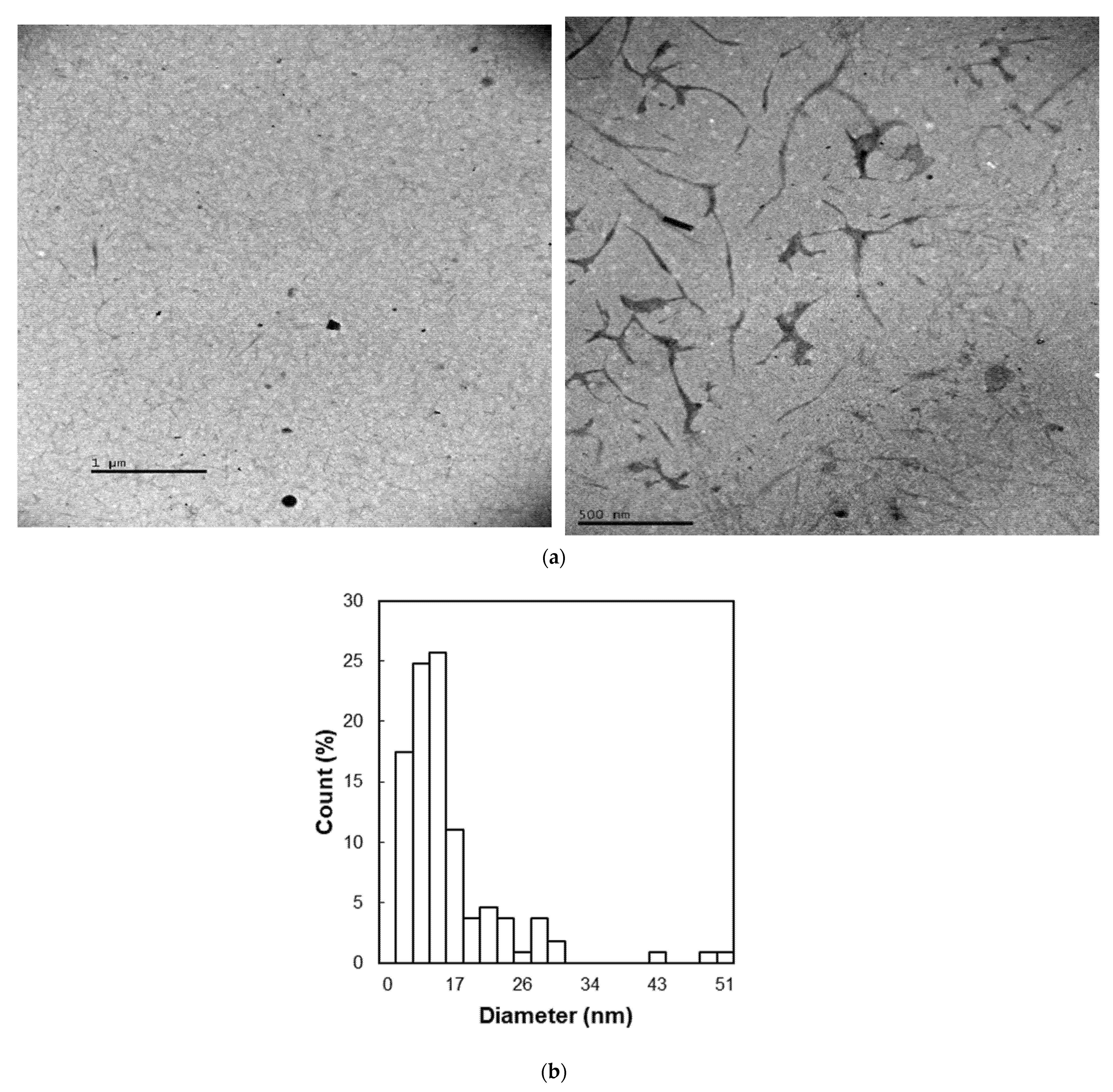
3.4. Particle Size Analysis of Cellulose Nanofibers
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshankhah, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M.; et al. Recent advances in cellulose-based structures as the wound-healing biomaterials: A clinically oriented review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Lam, W.S.; Lee, P.F.; Lam, W.H. Cellulose nanofiber for sustainable production: A bibliometric analysis. Mater. Today Proc. 2022, 62 Pt 12, 6460–6467. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48, 454–463. [Google Scholar] [CrossRef]
- Uusi-Tarkka, E.-K.; Skrifvars, M.; Haapala, A. Fabricating sustainable all-cellulose composites. Appl. Sci. 2021, 11, 10069. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose nanofibers and other biopolymers for biomedical applications. A review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Rosero, J.; Urbina-López, M.E.; Rodríguez-González, B.E.; León-Villegas, S.X.; Luna-Cruz, I.E.; Cárdenas-Chávez, D.L. Development and characterization of bioadsorbents derived from different agricultural wastes for water reclamation: A review. Appl. Sci. 2022, 12, 2740. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Chen, L. Applications of plant polymer-based solid foams: Current trends in the food industry. Appl. Sci. 2021, 11, 9605. [Google Scholar] [CrossRef]
- Phuong, H.T.; Thoa, N.K.; Tuyet, P.T.A.; Van, Q.N.; Hai, Y.D. Cellulose nanomaterials as a future, sustainable and renewable material. Crystals 2022, 12, 106. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.; Pereira, A.; Fajardo, A.; Rubira, A.; Muniz, E. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Kurita, H.; Ishigami, R.; Narita, F. Assessing the flexural properties of epoxy composites with extremely low addition of cellulose nanofiber content. Appl. Sci. 2020, 10, 1159. [Google Scholar] [CrossRef] [Green Version]
- Research and Markets. The Global Market for Cellulose Nanofibers to 2031; Future Markets, Inc.: Dublin, Ireland, 2022. [Google Scholar]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Hydrogels are reinforced with Colombian fique nanofibers to improve techno-functional properties for agricultural purposes. Agriculture 2022, 12, 117. [Google Scholar] [CrossRef]
- Guancha-Chalapud, M.A.; Gálvez, J.; Serna-Cock, L.; Aguilar, C.N. Valorization of Colombian fique (Furcraea bedinghausii) for production of cellulose nanofibers and its application in hydrogels. Sci. Rep. 2020, 10, 11637. [Google Scholar] [CrossRef] [PubMed]
