Influence of Finely Chopped Meat Addition on Quality Parameters of Minced Meat
Abstract
1. Introduction
2. Materials and Methods
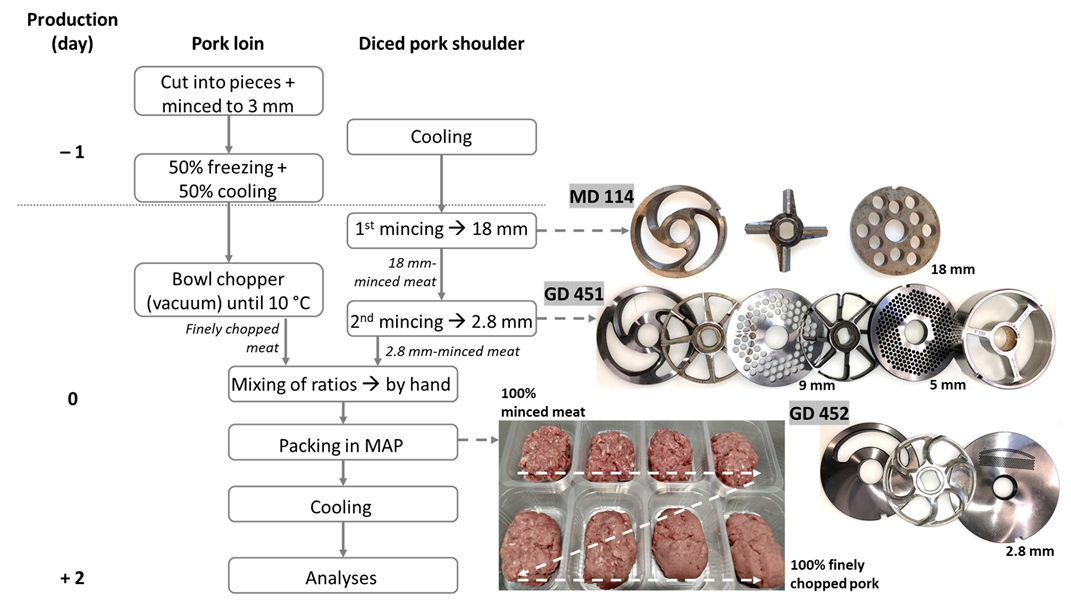
2.1. Meat Processing
2.1.1. Finely Chopped Pork Loin
2.1.2. Minced Pork Shoulder
2.1.3. Addition of Finely Chopped Pork Loin to Minced Pork Shoulder
2.2. Analyses of Proximate Composition
2.2.1. Fat
2.2.2. Protein
2.2.3. pH Values
2.2.4. Moisture
2.3. Determination of Water Holding Capacity
2.3.1. Filter Press
2.3.2. Cooking Loss
2.4. Histological Analysis of Amount of Non-Intact Cells
2.5. Images
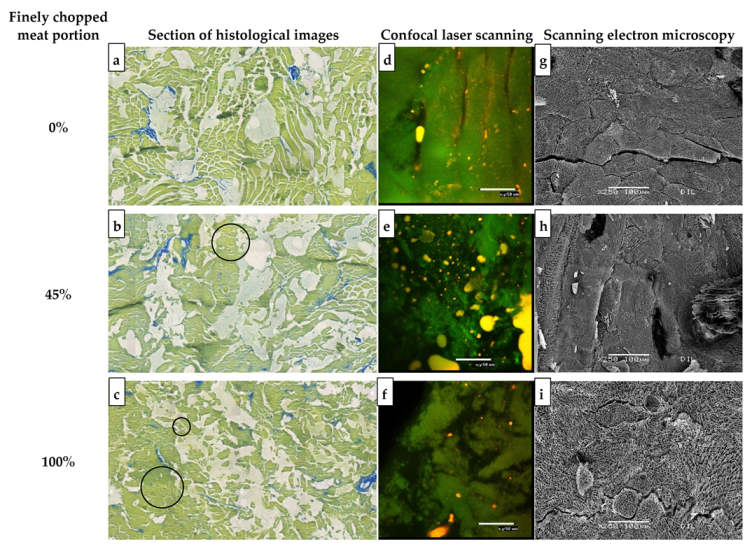
2.5.1. Confocal Laser Scanning Microscopy
2.5.2. Scanning Electron Microscopy
2.6. Texture Analyses
2.6.1. Mechanical
2.6.2. Sensory
2.7. Statistics
3. Results and Discussion
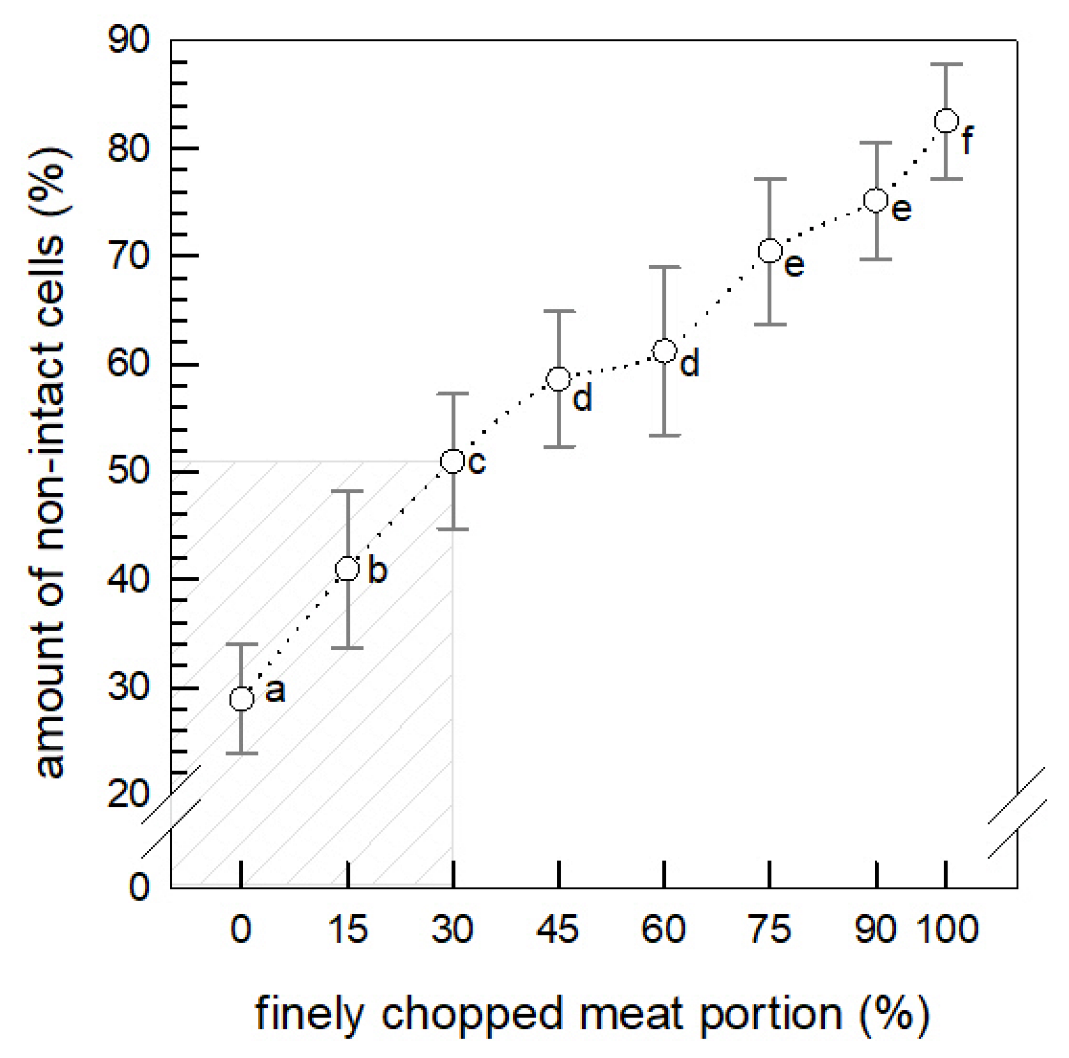
3.1. Cell Disintegration
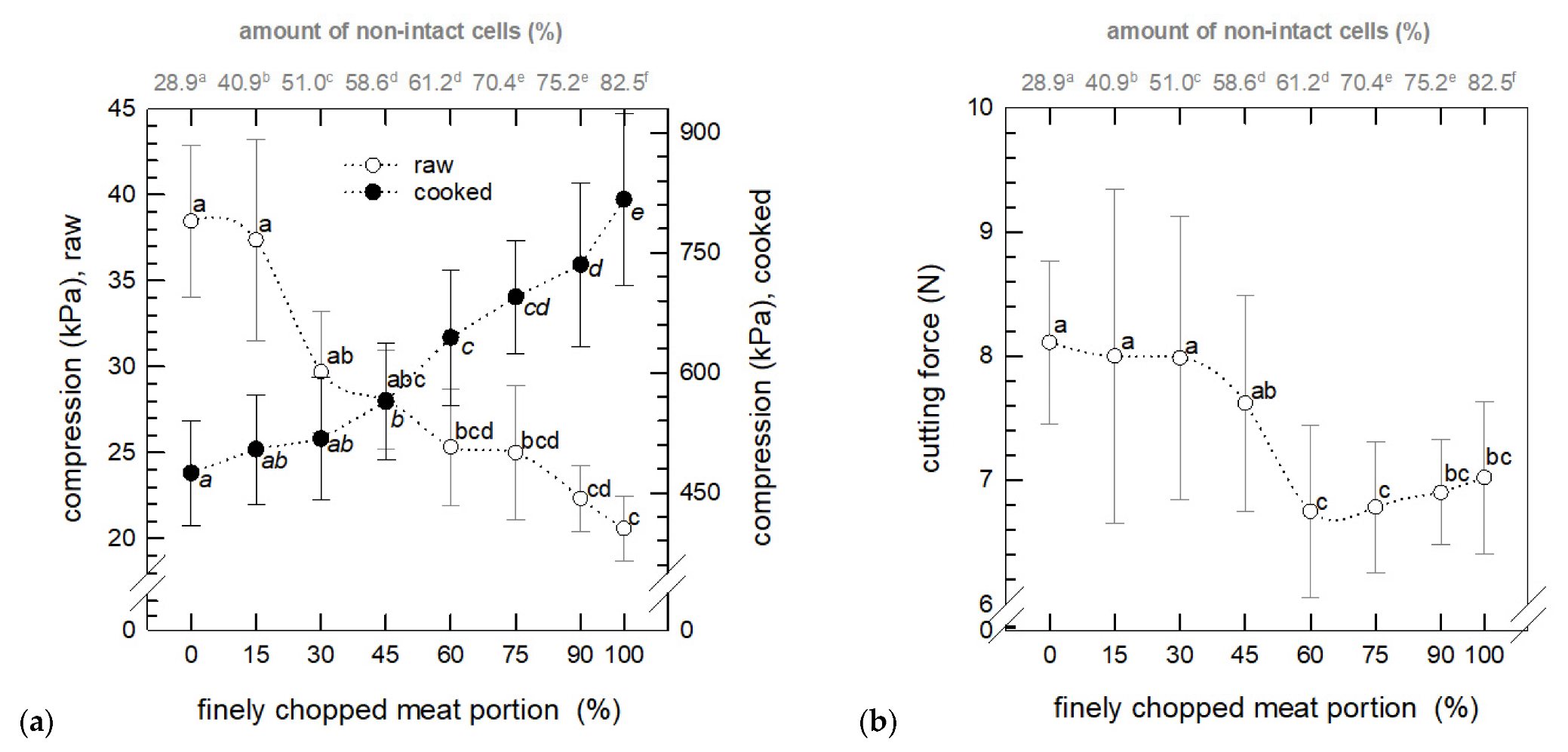
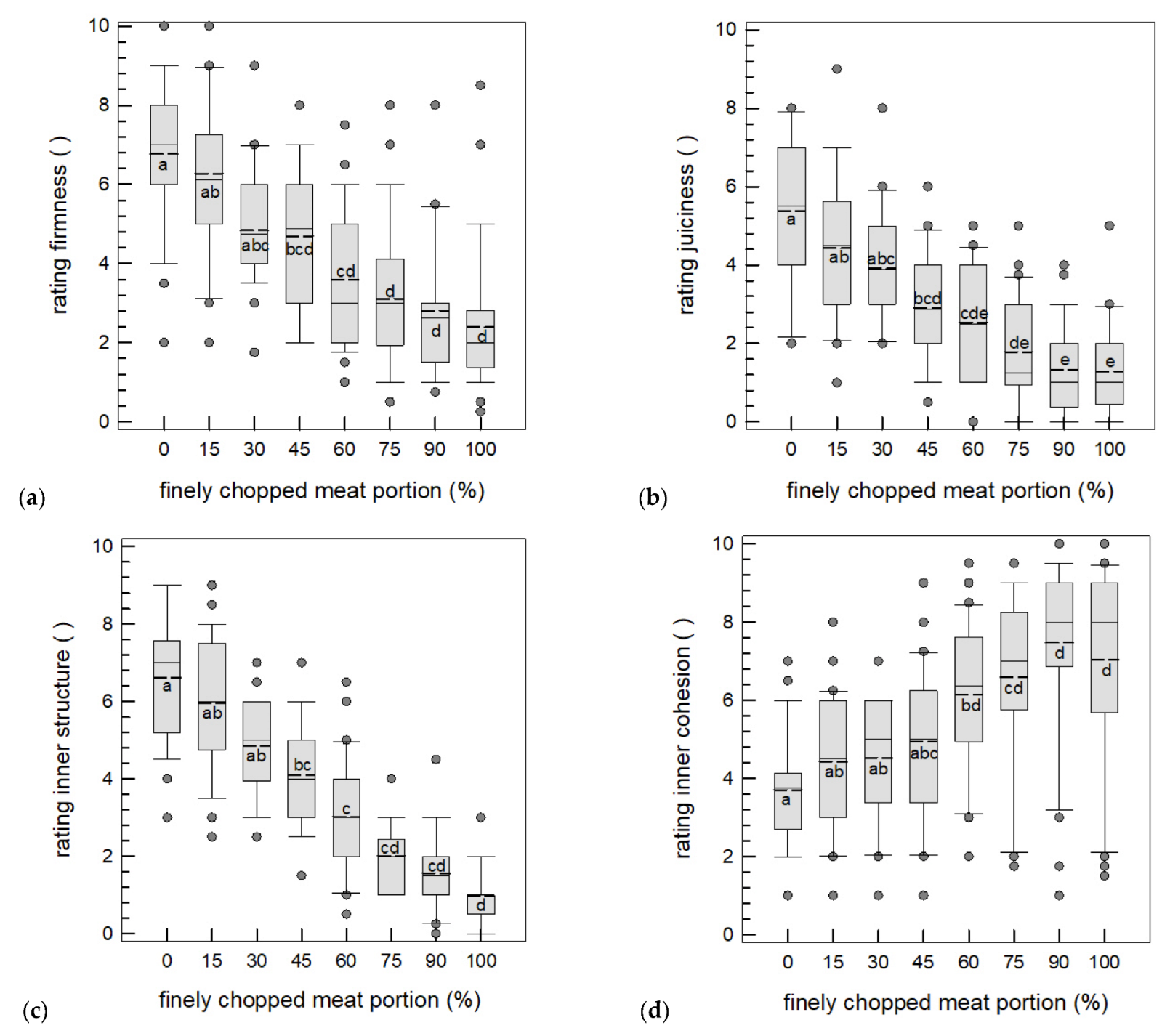
3.1.1. Amount of Non-Intact Cells
3.1.2. Imaging
