The Effect of Pre-Treatment of Arabica Coffee Beans with Cold Atmospheric Plasma, Microwave Radiation, Slow and Fast Freezing on Antioxidant Activity of Aqueous Coffee Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Treating Coffee Beans
2.3. Monitoring Temperature
2.4. Measuring Moisture Content
2.5. Chemicals and Reagents
2.6. Preparation of Aqueous Coffee Extracts
2.7. Measuring AOA
2.8. Statistical Analysis
3. Results and Discussion
3.1. MC Analysis
3.2. Selection of AOA Estimation Method
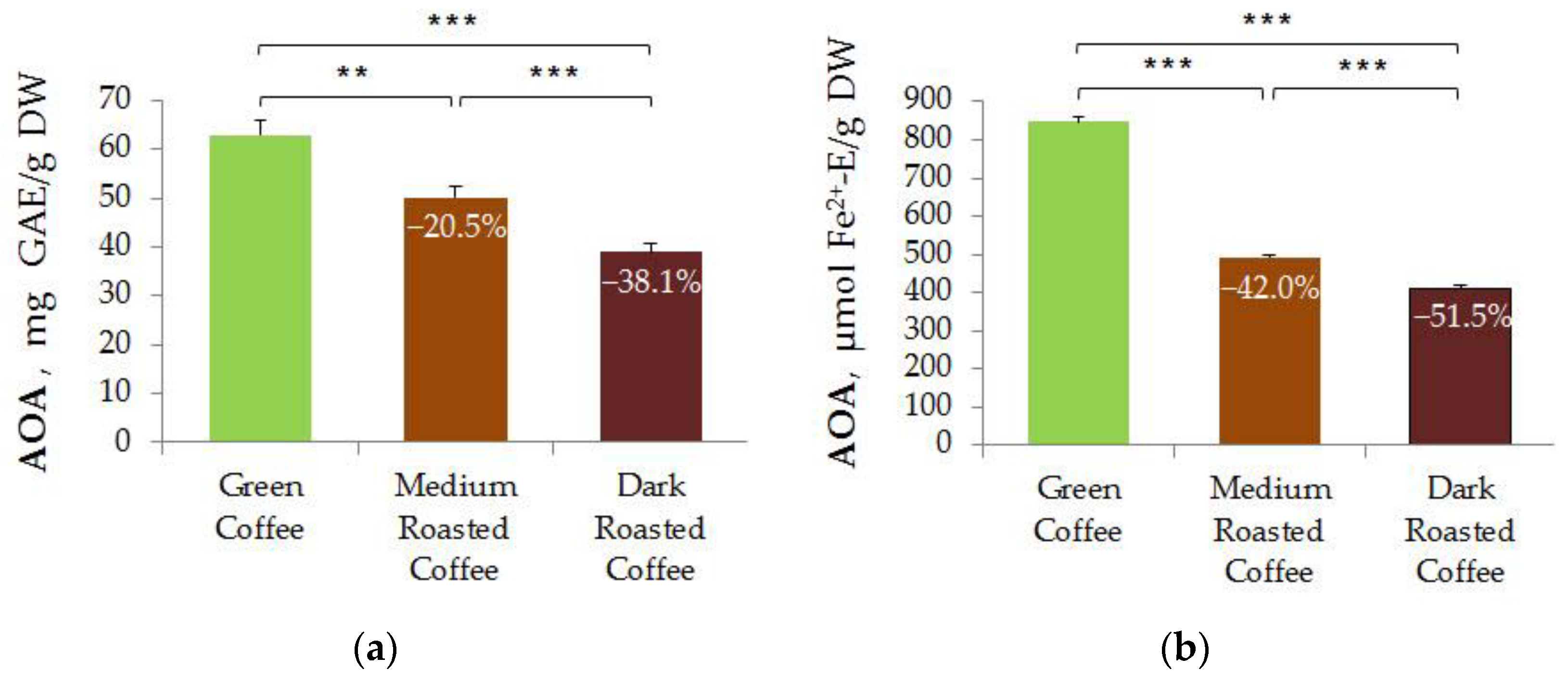
3.3. Comparative Analysis of the AOA of Aqueous Extracts of Treated and Untreated Coffee Beans
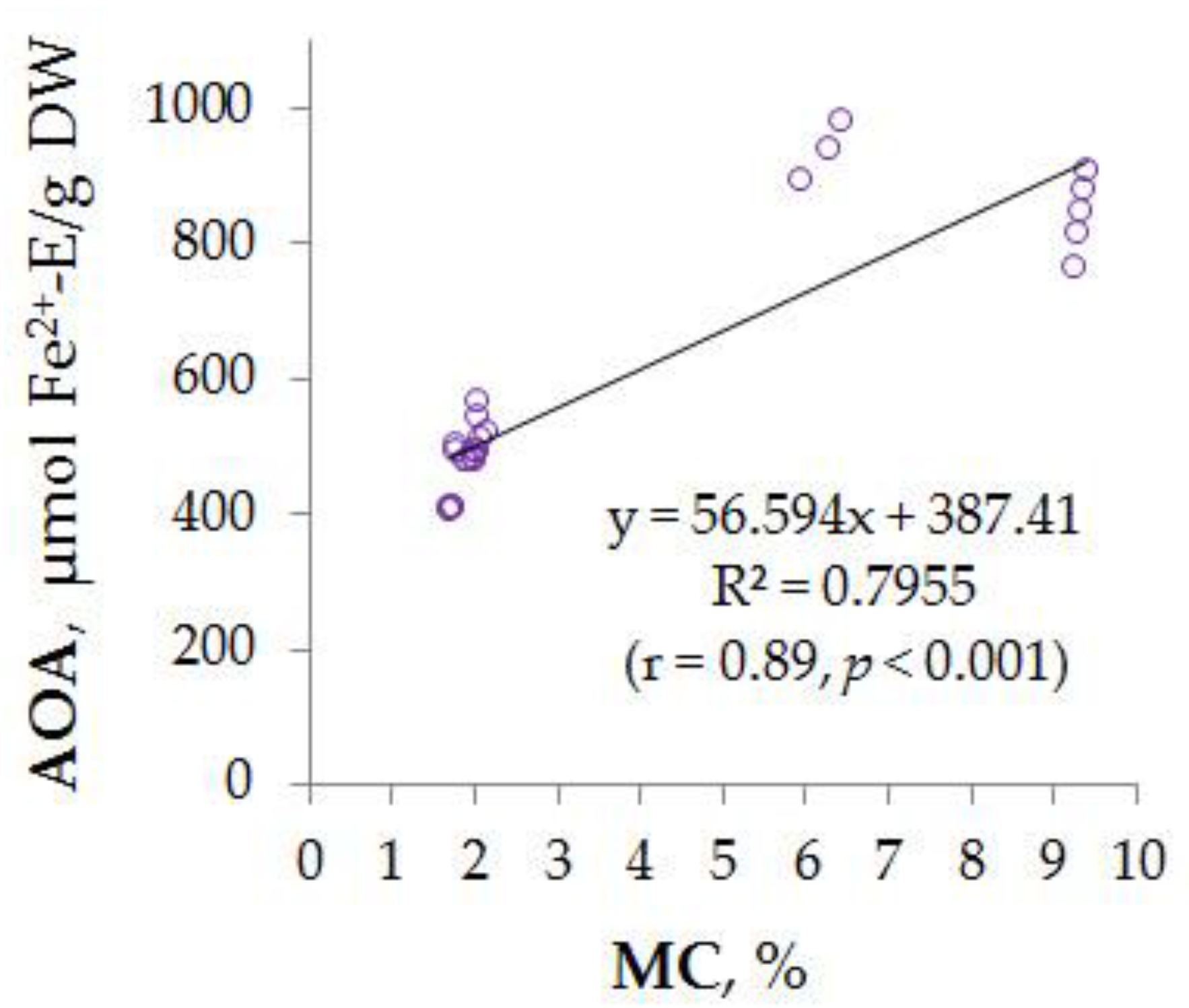
3.4. Interrelation between AOA and MC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
References
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. Br. Med. J. 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, caffeine, and health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Vu, T.-H.T.; Rydland, K.J.; Achenbach, C.J.; Van Horn, L.; Cornelis, M.C. Dietary behaviors and incident COVID-19 in the UK biobank. Nutrients 2021, 13, 2114. [Google Scholar] [CrossRef] [PubMed]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munyendo, L.M.; Njoroge, D.M.; Owaga, E.E.; Mugendi, B. Coffee phytochemicals and post-harvest handling–A complex and delicate balance. J. Food Compos. Anal. 2021, 102, 103995. