Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications
Abstract
:1. Introduction
2. Electrospinning of Carbon Precursor Polymer
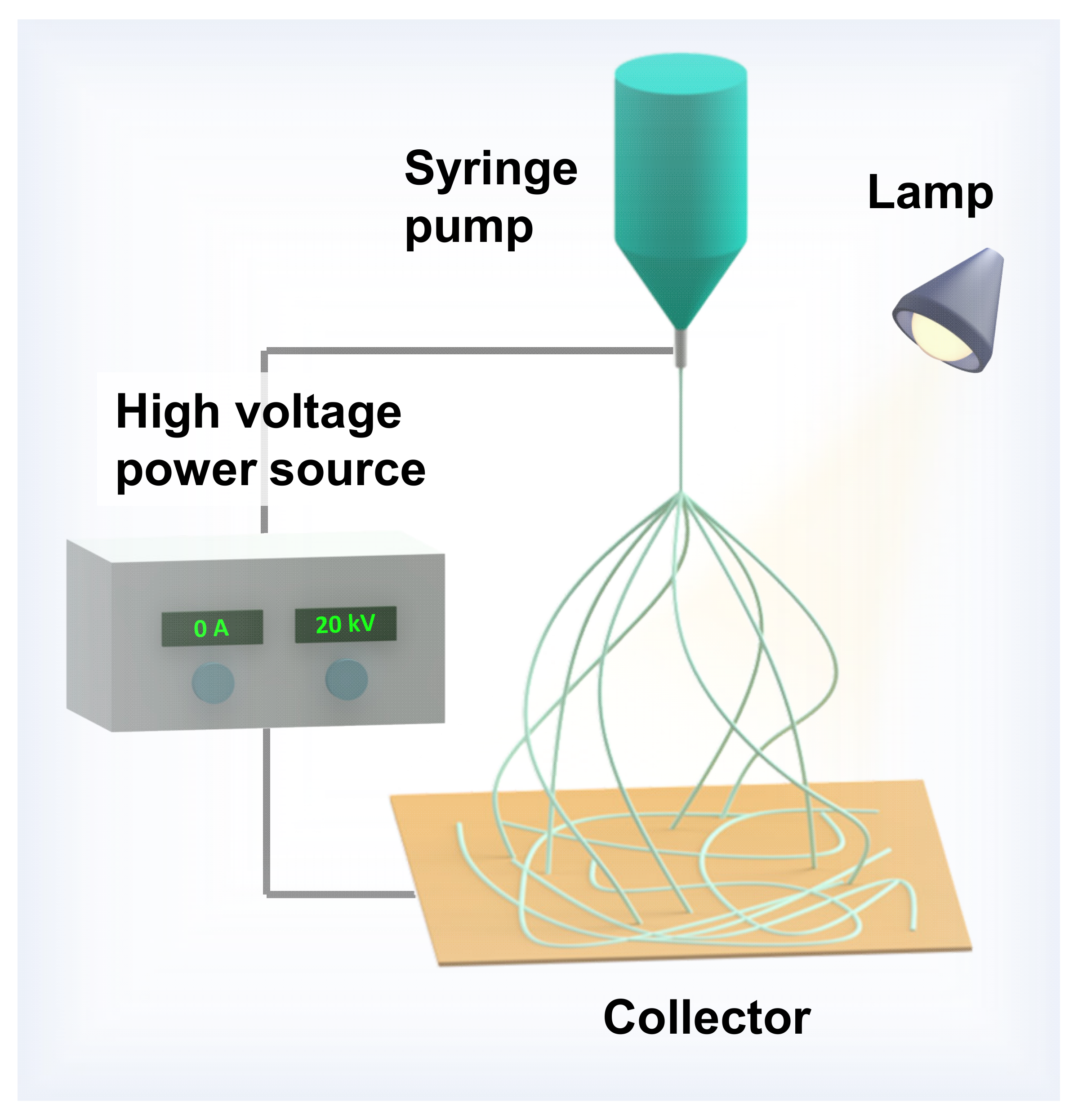
2.1. Setup and Conditions for Electrospinning
2.2. Polymer Nanofibers for Carbon Nanofibers
2.2.1. Polyacrylonitrile-Based Carbon Nanofibers
2.2.2. Polyvinyl Alcohol-Based Carbon Nanofibers
2.2.3. Pitch-Based Carbon Nanofibers
2.2.4. Polyimide-Based Carbon Nanofibers
2.2.5. Carbohydrate Polymer-Based Carbon Nanofibers
2.2.6. Poly(p-xylene tetrahydrothiophenium chloride)-Based Carbon Nanofibers
| Precursor | Temperature (°C) | Environment | Purpose | Ref. |
|---|---|---|---|---|
| PAN | 200–300 | Air | Oxidation stabilization | [38] |
| 900–1300 | Argon/Nitrogen | Carbonization | ||
| Up to 2500 | Argon/Nitrogen | Orientation Improvement | ||
| PVA | 180–300 | Air | Oxidation stabilization | [46] |
| 600–1000 | Argon/Nitrogen | Carbonization | ||
| Pitch | Before softening point of material | Air | Oxidation stabilization | [64] |
| Up to 1200 | Argon/Nitrogen | Carbonization | ||
| PI | Up to 1000 | Argon/Nitrogen | Carbonization | [50] |
| Carbohydrate/PEO | 105 | Air | Oxidation stabilization | [55,56] |
| Up to 800 | Argon/Nitrogen | Carbonization | ||
| PXTC/PPV | Up to 1800 | Argon/Nitrogen | Carbonization | [63] |
3. Different Carbon Nanofibers Obtained by Electrospinning
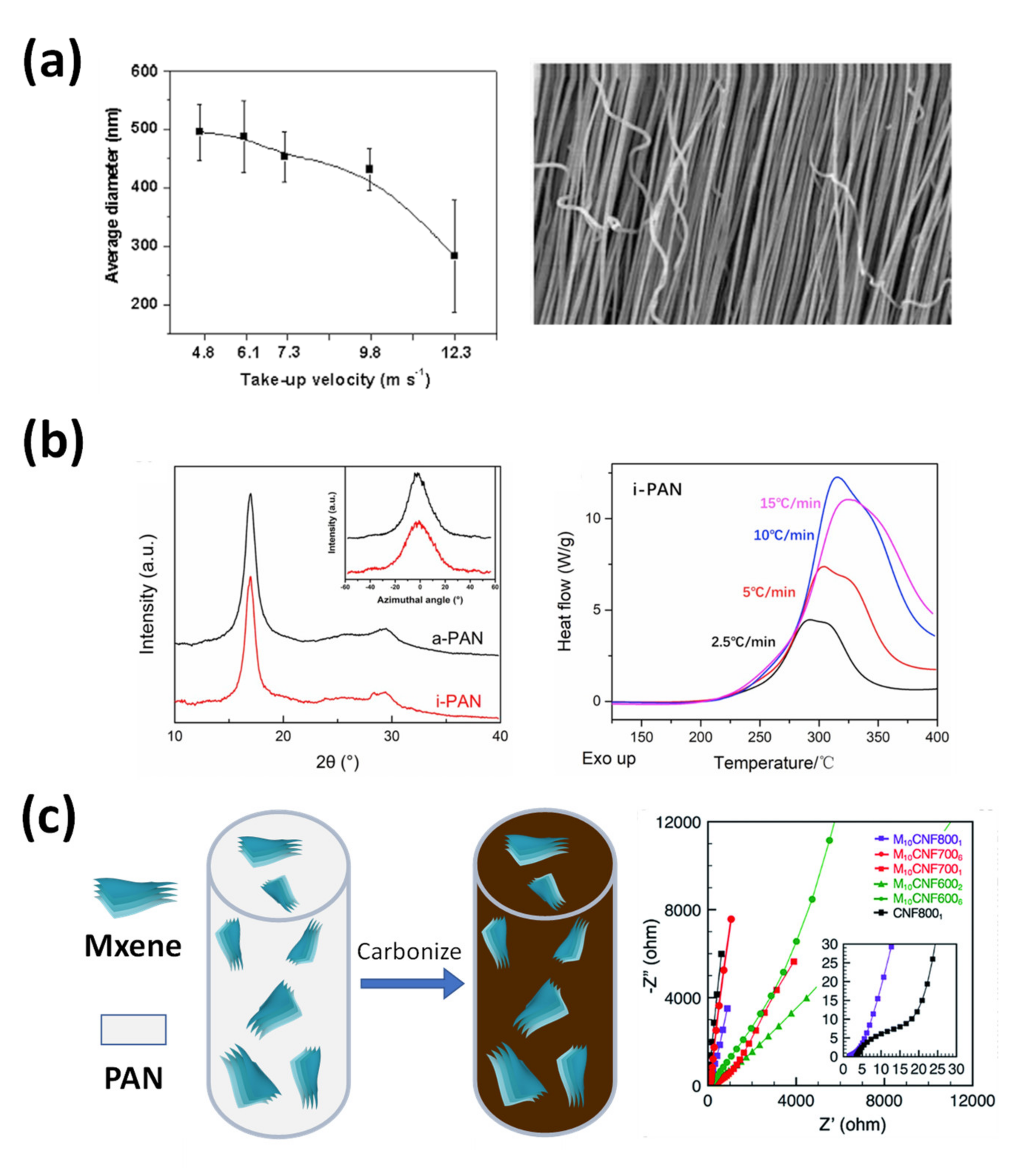
3.1. Carbon Nanofibers from Common Electrospinning
3.2. Carbon Nanofibers from Modified Single-Nozzle Electrospinning
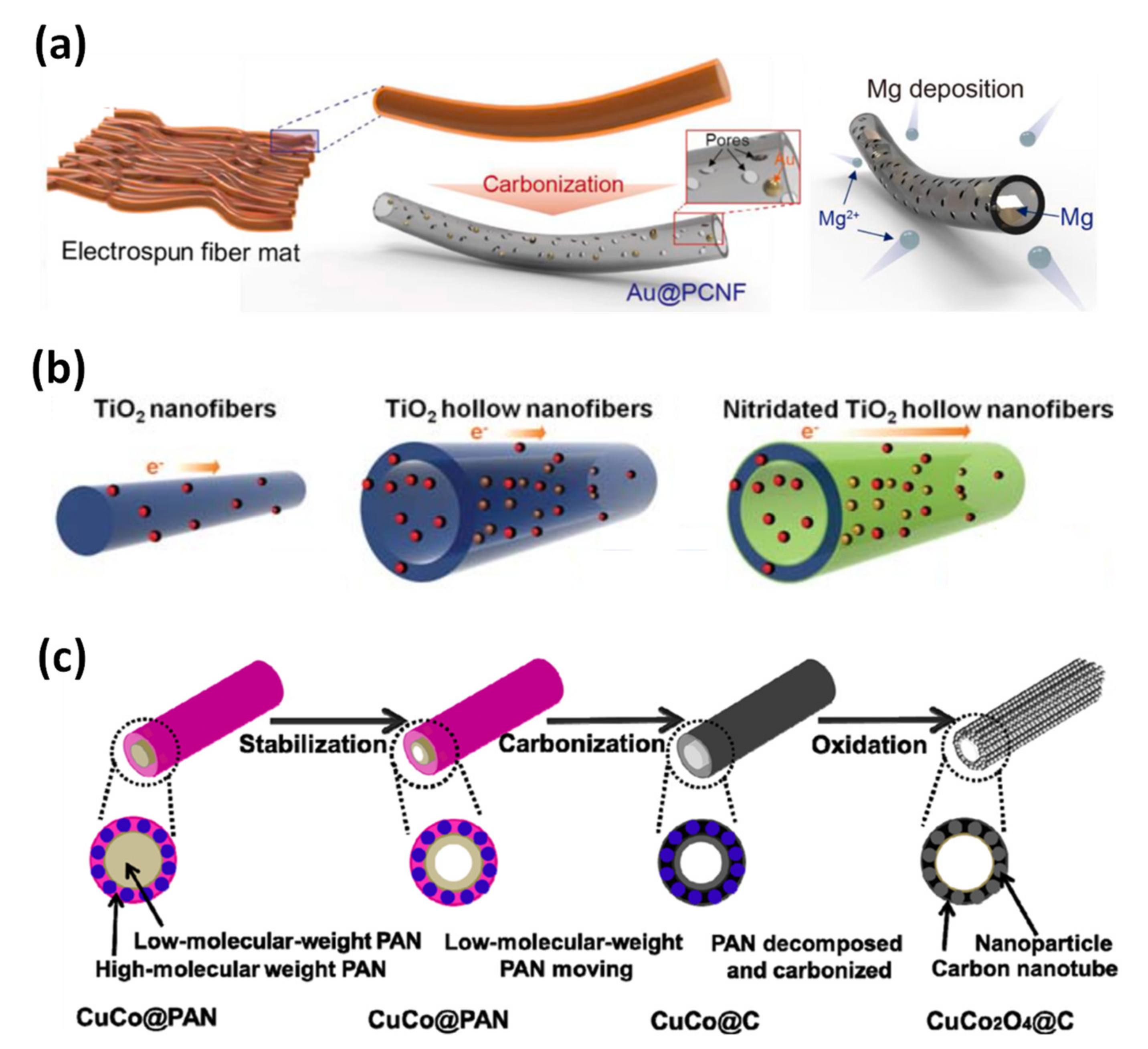
3.2.1. Porous Carbon Nanofibers
3.2.2. Metal-Oxide-Decorated Carbon Nanofibers
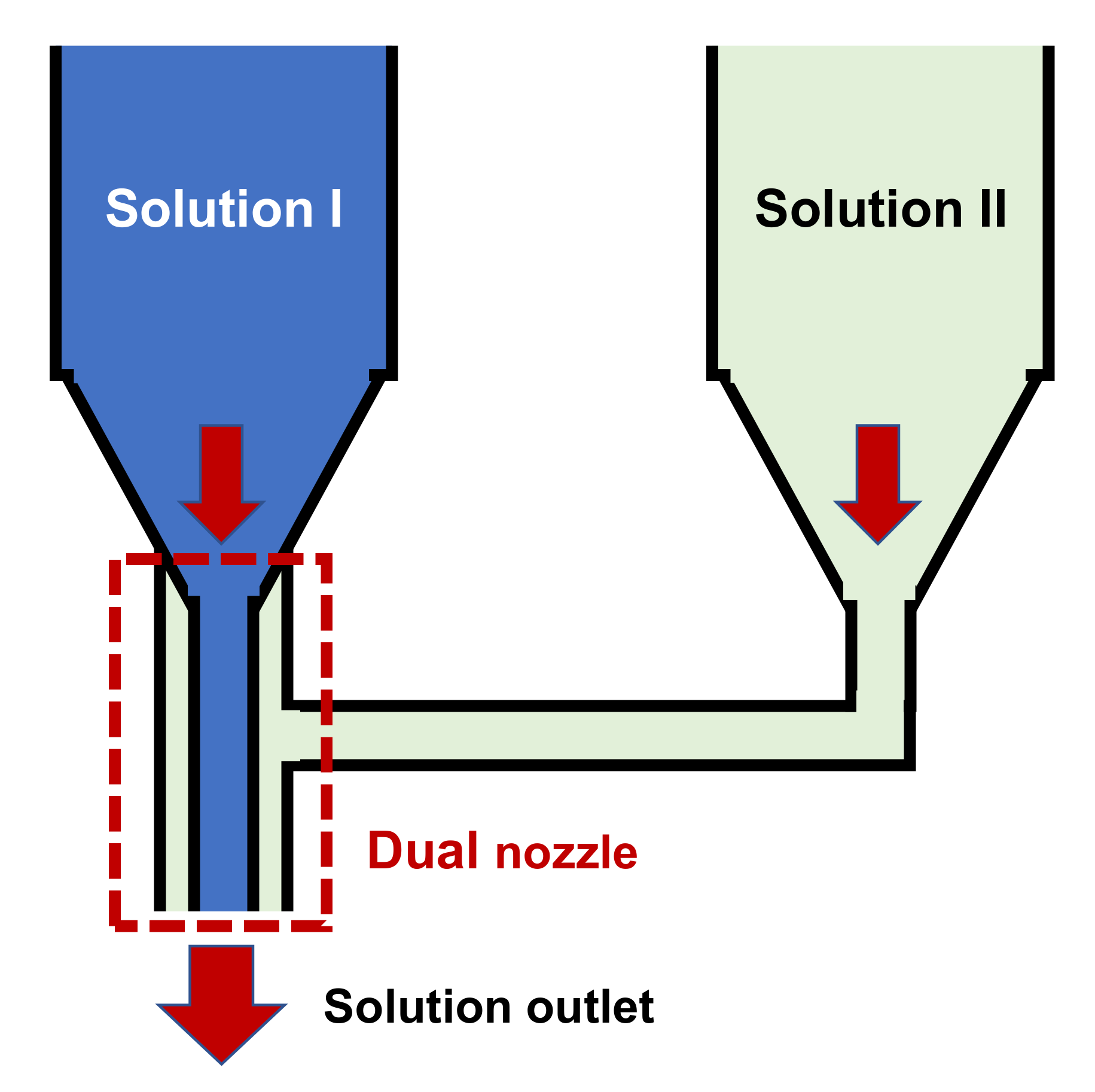
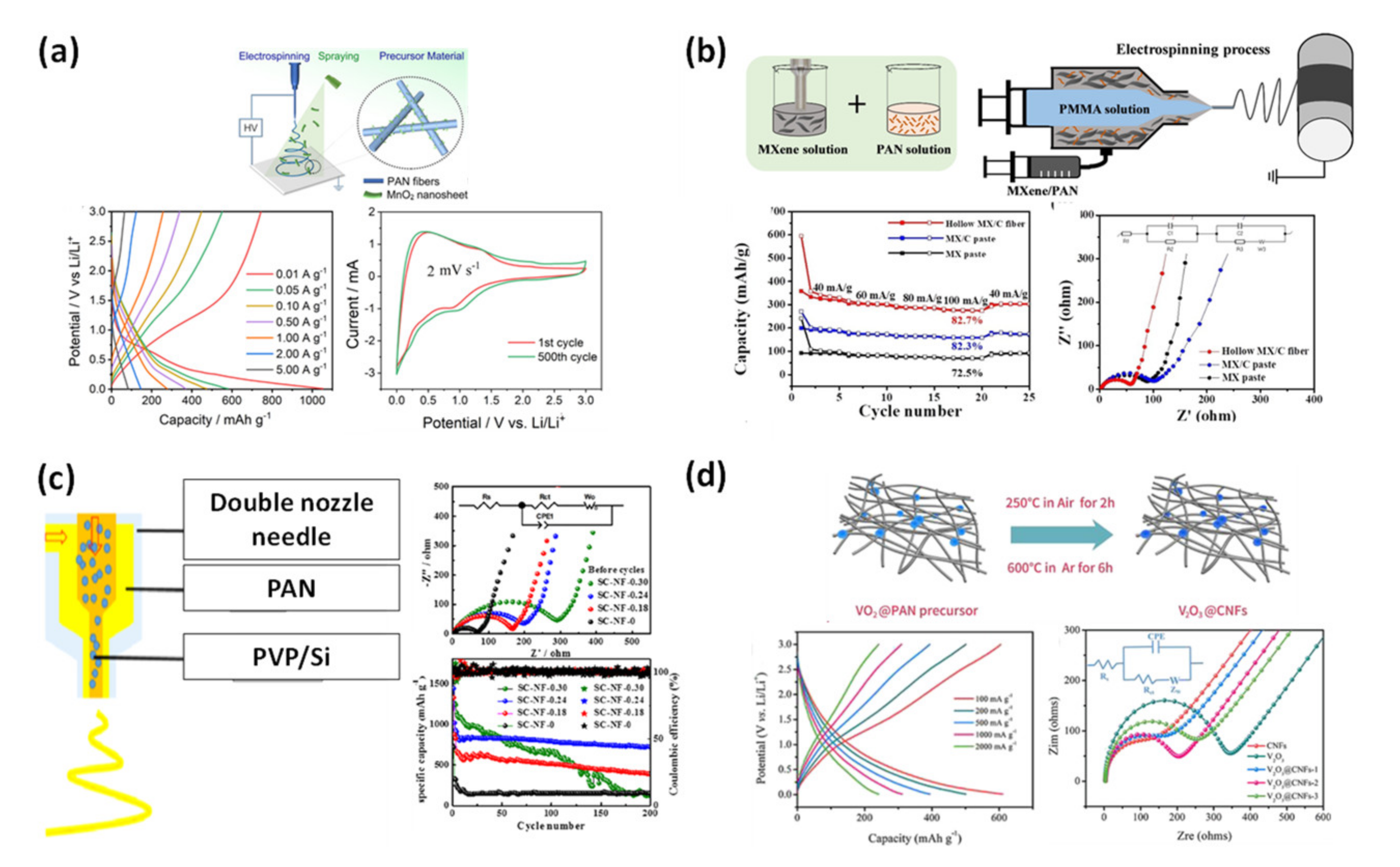
3.3. Carbon Nanofibers from Modified Dual-Nozzle Electrospinning
4. Application of Carbon Nanofibers
4.1. Energy Storage Applications
4.1.1. Supercapacitor
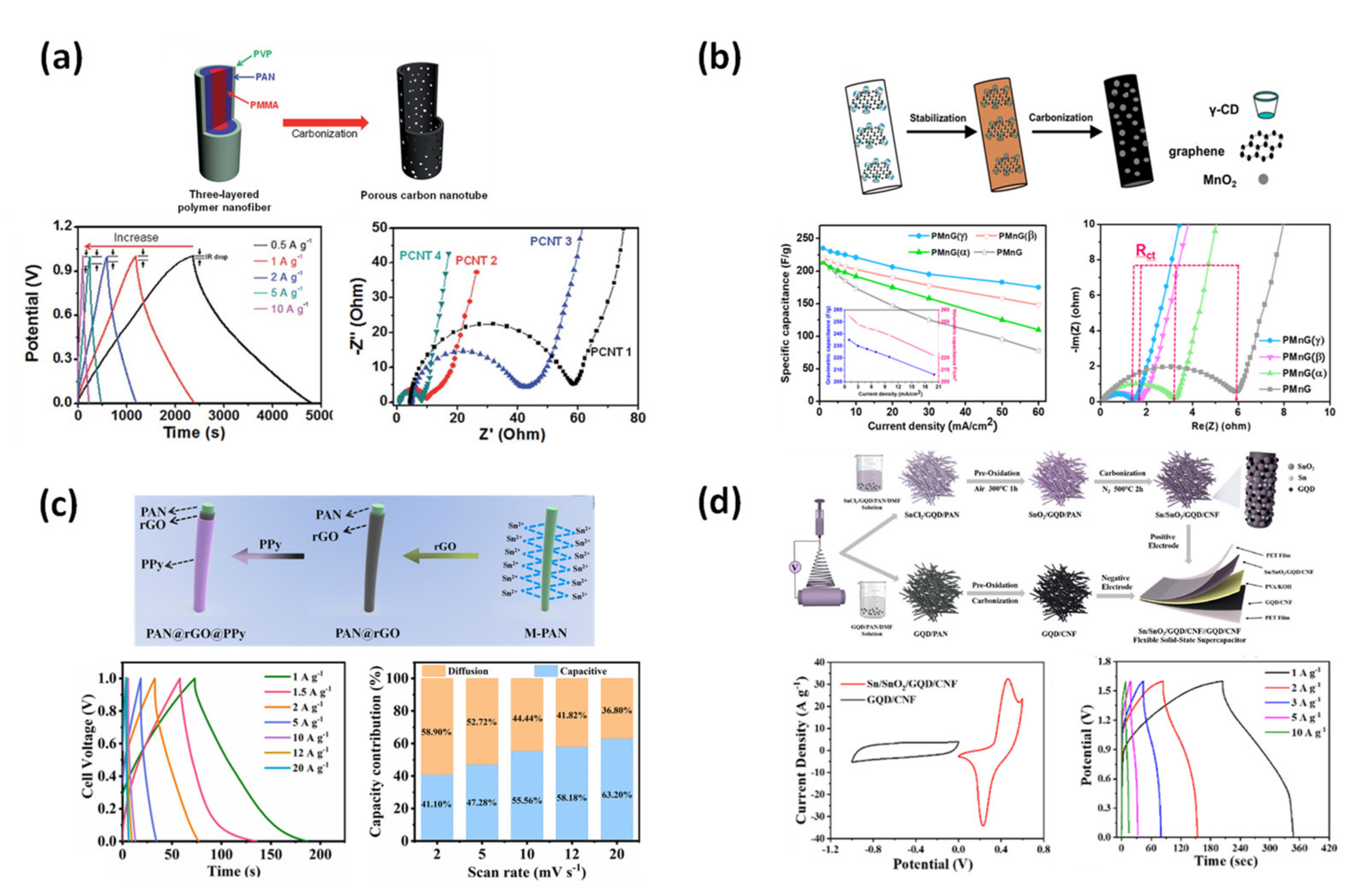
4.1.2. Li-Ion Battery Anode
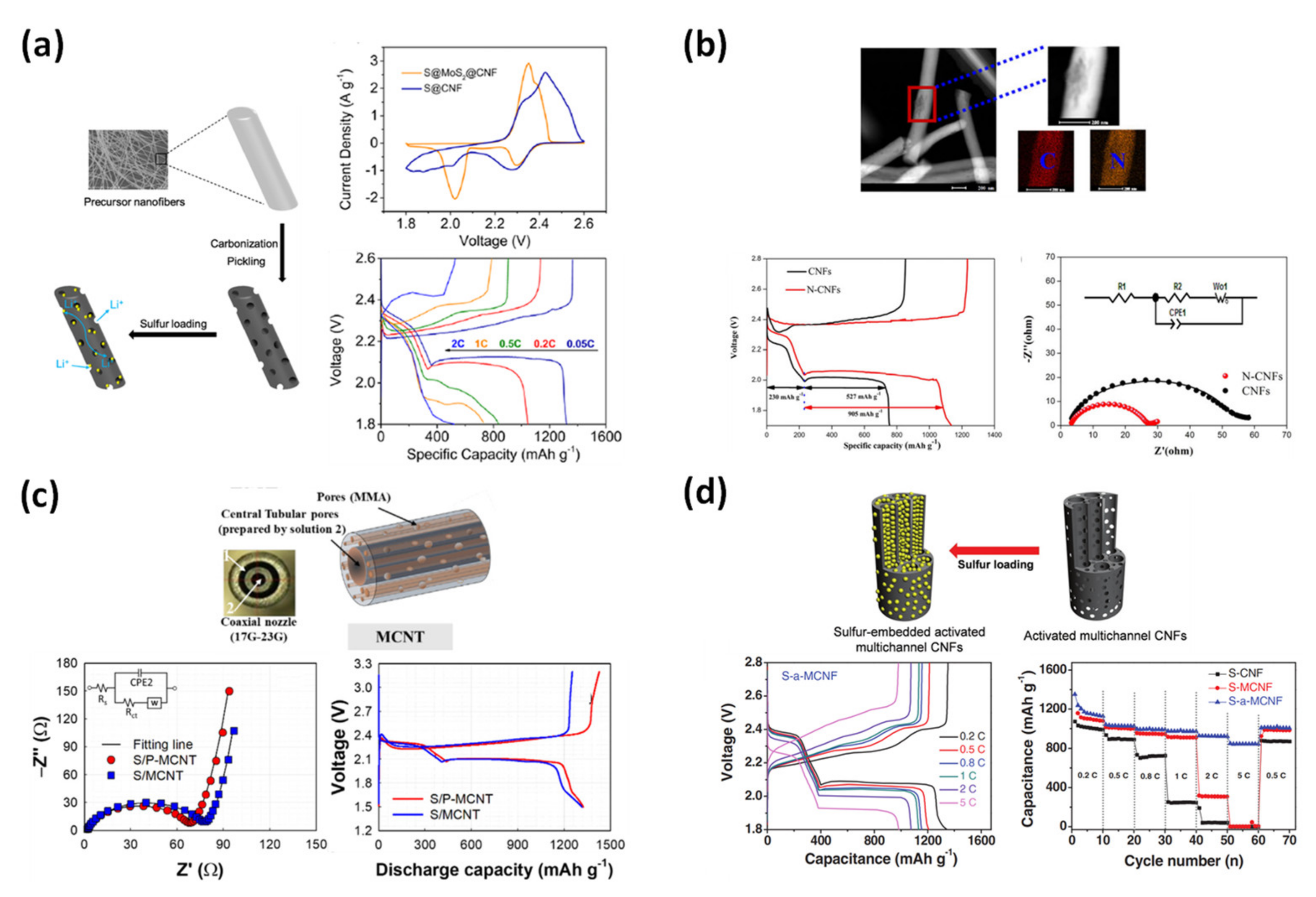
4.1.3. Lithium-Sulfur Battery
4.1.4. Dye-Sensitized Solar Cells (DSSC)
4.1.5. Catalyst
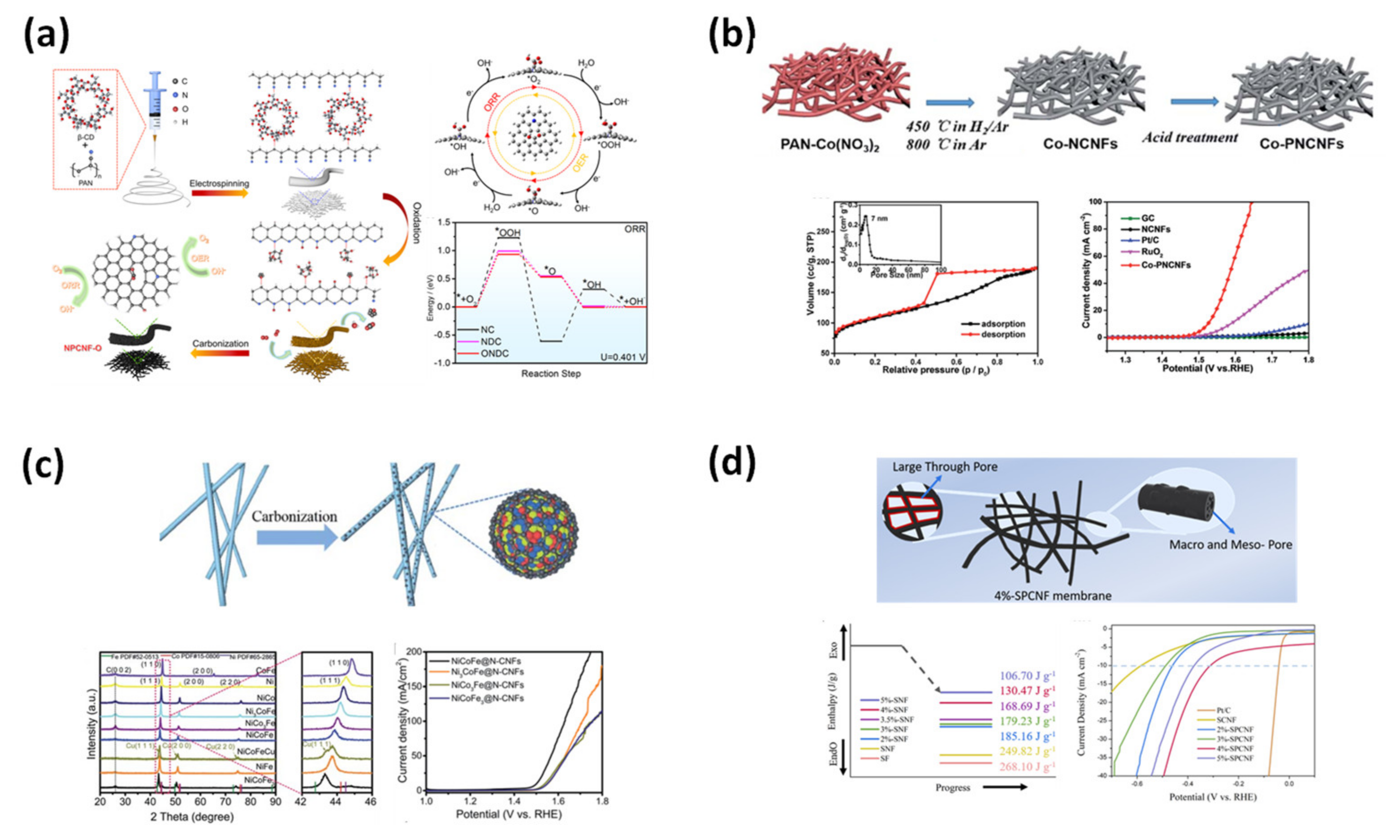
4.1.6. Membrane Electrode Assembly for Fuel Cells
4.2. Sensor Applications
4.2.1. Electrochemical Sensor
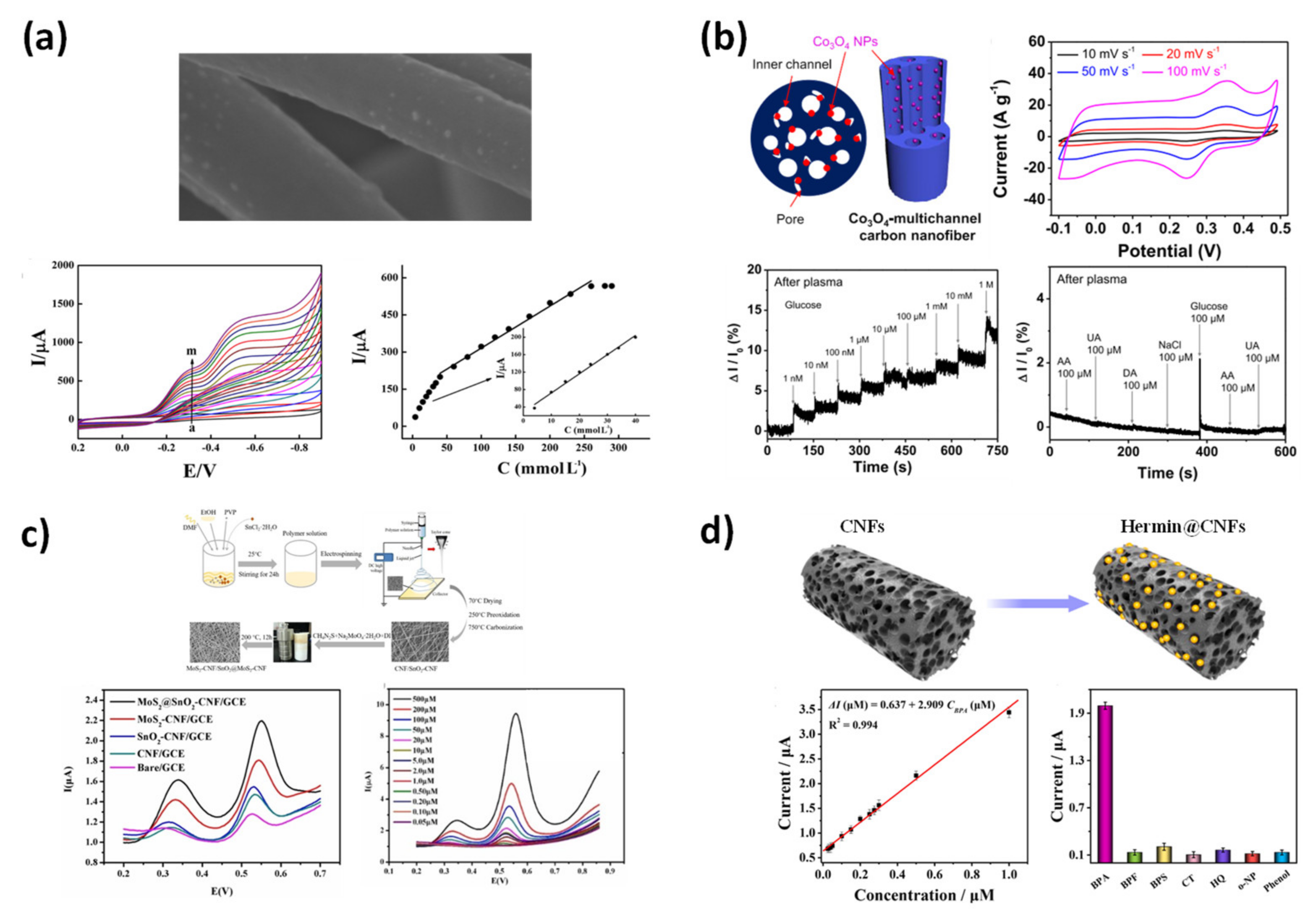
4.2.2. Warfare Agent Chemical Sensors
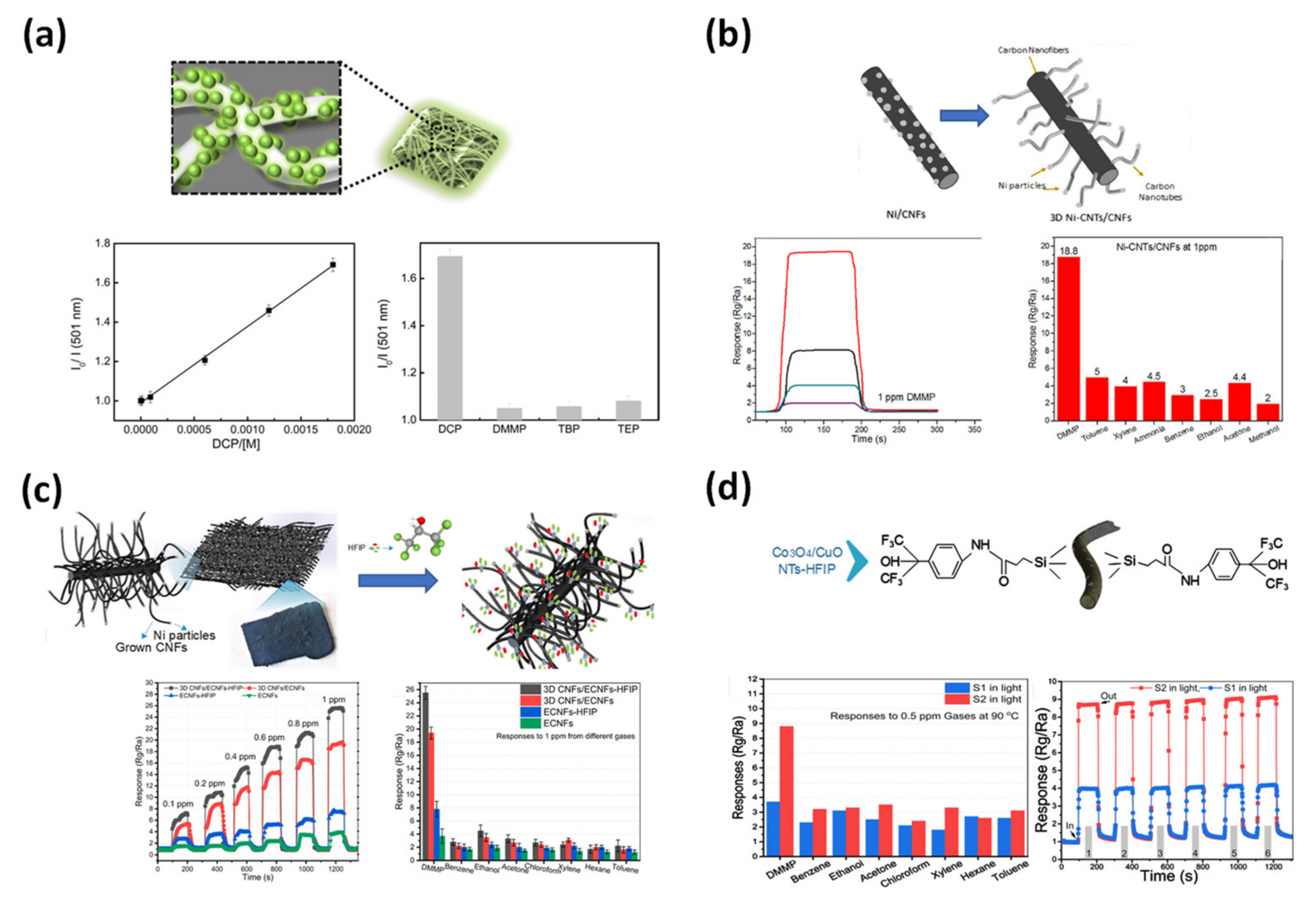
4.2.3. Biosensor
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2009, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Wei, C.-Y.; Lin, M.-C.; Pan, C.-J.; Chou, H.-L.; Chen, H.-A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for nonaqueous multivalent secondary batteries: Magnesium and beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.-S. A Novel Aluminum-Graphite Dual-Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Silva, S. Properties of Amorphous Carbon; The Institution of Engineering and Technology: London, UK, 2003. [Google Scholar]
- Beeman, D.; Silverman, J.; Lynds, R.; Anderson, M.R. Modeling studies of amorphous carbon. Phys. Rev. B 1984, 30, 870–875. [Google Scholar] [CrossRef]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Shen, C.; Sun, Y.; Yao, W.; Lu, Y. Facile synthesis of polypyrrole nanospheres and their carbonized products for potential application in high-performance supercapacitors. Polymer 2014, 55, 2817–2824. [Google Scholar] [CrossRef]
- Föllmer, M.; Jestin, S.; Neri, W.; Vo, V.S.; Derré, A.; Mercader, C.; Poulin, P. Wet-Spinning and Carbonization of Lignin-Polyvinyl Alcohol Precursor Fibers. Adv. Sustain. Syst. 2019, 3, e201900082. [Google Scholar] [CrossRef]
- Wang, Z.L.; Kang, Z.C. Pairing of Pentagonal and Heptagonal Carbon Rings in the Growth of Nanosize Carbon Spheres Synthesized by a Mixed-Valent Oxide-Catalytic Carbonization Process. J. Phys. Chem. 1996, 100, 17725–17731. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Chen, Y.; Yu, Z.-M.; Xie, Y.; Wang, H.; Yang, S.A.; Zhang, S. Three-dimensional Pentagon Carbon with a genesis of emergent fermions. Nat. Commun. 2017, 8, 15641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-X.; Yang, Y.-Q.; Niu, C.-Y.; Wang, J.-Q.; Jia, Y. C-57 carbon: A two-dimensional metallic carbon allotrope with pentagonal and heptagonal rings. Comput. Mater. Sci. 2019, 160, 115–119. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Fu, K.; White, R.; Zhao, C.; Bradford, P.D.; Zhang, X. Nanosized Ge@CNF, Ge@C@CNF and Ge@CNF@C composites via chemical vapour deposition method for use in advanced lithium-ion batteries. J. Power Sources 2014, 253, 366–372. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Helleu, C.; Behr, M.; Ba, H.; Romero, T.; Hébraud, A.; Schlatter, G.; Ersen, O.; Su, D.S.; et al. Hierarchical porous carbon fibers/carbon nanofibers monolith from electrospinning/CVD processes as a high effective surface area support platform. J. Mater. Chem. A 2016, 5, 2151–2162. [Google Scholar] [CrossRef]