3.2. Quality Characterization of Minced Meat
3.2.1. Water Holding Capacity
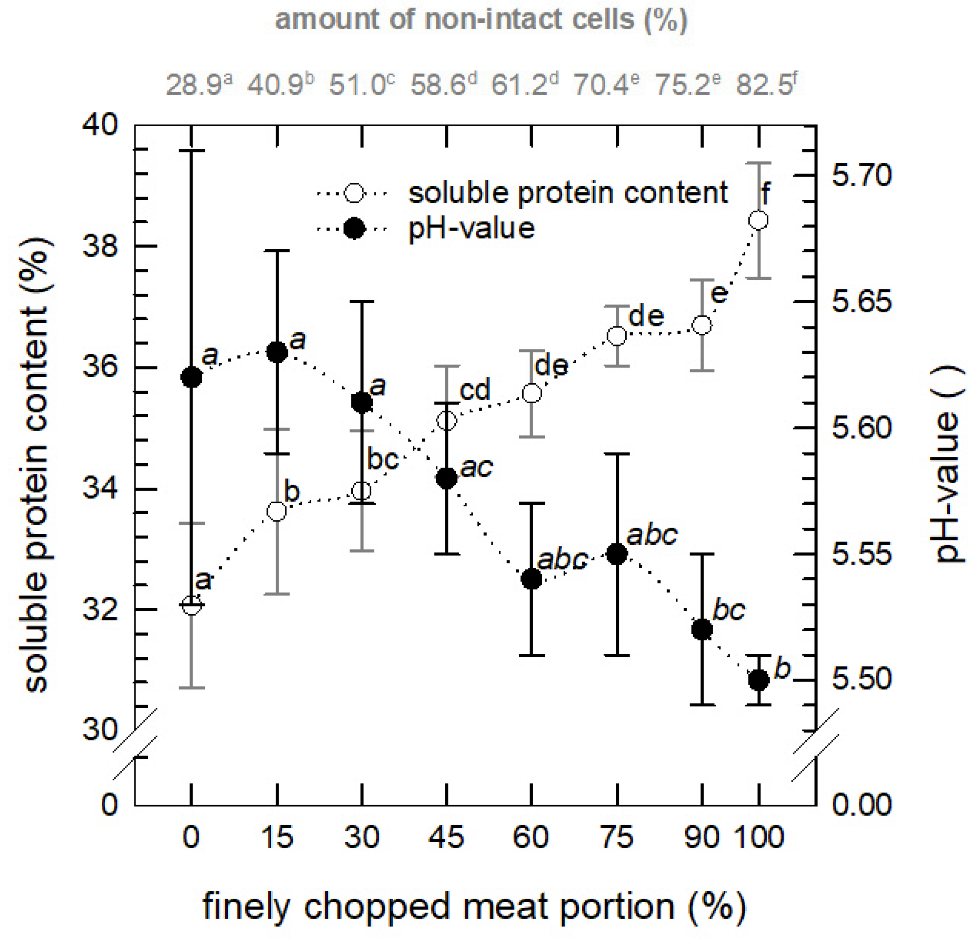
3.2.2. Soluble Protein and pH Value
3.3. Texture Analyses
3.3.1. Mechanical Texture Analysis
3.3.2. Sensory Texture Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreher, J.; König, M.; Herrmann, K.; Terjung, N.; Gibis, M.; Weiss, J. Varying the amount of solid fat in animal fat mimetics for plant-based salami analogues influences texture, appearance and sensory characteristics. LWT—J. Food Sci. Technol. 2021, 143, 111140. [Google Scholar] [CrossRef]
- Baune, M.-C.; Schroeder, S.; Witte, F.; Heinz, V.; Bindrich, U.; Weiss, J.; Terjung, N. Analysis of protein-network formation of different vegetable proteins during emulsification to produce solid fat substitutes. J. Food Meas. Charact. 2021, 15, 2399–2416. [Google Scholar] [CrossRef]
- Profeta, A.; Baune, M.-C.; Smetana, S.; Bornkessel, S.; Broucke, K.; Van Royen, G.; Enneking, U.; Weiss, J.; Heinz, V.; Hieke, S. Preferences of german consumers for meat products blended with plant-based proteins. Sustainability 2021, 13, 650. [Google Scholar] [CrossRef]
