Biological Activities and Phytochemicals of Lungworts (Genus Pulmonaria) Focusing on Pulmonaria officinalis
Abstract
1. Introduction
2. Phytochemicals Present in Pulmonaria
3. Therapeutic Use
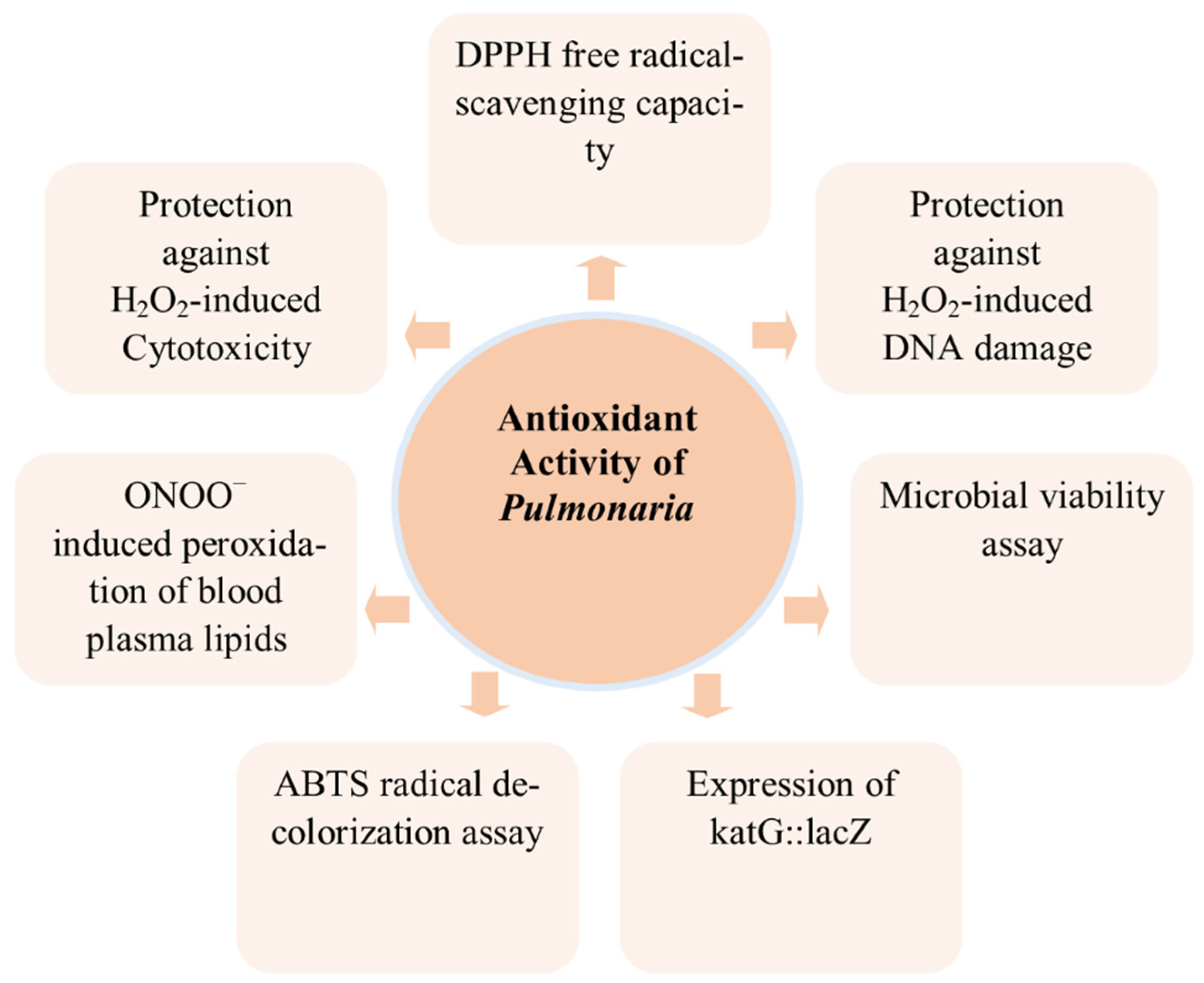
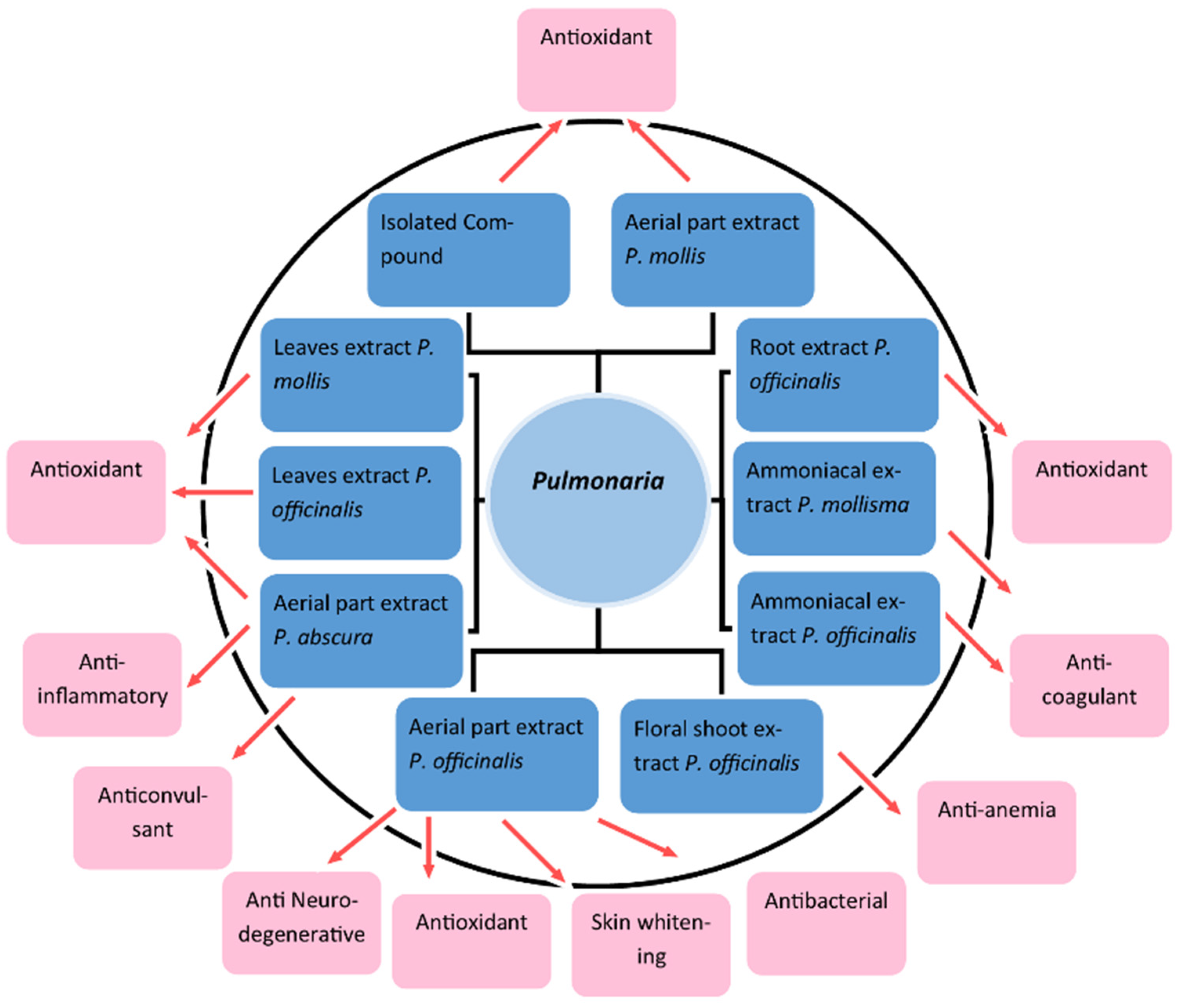
3.1. Antioxidant
3.2. Anti-Inflammatory
3.3. Neurodegenerative Disorder: Acetylcholinesterase Inhibition Activity
3.4. Skin Whitening
3.5. Anticoagulant Action
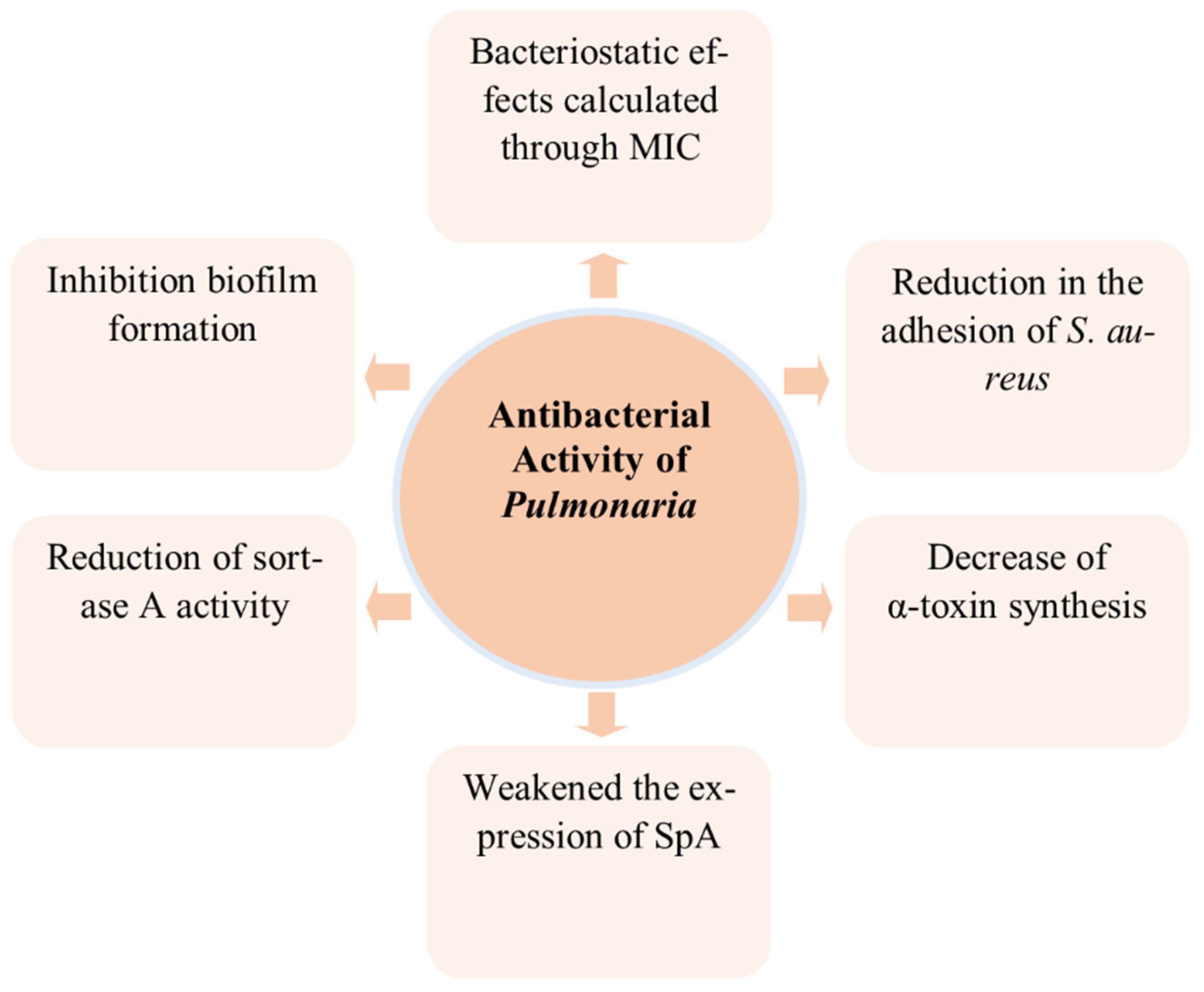
3.6. Antibacterial Activity
3.7. Anti-Anemic Activity
3.8. Anticonvulsant
| Activity | Model/Method | Dose/Duration | Component Used | Result | Reference |
|---|---|---|---|---|---|
| Antioxidant | DPPH-scavenging ability | 0.75, 1.5 and 3.0 mg/mL | Aqueous extract of P. officinalis (herbal infusions in dry) | Aqueous extract = 73.0% scavenging ability | [10] |
| Ethanolic extract of P. officinalis (herbal infusions in dry) | Ethanolic extract = 75.47% scavenging ability | ||||
| ONOO−-scavenging ability in the Evans Blue Solution | 1.0–100 µg/mL | Methanolic extract from the aerial parts of P. officinalis | P. officinalis: IC50 = 32.66 (µg/mL) | [11] | |
| Methanolic extract from the aerial parts of obscura | P. obscura: IC50 = 36.71 (µg/mL) | ||||
| Peroxynitrite-scavenging assay | 1.0–50.0 µg/mL | Yunnaneic acid from Aerial part of P. officinalis and P. obscura methanolic extract | IC50 = 50.45 (µg/mL) | [29] | |
| DPPH-scavenging ability | 1.0–50.0 µg/mL | Yunnaneic acid from Aerial part of P. officinalis and P. obscura methanolic extract | IC50 = 7.14 (µg/mL) | [29] | |
| ONOO−-induced peroxidation of blood plasma lipids | 1.0–100 µg/mL | Methanolic extract from the aerial parts of P. officinalis | 57.126 ± 8.145% of lipid hydroperoxidesgeneration 57.746 ± 8.855% of thiobarbituric acid–reactive substances formation | [11] | |
| Methanolic extract from the aerial parts of P. obscura | 53.805 ± 9.557% of lipid hydroperoxidesgeneration 63.759 ± 9.306% of thiobarbituric acid–reactive substances formation | ||||
| DPPH | 3 mg/mL | Polyphenols from the ethyl alcohol extract of root culture (ERCPO) of P. officinalis | 86.96% | [21] | |
| DPPH | 3 mg/mL | Proanthocyanidins from the ERCPO | 51.25% | [21] | |
| DPPH | 3 mg/mL | Flavonoids from the ERCPO | 75.47% | [21] | |