- Li, M.; Li, N.; Shao, W.; Zhou, C. Synthesis of carbon nanofibers by CVD as a catalyst support material using atomically ordered Ni3C nanoparticles. Nanotechnology 2016, 27, 505706. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Zuo, X.; Xu, P.; Zhang, C.; Li, M.; Jiang, X.; Yue, X. Porous magnetic carbon nanofibers (P-CNF/Fe) for low-frequency electromagnetic wave absorption synthesized by electrospinning. Ceram. Int. 2018, 45, 4474–4481. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, T.; Bian, Z.; Sun, H.; Pang, Y.; Peng, C.; Yang, J.; Zheng, S. Electrospun flexible Si/C@CNF nonwoven anode for high capacity and durable lithium-ion battery. Compos. Commun. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Guan, J.; Chen, W.; Fang, Y.; Wang, L.; Fu, Y.; Guo, B.; Zhang, M. Electrospinning derivative fabrication of sandwich-structured CNF/Co3S4/MoS2 as self-supported electrodes to accelerate electron transport in HER. Int. J. Hydrog. Energy 2022, 47, 14930–14941. [Google Scholar] [CrossRef]
- Zhai, H.; Jiang, H.; Qian, Y.; Cai, X.; Liu, H.; Qiu, Y.; Jin, M.; Xiu, F.; Liu, X.; Lai, L. Sb2S3 nanocrystals embedded in multichannel N-doped carbon nanofiber for ultralong cycle life sodium-ion batteries. Mater. Chem. Phys. 2019, 240, 122139. [Google Scholar] [CrossRef]
- Sebokolodi, T.I.; Sipuka, D.S.; Tsekeli, T.R.; Nkosi, D.; Arotiba, O.A. An electrochemical sensor for caffeine at a carbon nanofiber modified glassy carbon electrode. J. Food Meas. Charact. 2022, 1–9. [Google Scholar] [CrossRef]
- Guo, S.; Sun, Y.; Wang, J.; Peng, L.; Li, H.; Li, C. Bimetallic ZIF-derived cobalt nanoparticles anchored on N- and S-codoped porous carbon nanofibers as cathode catalyst for Li-O2 batteries. Electrochimica Acta 2022, 418, 140279. [Google Scholar] [CrossRef]
- Yao, X.; Wang, X.; Sun, L.; Li, L.; Kan, E.; Ouyang, B.; Zhang, W. Popcorn-like Co3O4 nanoparticles confined in a three-dimensional hierarchical N-doped carbon nanotube network as a highly-efficient trifunctional electrocatalyst for zinc–air batteries and water splitting devices. Inorg. Chem. Front. 2022, 9, 2517–2529. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, Z.; Liu, Y.; Wang, J.; Fu, C.; Yin, H.; Chen, J.; Zhao, L.; Pan, Z. One-Pot Rational Deposition of Coaxial Double-Layer MnO2/Ni(OH)2 Nanosheets on Carbon Nanofibers for High-Performance Supercapacitors. Adv. Fiber Mater. 2022, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.-E.; Zhang, M.-G. Investigation of the effect of anion/cation-modified cellulose nanofibers/MXene composite aerogels on the high-performance lithium-sulfur batteries. Ionics 2022, 28, 2805–2815. [Google Scholar] [CrossRef]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, A.; Sahatiya, P.; Badhulika, S. Template-Assisted Electrospinning of Bubbled Carbon Nanofibers as Binder-Free Electrodes for High-Performance Supercapacitors. ChemElectroChem 2017, 5, 531–539. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. Effects of Working Parameters on Electrospinning. In One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar]
- Bakar, S.S.S.; Fong, K.C.; Eleyas, A.; Nazeri, M.F.M. Effect of Voltage and Flow Rate Electrospinning Parameters on Polyacrylonitrile Electrospun Fibers. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Penang, Malaysia, 6–7 December 2017; p. 012076. [Google Scholar] [CrossRef] [Green Version]
- Ercolano, G.; Farina, F.; Cavaliere, S.; Jones, D.J.; Rozière, J. Nickel Based Electrospun Materials with Tuned Morphology and Composition. Nanomaterials 2016, 6, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Bahl, O.; Mathur, R. Effect of load on the mechanical properties of carbon fibres from pan precursor. Fibre Sci. Technol. 1979, 12, 31–39. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Saha, B.; Schatz, G.C. Carbonization in Polyacrylonitrile (PAN) Based Carbon Fibers Studied by ReaxFF Molecular Dynamics Simulations. J. Phys. Chem. B 2012, 116, 4684–4692. [Google Scholar] [CrossRef]
