The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Technological Treatment
2.2.1. Fermentation Process
2.2.2. Juice Pressing
2.2.3. Freeze-Drying
2.3. Analytical Method
2.3.1. Dry Matter
2.3.2. Total Acidity
2.3.3. Color Parameters
2.3.4. Thermal Properties
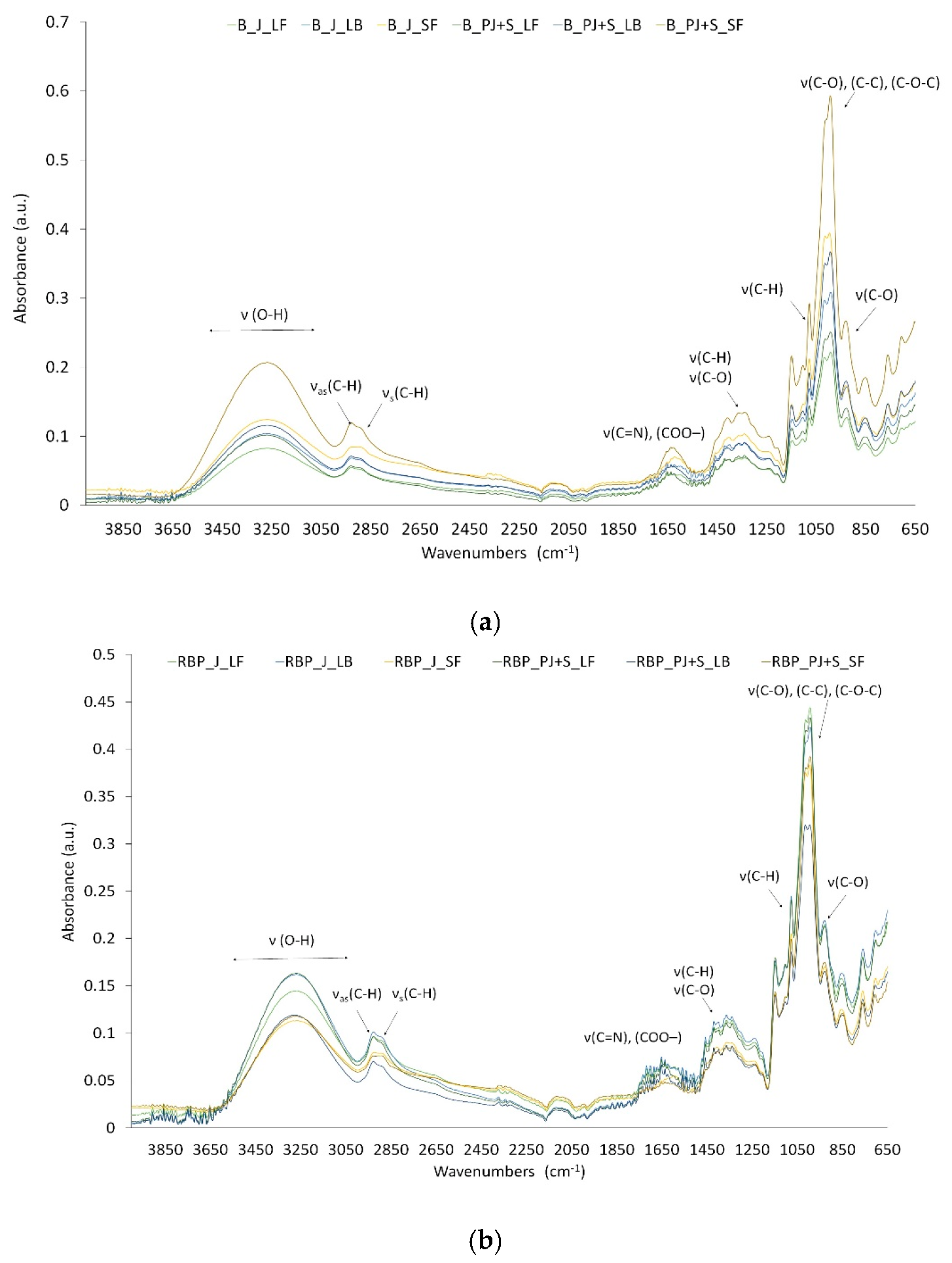
2.3.5. ATR-FTIR Spectroscopic Analysis
2.3.6. Determination of the Number of Lactic Acid Bacteria
2.3.7. Betalain Content
- (A)
- HPLC method
- (B)
- Spectrophotometric method
2.3.8. Carotenoids Analysis
2.4. Statistical Treatment
3. Results and Discussion
3.1. Juices
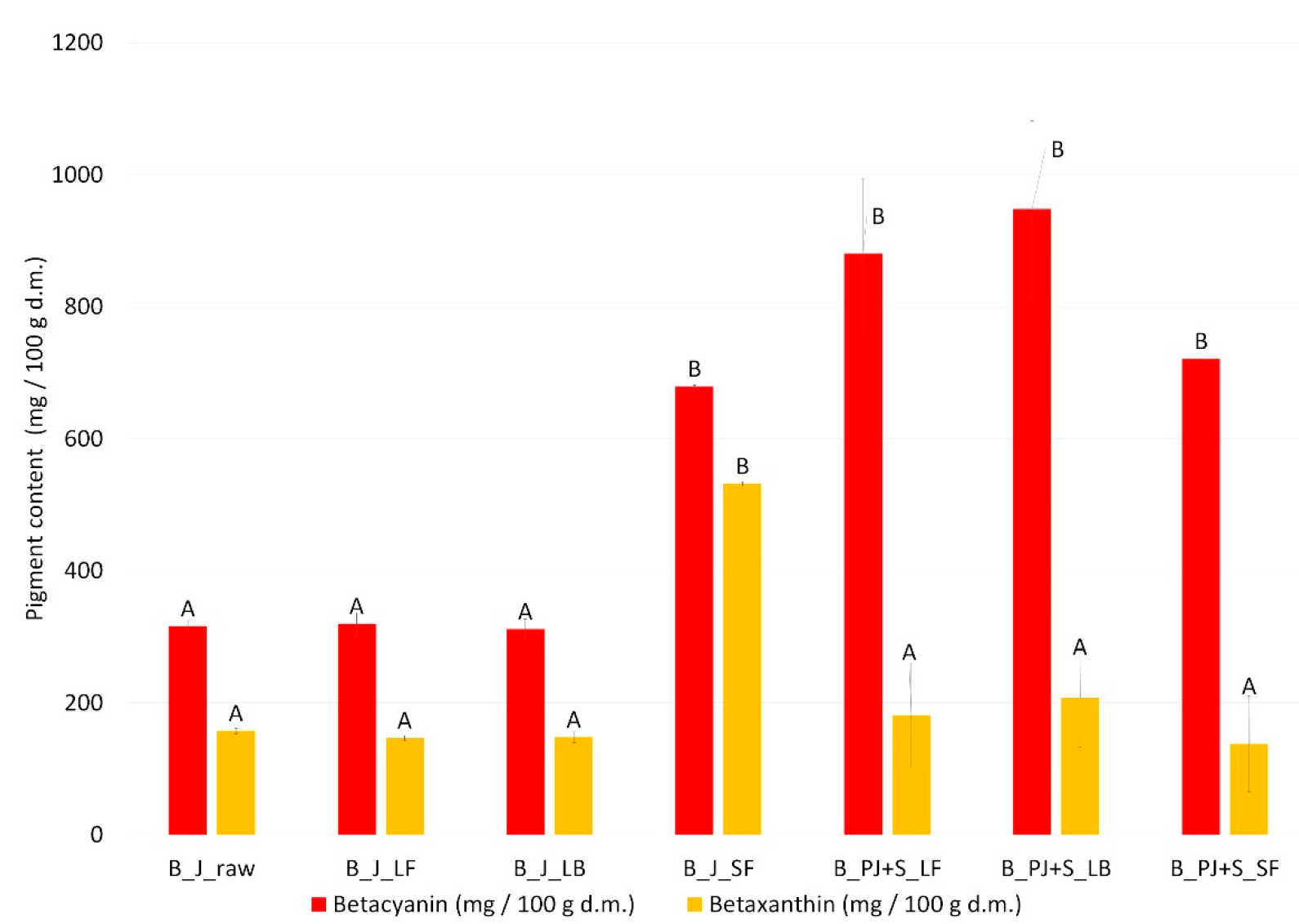
3.2. Freeze-Dried Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and Chemical Properties of Chokeberry Juice Fermented by Novel Lactic Acid Bacteria with Potential Probiotic Properties during Fermentation at 4 degrees C for 4 Weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Turak, E.; Walczak, M.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Witrowa-Rajchert, D. Influence of Fermentation Beetroot Juice Process on the Physico-Chemical Properties of Spray Dried Powder. Molecules 2022, 27, 1008. [Google Scholar] [CrossRef] [PubMed]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. Betalain Extracts from Beetroot as Food Colorants: Effect of Temperature and UV-Light on Storability. Plant Foods Hum. Nutr. 2021, 76, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Lombardelli, C.; Benucci, I.; Esti, M. Novel food colorants from tomatoes: Stability of carotenoid-containing chromoplasts under different storage conditions. LWT 2021, 140, 110725. [Google Scholar] [CrossRef]
- Lombardelli, C.; Liburdi, K.; Benucci, I.; Esti, M. Tailored and synergistic enzyme-assisted extraction of carotenoid-containing chromoplasts from tomatoes. Food Bioprod. Process. 2020, 121, 43–53. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, G.; Wang, D.; Hu, R.; Li, H.; Liu, S.; Zhang, Q.; Ming, J.; Chi, Y. Effects of dry-salting and brine-pickling processes on the physicochemical properties, nonvolatile flavour profiles and bacterial community during the fermentation of Chinese salted radishes. LWT 2022, 157, 113084. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Hornowska, Ł.; Pobiega, K.; Gniewosz, M.; Witrowa-Rajchert, D. The influence of Lactobacillus bacteria type and kind of carrier on the properties of spray-dried microencapsules of fermented beetroot powders. Int. J. Food Sci. Technol. 2021, 56, 2166–2174. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef] [PubMed]