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Meletis, C.D. Coffee—Functional food and medicinal herb. Altern. Complementary Ther. 2006, 12, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.C.; Casal, S.; Alves, M.R.; Oliveira, M.B. Discrimination between arabica and robusta coffee species on the basis of their tocopherol profiles. Food Chem. 2009, 114, 295–299. [Google Scholar] [CrossRef]
- Coffee, tea, cocoa. In Food Chemistry; Belitz, H.-D.; Grosch, W.; Schieberle, P. (Eds.) Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Data on coffee composition and mass spectrometry analysis of mixtures of coffee related carbohydrates, phenolic compounds and peptides. Data Brief 2017, 13, 145–161. [Google Scholar] [CrossRef]
- Brainina, K.H.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, methods, and future considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Saeed Alkaltham, M.; Musa Özcan, M.; Uslu, N.; Salamatullah, A.M.; Hayat, K. Effect of microwave and oven roasting methods on total phenol, antioxidant activity, phenolic compounds, and fatty acid compositions of coffee beans. J. Food Process. Preserv. 2020, 44, e14874. [Google Scholar] [CrossRef]
- Arzoo, S.; Badr, N.A.; Masri, S.A.; Obead, M.A.A.B. Study on the flavonoids, tocopherol and ascorbic acid content in raw and roasted Arabica coffee beans. J. Agric. Res. 2018, 4, 49–60. [Google Scholar]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gu, J.; BK, A.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of processing on bioaccessibility and bioavailability of bioactive compounds in coffee beans. Food Biosci. 2022, 46, 101373. [Google Scholar] [CrossRef]