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Fakhrhoseini, S.M.; Kumar, S.; Naebe, M. PAN precursor fabrication, applications and thermal stabilization process in carbon fiber production: Experimental and mathematical modelling. Prog. Mater. Sci. 2019, 107, 100575. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S. Freestanding and Flexible β-MnO2@Carbon Sheet for Application as a Highly Sensitive Dimethyl Methylphosphonate Sensor. ACS Omega 2021, 6, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Yu, H.-Q.; Feng, H.-M. PVA-based activated carbon fibers with lotus root-like axially porous structure. Carbon 2006, 44, 2059–2068. [Google Scholar] [CrossRef]
- Phong, N.T.; Gabr, M.H.; Okubo, K.; Chuong, B.; Fujii, T. Improvement in the mechanical performances of carbon fiber/epoxy composite with addition of nano-(Polyvinyl alcohol) fibers. Compos. Struct. 2013, 99, 380–387. [Google Scholar] [CrossRef]
- Wongtanakitcharoen, T.; Naaman, A.E. Unrestrained early age shrinkage of concrete with polypropylene, PVA, and carbon fibers. Mater. Struct. 2006, 40, 289–300. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Lee, D.-R.; Choi, K.-J. Preparation and characterization of nanoscaled poly(vinyl alcohol) fibers via electrospinning. Fibers Polym. 2002, 3, 73–79. [Google Scholar] [CrossRef]
- Fu, Q.; Jin, Y.; Song, X.; Gao, J.; Han, X.; Jiang, X.; Zhao, Q.; Yu, D. Size-dependent mechanical properties of PVA nanofibers reduced via air plasma treatment. Nanotechnology 2010, 21, 095703. [Google Scholar] [CrossRef]
- Ju, J.; Kang, W.; Deng, N.; Li, L.; Zhao, Y.; Ma, X.; Fan, L.; Cheng, B. Preparation and characterization of PVA-based carbon nanofibers with honeycomb-like porous structure via electro-blown spinning method. Microporous Mesoporous Mater. 2017, 239, 416–425. [Google Scholar] [CrossRef]
- Mora, E.; Blanco, C.; Prada, V.; Santamarıía, R.; Granda, M.; Menéndez, R. A study of pitch-based precursors for general purpose carbon fibres. Carbon 2002, 40, 2719–2725. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Otani, C.; Otani, S. Rheological study of eucalyptus tar pitches. J. Appl. Polym. Sci. 2002, 84, 900–908. [Google Scholar] [CrossRef]
- Yang, K.; Edie, D.D.; Lim, D.; Kim, Y.; Choi, Y. Preparation of carbon fiber web from electrostatic spinning of PMDA-ODA poly(amic acid) solution. Carbon 2003, 41, 2039–2046. [Google Scholar] [CrossRef]
- Kato, T.; Yamada, Y.; Nishikawa, Y.; Ishikawa, H.; Sato, S. Carbonization mechanisms of polyimide: Methodology to analyze carbon materials with nitrogen, oxygen, pentagons, and heptagons. Carbon 2021, 178, 58–80. [Google Scholar] [CrossRef]
- Kerisit, S.; Schwenzer, B.; Vijayakumar, M. Effects of Oxygen-Containing Functional Groups on Supercapacitor Performance. J. Phys. Chem. Lett. 2014, 5, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Sadati, S.M.; Mozafari, N.; Ashrafi, H.; Azadi, A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur. Polym. J. 2020, 128, 109607. [Google Scholar] [CrossRef]
- Xu, K.; Liu, C.; Kang, K.; Zheng, Z.; Wang, S.; Tang, Z.; Yang, W. Isolation of nanocrystalline cellulose from rice straw and preparation of its biocomposites with chitosan: Physicochemical characterization and evaluation of interfacial compatibility. Compos. Sci. Technol. 2017, 154, 8–17. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Bian, H.; Wang, W.; Wang, F.; Shao, Z. Dexterous and friendly preparation of N/P co-doping hierarchical porous carbon nanofibers via electrospun chitosan for high performance supercapacitors. J. Electroanal. Chem. 2020, 878, 114473. [Google Scholar] [CrossRef]
- Szabó, L.; Xu, X.; Ohsawa, T.; Uto, K.; Henzie, J.; Ichinose, I.; Ebara, M. Ultrafine self-N-doped porous carbon nanofibers with hierarchical pore structure utilizing a biobased chitosan precursor. Int. J. Biol. Macromol. 2021, 182, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Goepel, M.; Colmenares, J.C.; Gläser, R. Chitosan-Based N-Doped Carbon Materials for Electrocatalytic and Photocatalytic Applications. ACS Sustain. Chem. Eng. 2020, 8, 4708–4727. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Falco, C.; Weber, J.; White, R.J.; Howe, J.Y.; Titirici, M.-M. Carbohydrate-Derived Hydrothermal Carbons: A Thorough Characterization Study. Langmuir 2012, 28, 12373–12383. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Hu, P.; Dong, S.; Song, J.; Zhang, X. An efficient hydrothermal transformation approach for construction of controllable carbon coating on carbon fiber from renewable carbohydrate. Appl. Surf. Sci. 2019, 491, 478–487. [Google Scholar] [CrossRef]