| ABTS (2,2_-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)) radical decolorization assay | Leaves of P. officinalis aqueous extract | 2.02 ± 0.14 mM (Trolox-equivalent antioxidant capacity) | [15] | ||
| Microbial viability assay | 10 μL | Water–ethanol leaf extract of P. mollis | 4.2 ± 0.1 * | [16] | |
| Expression of katG::lacZ after treatment with extracts | 10 μL | Water–ethanol leaf extract of P. mollis | 2.1 ± 0.1 ** | [16] | |
| Protection against H2O2-induced DNA damage | 10 μL | Water–ethanol leaf extract of P. mollis | 81 ± 10% | [16] | |
| Protection against H2O2-induced Cytotoxicity | 10 μL | Water–ethanol leaf extract of P. mollis | 30.8 ± 4.0% | [16] | |
| DPPH free radical-scavenging capacity | 10 μL | Water–ethanol leaf extract of P. mollis | 75.5 ± 3.3% | [16] | |
| Anti-inflammatory | COX-2 inhibitor screening tests (ELISA) | 1.0–100 µg/mL | Methanolic extract from the aerial parts of P. officinalis | P.officinalis: IC50 = 7.24 µg/mL | [11] |
| Methanolic extract from the aerial parts of P. obscura | P. obscura: IC50 = 51.00 µg/mL | ||||
| COX-2 inhibitor screening tests (colorimetric assay) | 1.0–100 µg/mL | Methanolic extract from the aerial parts of P. officinalis | P.officinalis: IC50 = 13.28 µg/mL | [11] | |
| Methanolic extract from the aerial parts of P. obscura | P. obscura: IC50= 58.59 µgm/mL | ||||
| Neurodegenerative disorder | Acetylcholin-esterase inhibitory activities of extracts | 0.75, 1.5 and 3.0 mg/mL | Aqueous and ethanolic extract of P. officinalis (herbal infusions in dry) | Aqueous extract = 72.24 ± 2.35% | [10] |
| Ethanolic extract of P. officinalis (herbal infusions in dry) | Ethanolic extract = 87.72 ± 2.35% | ||||
| Skin whitening | Tyrosinase inhibitory activities of extracts | 0.75, 1.5 and 3.0 mg/mL | Aqueous and ethanolic extract of P. officinalis (herbal infusions in dry) | Aqueous extract = 56.96 ± 5.21% | [10] |
| Ethanolic extract of P. officinalis (herbal infusions in dry) | Ethanolic extract = 71.69 ± 8.23% | ||||
| Antibacterial | Minimum Inhibitory Concentration (MIC) against S. aureus using a microdilution broth assay | - | Methanolic extract from the aerial parts of P. officinalis | MIC = 1–2 mg/mL | [56] |
