Comparative Study of Fresh and Frozen Broiler Neck Skin Sampled for Process Hygiene Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbial Examination
2.3. Statistical Analysis
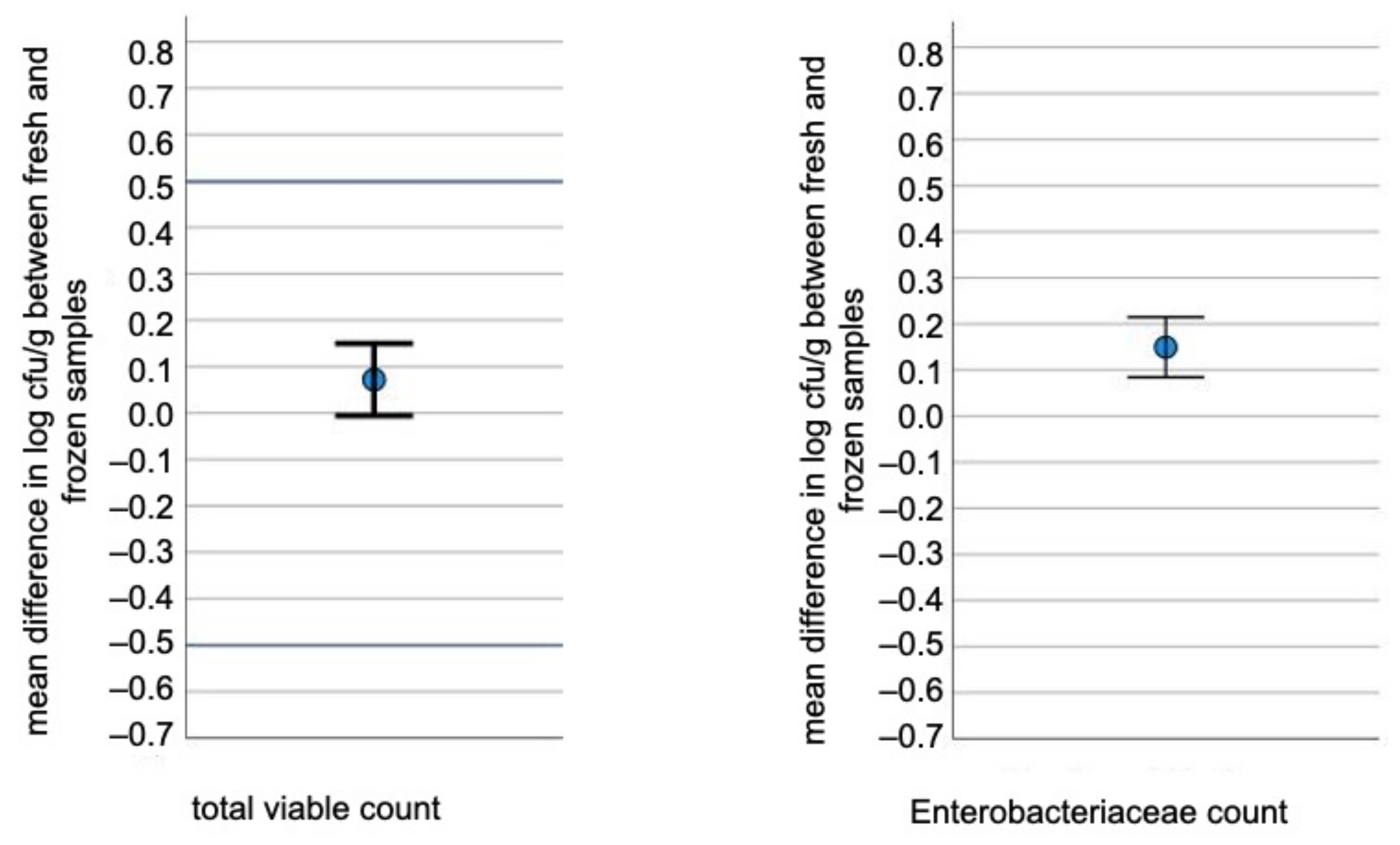
3. Results
3.1. TVC and EC Levels
3.2. Tests for Equivalence
3.3. Samples with Bacteria Counts for EC Below Minimum Limit of Detection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musgrove, M.; Cason, J.; Fletcher, D.; Stern, N.; Cox, N.; Bailey, J. Effect of cloacal plugging on microbial recovery from partially processed broilers. Poult. Sci. 1997, 76, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Kotula, K.L.; Pandya, Y. Bacterial contamination of broiler chickens before scalding. J. Food Prot. 1995, 58, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Corry, J.E.; Allen, V.; Hudson, W.; Breslin, M.; Davies, R. Sources of Salmonella on broiler carcasses during transportation and processing: Modes of contamination and methods of control. J. Appl. Microbiol. 2002, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ellerbroek, L.; Lienau, J.A.; Klein, G. Campylobacter spp. in broiler flocks at farm level and the potential for cross-contamination during slaughter. Zoonoses Public Health 2010, 57, e81–e88. [Google Scholar] [CrossRef]
- Izat, A.; Gardner, F.; Denton, J.; Golan, F. Incidence and level of Campylobacter jejuni in broiler processing. Poult. Sci. 1988, 67, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Ellerbroek, L.; Lox, C. Untersuchungen zur Eignung der Halshaut für die mikrobiologische Prozesskontrolle von frischem Geflügelfleisch mit dem Biolumineszenzverfahren. DTW. Dtsch. Tierärztliche Wochenschr. 2004, 111, 181–184. [Google Scholar]
- Commission of the European Communities. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 50, 1–26. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 15 June 2022).
- Hutchison, M.; Walters, L.; Mead, G.; Howell, M.; Allen, V. An assessment of sampling methods and microbiological hygiene indicators for process verification in poultry slaughterhouses. J. Food Prot. 2006, 69, 145–153. [Google Scholar] [CrossRef]
- Gill, C.; Badoni, M. Recovery of bacteria from poultry carcasses by rinsing, swabbing or excision of skin. Food Microbiol. 2005, 22, 101–107. [Google Scholar] [CrossRef]