- Noonim, P.; Mahakarnchanakul, W.; Varga, J.; Samson, R.A. Aspergilli and Ochratoxin A in coffee. In Aspergillus in the Genomic Era; Varga, J., Samson, R.A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2008; pp. 213–231. [Google Scholar] [CrossRef]
- Hollingsworth, R.G.; Jang, E.B.; Follett, P.A. Freezing as a treatment to prevent the spread of Hypothenemus hampei (Coleoptera: Curculionidae), in coffee. J. Econ. Entomol. 2013, 106, 653–660. [Google Scholar] [CrossRef] [Green Version]
- International Coffee Organization. Available online: https://www.ico.org/ (accessed on 27 April 2022).
- Dutra, E.R.; Oliveira, L.S.; Franca, A.S.; Ferraz, V.P.; Afonso, R.J.C.F. A Preliminary study on the feasibility of using the composition of coffee roasting exhaust gas for the determination of the degree of roast. J. Food Eng. 2001, 47, 241–246. [Google Scholar] [CrossRef]
- Souza, L.S.; Carrero Horta, I.P.; Souza Rosa, L.; Barbosa Lima, L.G.; Santos da Rosa, J.; Montenegro, J.; Silva Santos, L.; Nana de Castro, R.B.; Freitas-Silva, O.; Teodoro, A.J. Effect of the roasting levels of Coffea arabica L. extracts on their potential antioxidant capacity and antiproliferative activity in human prostate cancer cells. RSC Adv. 2020, 10, 30115–30126. [Google Scholar] [CrossRef]
- Jung, S.; Gu, S.; Lee, S.-H.; Jeong, Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee extracted from Coffea arabica cv. Java. Appl. Sci. 2021, 11, 7025. [Google Scholar] [CrossRef]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, L.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health Part B 2020, 55, 495–500. [Google Scholar] [CrossRef]