- Okuzaki, H.; Takahashi, T.; Miyajima, N.; Suzuki, A.Y.; Kuwabara, T. Spontaneous Formation of Poly(p-phenylenevinylene) Nanofiber Yarns through Electrospinning of a Precursor. Macromolecules 2006, 39, 4276–4278. [Google Scholar] [CrossRef]
- Okuzaki, H.; Takahashi, T.; Hara, Y.; Yan, H. Uniaxially aligned carbon nanofibers derived from electrospun precursor yarns. J. Polym. Sci. Part B: Polym. Phys. 2007, 46, 305–310. [Google Scholar] [CrossRef]
- Yousaf, S.M.; Bower, K.E.; Sychov, M.M.; Yousaf, M.R. Poly(p-xylene tetrahydrothiophenium chloride) doped photoluminescent sol-gel composite. J. Mater. Sci. 2005, 40, 5507–5510. [Google Scholar] [CrossRef]
- Burn, P.L.; Bradley, D.D.C.; Friend, R.H.; Halliday, D.A.; Holmes, A.B.; Jackson, R.W.; Kraft, A. Precursor route chemistry and electronic properties of poly(p-phenylenevinylene), poly[(2,5-dimethyl-p-phenylene)vinylene] and poly[(2,5-dimethoxy-p-phenylene)vinylene]. J. Chem. Soc. Perkin Trans. 1992, 3225–3231. [Google Scholar] [CrossRef]
- Buchmeiser, M.R.; Unold, J.; Schneider, K.; Anderson, E.B.; Hermanutz, F.; Frank, E.; Müller, A.; Zinn, S. A new carbon precursor: Synthesis and carbonization of triethylammonium-based poly(p-phenylenevinylene) (PPV) progenitors. J. Mater. Chem. A 2013, 1, 13154–13163. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Guan, Y.; Zhu, H.; Yuan, G.; Dong, Z.; Li, X.; Zhang, Q.; Cong, Y. Preparation of pitch-based activated carbon fibers with high specific surface area and excellent adsorption properties. Res. Chem. Intermed. 2022, 48, 1733–1746. [Google Scholar] [CrossRef]
- Moon, S.; Farris, R.J. Strong electrospun nanometer-diameter polyacrylonitrile carbon fiber yarns. Carbon 2009, 47, 2829–2839. [Google Scholar] [CrossRef]
- Hao, J.; Li, W.; Suo, X.; Wei, H.; Lu, C.; Liu, Y. Highly isotactic (>60%) polyacrylonitrile-based carbon fiber: Precursor synthesis, fiber spinning, stabilization and carbonization. Polymer 2018, 157, 139–150. [Google Scholar] [CrossRef]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2018, 7, 269–277. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, T.; Khan, A.U.; Liu, G. Controlling the physical and electrochemical properties of block copolymer-based porous carbon fibers by pyrolysis temperature. Mol. Syst. Des. Eng. 2019, 5, 153–165. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, T.; Khan, A.U.; Liu, G. Block copolymer-based porous carbon fibers. Sci. Adv. 2019, 5, eaau6852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Lee, J.S. Multiscale pore contained carbon nanofiber-based field-effect transistor biosensors for nesfatin-1 detection. J. Mater. Chem. B 2021, 9, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, J.S.; Jun, J.; Shin, D.H.; Jang, J. Ultrasensitive Bisphenol A Field-Effect Transistor Sensor Using an Aptamer-Modified Multichannel Carbon Nanofiber Transducer. ACS Appl. Mater. Interfaces 2016, 8, 6602–6610. [Google Scholar] [CrossRef]
- Yan, J.; Dong, K.; Zhang, Y.; Wang, X.; AboAlhassan, A.A.; Yu, J.; Ding, B. Multifunctional flexible membranes from sponge-like porous carbon nanofibers with high conductivity. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-C.; Zhang, Y.; Kong, J.-H.; Wong, S.Y.; Li, X.; Wang, J. Hollow Carbon Nanoparticles of Tunable Size and Wall Thickness by Hydrothermal Treatment of α-Cyclodextrin Templated by F127 Block Copolymers. Chem. Mater. 2013, 25, 704–710. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Z.; Wang, Y.; Shang, X.; Nie, P.; Liu, J. Electrospun polyacrylonitrile/β-cyclodextrin based porous carbon nanofiber self-supporting electrode for capacitive deionization. RSC Adv. 2017, 7, 55224–55231. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Zhang, G.; Yu, L.; Lou, X.W.D. One-dimensional metal oxide–carbon hybrid nanostructures for electrochemical energy storage. Nanoscale Horiz. 2016, 1, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, J.S. Ruthenium Nanoparticle-Immobilized Porous Carbon Nanofibers for Nonenzymatic Dopamine Sensing. ACS Appl. Nano Mater. 2021, 4, 13683–13691. [Google Scholar] [CrossRef]