- Avens, J.; Miller, B. Quantifying bacteria on poultry carcass skin. Poult. Sci. 1970, 49, 1309–1315. [Google Scholar] [CrossRef]
- Barer, M.R. Bacterial growth, physiology and death. In Medical Microbiology. A Guide to Microbial Infections: Pathogenesis, Immunity, Laboratory Diagnosis and Control, 18th ed.; Greenwood, D., Barer, M., Slack, R., Irving, W., Eds.; Churchill Livingstone/Elsevier: Edinburgh, NY, USA, 2012; pp. 39–53. [Google Scholar]
- Rahman, M.S.; Velez-Ruiz, J.F. Food preservation by freezing. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 636–665. [Google Scholar]
- Zhou, G.; Xu, X.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Speck, M.; Ray, B. Effects of freezing and storage on microorganisms in frozen foods: A review. J. Food Prot. 1977, 40, 333–336. [Google Scholar] [CrossRef] [PubMed]
- ICMSF. Temperature. In Microorganisms in Foods 3: Microbial Ecology of Foods; Silliker, H.J., Elliott, R.P., Baird-Parker, A.C., Bryan, F.L., Christian, J.H.B., Clark, D.S., Olson, J.C., Roberts, T.A., Eds.; Academic Press Inc.: New York, NY, USA, 1980; pp. 1–37. [Google Scholar]
- Perez-Chabela, M.; Mateo-Oyagüe, J. Frozen meat: Quality and shelf life. In Handbook of Frozen Foods; Hui, Y.H., Cornillon, P., Legaretta, I.G., Lim, M.H., Murrell, K.D., Nip, W.-K., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 201–214. [Google Scholar]
- Rose, A. Physiology of Micro-organisms at Low Temperatures. J. Appl. Bacteriol. 1968, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Schmidt-Lorenz, W.; Gutschmidt, J. Mikrobielle und sensorische Veränderungen gefrorener Brathähnchen und Poularden bei Lagerung im Temperaturbereich von −2,5° bis −10 °C. Fleischwirtschaft 1969, 8, 1033–1041. [Google Scholar]
- Mossel, D.A.A. Microbiology of Foods: The Ecological Essentials of Assurance and Assessment of Safety and Quality, 3rd ed.; University of Utrecht, Faculty of Veterinary Medicine: Utrecht, The Netherlands, 1982; 188p. [Google Scholar]
- Reinartz, M. Einfluss des Tiefgefrierens und der Tiefkühllagerung auf die Mikroflora von vier handelsüblichen Tiefkühlprodukten. Doctor of veterinary medicine, Freie Universität Berlin, Berlin, Germany, 9 November 2011. Available online: https://refubium.fu-berlin.de/handle/fub188/5243 or https://refubium.fu-berlin.de/bitstream/handle/fub188/5243/Reinartz_online.pdf?sequence=1&isAllowed=y (accessed on 30 June 2022).
