Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Materials
2.3. Experimental Set-Up
2.4. Calculation Methods
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results
3.1. Feedstock Properties and Parameters
3.2. Biogas Yield and Composition
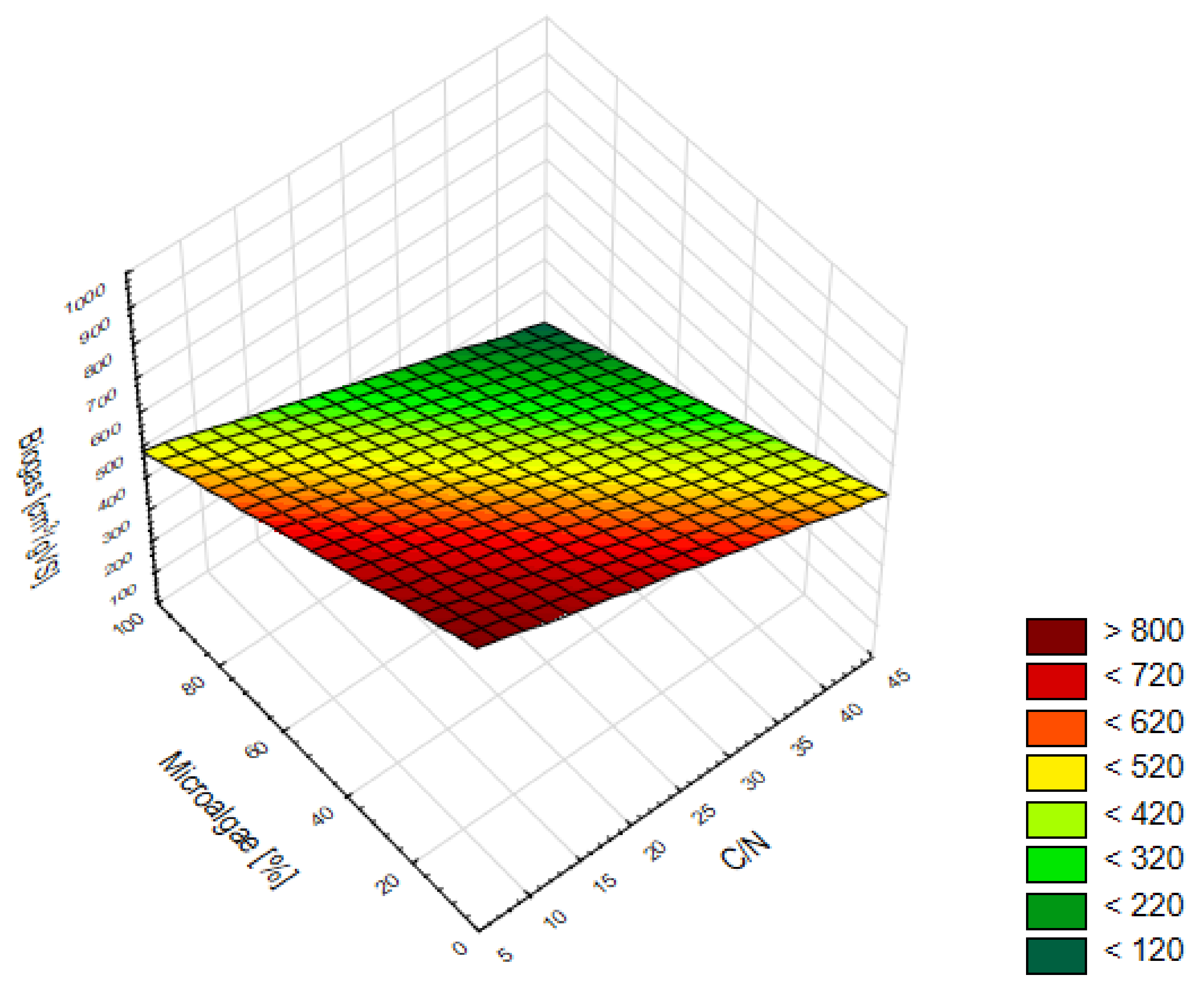
3.3. Forecast of Biogas and Methane Production
3.4. Bacterial Community
3.5. Correlations
3.6. Digestate Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, P.K.; Das, B.P.; Dash, P. Role of Energy Crops to Meet the Rural Energy Needs: An Overview. In Biomass Valorization Bioenergy; Springer: Singapore, 2019; pp. 11–30. [Google Scholar] [CrossRef]
- Meena, M.; Shubham, S.; Paritosh, K.; Pareek, N.; Vivekanand, V. Production of Biofuels from Biomass: Predicting the Energy Employing Artificial Intelligence Modelling. Bioresour. Technol. 2021, 340, 125642. [Google Scholar] [CrossRef] [PubMed]
- Manolis, E.; Zagas, T.D.; Karetsos, G.K.; Poravou, C.A. Ecological restrictions in forest biomass extraction for a sustainable renewable energy production. Renew. Sustain. Energy Rev. 2019, 110, 290–297. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Secur. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Knápek, J.; Králík, T.; Vávrová, K.; Weger, J. Dynamic biomass potential from agricultural land. Renew. Sustain. Energy Rev. 2020, 134, 110319. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Is the use of renewable energy sources an answer to the problems of global warming and pollution? Crit. Rev. Environ. Sci Technol 2012, 42, 99–154. [Google Scholar] [CrossRef]
- Perea-Moreno, M.-A.; Samerón-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- Reid, W.V.; Ali, M.K.; Field, C.B. The Future of Bioenergy; Stanford University: Stanford, CA, USA, 2020. [Google Scholar] [CrossRef] [Green Version]
- Bastos, R.G. Biofuels from Microalgae: Bioethanol. In Energy from Microalgae. Green Energy and Technology; Jacob-Lopes, E., Queiroz Zepka, L., Queiroz, M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Agrawal, K.; Verma, P. Algal Biofuels: An Economic and Effective Alternative of Fossil Fuels. In Microbial Strategies for Techno-Economic Biofuel Production. Clean Energy Production Technologies; Srivastava, N., Srivastava, M., Mishra, P.K., Gupta, V.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 207–227. [Google Scholar] [CrossRef]
- Marangon, B.B.; Calijuri, M.L.; de Siqueira Castro, J.; Assemany, P.P. A life cycle assessment of energy recovery using briquette from wastewater grown microalgae biomass. J. Environ. Manag. 2021, 285, 112171. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. N. Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, F.G. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies 2018, 11, 664. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, R.; Mousavi, S.E.; Goyette, B.; Adhikary, S. Coupling of Microalgae Cultivation with Anaerobic Digestion of Poultry Wastes: Toward Sustainable Value Added Bioproducts. Bioengineering 2021, 8, 57. [Google Scholar] [CrossRef]