- Farah, A.; Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting non-thermal food processing and preservation methods–Action mechanisms, pros and cons: A technological update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Casas-Junco, P.P.; Solís-Pacheco, J.R.; Ragazzo-Sánchez, J.A.; Aguilar-Uscanga, B.R.; Bautista-Rosales, P.U.; Calderón-Santoyo, M. Cold plasma treatment as an alternative for Ochratoxin A detoxification and inhibition of mycotoxigenic fungi in roasted coffee. Toxins 2019, 11, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Cheng, K.; Hu, R.; Chu, Z.; Zhao, J.; Long, Y. Effect of microwave vacuum drying on the drying characteristics, color, microstructure, and antioxidant activity of green coffee beans. Molecules 2018, 23, 1146. [Google Scholar] [CrossRef] [Green Version]
- Casas-Junco, P.P.; Ragazzo-Sánchez, J.A.; Solís-Pacheco, J.R.; Aguilar-Uscanga, B.R.; Sáyago-Ayerdi, S.G.; Calderón-Santoyo, M. Physicochemical, aromatic, sensory properties and antioxidant activity of roasted coffee (Coffea arabica L.) treated with cold plasma technology. Biotecnia 2021, 23, 120–126. [Google Scholar] [CrossRef]
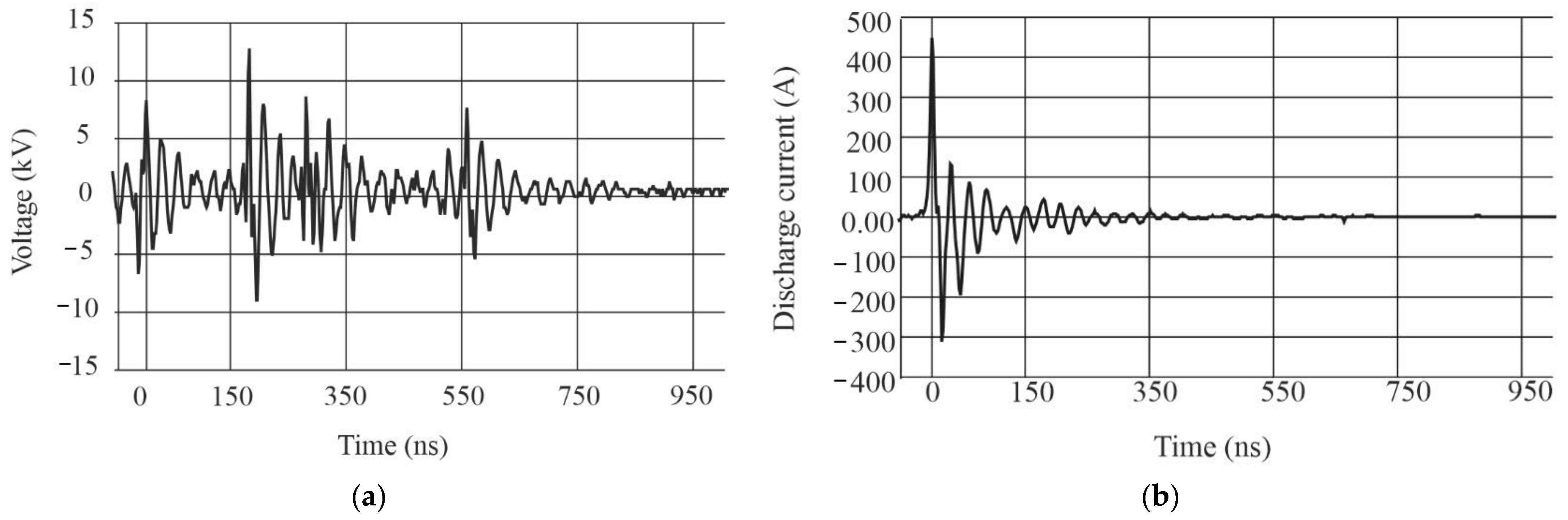
- Muzyukin, I.L. Experimental investigation of plasma cloud scattering initiated by an accelerated electron beam. IEEE Trans. Plasma Sci. 2015, 43, 3744–3748. [Google Scholar] [CrossRef]
- ISO 1446:2001; Green Coffee. Determination of Water Content. Basic Reference Method. International Organization for Standardization: Geneva, Switzerland, 2018; pp. 1–8.