- Singh, K.; Lou, B.-S.; Her, J.-L.; Pang, S.-T.; Pan, T.-M. Super Nernstian pH response and enzyme-free detection of glucose using sol-gel derived RuOx on PET flexible-based extended-gate field-effect transistor. Sensors Actuators B Chem. 2019, 298, e126837. [Google Scholar] [CrossRef]
- Ryazantsev, M.N.; Jamal, A.; Maeda, S.; Morokuma, K. Global investigation of potential energy surfaces for the pyrolysis of C1–C3 hydrocarbons: Toward the development of detailed kinetic models from first principles. Phys. Chem. Chem. Phys. 2015, 17, 27789–27805. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, O.S.; Park, S.J.; Park, E.Y.; You, S.A.; Yoon, H.; Jang, J. Fabrication of Ultrafine Metal-Oxide-Decorated Carbon Nanofibers for DMMP Sensor Application. ACS Nano 2011, 5, 7992–8001. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Zhao, Y.; Zhang, J.; Li, X.; Fang, L.; Li, L. Mo-Doped Zn, Co Zeolitic Imidazolate Framework-Derived Co9S8 Quantum Dots and MoS2 Embedded in Three-Dimensional Nitrogen-Doped Carbon Nanoflake Arrays as an Efficient Trifunctional Electrocatalysts for the Oxygen Reduction Reaction, Oxygen Evolution Reaction, and Hydrogen Evolution Reaction. ACS Appl. Mater. 2020, 12, 10280–10290. [Google Scholar]
- Lee, S.; Kang, D.W.; Kwak, J.H.; Shin, S.; Park, J.-W.; Yu, S.-H.; Jung, H.-G.; Kim, B.G.; Lim, H.-D. Gold-incorporated porous hollow carbon nanofiber for reversible magnesium-metal batteries. Chem. Eng. J. 2021, 431, 133968. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Bae, J.-Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 hollow nanofibers as an anode material for high power lithium ion batteries. Energy Environ. Sci. 2011, 4, 4532–4536. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, T.; Meng, J.; Jiao, L.; Zhu, M.; Chen, J. Electrospun Thin-Walled CuCo2O4@C Nanotubes as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn–Air Batteries. Nano Lett. 2017, 17, 7989–7994. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent advances in carbon-based supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Bi, A.T.; Chen, H.L.; Pei, Y.R.; Zhao, M.; Yang, C.C.; Jiang, Q. Rational design of porous Sn nanospheres/N-doped carbon nanofibers as an ultra-stable potassium-ion battery anode material. J. Mater. Chem. A 2021, 9, 5740–5750. [Google Scholar] [CrossRef]
- Belgibayeva, A.; Taniguchi, I. Insights into the improved electrochemical performance of lithium–sulfur battery with free-standing SiO2/C composite nanofiber mat interlayer. J. Power Sources 2021, 484, 229308. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous Carbons: Structure-Oriented Design and Versatile Applications. Adv. Funct. Mater. 2020, 30, e201909265. [Google Scholar] [CrossRef]
- Luo, J.; Guan, K.; Lei, W.; Zhang, S.; Jia, Q.; Zhang, H. One dimensional carbon-based composites as cathodes for lithium-sulfur battery. J. Mater. Sci. Technol. 2022, 122, 101–120. [Google Scholar] [CrossRef]
- Lee, J.S.; Jun, J.; Cho, S.; Kim, W.; Jang, J. Electrospun three-layered polymer nanofiber-based porous carbon nanotubes for high-capacity energy storage. RSC Adv. 2016, 7, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shi, X.; Mao, Z.; Luo, C.; Li, G.; Wang, R.; He, B.; Jin, J.; Gong, Y.; Wang, H. Sulfur covalently linked TiO2/C nanofiber as a high-capacity, ultrastable, and self-supported anode for sodium-ion capacitors. Electrochim. Acta 2021, 399, 139377. [Google Scholar] [CrossRef]
- Yun, S.I.; Song, J.-W.; Kim, B.-H. Cyclodextrin/Graphene-Based Porous Carbon Nanofibers with Embedded MnO2 Nanoparticles for Supercapacitor Applications. ACS Appl. Nano Mater. 2022, 5, 5688–5698. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Gao, Y.; Dai, J.; Sun, X. The construction of carbon nanofiber composites modified by graphene/polypyrrole for flexible supercapacitors. J. Energy Storage 2022, 51, 104581. [Google Scholar] [CrossRef]
- Caturla, F.; Molina-Sabio, M.; Rodriguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Bu, J.; Zhang, L.; Cheng, L.; Wu, M. Constructing a novel carbon skeleton to anchor Sn/SnO2 nanodots for flexible supercapacitor with excellent rate capability. Carbon 2022, 194, 197–206. [Google Scholar] [CrossRef]