- Serna Cock, L.; Guancha-Chalapud, M.A. Natural fibers for hydrogels production and their applications in agriculture. Acta Agronómica 2017, 66, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzoli, P.; Sorlini, M.; Rovere, D.; Lazaro, O.; Malò, P.; Fiorello, M. Challenges and founding pillars for a manufacturing platform to support value networks operating in a circular economy framework. Appl. Sci. 2022, 12, 2995. [Google Scholar] [CrossRef]
- Righi, C.; Barbieri, F.; Sgarbi, E.; Maistrello, L.; Bertacchini, A.; Andreola, F.N.; D’Angelo, A.; Catauro, M.; Barbieri, L. Suitability of porous inorganic materials from industrial residues and bioproducts for use in horticulture: A multidisciplinary approach. Appl. Sci. 2022, 12, 5437. [Google Scholar] [CrossRef]
- Adler, I.; Kotta, J.; Tuvikene, R.; Kaldre, K. Optimizing the processing of shellfish (Mytilus edulis and M. trossulus hybrid) miomass cultivated in the low salinity region of the Baltic sea for the extraction of meat and proteins. Appl. Sci. 2022, 12, 5163. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Evtuguin, D.; Esteves, B. Eco valorization of Eucalyptus globulus bark and branches through liquefaction. Appl. Sci. 2022, 12, 3775. [Google Scholar] [CrossRef]
- Martinez, C.; Menjívar, J.; Saavedra, R. Soils erosion in pineapple (Ananas comosus L. Merr) producing areas. Rev. Ciencias Agrícolas 2022, 39, 6366. [Google Scholar]
- Nashiruddin, N.I.; Mansor, A.F.; Rahman, R.A.; Ilias, R.M.; Yussof, H.W. Process parameter optimization of pretreated pineapple leaves fiber for enhancement of sugar recovery. Ind. Crops Prod. 2020, 152, 112514. [Google Scholar] [CrossRef]
- Cheng, S.; Panthapulakkal, S.; Sain, M.; Asiri, A. Aloe vera rind cellulose nanofibers-reinforced films. J. Appl. Polym. Sci. 2014, 131, 40592. [Google Scholar] [CrossRef]
- Ramezani Kakroodi, A.; Cheng, S.; Sain, M.; Asiri, A. Mechanical, thermal, and morphological properties of nanocomposites based on polyvinyl alcohol and cellulose nanofiber from Aloe vera rind. J. Nanomater. 2014, 2014, 903498. [Google Scholar] [CrossRef] [Green Version]
- Julie Chandra, C.S.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Li, C.; Shupe, T.F.; Hu, T.; Qi, J.; De Hoop, C.F. Characterization of microwave liquefied bamboo residue and its potential use in the generation of nanofibrillated cellulosic fiber. ACS Sustain. Chem. Eng. 2016, 4, 3477–3485. [Google Scholar] [CrossRef]
- Zhang, P.P.; Tong, D.S.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Yu, W.H.; Wang, H.; Zhou, C.H. Effects of acid treatments on bamboo cellulose nanocrystals. Asia-Pacific J. Chem. Eng. 2014, 9, 686–695. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Célino, A.; Gonçalves, O.; Jacquemin, F.; Fréour, S. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.R.; Cranston, E.D. Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 2019, 26, 507–528. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yaschenko, O.V.; Alushkin, S.V.; Kondratyuk, A.S.; Posudievsky, O.Y.; Koshechko, V.G. Effect of mechanochemical treatment of cellulose on characteristics of nanocellulose films. Springer Proc. Phys. 2016, 183, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Hashaikeh, R.; Abushammala, H. Acid mediated networked cellulose: Preparation and characterization. Carbohydr. Polym. 2011, 83, 1088–1094. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulphate groups from sulphuric acid hydrolysis on the thermal degradation behaviour of bacterial cellulose. Biomacromolecules 2004, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Rämänen, P.; Penttilä, P.A.; Svedström, K.; Maunu, S.L.; Serimaa, R. The effect of drying method on the properties and nanoscale structure of cellulose whiskers. Cellulose 2012, 19, 901–912. [Google Scholar] [CrossRef]
- Song, Y.K.; Leng Chew, I.M.; Yaw Choong, T.S.; Tan, J.; Tan, K.W. Isolation of Nanocrystalline Cellulose from oil palm empty fruit bunch—A response surface methodology study. MATEC Web Conf. 2016, 60, 04009. [Google Scholar] [CrossRef]
- Vieyra, H.; Figueroa-López, U.; Guevara-Morales, A.; Vergara-Porras, B.; San Martín-Martínez, E.; Aguilar-Mendez, M.Á. Optimized monitoring of production of cellulose nanowhiskers from Opuntia ficus-indica (nopal cactus). Int. J. Polym. Sci. 2015, 2015, 871345. [Google Scholar] [CrossRef]
- Csiszar, E.; Kalic, P.; Kobol, A.; de Paulo Ferreira, E. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason. Sonochem. 2016, 31, 473–480. [Google Scholar] [CrossRef]
- Ni, Y.; Li, J.; Fan, L. Effects of ultrasonic conditions on the interfacial property and emulsifying property of cellulose nanoparticles from ginkgo seed shells. Ultrason. Sonochem. 2021, 70, 105335. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Costa, L.M.M.; de Olyveira, G.M.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Valorization of Pineapple Residues from the Colombian Agroindustry to Produce Cellulose Nanofibers. Appl. Sci. 2022, 12, 6956. https://doi.org/10.3390/app12146956
Guancha-Chalapud MA, Serna-Cock L, Tirado DF. Valorization of Pineapple Residues from the Colombian Agroindustry to Produce Cellulose Nanofibers. Applied Sciences. 2022; 12(14):6956. https://doi.org/10.3390/app12146956
Chicago/Turabian StyleGuancha-Chalapud, Marcelo A., Liliana Serna-Cock, and Diego F. Tirado. 2022. "Valorization of Pineapple Residues from the Colombian Agroindustry to Produce Cellulose Nanofibers" Applied Sciences 12, no. 14: 6956. https://doi.org/10.3390/app12146956
APA StyleGuancha-Chalapud, M. A., Serna-Cock, L., & Tirado, D. F. (2022). Valorization of Pineapple Residues from the Colombian Agroindustry to Produce Cellulose Nanofibers. Applied Sciences, 12(14), 6956. https://doi.org/10.3390/app12146956