- He, J.; Evans, N.M.; Liu, H.; Shao, S. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Terjung, N.; Witte, F.; Heinz, V. The dry aged beef paradox: Why dry aging is sometimes not better than wet aging. Meat Sci. 2021, 172, 108355. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Smetana, S.; Heinz, V.; Terjung, N. High-pressure processing of usually discarded dry aged beef trimmings for subsequent processing. Meat Sci. 2020, 170, 108241. [Google Scholar] [CrossRef]
- Berger, L.; Gibis, M.; Witte, F.; Terjung, N.; Weiss, J. Influence of meat batter addition in ground beef on structural properties and quality parameters. Eur. Food Res. Technol. 2022, 248, 2509–2520. [Google Scholar] [CrossRef]
- Berger, L.; Witte, F.; Terjung, N.; Weiss, J.; Gibis, M. Influence of Processing Steps on Structural, Functional, and Quality Properties of Beef Hamburgers. Appl. Sci. 2022, 12, 7377. [Google Scholar] [CrossRef]
- Zerabruk, K.; Retta, N.; Muleta, D.; Tefera, A.T. Assessment of microbiological safety and quality of minced meat and meat contact surfaces in selected butcher shops of Addis Ababa, Ethiopia. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef]
- Nortjé, G.; Nel, L.; Jordaan, E.; Naudé, R.; Holzapfel, W.; Grimbeek, R. A microbiological survey of fresh meat in the supermarket trade. Part 2: Beef retail cuts. Meat Sci. 1989, 25, 99–112. [Google Scholar] [CrossRef]
- Davey, C.L.; Gilbert, K.V. Temperature-dependent cooking toughness in beef. J. Sci. Food Agric. 1974, 25, 931–938. [Google Scholar]
- Ranken, M.D. Handbook of Meat Product Technology; Blackwell Science Oxford: Oxford, UK, 2000; Volume 246. [Google Scholar]
- Foegeding, E.A. Gelation in meat batters. In Proceedings of the Reciprocal Meat Conference Proceedings, Laramie, WY, USA, 12–15 June 1988; pp. 44–47. [Google Scholar]
- Beneke, B. Technologie verändert die Muskelstruktur: Histologische Identifikation und Beurteilung bei Fleisch und Fleischerzeugnissen. Fleischwirtschaft 2018, 2018, 62–68. [Google Scholar]
- Tornberg, E. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Knipe, C. Types of sausages—Emulsion. In Encyclopedia of Meat Sciences; Jensen, W., Devine, C., Dikemann, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1126–1220. [Google Scholar]
- Raudsepp, P.; Brüggemann, D.A.; Henckel, P.; Vyberg, M.; Groves, K.; Oksbjerg, N.; Therkildsen, M. Performance of conventional histochemical methods relative to a novel immunolabeling technique in assessing degree of degradation in comminuted chicken meat. Food Control 2017, 73, 133–139. [Google Scholar] [CrossRef]
- Haack, E.; Haack, O.; Schnäckel, W. Probleme, Ursachen und Lösungen—Grundlagen und Vorgänge bei der Fleischbearbeitung mit Maschinen der Wolftechnologie-1. Teil. Fleischwirtschaft 2003, 83. Available online: https://cloud.hs-anhalt.de/index.php/s/A2drwDnJS2GTrCd (accessed on 28 September 2022).