- Zheng, S.; Li, D.; Li, W.; Chen, J.; Rao, X.; Wang, N.; Qi, J.; Wang, B.; Luo, S.; Zhao, Y. MnO2 Nanosheets on a Carbon Nanofiber Freestanding Film by Electrospinning and In Situ Spraying for Lithium and Sodium Storage. ACS Appl. Energy Mater. 2022, 5, 3587–3594. [Google Scholar] [CrossRef]
- Seo, D.; Kim, M.; Song, J.K.; Kim, E.; Koo, J.; Kim, K.; Han, H.; Lee, Y.; Ahn, C.W. Hollow Ti3C2 MXene/Carbon Nanofibers as an Advanced Anode Material for Lithium-Ion Batteries. ChemElectroChem 2021, 9, e202101344. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.; Li, H.; Wu, Z.; Huang, Y.; Chang, B.; Guo, X.; Chen, G.; Wang, X. Encapsulating Nanoscale Silicon inside Carbon Fiber as Flexible Self-Supporting Anode Material for Lithium-Ion Battery. ACS Appl. Energy Mater. 2021, 4, 8529–8537. [Google Scholar] [CrossRef]
- Liang, P.; Zhu, K.; Rao, Y.; Zheng, H.; Yao, Z.; Wu, M.; Zhang, J.; Liu, J.; Yan, K.; Wang, J.; et al. Flexible and Self-Standing Urchinlike V2O3@Carbon Nanofibers toward Ultralong Cycle Lifespan Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 3242–3251. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, J.; Zhang, B.; Ling, M.; Hou, Y.; Liang, C. Electrospinning MoS2-Decorated Porous Carbon Nanofibers for High-Performance Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 11893–11899. [Google Scholar] [CrossRef]
- Deacon, A.; Briquet, L.; Malankowska, M.; Massingberd-Mundy, F.; Rudić, S.; Hyde, T.L.; Cavaye, H.; Coronas, J.; Poulston, S.; Johnson, T. Understanding the ZIF-L to ZIF-8 transformation from fundamentals to fully costed kilogram-scale production. Commun. Chem. 2022, 5, 1–10. [Google Scholar] [CrossRef]
- Yao, S.; Xue, S.; Peng, S.; Jing, M.; Shen, X.; Li, T.; Yiliu, Z. Electrospun zeolitic imidazolate framework-derived nitrogen-doped carbon nanofibers with high performance for lithium-sulfur batteries. Int. J. Energy Res. 2019, 43, 1892–1902. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, J.; Jin, B.; Jiang, Q.; Jin, E.M.; Jeong, S.M. Electrochemical performance of electrospun lotus–root–structure porous multichannel carbon nanotubes for lithium–sulfur battery applications. J. Electroanal. Chem. 2020, 878, 114564. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.; Jang, J.; Manthiram, A. Sulfur-Embedded Activated Multichannel Carbon Nanofiber Composites for Long-Life, High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2016, 7, 1601943. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.K.; Rahim, N.A. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Kabir, F.; Sakib, S.N.; Uddin, S.S.; Efaz, E.T.; Himel, T.F. Enhance cell performance of DSSC by dye mixture, carbon nanotube and post TiCl4 treatment along with degradation study. Sustain. Energy Technol. Assessments 2019, 35, 298–307. [Google Scholar] [CrossRef]
- Narudin, N.; Ekanayake, P.; Soon, Y.W.; Nakajima, H.; Lim, C.M. Enhanced properties of low-cost carbon black-graphite counter electrode in DSSC by incorporating binders. Sol. Energy 2021, 225, 237–244. [Google Scholar] [CrossRef]
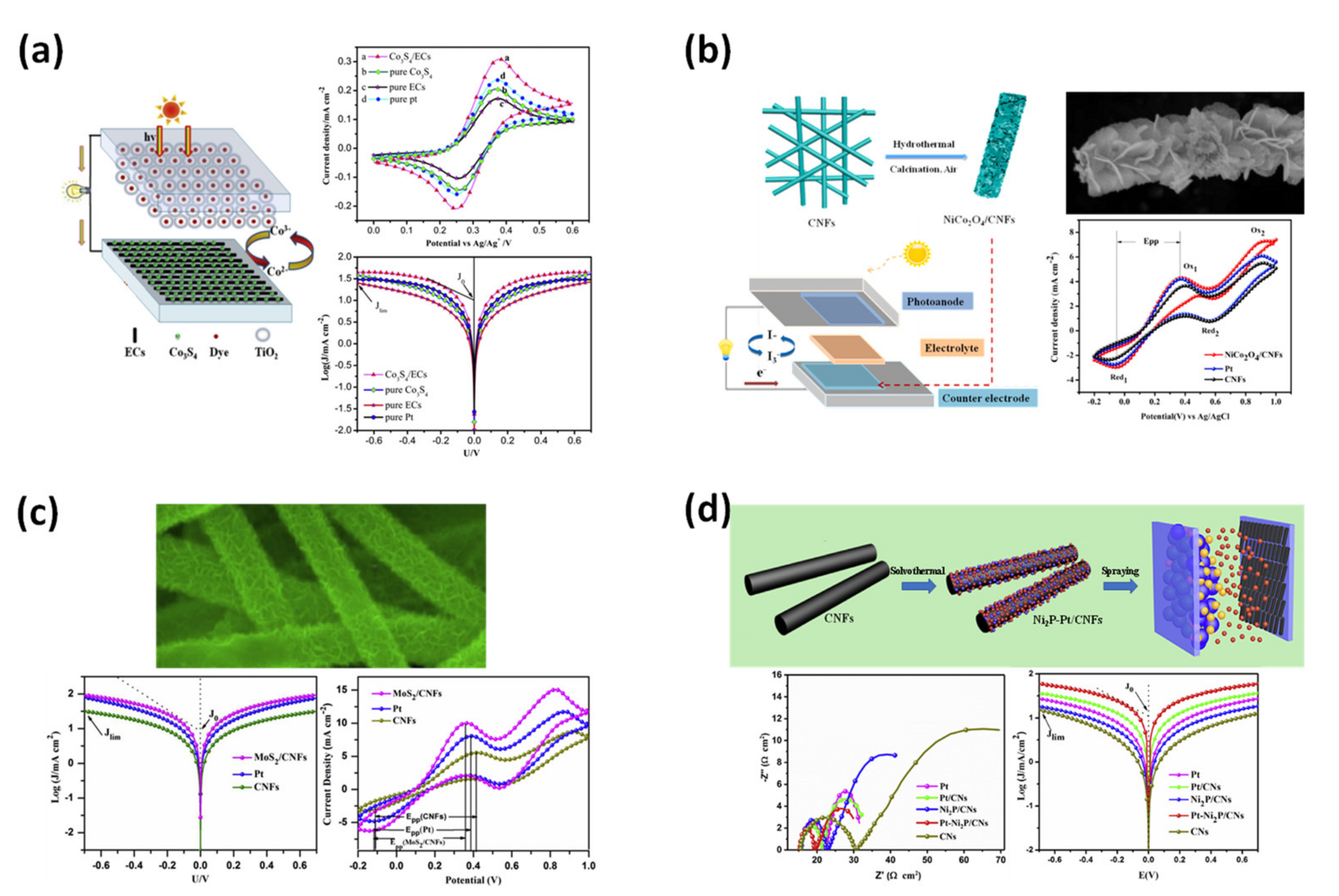
- Li, L.; Xiao, J.; Sui, H.; Yang, X.; Zhang, W.; Li, X.; Hagfeldt, A.; Wu, M. Highly effective Co3S4/electrospun-carbon-nanofibers composite counter electrode synthesized with electrospun technique for cobalt redox electrolyte based on dye-sensitized solar cells. J. Power Sources 2016, 326, 6–13. [Google Scholar] [CrossRef]
- Li, L.; Ma, Z.; Liu, L.; Wang, X.; Wang, J.; Cao, L.; Liu, S.; Zhang, W. NiCo2O4/carbon nanofibers composite as an efficient counter electrode for dye-sensitized solar cells. Mater. Res. Bull. 2022, 145, 111528. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Wang, D.; Zhang, W.; Li, X.; Zhao, X.; Zhang, Q.; Gu, L.; Yu, Z.; Wu, M. Electrospinning synthesis of high performance carbon nanofiber coated flower-like MoS2 nanosheets for dye-sensitized solar cells counter electrode. Electrochimica Acta 2018, 280, 94–100. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, X.; Wang, M.; Zhang, W.; Li, X.; Wang, H.; Li, L. Electrospun carbon nanofibers decorated with Pt-Ni2P nanoparticles as high efficiency counter electrode for dye-sensitized solar cells. J. Alloy. Compd. 2019, 786, 50–55. [Google Scholar] [CrossRef]