- Haines, R. The effect of freezing on bacteria. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1938, 124, 451–463. [Google Scholar]
- Fries, R.; Eggerding, B. Bacterial reduction in deep-frozen sterile poultry meat. Arch. Lebensm. 1997, 48, 121–144. [Google Scholar]
- Vieira, C.; Diaz, M.; Martínez, B.; García-Cachán, M. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef]
- Alrabadi, N.I. The effect of freezing on different bacterial counts in raw milk. Int. J. Biol. 2015, 7, 9–12. [Google Scholar] [CrossRef]
- Hubáčková, M.; Ryšánek, D. Effects of freezing milk samples on the recovery of alimentary pathogens and indicator microorganisms. Acta Vet. Brno 2007, 76, 301–307. [Google Scholar] [CrossRef]
- Medić, H.; Kušec, I.D.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef]
- Schukken, Y.; Smit, J.; Grommers, F.; Vandegeer, D.; Brand, A. Effect of freezing on bacteriologic culturing of mastitis milk samples. J. Dairy Sci. 1989, 72, 1900–1906. [Google Scholar] [CrossRef]
- Green, H.P.; Johnson, J.A.; Furuno, J.P.; Strauss, S.M.; Perencevich, E.N.; Lautenbach, E.; Lee, D.; Harris, A.D. Impact of freezing on the future utility of archived surveillance culture specimens. Infect. Control Hosp. Epidemiol. 2007, 28, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Regnath, T. Stammhaltung. In Mikrobiologische Diagnostik: Bakteriologie-Mykologie-Virologie-Parasitologie, Teil II: Mikrobiologische Untersuchungsmethoden; Neumeister, B., Geiss, H.K., Braun, R., Kimmig, P., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2009; pp. 171–173. [Google Scholar]
- Schrödel, A. Wie werden Bakterien optimal gelagert? Biol. Unserer Zeit 2009, 39, 372. [Google Scholar] [CrossRef]
- Redmond, G.; Gormley, T.; Butler, F. The effect of short-and long-term freeze-chilling on the quality of mashed potato. Innov. Food Sci. Emerg. Technol. 2003, 4, 85–97. [Google Scholar] [CrossRef]
- Redmond, G.; Gormley, T.; Butler, F. The effect of short-and long-term freeze-chilling on the quality of cooked green beans and carrots. Innov. Food Sci. Emerg. Technol. 2004, 5, 65–72. [Google Scholar] [CrossRef]
- Böhmler, G.; Seide, K.; Thiem, I. Einfrieren von Lebensmittelproben—Ermittlung des Einflusses auf den Keimgehalt. In Proceedings of the 49th Arbeitstagung des Arbeitsgebietes Lebensmittelhygiene—Dreiländertagung, Garmisch-Partenkirchen, Germany, 29 September–2 October 2008; p. 135. [Google Scholar]
- DIN EN ISO 7218:2014-02: Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations (ISO 7218:2007 + Amd 1:2013); German Version EN ISO 7218:2007 + A1:2013 (Mikrobiologie von Lebensmitteln und Futtermitteln—Allgemeine Anforderungen und Leitlinien für Mikrobiologische Untersuchungen (ISO 7218:2007 + Amd 1:2013, Korrigierte Fassung 2014-04-15); Deutsche Fassung EN ISO 7218:2007 + A1:2013), 2014. Available online: https://www.beuth.de/de/norm/din-en-iso-7218/216839549 (accessed on 15 June 2022).
- DIN 10161:2016-12: Microbiological Analysis of Meat and Meat Products—Aerobic Counts at 30 °C—Drop Plating Method (German Norm, Original Title: DIN 10161:2016-12 Mikrobiologische Untersuchung von Fleisch und Fleischprodukten—Bestimmung der Aeroben Keimzahl bei 30 °C—Tropfplattenverfahren). 2016. Available online: https://www.beuth.de/de/norm/din-10161/263799680 (accessed on 15 June 2022).