- Haack, E.; Haack, O.; Schnäckel, W. Optimal Fördern und Zerkleinern—Grundlagen und Vorgänge bei der Fleischbearbeitung mit Maschinen der Wolftechnologie-3. Teil. Fleischwirtschaft 2003, 83. Available online: https://cloud.hs-anhalt.de/index.php/s/Z2zbPpieGd283wm (accessed on 28 September 2022).
- Tyszkiewicz, I.; Kłossowska, B.M.; Wieczorek, U.; Jakubiec-Puka, A. Mechanical tenderisation of pork meat: Protein and water release due to tissue damage. J. Sci. Food Agric. 1997, 73, 179–185. [Google Scholar] [CrossRef]
- BMEL. Leitsätze für Fleisch und Fleischerzeugnisse, Neufassung Vom 25.11.2015. Bundesanzeiger AT 23.12.2015 B4. 2015. Available online: https://www.bmel.de/SharedDocs/Downloads/Ernaehrung/Lebensmittelbuch/LeitsaetzeFleisch.pdf?__blob=publicationFile (accessed on 16 October 2022).
- Honikel, K.O.; Hamm, R. Measurement of water-holding capacity and juiciness. In Quality Attributes and Their Measurement in Meat, Poultry and Fish Products; Pearson, A., Dutson, T., Eds.; Springer: Boston, MA, USA, 1994; Volume 8, pp. 125–161. [Google Scholar]
- De Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner–Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar]
- Irmscher, S.B.; Gibis, M.; Herrmann, K.; Kohlus, R.; Weiss, J. Development of a novel homogenizer using the vane pump-grinder technology for the production of meat batter. J. Food Eng. 2016, 169, 10–17. [Google Scholar]
- Schiel, L.; Wind, C.; Krueger, S.; Braun, P.G.; Koethe, M. Applicability of analytical methods for determining the composition of edible insects in German Food Control. J. Food Compos. Anal. 2022, 122, 104676. [Google Scholar] [CrossRef]
- Grau, R.; Hamm, R. Über das Wasserbindevermögen des Säugetiermuskels. Z. Für Lebensm. Und-Forsch. 1957, 105, 446. [Google Scholar] [CrossRef]
- Irmscher, S.B.; Rühl, S.; Herrmann, K.; Gibis, M.; Kohlus, R.; Weiss, J. Determination of process-structure relationship in the manufacturing of meat batter using vane pump-grinder systems. Food Bioprocess Technol. 2015, 8, 1512–1523. [Google Scholar] [CrossRef]
- Friedelsheimer, B.; Büchl-Zimmermann, S.; Welsch, U. Schnittpräparation für die Lichtmikroskopie. In Romeis Mikroskopische Technik; Mulisch, M., Welsch, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- BVL, Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Amtliche Sammlung von Untersuchungsverfahren nach § 64 Lebensmittel- und Futtermittelgesetzbuch (LFGB), § 38 Tabakerzeugnisgesetz (TabakerzG), §28b Gentechnikgesetz (GenTG)-Verfahren zur Probennahme und Untersuchung von Lebensmitteln; Beuth Verlag GmbH: Berlin, Germany, 2017. [Google Scholar]
- Smetana, S.; Larki, N.A.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- Hildebrandt, G.; Jöckel, J. Die Nachweismöglichkeit von wie Brühwurstbrät fein zerkleinertem Fleisch in Modellversuchen. Fleischwirtschaft 1980, 60, 392–403. [Google Scholar]
- Irmscher, S.B.; Böjthe, Z.; Herrmann, K.; Gibis, M.; Kohlus, R.; Weiss, J. Influence of filling conditions on product quality and machine parameters in fermented coarse meat emulsions produced by high shear grinding and vacuum filling. J. Food Eng. 2013, 117, 316–325. [Google Scholar] [CrossRef]
- Otto-Kuhn, D.; Tichaczek-Dischinger, P. Hackfleisch aus handwerklicher Herstellung oder aus der Fertigpackung—ein qualitativer Vergleich. Fleischwirtschaft 2015, 95, 104–107. [Google Scholar]
- Project-Attending Committee (Quakenbrück, Stuttgart). Personal communication, 2019.