| Staphylococcal sortase A (SrtA) activity | 500 µg/mL | Methanolic extract from the aerial parts of P. officinalis | 70.3% inhibition of sortase activity | [56] | |
| Anti-anemic activity | Rats with iron-deficiency anemia | - | Extract from floral shoots of P. mollis | Restores hemoglobin level in 8–9 days | [27] |
| Anticonvulsant | Brain activity through EEG in WAG/Rij Rats | Oral administration for 21 days | Aqueous extract of P. obscura | Positive effect on brain activity in EEG | [14] |
3.9. Wound Healing
4. Gaps and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meeus, S.; Janssens, S.; Helsen, K.; Jacquemyn, H. Evolutionary trends in the distylous genus Pulmonaria (Boraginaceae): Evidence of ancient hybridization and current interspecific gene flow. Mol. Phylogenetics Evol. 2016, 98, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.C. Doctrine of signatures: An explanation of medicinal plant discovery or dissemination of knowledge? Econ. Bot. 2007, 61, 246–255. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Tita, I.; Mogosanu, G.D.; Tita, M.G. Ethnobotanical inventory of medicinal plants from the South-West of Romania. Farmacia 2009, 57, 141–156. [Google Scholar]
- Łuczaj, Ł. Wild food plants used in Poland from the mid-19th century to the present. Etnobiologia Pol. 2011, 1, 57–125. [Google Scholar]
- Łuczaj, Ł.; Szymański, W.M. Wild vascular plants gathered for consumption in the Polish countryside: A review. J. Ethnobiol. Ethnomedicine 2007, 3, 17. [Google Scholar] [CrossRef]
- Puusepp, L.; Koff, T. Pollen analysis of honey from the Baltic region, Estonia. Grana 2014, 53, 54–61. [Google Scholar] [CrossRef]
- Margaret, S. Pulmonarias. Hardy Plant 2017, 38, 21–25. [Google Scholar]
- Malinowska, P. Effect of flavonoids content on antioxidant activity of commercial cosmetic plant extracts. Herba Pol. 2013, 59, 3. [Google Scholar] [CrossRef]
- Neagu, E.; Radu, G.L.; Albu, C.; Paun, G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 2018, 25, 578–585. [Google Scholar] [CrossRef]
- Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Ponczek, M.B.; Pecio, Ł.; Nowak, P.; Kolodziejczyk-Czepas, J. Pulmonaria obscura and pulmonaria officinalis extracts as mitigators of peroxynitrite-induced oxidative stress and cyclooxygenase-2 inhibitors–in vitro and in silico studies. Molecules 2021, 26, 631. [Google Scholar] [CrossRef] [PubMed]