- DIN 10164-2:2019-06: Microbiological Examination of Meat and Meat Products—Determination of Enterobacteriaceae—Part 2: Drop Plating Method (German Norm: Original Title: Mikrobiologische Untersuchung von Fleisch und Fleischprodukten—Bestimmung von Enterobacteriaceae—Teil 2: Tropfplatten-Verfahren), 2019. Available online: https://www.beuth.de/de/norm/din-10164-2/302005342 (accessed on 15 June 2022).
- Thrusfield, M. 17 Clinical trials. In Veterinary Epidemiology, 4th ed.; Thrusfield, M., Christley, R., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 2018; pp. 361–382. [Google Scholar]
- Hübner, P.; Gautsch, S.; Jemmi, T. In house validation (single laboratory validation) of microbiological methods. Mittelungen Aus Lebensm. Und Hyg. 2002, 93, 118–139. [Google Scholar]
- Anonymous. PLQML-5010-09 Entscheidungsregeln nach DIN EN ISO-IEC 17025-2018. Allgemeines Vorgehen, 2020. Available online: https://www.oslab.de/fileadmin/content/pdf/diverse_Scheine/PLQML-5010-09_Entscheidungsregeln_nach_DIN_EN_ISO-IEC_17025-2018._Allgemeines_Vorgehen.pdf (accessed on 9 May 2022).
- SAS. Leitfaden zur Validierung Mikrobiologischer Prüfverfahren und zur Abschätzung der Messunsicherheit im Bereich Lebensmittel-und Umweltmikrobiologie, Dokument 328dw. Available online: https://www.sas.admin.ch/dam/sas/de/dokumente/Wie%20wird%20meine%20Stelle%20akkreditiert/Pr%C3%BCflaboratorien%20STS/328.pdf.download.pdf/328d.pdf (accessed on 9 May 2022).
- Kluth, I.-K.; Teuteberg, V.; Ploetz, M.; Krischek, C. Effects of freezing temperatures and storage times on the quality and safety of raw turkey meat and sausage products. Poult. Sci. 2021, 100, 101305. [Google Scholar] [CrossRef]
- Krischek, C.; Teuteberg, V.; Kluth, I.-K.; Plötz, M. Gefrieren von Fleisch. Fleischwirtschaft 2021, 11, 89–94. [Google Scholar]
- Georgsson, F.; Þorkelsson, Á.E.; Geirsdóttir, M.; Reiersen, J.; Stern, N.J. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 2006, 23, 677–683. [Google Scholar] [CrossRef]
- Petzer, I.-M.; Karzis, J.; Van der Schans, T.J.; Watermeyer, J.C.; Eloff, S.; Fosgate, G.T. Comparing effects of freezing at −196 °C and −20 °C on the viability of mastitis pathogens. Onderstepoort J. Vet. Res. 2012, 79, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langkabel, N.; Oswaldi, V.; Merle, R.; Dzierzon, J.; Meemken, D. Comparative Study of Fresh and Frozen Broiler Neck Skin Sampled for Process Hygiene Purposes. Appl. Sci. 2022, 12, 6701. https://doi.org/10.3390/app12136701
Langkabel N, Oswaldi V, Merle R, Dzierzon J, Meemken D. Comparative Study of Fresh and Frozen Broiler Neck Skin Sampled for Process Hygiene Purposes. Applied Sciences. 2022; 12(13):6701. https://doi.org/10.3390/app12136701
Chicago/Turabian StyleLangkabel, Nina, Verena Oswaldi, Roswitha Merle, Janine Dzierzon, and Diana Meemken. 2022. "Comparative Study of Fresh and Frozen Broiler Neck Skin Sampled for Process Hygiene Purposes" Applied Sciences 12, no. 13: 6701. https://doi.org/10.3390/app12136701
APA StyleLangkabel, N., Oswaldi, V., Merle, R., Dzierzon, J., & Meemken, D. (2022). Comparative Study of Fresh and Frozen Broiler Neck Skin Sampled for Process Hygiene Purposes. Applied Sciences, 12(13), 6701. https://doi.org/10.3390/app12136701