- Coh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Taşkan, B.; Köroğlu, E.O.; Taşkan, E. Cladophora sp. as a sustainable feedstock for dark fermentative biohydrogen production. Int. J. Hydrog. Energy 2022, 47, 15410–15418. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Priya, A.K.; Dutta, K.; Rajendran, S.; Vasseghian, Y. Engineering strategies and opportunities of next generation biofuel from microalgae: A perspective review on the potential bioenergy feedstock. Fuel 2022, 312, 122827. [Google Scholar] [CrossRef]
- Avila, R.; Peris, A.; Eljarrat, E.; Vicent, T.; Blánquez, P. Biodegradation of Hydrophobic Pesticides by Microalgae: Transformation Products and Impact on Algae Biochemical Methane Potential. Sci. Total Environ. 2021, 754, 142114. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Korzeniewska, E.; Harnisz, M.; Czatzkowska, M. Intensification of biogas production using various technologies: A review. Int. J. Energy Res. 2020, 44, 6240–6258. [Google Scholar] [CrossRef]
- Wang, M.; Park, C. Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 2015, 80, 30–37. [Google Scholar] [CrossRef]
- Ramos-Ibarra, J.R.; Snell-Castro, R.; Neria-Casillas, J.A.; Choix, F.J. Biotechnological potential of Chlorella sp. And Scenedesmus sp. microalgae to endure high CO2 and methane concentrations from biogas. Bioprocess. Biosyst. Eng. 2019, 42, 1603–1610. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic digestion of microalgal biomass for bioenergy production, removal of nutrients and microcystin: Current status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Optimization of methane production during anaerobic co-digestion of rice straw and hydrilla verticillata using response surface methodology. Fuel 2019, 235, 92–99. [Google Scholar] [CrossRef]
- Suksong, W.; Promnuan, K.; Seengenyoung, J.; Sompong, S. Anaerobic Co-Digestion of Palm Oil Mill Waste Residues with Sewage Sludge for Biogas Production. Energy Procedia 2017, 138, 789–794. [Google Scholar] [CrossRef]
- Lu, D.; Liu, X.; Apul, O.G.; Zhang, L.; Ryan, D.K.; Zhang, X. Optimization of biomethane production from anaerobic Co-digestion of microalgae and septic tank sludge. Biomass Bioenergy 2019, 127, 105266. [Google Scholar] [CrossRef]
- Dach, J.; Pulka, J.; Janczak, D.; Lewicki, A.; Pochwatka, P.; Oniszczuk, T. Energetic Assessment of Biogas Plant Projects Based on Biowaste and Maize Silage Usage. IOP Conf. Ser. Earth Environ. Sci 2020, 505, 012029. [Google Scholar] [CrossRef]