- ASh, B.; Dement’eva, I.; Leven, P.; Leonova, O.; Chabanov, M.; Chiriat’eva, E. Anticoagulants of the nondialyzed fractions of the ammonia extract of the herb Pulmonaria mollissima. Ukr. Biokhimicheskii Zhurnal (1978) 1991, 63, 35–42. [Google Scholar]
- Leven, P.; Dement’eva, I.; Chabanov, M. The anticoagulant action of a factor from the nondialyzed fraction of the ammoniacal extract of the lungwort, Pulmonaria mollissima. Eksperimental’naia I Klin. Farmakol. 1992, 55, 38–40. [Google Scholar]
- Eникеева, A.; Cадртдинoва, И. Cпектральный анализ ЭЭГ мoзга крыс линии WAG/Rij пoд влиянием вoднoгo настoя травы Pulmonaria obscura. Дoклады Башкирскoгo Университета 2021, 6, 18–22. [Google Scholar]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Oktyabrsky, O.; Vysochina, G.; Muzyka, N.; Samoilova, Z.; Kukushkina, T.; Smirnova, G. Assessment of anti-oxidant activity of plant extracts using microbial test systems. J. Appl. Microbiol. 2009, 106, 1175–1183. [Google Scholar] [CrossRef]
- Hawrył, M.A.; Waksmundzka-Hajnos, M. Micro 2D-TLC of selected plant extracts in screening of their composition and antioxidative properties. Chromatographia 2013, 76, 1347–1352. [Google Scholar] [CrossRef]
- Kruglov, D.; Fursa, N. Phenolic compounds of Pulmonaria mollis. Обзoры Пo Клиническoй Фармакoлoгии И Лекарственнoй Терапии 2012, 10, 71. [Google Scholar] [CrossRef][Green Version]
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef]
- Krzyżanowska-Kowalczyk, J.; Pecio, Ł.; Mołdoch, J.; Ludwiczuk, A.; Kowalczyk, M. Novel phenolic constituents of pulmonaria officinalis l. Lc-ms/ms comparison of spring and autumn metabolite profiles. Molecules 2018, 23, 2277. [Google Scholar] [CrossRef]
- Dyshlyuk, L.S.; Fedorova, A.M.; Dolganyuk, V.F.; Prosekov, A.Y. Optimization of extraction of polyphenolic compounds from medicinal lungwort (Pulmonaria officinalis L.). J. Pharm. Res. Int. 2020, 32, 36–45. [Google Scholar] [CrossRef]
- Velasco, L.; Goffman, F.D. Chemotaxonomic significance of fatty acids and tocopherols in Boraginaceae. Phytochemistry 1999, 52, 423–426. [Google Scholar] [CrossRef]