- Li, G.; Feng, L.; Chang, J.; Wickman, B.; Grönbeck, H.; Liu, C.; Xing, W. Activity of Platinum/Carbon and Palladium/Carbon Catalysts Promoted by Ni2P in Direct Ethanol Fuel Cells. ChemSusChem 2014, 7, 3374–3381. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Feng, L.; Liu, C.; Xing, W.; Hu, X. An Effective Pd-Ni2P/C Anode Catalyst for Direct Formic Acid Fuel Cells. Angew. Chem. Int. Ed. 2013, 53, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Kang, H.; Yang, X.; Li, Y.; Cui, K.; Wu, X.; Qin, W.; Wu, G. Amorphous Ni(III)-based sulfides as bifunctional water and urea oxidation anode electrocatalysts for hydrogen generation from urea-containing water. Appl. Catal. B 2022, 312, 121389. [Google Scholar] [CrossRef]
- Kumar, A.; Yasin, G.; Ajmal, S.; Ali, S.; Mushtaq, M.A.; Makhlouf, M.M.; Nguyen, T.A.; Bocchetta, P.; Gupta, R.K.; Ibraheem, S. Molecular MnN4-Complex immobilized on carbon black as efficient electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 17621–17629. [Google Scholar] [CrossRef]
- Qin, X.; Dai, L.; Li, H.; Qu, K.; Li, R. Enhanced HER catalysis based on MXene/N-doped graphene heterostructures: A first-principles study. Int. J. Hydrogen Energy 2022, 47, 15775–15782. [Google Scholar] [CrossRef]
- Qiang, F.; Feng, J.; Wang, H.; Yu, J.; Shi, J.; Huang, M.; Shi, Z.; Liu, S.; Li, P.; Dong, L. Oxygen Engineering Enables N-Doped Porous Carbon Nanofibers as Oxygen Reduction/Evolution Reaction Electrocatalysts for Flexible Zinc–Air Batteries. ACS Catal. 2022, 12, 4002–4015. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, D.; Wang, Z.; Zheng, X.; Fang, P.; Zhu, J.; Yu, M.; Tong, Y.; Lu, X. A Confinement Strategy for Stabilizing ZIF-Derived Bifunctional Catalysts as a Benchmark Cathode of Flexible All-Solid-State Zinc-Air Batteries. Adv. Mater. 2018, 30, e1805268. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, J.; Li, K.; Ao, Z.; Wang, C.; Liu, H.; Sun, K.; Wang, G. Electrospun cobalt embedded porous nitrogen doped carbon nanofibers as an efficient catalyst for water splitting. J. Mater. Chem. A 2016, 4, 12818–12824. [Google Scholar] [CrossRef]
- Cao, F.; Yang, X.; Shen, C.; Li, X.; Wang, J.; Qin, G.W.; Li, S.; Pang, X.; Li, G. Electrospinning synthesis of transition metal alloy nanoparticles encapsulated in nitrogen-doped carbon layers as an advanced bifunctional oxygen electrode. J. Mater. Chem. A 2020, 8, 7245–7252. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Ma, F.; Ou, Y.; Chen, Q.N.; Xie, S.; Li, J. Mesoporous carbon nanofibers with a high surface area electrospun from thermoplastic polyvinylpyrrolidone. Nanoscale 2012, 4, 7199–7204. [Google Scholar] [CrossRef]
- Ekabutr, P.; Ariyathanakul, T.; Chaiyo, S.; Niamlang, P.; Rattanaveeranon, S.; Chailapakul, O.; Supaphol, P. Carbonized electrospun polyvinylpyrrolidone/metal hybrid nanofiber composites for electrochemical applications. J. Appl. Polym. Sci. 2017, 135, 45639. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Zhang, W.; Li, Y.; Zhu, X.; Wang, P.; Hu, D. Porous Carbon Nanofibers Derived from Silk Fibroin through Electrospinning as N-Doped Metal-Free Catalysts for Hydrogen Evolution Reaction in Acidic and Alkaline Solutions. ACS Appl. Mater. Interfaces 2021, 14, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, G.; Qiao, J. Ultrafine porous carbon fiber and its supported platinum catalyst for enhancing performance of proton exchange membrane fuel cells. Electrochim. Acta 2015, 177, 174–180. [Google Scholar] [CrossRef]
- Chan, S.; Jankovic, J.; Susac, D.; Saha, M.S.; Tam, M.; Yang, H.; Ko, F. Electrospun carbon nanofiber catalyst layers for polymer electrolyte membrane fuel cells: Structure and performance. J. Power Sources 2018, 392, 239–250. [Google Scholar] [CrossRef]
- Di, Y.; Yang, W.; Li, X.; Zhao, Z.; Wang, M.; Dai, J. Preparation and characterization of continuous carbon nanofiber-supported SPEEK composite membranes for fuel cell application. RSC Adv. 2014, 4, 52001–52007. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Zhang, Y.; Li, C.; Dong, J.; Liu, Y.; Cheng, H. Electrospun multifunctional sulfonated carbon nanofibers for design and fabrication of SPEEK composite proton exchange membranes for direct methanol fuel cell application. Int. J. Hydrog. Energy 2017, 42, 10275–10284. [Google Scholar] [CrossRef]
- Karadurmus, L.; Cetinkaya, A.; Kaya, S.I.; Ozkan, S.A. Recent trends on electrochemical carbon-based nanosensors for sensitive assay of pesticides. Trends Environ. Anal. Chem. 2022, 34, e00158. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