- Kasprzycka, A.; Lalak, J.; Tys, J. Impact of fragmentation on biogas production from plant biomass. Acta Agrophys. 2015, 22, 139–149. [Google Scholar]
- Wang, C.; Kong, Y.; Hu, R.; Zhou, G. Miscanthus: A fast-growing crop for environmental remediation and biofuel production. Glob. Chang. Biol. Bioenergy 2020, 13, 58–69. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Solé-Bundo, M.; Passos, F.; Romero-Guiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Herrmann, C.; Kalita, N.; Wall, D.; Xia, A.; Murphy, J.D. Optimised biogas production from microalgae through co-digestion with carbon-rich co-substrates. Bioresour. Technol. 2016, 214, 328–337. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, Y.; Wang, J. Co-fermentation of sewage sludge and algae and Fe2+ addition for enhancing hydrogen production. Int. J. Hydrog. Energy 2021, 46, 8950–8960. [Google Scholar] [CrossRef]
- Sheehan, N.P.; Plante, L.; Martinez, E.; Murray, K.; Bier, P.; Ouellette, C. Sustainable bioenergy from biofuel residues and waste. Water Environ. Res. 2018, 90, 1073–1090. [Google Scholar] [CrossRef] [Green Version]
- Shobana, S.; Saratale, G.D.; Pugazhendhi, A.; Arvindnarayan, S.; Periyasamy, S.; Kumar, G.; Kim, S.H. Fermentative hydrogen production from mixed and pure microalgae biomass: Key challenges and possible opportunities. Int. J. Hydrog. Energy 2017, 42, 26440–26453. [Google Scholar] [CrossRef]
- Sun, C.; Xia, A.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X. Life-cycle assessment of biohythane production via two-stage anaerobic fermentation from microalgae and food waste. Renew. Sustain. Energy Rev. 2019, 112, 395–410. [Google Scholar] [CrossRef]
- Wirth, R.; Böjti, T.; Lakatos, G.; Maroti, G.; Bagi, Z.; Rakhely, G.; Kovacs, K.L. Characterization of core microbiomes and functional profiles of mesophilic anaerobic digesters fed with Chlorella vulgaris green microalgae and maize silage. Front. Energy Res. 2019, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Ray, R.C.; Ramachandran, S. Bioethanol from Biorenewable Feedstocks: Technology, Economics, and Challenges. In Bioethanol Production from Food Crops; Academic Press: Cambridge, MA, USA, 2019; pp. 3–27. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Aboudi, K.; Gómez-Quiroga, X.; Álvarez-Gallego, C.J.; Romero-García, L.I. Insights into Anaerobic Co-Digestion of Lignocellulosic Biomass (Sugar Beet By-Products) and Animal Manure in Long-Term Semi-Continuous Assays. Appl. Sci. 2020, 10, 5126. [Google Scholar] [CrossRef]
- Wilinska-Lisowska, A.; Ossowska, M.; Czerwionka, K. The Influence of Co-Fermentation of Agri-Food Waste with Primary Sludge on Biogas Production and Composition of the Liquid Fraction of Digestate. Energies 2021, 14, 1907. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Elkatory, M.R.; El Nemr, A.; Pantaleo, A. Eco-friendly biogas production from algal biomass. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–249. [Google Scholar]