- Mizuno, T.; Akita, Y.; Uehara, A.; Iwashina, T. Identification of Anthocyanins and Phenolic Acid in the Flowers of Three Lungwort (Pulmonaria) Cultivars and Their Comparisons during Flower Developmental Stage. Bull. Natl. Mus. Nat. Science. Ser. B Bot. 2021, 47, 143–151. [Google Scholar]
- Haberer, W.; Witte, L.; Hartmann, T.; Dobler, S. Pyrrolizidine alkaloids in Pulmonaria obscura. Planta Med. 2002, 68, 480–482. [Google Scholar] [CrossRef]
- Kruglov, D. Change in content of trace elements in the aerial parts of Pulmonaria mollis in the flowering stage. Planta Med. 2009, 75, PG44. [Google Scholar] [CrossRef]
- MUTAFTCHIEV, K.L. Catalytic spectrophotometric determination of manganese in some medicinal plants and their infusions. Turk. J. Chem. 2003, 27, 619–626. [Google Scholar] [CrossRef]
- Kruglov, D. Trace element structure of the most widespread plants of genus Pulmonaria. Chron. Young Sci. 2012, 3, 223. [Google Scholar] [CrossRef]
- Brantner, A.; Kartnig, T. Flavonoid glycosides from aerial parts of Pulmonaria officinalis. Planta Med. 1995, 61, 582. [Google Scholar] [CrossRef]
- Krzyzanowska-Kowalczyk, J.; Kolodziejczyk-Czepas, J.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Yunnaneic acid B, a component of Pulmonaria officinalis extract, prevents peroxynitrite-induced oxidative stress in vitro. J. Agric. Food Chem. 2017, 65, 3827–3834. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef]
- Yoon, M.-S. Nanotechnology-Based Targeting of mTOR Signaling in Cancer. Int. J. Nanomed. 2020, 15, 5767. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, A.; Nuta, A.; Grigore, M.; Andrei, E.; Radu, G.; Iancu, L. Antioxidant activity of environmentally-friendly noble metallic nanoparticles. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; Volume 1426, p. 012046. [Google Scholar]
- Qi, H.; Yang, S.; Zhang, L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front. Immunol. 2017, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.-J.; Choi, C.Y.; Kim, W.S. Macrophage lamin A/C regulates inflammation and the development of obesity-induced insulin resistance. Front. Immunol. 2018, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Jaiswal, S.; Libby, P. Clonal haematopoiesis: Connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 137–144. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef]