- Schering, B. Zur aktuellen Bedeutung der Lebensmittel-Histologie—Entwicklung der Fragestellungen, §64 Methoden und Validierung—Teil 1: Bezug auf Fleischerzeugnisse. Fleischwirtschaft 2015, 95, 103–107. [Google Scholar]
- Schering, B. Zur aktuellen Bedeutung der Lebensmittel-Histologie—Entwicklung der Fragestellungen, § 64 Methoden und Validierung—Teil 2: Die Entwicklung der Fragestellungen für die Lebensmittel-Histologie. Fleischwirtschaft 2015, 95, 94–98. [Google Scholar]
- Beck, K.; Eißner, A.; Rien, K.; Wenisch, S.; Lücker, E. Der Nachweis des Gefrierens bei Hackfleisch im Rahmen der lebensmittel-histologischen Untersuchung; Arbeitstagung der DVG: Garmisch, Germany, 2011. [Google Scholar]
- Herrero, A.M.; Carmona, P.; LóPez-LóPez, I.; JiméNez-Colmenero, F. Raman spectroscopic evaluation of meat batter structural changes induced by thermal treatment and salt addition. J. Agric. Food Chem. 2008, 56, 7119–7124. [Google Scholar] [CrossRef]
- Souci, S.; Fachmann, W.; Kraut, H. Fleisch. In Die Zusammensetzung der Lebensmittel Nährwert-Tabellen 1989/90; Souci, S., Fachmann, W., Kraut, H., Eds.; Wissenschaftliche Verlags-GmbH: Stuttgart, Germany, 1989. [Google Scholar]
- Leseigneur-Meynier, A.; Gandemer, G. Lipid composition of pork muscle in relation to the metabolic type of the fibres. Meat Sci. 1991, 29, 229–241. [Google Scholar] [CrossRef]
- Offer, G.; Knight, P.; Jeacocke, R.; Almond, R.; Cousins, T.; Elsey, J.; Parsons, N.; Sharp, A.; Starr, R.; Purslow, P. The structural basis of the water-holding, appearance and toughness of meat and meat products. Food Struct. 1989, 8, 17. [Google Scholar]
- Hamm, R. Water-holding capacity of meat. In Meat; Cole, D., Lawrie, R., Eds.; Butterworths: London, UK, 1975; p. 321. [Google Scholar]
- Warner, R.D. The eating quality of meat—IV water-holding capacity and juiciness. In Lawrie´ s Meat Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 419–459. [Google Scholar]
- Hughes, J.; Oiseth, S.; Purslow, P.; Warner, R. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Krickmeier, J. Modellierung der Bedingungen beim Schneiden insbesondere in einer Wolfmaschine mit dem Ziel der Optimierung des Zerkleinerungsprozesses sowie der Erhöhung der Produktqualität. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2015. [Google Scholar]
- Palka, K.; Wesierska, E. Physics and Chemistry. In Encyclopedia of Meat Sciences, 2nd ed.; Devine, C., Dikeman, M., Eds.; Elsevier: London, UK; Waltham, UK; San Diego, CA, USA, 2014; Volume 1, pp. 404–409. [Google Scholar]
- Bejerholm, C.; Tørngren, M.; Aaslyng, M. Cooking of Meat. In Encyclopedia of Meat Sciences, 2nd ed.; Devine, C., Dikeman, M., Eds.; Elsevier: London, UK; Waltham, UK; San Diego, CA, USA, 2014; Volume 1, pp. 370–376. [Google Scholar]
- Hamm, R. Die Mikrostruktur des Muskels und ihre Beziehung zum Wasser-bindungsvermögen. Fleischwirtschaft 1963, 43, 298–301. [Google Scholar]
- Branscheid, W.; Honikel, K.; Von Lengerken, G.; Troeger, K. Qualität von Fleisch und Fleischwaren–Band 1. 2. edition. Dtsch. Fachverlag GmbH Frankf. Am Main 2007, 157–197. [Google Scholar]
- Bertram, H.C.; Kristensen, M.; Andersen, H.J. Functionality of myofibrillar proteins as affected by pH, ionic strength and heat treatment–a low-field NMR study. Meat Sci. 2004, 68, 249–256. [Google Scholar]