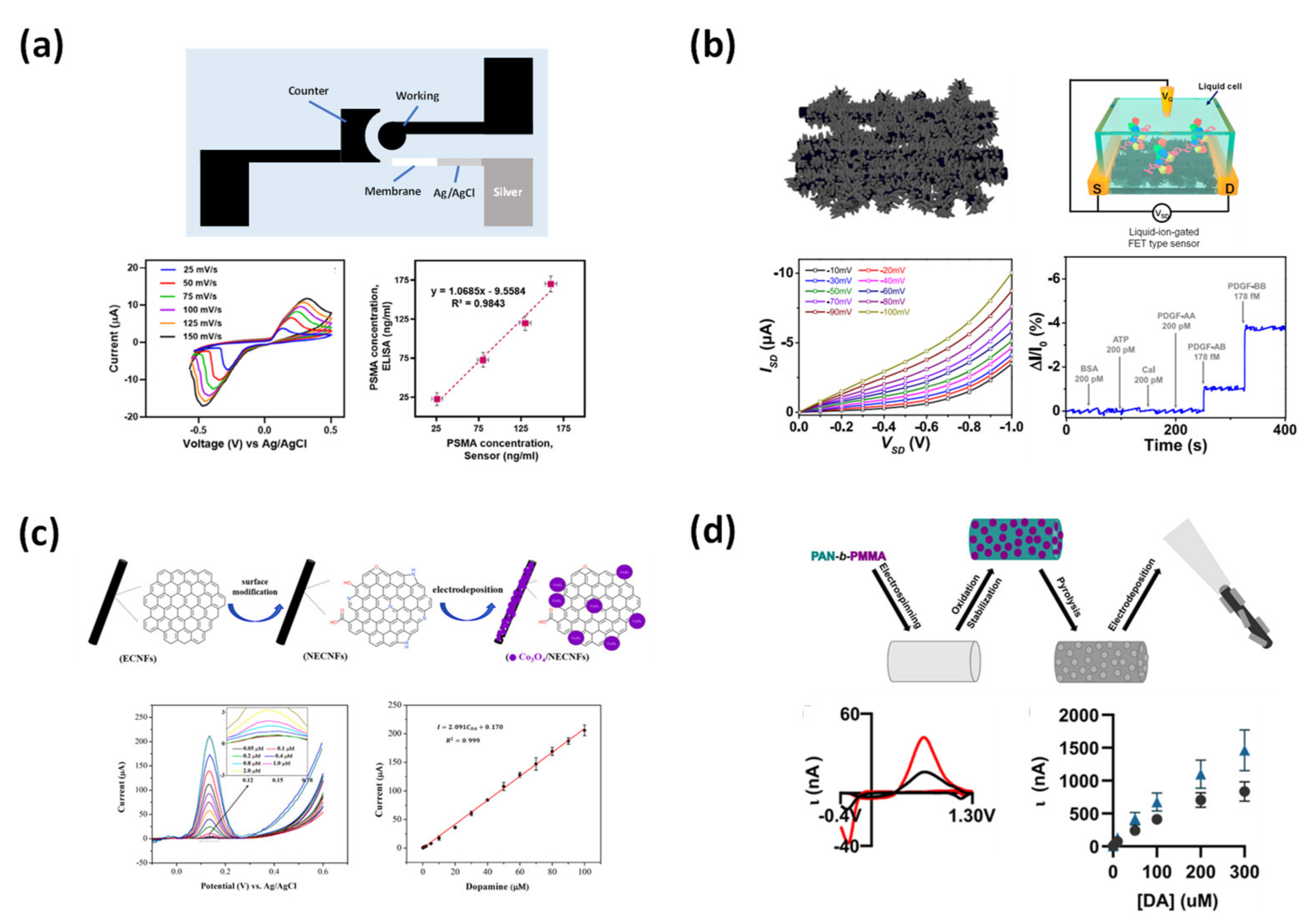
- Xie, H.; Luo, G.; Niu, Y.; Weng, W.; Zhao, Y.; Ling, Z.; Ruan, C.; Li, G.; Sun, W. Synthesis and utilization of Co3O4 doped carbon nanofiber for fabrication of hemoglobin-based electrochemical sensor. Mater. Sci. Eng. C 2019, 107, 110209. [Google Scholar] [CrossRef]
- Kim, S.G.; Jun, J.; Kim, Y.K.; Kim, J.; Lee, J.S.; Jang, J. Facile Synthesis of Co3O4-Incorporated Multichannel Carbon Nanofibers for Electrochemical Applications. ACS Appl. Mater. Interfaces 2020, 12, 20613–20622. [Google Scholar] [CrossRef]
- Hu, W.; Lu, H.; Duan, Y.; Li, L.; Ding, Y.; An, J.; Duan, D. An electrochemical sensor based on electrospun MoS2@SnO2 modified carbon nanofiber composite materials for simultaneously detection of phenacetin and indomethacin. Chem. Asian J. 2022, 17, e202101372. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Sangaranarayanan, M.V. Electrochemical Sensing of Anesthetics using Polythiophene Coated Glassy Carbon Electrodes. Chem. Sel. 2019, 4, 9776–9783. [Google Scholar] [CrossRef]
- Chai, A.-W.; Wang, C.-C.; Chen, C.-Y. Magnetic-field-induced acicular nickel immobilized on carbon nanofibers as electrodes for electrochemical glucose sensing. J. Taiwan Inst. Chem. Eng. 2021, 129, 237–245. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, J.; Noh, J.; Kim, D.; Jang, G.; Lee, N.; Lee, E.; Lee, T.S. Conjugated Polymer Dots-on-Electrospun Fibers as a Fluorescent Nanofibrous Sensor for Nerve Gas Stimulant. ACS Appl. Mater. Interfaces 2014, 6, 22884–22893. [Google Scholar] [CrossRef] [PubMed]
- Tuccitto, N.; Riela, L.; Zammataro, A.; Spitaleri, L.; Li-Destri, G.; Sfuncia, G.; Nicotra, G.; Pappalardo, A.; Capizzi, G.; Sfrazzetto, G.T. Functionalized Carbon Nanoparticle-Based Sensors for Chemical Warfare Agents. ACS Appl. Nano Mater. 2020, 3, 8182–8191. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Aljebawi, K.; Liu, Q.; Chen, R.; Yu, J.; Zhang, M.; Wang, J. 3D hybrid Ni-Multiwall carbon nanotubes/carbon nanofibers for detecting sarin nerve agent at room temperature. J. Alloy. Compd. 2018, 780, 680–689. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Moharram, D.; Yu, J.; Liu, Q.; Zhu, J.; Li, R.; Wang, J. HFIP-functionalized 3D carbon nanostructure as chemiresistive nerve agents sensors under visible light. Sensors Actuators B Chem. 2022, 358, e131475. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Moharram, D.; Liu, Q.; Yu, J.; Chen, R.; Li, R.; Wang, J. Fabrication of electrospun Co3O4/CuO p-p heterojunctions nanotubes functionalized with HFIP for detecting chemical nerve agent under visible light irradiation. Sens. Actuators B Chem. 2020, 314, e128076. [Google Scholar] [CrossRef]
- Rezaei, Z.; Alemzadeh, I.; Vossoughi, M. Design and fabrication of an electrochemical-based nanofibrous immunosensor for detection of prostate cancer biomarker, PSMA. Polym. Adv. Technol. 2022, 33, 1967–1977. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Jang, J. Aptamer-Functionalized Three-Dimensional Carbon Nanowebs for Ultrasensitive and Free-Standing PDGF Biosensor. ACS Appl. Mater. Interfaces 2020, 12, 20882–20890. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ji, Z.; Bloom, B.P.; Jayapalan, A.; Liu, M.; Zeng, X.; Waldeck, D.H.; Wei, J. Manipulating cobalt oxide on N-doped aligned electrospun carbon nanofibers towards instant electrochemical detection of dopamine secreted by living cells. Appl. Surf. Sci. 2021, 577, 151912. [Google Scholar] [CrossRef]
- Ostertag, B.J.; Cryan, M.T.; Serrano, J.M.; Liu, G.; Ross, A.E. Porous Carbon Nanofiber-Modified Carbon Fiber Microelectrodes for Dopamine Detection. ACS Appl. Nano Mater. 2022, 5, 2241–2249. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.D.; Lee, J.S. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Appl. Sci. 2022, 12, 6048. https://doi.org/10.3390/app12126048
Nguyen TD, Lee JS. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Applied Sciences. 2022; 12(12):6048. https://doi.org/10.3390/app12126048
Chicago/Turabian StyleNguyen, Trong Danh, and Jun Seop Lee. 2022. "Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications" Applied Sciences 12, no. 12: 6048. https://doi.org/10.3390/app12126048
APA StyleNguyen, T. D., & Lee, J. S. (2022). Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Applied Sciences, 12(12), 6048. https://doi.org/10.3390/app12126048