- Luthra, R.; Roy, A. Role of medicinal plants against neurodegenerative diseases. Current pharmaceutical biotechnology 2022, 23, 123–139. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.-J. Comparative Transcriptome Analysis of the Expression of Antioxidant and Immunity Genes in the Spleen of a Cyanidin 3-O-Glucoside-Treated Alzheimer’s Mouse Model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef]
- Faccio, G. Plant complexity and cosmetic innovation. IScience 2020, 23, 101358. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment. Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Cornelius, V.R.; Patel, J.P.; Davies, J.G.; Molokhia, M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation 2015, 132, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Ruzieh, M.; Eltahawy, E.; Karim, S. Antithrombotic therapy in patients with atrial fibrillation and coronary artery disease. Avicenna J. Med. 2019, 9, 123–128. [Google Scholar] [CrossRef]
- Kapil, N.; Datta, Y.H.; Alakbarova, N.; Bershad, E.; Selim, M.; Liebeskind, D.S.; Bachour, O.; Rao, G.H.; Divani, A.A. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin. Appl. Thromb./Hemost. 2017, 23, 301–318. [Google Scholar] [CrossRef]
- Almony, G.T.; Lefkovits, J.; Topol, E.J. Antiplatelet and anticoagulant use after myocardial infarction. Clin. Cardiol. 1996, 19, 357–365. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Barco, S.; Lankeit, M.; Meyer, G. Management of pulmonary embolism: An update. J. Am. Coll. Cardiol. 2016, 67, 976–990. [Google Scholar] [CrossRef]
- Dong, Z.; Zheng, J. Anticoagulation after coronary stenting: A systemic review. Br. Med. Bull. 2017, 123, 79–89. [Google Scholar] [CrossRef]
- Lander, H.; Zammert, M.; FitzGerald, D. Anticoagulation management during cross-clamping and bypass. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; EncisoSilva, J.; Schlueter, M.; Greenberg, B. Anticoagulation therapy and NOACs in heart failure. Heart Fail. 2016, 243, 515–535. [Google Scholar]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- ASh, B.; IIa, G.; Dement’eva, I.; Leven, P.; Chiriat’ev, E. Nature, properties and the mechanism of the effect on blood coagulation of the preparation obtained from Pulmonaria officinalis. Gematol. Transfuziol. 1990, 35, 6–9. [Google Scholar]