- ISO 11294:1994; Roasted Ground Coffee. Determination of Moisture Content. Method by Determination of Loss in Mass at 103 Degrees C (Routine Method). International Organization for Standardization: Geneva, Switzerland, 2018; pp. 1–3.
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of brewing process parameters on antioxidant activity and caffeine content in infusions of roasted and unroasted Arabica coffee beans originated from different countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- SCAA Standard. Golden Cup; Specialty Coffee Association of America: Santa Ana, CA, USA, 2015; pp. 1–2. Available online: http://www.scaa.org/PDF/resources/golden-cup-standard.pdf (accessed on 27 April 2022).
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substances and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.H.Z.; Tarasov, A.V.; Kazakov, Y.A.E.; Vidrevich, M.B. Platinum electrode regeneration and quality control method for chronopotentiometric and chronoamperometric determination of antioxidant activity of biological fluids. J. Electroanal. Chem. 2018, 808, 14–20. [Google Scholar] [CrossRef]
- GNU PSPP. Available online: https://www.gnu.org/software/pspp/ (accessed on 27 April 2022).
- Li, D.; Zhu, Z.; Sun, D.-W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Jha, P.K.; Xanthakis, E.; Chevallier, S.; Jury, V.; Le-Bail, A. Assessment of freeze damage in fruits and vegetables. Food Res. Int. 2019, 121, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, D.; Chang, Y.; Xiao, Y. Effect of freeze-thaw pretreatment on extraction yield and antioxidant bioactivity of corn carotenoids (lutein and zeaxanthin). J. Food Qual. 2018, 2018, 9843503. [Google Scholar] [CrossRef]
- Schenker, S.; Handschin, S.; Frey, B.; Perren, R.; Escher, F. Pore structure of coffee beans affected by roasting conditions. J. Food Sci. 2000, 65, 452–457. [Google Scholar] [CrossRef]
- Wang, X.; Hong, D.-F.; Hu, G.-L.; Li, Z.-R.; Peng, X.-R.; Shi, Q.-Q.; Qiu, M.-H. Morphological changes and component characterization of coffee silverskin. Molecules 2021, 26, 4914. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Abert-Vian, M.; Chemat, F. Microwave-assisted extraction of antioxidants and food colors. In Microwave-Assisted Extraction for Bioactive Compounds. Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2012; Chapter 5; pp. 103–125. [Google Scholar] [CrossRef]
- Hu, Q.; He, Y.; Wang, F.; Wu, J.; Ci, Z.; Chen, L.; Xu, R.; Yang, M.; Lin, J.; Han, L.; et al. Microwave technology: A novel approach to the transformation of natural metabolites. Chin. Med. 2021, 16, 87. [Google Scholar] [CrossRef]
- Jafari, M.; Chegini, G.; Rezaeealam, B.; Akmal, A.A.S. Experimental determination of the dielectric constant of wheat grain and cluster straw in different moisture contents. Food Sci. Nutr. 2020, 8, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Solyom, K.; López, P.R.; Esquivel, P.; Vásquez-Caicedo, A.L. Effect of temperature and moisture contents on dielectric properties at 2.45 GHz of fruit and vegetable processing by-products. RSC Adv. 2020, 10, 16783–16790. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Tarasov, A.V.; Chugunova, O.V.; Stozhko, N.Y. Potentiometric sensor system based on modified thick-film electrodes for determining the antioxidant activity of beverages. Food Ind. 2020, 5, 85–96. [Google Scholar] [CrossRef]