- Kannah, R.Y.; Kavitha, S.; Karthikeyan, O.P.; Rene, E.R.; Kumar, G.; Banu, J.R. A review on anaerobic digestion of energy and cost effective microalgae pretreatment for biogas production. Bioresour. Technol. 2021, 332, 125055. [Google Scholar] [CrossRef]
- Wu, X.; Yao, W.; Zhu, J.; Miller, C. Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef]
- Kumar, P.; Prajapati, S.K.; Malik, A.; Vijay, V.K. Evaluation of biomethane potential of waste algal biomass collected from eutrophied lake: Effect of source of inocula, co-substrate, and VS loading. J. Appl. Phycol. 2019, 31, 533–545. [Google Scholar] [CrossRef]
- Naaz, F.; Bhattacharya, A.; Pant, K.K.; Malik, A. Investigations on energy efficiency of biomethane/biocrude production from pilot scale wastewater grown algal biomass. Appl. Energy 2019, 254, 113656. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; Castro, J.S.; Couto, E.A.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci. Total. Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]
- Peng, S.; Hou, C.; Wang, J.; Chen, T.; Liu, X.; Yue, Z. Performance of anaerobic co-digestion of corn straw and algae biomass from lake Chaohu. Trans. Chin. Soc. Agric. Eng. 2012, 28, 173–178. [Google Scholar]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- Schwede, S.; Kowalczyk, A.; Gerber, M.; Span, R. Anaerobic co-digestion of the marine microalga Nannochloropsis salina with energy crops. Bioresour. Technol. 2013, 148, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes: An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Matsui, T.; Koike, Y. Methane fermentation of a mixture of seaweed and milk at a pilot-scale plant. J. Biosci. Bioeng. 2010, 110, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, X.; Wen, B.; Wang, X.; Zhu, W.; Cui, Z. Methane potential and microbial community dynamics in anaerobic digestion of silage and dry cornstalks: A substrate exchange study. Appl. Biochem. Biotechnol. 2017, 181, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Nikolausz, M.; Zhang, J.; Riya, S.; Terada, A.; Hosomi, M. Variation of the microbial community in thermophilic anaerobic digestion of pig manure mixed with different ratios of rice straw. J. Biosci. Bioeng. 2016, 122, 334–340. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Benn, N.; Seyedi, S.; Zitomer, D. Methane yield and lag correlate with bacterial community shift following bioplastic anaerobic co-digestion. Bioresour. Technol. Rep. 2019, 7, 100198. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, W.; Zheng, Y.; Lian, T.; Dong, H. Production of short-chain carboxylic acids by co-digestion of swine manure and corn silage: Effect of carbon-nitrogen ratio. Trans. ASABE 2020, 63, 445–454. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Dudek, M.; Grala, A. Effect of algae biomass addition on the effectiveness of methane fermentation of hay silage. Monogr. Kom. Inżynierii Sr. 2012, 100, 115–124. (In Polish) [Google Scholar]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M. Effect of algae biomass addition on the effectiveness of methane fermentation of maize silage. In Proceedings of the 4th International Symposium on Energy from Biomass and Waste 2012, San Servolo, Venice, Italy, 12–15 November 2012. [Google Scholar]
- Catenacci, A.; Boniardi, G.; Mainardis, M.; Gievers, F.; Farru, G.; Asunis, F.; Canziani, R. Processes, applications and legislative framework for carbonized anaerobic digestate: Opportunities and bottlenecks. A critical review. Energy Convers. Manag. 2022, 263, 115691. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.Y. Review Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef]
- Oehmichen, K.; Thrän, D. Fostering renewable energy provision from manure in Germany—Where to implement GHG emission reduction incentives. Energy Policy 2017, 110, 471–477. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural use of biogas digestate as a replacement fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Villa, R.; Ortega Rodriguez, L.; Fenech, C.; Anika, O.C. Ensiling for anaerobic digestion: A review of key considerations to maximise methane yields. Renew. Sustain. Energy Rev. 2020, 134, 110401. [Google Scholar] [CrossRef]
- Camacho-Muñoz, R.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Anaerobic biodegradation under slurry thermophilic conditions of poly(lactic acid)/starch blend compatibilized by maleic anhydride. Int. J. Biol. Macromol. 2020, 163, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.-H.; Kim, D.-H. Fermentative hydrogen production using lignocellulose biomass: An overview of pre-treatment methods, inhibitor effects and detoxification experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manag. Res. 2019, 37, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameter | Unit | Variant | ||||||
|---|---|---|---|---|---|---|---|---|
| 1—MA100+MS0 | 2—MA80+MS20 | 3—MA60+MS40 | 4—MA40+MS60 | 5—MA20+MS80 | 6—MA0 + MS100 | Inoculum | ||
| Dry matter (TS) | % | 10.4 ± 1.5 | 12.0 ± 1.4 | 14.0 ± 1.2 | 17.1 ± 1.1 | 21.8 ± 1.0 | 30.2 ± 0.9 | 3.8 ± 0.2 |
| Dry organic matter (VS) | % TS | 87.7 ± 1.1 | 88.9 ± 0.9 | 90.2 ± 0.7 | 91.4 ± 0.5 | 92.6 ± 0.4 | 93.8 ± 0.2 | 68.5 ± 2.5 |
| Total nitrogen (TN) | mg/gTS | 46.0 ± 3.9 | 39.0 ± 3.3 | 32.0 ± 2.7 | 25.1 ± 2.1 | 18.1 ± 1.5 | 11.1 ± 0.9 | 33.1 ± 3.4 |
| Total phosphorus (TP) | mg/gTS | 4.4 ± 0.9 | 4.0 ± 0.8 | 3.6 ± 0.7 | 3.2 ± 0.5 | 2.8 ± 0.4 | 2.4 ± 0.3 | 1.7 ± 0.2 |