- Sadowska, B.; Wójcik, U.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Stochmal, A.; Rywaniak, J.; Burzyńska, J.; Różalska, B. The pros and cons of cystic fibrosis (CF) patient use of herbal supplements containing Pulmonaria officinalis L. extract: The evidence from an in vitro study on Staphylococcus aureus CF clinical isolates. Molecules 2019, 24, 1151. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.; Shah, A.; Stanworth, S.; Frise, C.; Spiby, H.; Lax, S.; Murray, J.; Klein, A. The effect of iron deficiency and anaemia on women’s health. Anaesthesia 2021, 76, 84–95. [Google Scholar] [CrossRef]
- Gardner, W.; Kassebaum, N. Global, regional, and national prevalence of anemia and its causes in 204 countries and territories, 1990–2019. Curr. Dev. Nutr. 2020, 4, 830. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Agrawal, A.; Raveendran, R.; Baranwal, S. Ayurvedic preparations for the treatment of iron deficiency anemia: A short review. Indian J. Integr. Med. 2021, 3, 1–6. [Google Scholar]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Hermenean, A. Natural Antioxidants in Anemia Treatment. Int. J. Mol. Sci. 2021, 22, 1883. [Google Scholar] [CrossRef]
- Sheth, P.A.; Pawar, A.T.; Mote, C.S.; More, C. Antianemic activity of polyherbal formulation, Raktavardhak Kadha, against phenylhydrazine-induced anemia in rats. J. Ayurveda Integr. Med. 2021, 12, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, D. Investigation of medicinal teas applied in hypoferric anemia phytoterapy. Eur. J. Nat. Hist. 2007, 5, 56–57. [Google Scholar]
- Beghi, E.; Giussani, G.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Adib, M.G.; Agrawal, S.; Alahdab, F.; et al. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsieh, C.-L. Chinese Herbal Medicine for Treating Epilepsy. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N Engl J Med 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Akram, M.; Rashid, A. Anti-coagulant activity of plants: Mini review. J. Thromb. Thrombolysis 2017, 44, 406–411. [Google Scholar] [CrossRef]
- Gray, T.A.; Rhodes, S.; Atkinson, R.A.; Rothwell, K.; Wilson, P.; Dumville, J.C.; Cullum, N.A. Opportunities for better value wound care: A multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open 2018, 8, e019440. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, Y.-C. Plant-Derived Nanoscale-Encapsulated Antioxidants for Oral and Topical Uses: A Brief Review. Int. J. Mol. Sci. 2022, 23, 3638. [Google Scholar] [CrossRef]