- Neri, L.; Faieta, M.; Di Mattia, C.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Antioxidant activity in frozen plant foods: Effect of cryoprotectants, freezing process and frozen storage. Foods 2020, 9, 1886. [Google Scholar] [CrossRef]
- Chang, M.B.; Wu, S.-J. Experimental study on ozone synthesis via dielectric barrier discharges. Ozone Sci. Eng. 1997, 19, 241–254. [Google Scholar] [CrossRef]
- Pekárek, S. Non-thermal plasma ozone generation. Acta Polytech. 2003, 43, 47–51. [Google Scholar] [CrossRef]
- Shimizu, T.; Sakiyama, Y.; Graves, D.B.; Zimmermann, J.L.; Morfill, G.E. The dynamics of ozone generation and mode transition in air surface micro-discharge plasma at atmospheric pressure. New J. Phys. 2012, 14, 103028. [Google Scholar] [CrossRef] [Green Version]
- Locke, B.R.; Shih, K.-Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci. Technol. 2011, 20, 034006. [Google Scholar] [CrossRef]
- Feizollahi, E.; Iqdiam, B.; Vasanthan, T.; Thilakarathna, M.S.; Roopesh, M.S. Effects of atmospheric-pressure cold plasma treatment on deoxynivalenol degradation, quality parameters, and germination of barley grains. Appl. Sci. 2020, 10, 3530. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Rohn, S.; Kroh, L.W.; Geyer, M.; Schlüter, O. Surface morphology and chemical composition of lamb’s lettuce (Valerianella locusta) after exposure to a low-pressure oxygen plasma. Food Chem. 2010, 122, 1145–1152. [Google Scholar] [CrossRef]


| Treatment | Green Coffee | Medium Roasted Coffee | Dark Roasted Coffee | |||
|---|---|---|---|---|---|---|
| MC, % | ΔMC, % | MC, % | ΔMC, % | MC, % | ΔMC, % | |
| No Treatment | 9.33 ± 0.15 | - | 2.02 ± 0.08 | - | 1.72 ± 0.07 | - |
| SF | 9.42 ± 0.29 | +0.09 | 2.15 ± 0.12 * | +0.13 | 1.92 ± 0.14 * | +0.20 |
| FF | 9.37 ± 0.25 | +0.04 | 2.08 ± 0.13 | +0.06 | 1.87 ± 0.12 * | +0.15 |
| MWR | 5.95 ± 0.37 ** | −3.38 | 1.99 ± 0.07 | −0.03 | 1.70 ± 0.06 | −0.02 |
| SF + MWR | 6.44 ± 0.45 ** | −2.89 | 2.04 ± 0.14 | +0.02 | 1.79 ± 0.13 | +0.07 |
| FF + MWR | 6.28 ± 0.41 ** | −3.05 | 2.02 ± 0.14 | 0 | 1.77 ± 0.15 | +0.05 |
| CAP–5 | 9.31 ± 0.17 | −0.02 | 2.01 ± 0.09 | −0.01 | 1.73 ± 0.08 | +0.01 |
| CAP–15 | 9.25 ± 0.19 | −0.08 | 2.00 ± 0.09 | −0.02 | 1.71 ± 0.08 | −0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasov, A.; Bochkova, A.; Muzyukin, I.; Chugunova, O.; Stozhko, N. The Effect of Pre-Treatment of Arabica Coffee Beans with Cold Atmospheric Plasma, Microwave Radiation, Slow and Fast Freezing on Antioxidant Activity of Aqueous Coffee Extract. Appl. Sci. 2022, 12, 5780. https://doi.org/10.3390/app12125780
Tarasov A, Bochkova A, Muzyukin I, Chugunova O, Stozhko N. The Effect of Pre-Treatment of Arabica Coffee Beans with Cold Atmospheric Plasma, Microwave Radiation, Slow and Fast Freezing on Antioxidant Activity of Aqueous Coffee Extract. Applied Sciences. 2022; 12(12):5780. https://doi.org/10.3390/app12125780
Chicago/Turabian StyleTarasov, Aleksey, Anastasia Bochkova, Ilya Muzyukin, Olga Chugunova, and Natalia Stozhko. 2022. "The Effect of Pre-Treatment of Arabica Coffee Beans with Cold Atmospheric Plasma, Microwave Radiation, Slow and Fast Freezing on Antioxidant Activity of Aqueous Coffee Extract" Applied Sciences 12, no. 12: 5780. https://doi.org/10.3390/app12125780
APA StyleTarasov, A., Bochkova, A., Muzyukin, I., Chugunova, O., & Stozhko, N. (2022). The Effect of Pre-Treatment of Arabica Coffee Beans with Cold Atmospheric Plasma, Microwave Radiation, Slow and Fast Freezing on Antioxidant Activity of Aqueous Coffee Extract. Applied Sciences, 12(12), 5780. https://doi.org/10.3390/app12125780