| Total carbon (TC) | mg/gTS | 464 ± 25 | 463 ± 23 | 462 ± 20 | 462 ± 18 | 461 ± 15 | 460 ± 13 | 309 ± 28 |
| Total organic carbon (TOC) | mg/gTS | 437 ± 20 | 438 ± 19 | 439 ± 18 | 440 ± 17 | 440 ± 16 | 441 ± 15 | 199 ± 34 |
| C:N ratio | - | 9.5 ± 0.4 | 11.2 ± 0.7 | 13.7 ± 0.9 | 17.5 ± 1.2 | 24.3 ± 1.4 | 39.6 ± 1.7 | 9.4 ± 0.1 |
| pH | - | 8.1 ± 0.8 | 7.8 ± 0.6 | 7.4 ± 0.5 | 6.9 ± 0.4 | 6.0 ± 0.2 | 4.4 ± 0.1 | 7.2 ± 0.3 |
| Protein | % TS | 28.7 ± 2.5 | 20.9 ± 2.1 | 13.1 ± 1.7 | 24.8 ± 1.4 | 17.0 ± 1.0 | 9.2 ± 0.6 | 20.7 ± 2.8 |
| Lipids | % TS | 20.0 ± 1.4 | 12.9 ± 1.2 | 5.8 ± 1.0 | 16.4 ± 0.9 | 9.3 ± 0.7 | 2.2 ± 0.5 | 3.1 ± 0.5 |
| Sugars | % TS | 15.8 ± 2.6 | 33.7 ± 2.3 | 51.5 ± 2.0 | 24.8 ± 1.6 | 42.6 ± 1.3 | 60.4 ± 1.0 | 1.6 ± 0.4 |
| Variant | Reactor Active Volume (dm3) | OLR (gVS/dm3·d) | MA Wet Mass (g/d) | MS Wet Mass (g/d) | Initial Water Content of MA + MS Mixture (%) | Volume of Water Used for Dilution (cm3) | Daily Volume Supply of Feedstock (cm3) | Mixture Water Content (%) | VS (% TS) | HRT (d) | Days of Experiment (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1: MA100/MS0 | 4.0 | 2.0 | 87.7 | 0 | 89.6 | 15.7 | 100 | 90.9 | 87.7 | 40 | 80 |
| 2: MA80/MS20 | 70.2 | 5.6 | 88.1 | 29.8 | 91.0 | 87.8 | |||||
| 3: MA60/MS40 | 52.6 | 11.3 | 85.9 | 41.4 | 91.0 | 87.9 | |||||
| 4: MA40/MS60 | 35.0 | 16.9 | 82.9 | 53.3 | 91.2 | 88.1 | |||||
| 5: MA20/MS80 | 17.5 | 22.6 | 78.2 | 64.5 | 91.3 | 88.4 | |||||
| 6: MA0/MS100 | 0 | 28.2 | 69.8 | 76.7 | 91.5 | 89.0 |
| Parameter | Unit | Variant | |||||
|---|---|---|---|---|---|---|---|
| 1—MA100+MS0 | 2—MA80+MS20 | 3—MA60+MS40 | 4—MA40+MS60 | 5—MA20+MS80 | 6—MA0 + MS100 | ||
| Biogas production | cm3/gTS | 384.72 ± 22.11 | 453.06 ± 26.05 | 511.42 ± 28.11 | 573.83 ± 12.54 | 567.11 ± 17.66 | 534.29 ± 7.74 |
| cm3/gVS | 438.73 ± 25.21 | 509.53 ± 11.71 | 567.32 ± 22.31 | 628.00 ± 20.05 | 612.42 ± 19.07 | 569.42 ± 8.25 | |
| cm3/d | 3509.82 ± 201.71 | 4076.21 ± 114.24 | 4538.59 ± 125.73 | 5024.01 ± 185.64 | 4899.33 ± 152.57 | 4555.39 ± 65.99 | |
| cm3/gVSremoved | 2290.00 ± 99.73 | 2117.10 ± 149.14 | 2122.07 ± 152.22 | 2608.32 ± 139.44 | 2206.65 ± 125.16 | 1780.97 ± 100.64 | |
| Methane production | cm3/gTS | 250.53 ± 18.15 | 289.60 ± 20.01 | 317.44 ± 25.21 | 347.86 ± 25.55 | 325.41 ± 31.06 | 274.36 ± 19.36 |
| cm3/gVS | 285.70 ± 18.69 | 325.69 ± 25.88 | 352.14 ± 29.46 | 380.69 ± 28.55 | 351.40 ± 33.54 | 292.40 ± 20.64 | |
| cm3/d | 2285.59 ± 199.46 | 2605.51 ± 272.94 | 2817.10 ± 256.62 | 3045.56 ± 274.06 | 2811.24 ± 268.35 | 2339.19 ± 165.09 | |
| cm3/gVSremoved | 1491.25 ± 54.62 | 1353.25 ± 40.62 | 1317.17 ± 39.99 | 1581.16 ± 69.46 | 1266.18 ± 55.94 | 914.53 ± 50.35 | |
| % | 65.12 ± 1.94 | 63.92 ± 3.02 | 62.07 ± 2.76 | 60.62 ± 4.13 | 57.38 ± 3.69 | 51.35 ± 2.88 | |