- USDA1. Beet Raw, FDC ID: 169145. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169145/nutrients (accessed on 25 April 2022).
- USDA2. Peppers, Sweet, Red, Raw FDC ID: 170108. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170108/nutrients (accessed on 25 April 2022).
- USDA3; Carrots Raw FDC ID: 170393. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170393/nutrients (accessed on 25 April 2022).
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Nowacka, M.; Dadan, M.; Janowicz, M.; Wiktor, A.; Witrowa-Rajchert, D.; Mandal, R.; Pratap-Singh, A.; Janiszewska-Turak, E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5097–5144. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability-a review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petito, N.D.L.; Devens, J.M.; Falcão, D.Q.; Dantas, F.M.L.; Passos, T.S.; Araujo, K.G.D.L. Nanoencapsulation of Red Bell Pepper Carotenoids: Comparison of Encapsulating Agents in an Emulsion Based System. Colorants 2022, 1, 132–148. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Polak, R.; Biernacka, B. Effect of pre-treatment conditions and freeze-drying temperature on the process kinetics and physicochemical properties of pepper. LWT 2018, 98, 25–30. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Jaskulska, A. The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups. Appl. Sci. 2021, 11, 654. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, L. High efficient freeze-drying technology in food industry. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–19. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Appl. Sci. 2021, 11, 7864. [Google Scholar] [CrossRef]