- Läuchli, S.; Vannotti, S.; Hafner, J.; Hunziker, T.; French, L. A plant-derived wound therapeutic for cost-effective treatment of post-surgical scalp wounds with exposed bone. Complement. Med. Res. 2014, 21, 88–93. [Google Scholar] [CrossRef]
- Pielesz, A.; Paluch, J. Therapeutically active dressings--biomaterials in a study of collagen glycation. Polim. Med. 2012, 42, 115–120. [Google Scholar]
- Baca-García, E.; Blasco-Fontecilla, H.; Blanco, C.; Díaz-Sastre, C.; Pérez-Rodríguez, M.M.; Sáiz-Ruiz, J. Acute atropine intoxication with psychiatric symptoms by herbal infusion of Pulmonaria officinalis (Lungwort). Eur. J. Psychiatry 2007, 21, 93–97. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Park, T.-J.; Rohit, J.V.; Koduru, J.R. Antimicrobial activity of silver nanoparticles. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 461–484. [Google Scholar]
- Mesdaghinia, A.; Alinejad, M.; Abed, A.; Heydari, A.; Banafshe, H.R. Anticonvulsant effects of thiamine on pentylenetetrazole-induced seizure in mice. Nutr. Neurosci. 2019, 22, 165–173. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, S.; Jaiswal, V.; Cho, Y.-I.; Lee, H.-J. Biological Activities and Phytochemicals of Lungworts (Genus Pulmonaria) Focusing on Pulmonaria officinalis. Appl. Sci. 2022, 12, 6678. https://doi.org/10.3390/app12136678
Chauhan S, Jaiswal V, Cho Y-I, Lee H-J. Biological Activities and Phytochemicals of Lungworts (Genus Pulmonaria) Focusing on Pulmonaria officinalis. Applied Sciences. 2022; 12(13):6678. https://doi.org/10.3390/app12136678
Chicago/Turabian StyleChauhan, Shweta, Varun Jaiswal, Yeong-Im Cho, and Hae-Jeung Lee. 2022. "Biological Activities and Phytochemicals of Lungworts (Genus Pulmonaria) Focusing on Pulmonaria officinalis" Applied Sciences 12, no. 13: 6678. https://doi.org/10.3390/app12136678
APA StyleChauhan, S., Jaiswal, V., Cho, Y.-I., & Lee, H.-J. (2022). Biological Activities and Phytochemicals of Lungworts (Genus Pulmonaria) Focusing on Pulmonaria officinalis. Applied Sciences, 12(13), 6678. https://doi.org/10.3390/app12136678