- Chen, X.; Tume, R.K.; Xu, X.; Zhou, G. Solubilization of myofibrillar proteins in water or low ionic strength media: Classical techniques, basic principles, and novel functionalities. Crit. Rev. Food Sci. Nutr. 2017, 57, 3260–3280. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, J.; Liu, S.; Gao, Y.; Shao, J.H. Effect of fatty acid saturation on gel properties of salt-soluble protein in pork. J. Food Sci. 2021, 86, 4037–4044. [Google Scholar]
- Honikel, K.O. Minced meats. In Encyclopedia of Meat Sciences; Academic Press: Cambridge, MA, USA, 2014; pp. 422–424. [Google Scholar]
- Cardoso, C.M.; Mendes, R.; Nunes, M.L. Instrumental texture and sensory characteristics of cod frankfurter sausages. Int. J. Food Prop. 2009, 12, 625–643. [Google Scholar]
- Rahman, M.S.; Al-Waili, H.; Guizani, N.; Kasapis, S. Instrumental-sensory evaluation of texture for fish sausage and its storage stability. Fish. Sci. 2007, 73, 1166–1176. [Google Scholar] [CrossRef]
- Bejerholm, C.; Aaslyng, M.D. The influence of cooking technique and core temperature on results of a sensory analysis of pork—Depending on the raw meat quality. Food Qual. Prefer. 2004, 15, 19–30. [Google Scholar] [CrossRef]
- Sifre, L.; André, B.; Coton, J.-P. Development of a system to quantify muscle fibre destructuration. Meat Sci. 2009, 81, 515–522. [Google Scholar] [CrossRef]
| Finely Chopped Meat Portion (%) | Moisture Content (%) | Drip Loss (%) (Filter Press Method) | Cooking Loss (%) |
|---|---|---|---|
| 0 | 72.73 a ± 0.93 | 17.05 a ± 1.50 | 28.27 a ± 1.54 |
| 15 | 72.66 a ± 0.75 | 17.22 a ± 2.14 | 28.33 a ± 1.24 |
| 30 | 72.78 a ± 0.67 | 16.91 a ± 1.95 | 26.82 ab ± 1.03 |
| 45 | 73.19 ab ± 1.00 | 17.40 a ± 2.51 | 27.09 ab ± 1.07 |
| 60 | 73.37 ab ± 0.67 | 17.07 a ± 2.53 | 27.45 ab ± 1.60 |
| 75 | 73.80 ab ± 0.39 | 16.82 a ± 2.30 | 26.66 ab ± 1.29 |
| 90 | 74.12 b ± 0.37 | 17.07 a ± 2.95 | 26.57 ab ± 1.26 |
| 100 | 74.14 b ± 0.53 | 16.12 a ± 2.25 | 25.70 b ± 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, F.; Sawas, E.; Berger, L.M.; Gibis, M.; Weiss, J.; Röser, A.; Upmann, M.; Joeres, E.; Juadjur, A.; Bindrich, U.; et al. Influence of Finely Chopped Meat Addition on Quality Parameters of Minced Meat. Appl. Sci. 2022, 12, 10590. https://doi.org/10.3390/app122010590
Witte F, Sawas E, Berger LM, Gibis M, Weiss J, Röser A, Upmann M, Joeres E, Juadjur A, Bindrich U, et al. Influence of Finely Chopped Meat Addition on Quality Parameters of Minced Meat. Applied Sciences. 2022; 12(20):10590. https://doi.org/10.3390/app122010590
Chicago/Turabian StyleWitte, Franziska, Erik Sawas, Lisa M. Berger, Monika Gibis, Jochen Weiss, Anja Röser, Matthias Upmann, Eike Joeres, Andreas Juadjur, Ute Bindrich, and et al. 2022. "Influence of Finely Chopped Meat Addition on Quality Parameters of Minced Meat" Applied Sciences 12, no. 20: 10590. https://doi.org/10.3390/app122010590
APA StyleWitte, F., Sawas, E., Berger, L. M., Gibis, M., Weiss, J., Röser, A., Upmann, M., Joeres, E., Juadjur, A., Bindrich, U., Heinz, V., & Terjung, N. (2022). Influence of Finely Chopped Meat Addition on Quality Parameters of Minced Meat. Applied Sciences, 12(20), 10590. https://doi.org/10.3390/app122010590