| Consortium | Variant | |||||
|---|---|---|---|---|---|---|
| 1: MA100 + MS0 | 2: MA80 + MS20 | 3: MA60 + MS40 | 4: MA40 + MS60 | 5: MA20 + MS80 | 6: MA0 + MS100 | |
| Bacteria (EUB338) | 72 ± 2 | 68 ± 3 | 70 ± 3 | 69 ± 4 | 69 ± 3 | 71 ± 4 |
| Archaea (ARC915) | 24 ± 1 | 26 ± 2 | 25 ± 2 | 27 ± 3 | 26 ± 3 | 23 ± 3 |
| Methanosarcinaceae (MSMX860) | 11 ± 2 | 12 ± 2 | 11 ± 1 | 14 ± 2 | 15 ± 2 | 12 ± 1 |
| Methanosaeta (MX825) | 8 ± 1 | 9 ± 1 | 9 ± 1 | 11 ± 1 | 10 ± 1 | 9 ± 1 |
| Parameter | Unit | Variant | |||||
|---|---|---|---|---|---|---|---|
| 1: MA100 + MS0 | 2: MA80 + MS20 | 3: MA60 + MS40 | 4: MA40 + MS60 | 5: MA20 + MS80 | 6: MA0 + MS100 | ||
| TS | % | 4.7 ± 1.3 | 4.1 ± 1.4 | 4.0 ± 1.0 | 5.0 ± 0.9 | 4.3 ± 0.8 | 3.9 ± 0.9 |
| VS | % TS | 70.9 ± 2.5 | 67.5 ± 3.6 | 66.1 ± 2.8 | 69.4 ± 1.3 | 66.9 ± 1.9 | 63.8 ± 3.2 |
| TN | mg/gTS | 45.3 ± 3.1 | 40.0 ± 1.2 | 29.9 ± 3.6 | 21.5 ± 1.7 | 18.1 ± 3.1 | 13.1 ± 2.0 |
| TP | mg/gTS | 4.0 ± 1.0 | 3.6 ± 0.7 | 3.1 ± 1.1 | 2.8 ± 0.2 | 2.0 ± 0.8 | 2.3 ± 1.4 |
| TK | mg/gTS | 9.1 ± 2.4 | 8.7 ± 1.2 | 8.3 ± 1.9 | 7.9 ± 0.7 | 7.3 ± 1.6 | 7.5 ± 1.2 |
| TC | mg/gTS | 384 ± 29 | 378 ± 35 | 336 ± 18 | 381 ± 18 | 372 ± 30 | 328 ± 42 |
| TOC | mg/gTS | 316 ± 30 | 315 ± 30 | 300 ± 22 | 333 ± 22 | 330 ± 31 | 315 ± 30 |
| C:N ratio | - | 6.9 ± 0.2 | 7.8 ± 0.5 | 10.3 ± 0.1 | 15.4 ± 0.1 | 18.2 ± 0.1 | 24.0 ± 0.6 |
| pH | - | 6.7 ± 0.2 | 6.9 ± 0.4 | 7.3 ± 0.1 | 6.8 ± 0.4 | 7.0 ± 0.3 | 7.5 ± 0.2 |
| Protein | % TS | 28.3 ± 1.9 | 25.0 ± 0.8 | 18.1 ± 2.2 | 13.1 ± 1.1 | 11.2 ± 2.0 | 8.1 ± 1.3 |
| Lipids | % TS | 6.1 ± 0.8 | 4.2 ± 1.1 | 2.7 ± 0.8 | 3.0 ± 0.3 | 3.7 ± 0.7 | 3.0 ± 0.5 |
| Sugars | % TS | 1.8 ± 0.5 | 2.0 ± 0.8 | 1.8 ± 0.4 | 2.0 ± 0.3 | 1.3 ± 0.1 | 2.5 ± 0.5 |
| ɳFMSS | % | 60.43 ± 1.68 | 68.92 ± 1.55 | 70.35 ± 2.31 | 70.27 ± 1.82 | 67.38 ± 1.52 | 71.84 ± 2.12 |
| ɳVS | % | 58.25 ± 1.22 | 64.98 ± 2.01 | 66.58 ± 1.89 | 65.24 ± 1.95 | 62.60 ± 2.23 | 67.11 ± 1.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Bartkowska, I. Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics. Appl. Sci. 2022, 12, 7291. https://doi.org/10.3390/app12147291
Dębowski M, Kazimierowicz J, Zieliński M, Bartkowska I. Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics. Applied Sciences. 2022; 12(14):7291. https://doi.org/10.3390/app12147291
Chicago/Turabian StyleDębowski, Marcin, Joanna Kazimierowicz, Marcin Zieliński, and Izabela Bartkowska. 2022. "Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics" Applied Sciences 12, no. 14: 7291. https://doi.org/10.3390/app12147291
APA StyleDębowski, M., Kazimierowicz, J., Zieliński, M., & Bartkowska, I. (2022). Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics. Applied Sciences, 12(14), 7291. https://doi.org/10.3390/app12147291