- Cierach, M.; Niedźwiedź, J. Effects of three lighting intensities during display on discolouration of beef semitendinosus muscle. Eur. Food Res. Technol. 2014, 239, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Fernandes, G.; Bastos, M.C.; Mondamert, L.; Labanowski, J.; Burrow, R.A.; Rheinheimer, D.D.S. Organic composition of epilithic biofilms from agricultural and urban watershed in South Brazil. Environ. Sci. Pollut. Res. 2021, 28, 28808–28824. [Google Scholar] [CrossRef]
- Sokołowska, B.; Woźniak, Ł.; Skąpska, S.; Porębska, I.; Nasiłowska, J.; Rzoska, S.J. Evaluation of quality changes of beetroot juice after high hydrostatic pressure processing. High Press. Res. 2017, 37, 214–222. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules 2021, 26, 3683. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Witrowa-Rajchert, D. The influence of carrot pretreatment, type of carrier and disc speed on the physical and chemical properties of spray-dried carrot juice microcapsules. Dry. Technol. 2021, 39, 439–449. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Pobiega, K.; Witrowa-Rajchert, D.; Nowacka, M. Impact of pulsed light treatment on the quality properties and microbiological aspects of red bell pepper fresh-cuts. LWT 2021, 149, 111906. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Chauhan, D.S.; Thakur, D.; Chhikara, S.; Prabhakar, P.K. Status of beetroot processing and processed products: Thermal and emerging technologies intervention. Trends Food Sci. Technol. 2021, 114, 443–458. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Siemianowska, K.; Śniadowska, M.; Nowak, A. Bioactive Compounds and Microbial Quality of Stored Fermented Red Beetroots and Red Beetroot Juice. Pol. J. Food Nutr. Sci. 2020, 70, 35–44. [Google Scholar] [CrossRef]
- Massimo, C.; Marina, C.; Riccardo, M.; Danilo, M.; Roberto, M. Effects of controlled atmospheres and low temperature on storability of chestnuts manually and mechanically harvested. Postharvest Biol. Technol. 2011, 61, 131–136. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Wolkers-Rooijackers, J.C.M.; Thomas, S.M.; Nout, M.J.R. Effects of sodium reduction scenarios on fermentation and quality of sauerkraut. LWT-Food Sci. Technol. 2013, 54, 383–388. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Ua-arak, T.; Adhikari, B.P.; Howes, T.; Bhandari, B.R. Glass Transition Behavior of Spray Dried Orange Juice Powder Measured by Differential Scanning Calorimetry (DSC) and Thermal Mechanical Compression Test (TMCT). Int. J. Food Prop. 2007, 10, 661–673. [Google Scholar] [CrossRef]
- Otalora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhong, C.; Langrish, T. Encapsulation of caffeine in spray-dried micro-eggs for controlled release: The effect of spray-drying (cooking) temperature. Food Hydrocoll. 2020, 108, 105979. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruiz-Gutierrez, M.G.; Sanchez-Vega, R.; Santellano-Estrada, E.; Chavez-Martinez, A. Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin. Molecules 2020, 25, 5498. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.D.; Bronlund, J.E.; Paterson, A.H.J. Glass transition related cohesion of amorphous sugar powders. J. Food Eng. 2006, 77, 997–1006. [Google Scholar] [CrossRef]
- Avaltroni, F.; Bouquerand, P.; Normand, V. Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohydr. Polym. 2004, 58, 323–334. [Google Scholar] [CrossRef]
- Małecka, B. Metody analizy termicznej połączone z analizą produktów gazowych (TG-DSC-MS). LAB Lab. Apar. Bad. 2012, 17, 6–16. [Google Scholar]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Macêdo, R.; de Moura, O.; de Souza, A.; Macêdo, A. Comparative studies on some analytical methods: Thermal decomposition of powder milk. J. Therm. Anal. Calorim. 1997, 49, 857–862. [Google Scholar] [CrossRef]
- Carmo, E.L.D.; Teodoro, R.A.R.; Felix, P.H.C.; Fernandes, R.V.B.; Oliveira, E.R.; Veiga, T.; Borges, S.V.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 249, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Siemons, I.; Politiek, R.; Boom, R.; van der Sman, R.; Schutyser, M. Dextrose equivalence of maltodextrins determines particle morphology development during single sessile droplet drying. Food Res. Int. 2020, 131, 108988. [Google Scholar] [CrossRef] [PubMed]
- Aztatzi-Rugerio, L.; Granados-Balbuena, S.Y.; Zainos-Cuapio, Y.; Ocaranza-Sánchez, E.; Rojas-López, M. Analysis of the degradation of betanin obtained from beetroot using Fourier transform infrared spectroscopy. J. Food Sci. Technol. 2019, 56, 3677–3686. [Google Scholar] [CrossRef]
- Mocanu, G.-D.; Chirilă, A.C.; Vasile, A.M.; Andronoiu, D.G.; Nistor, O.-V.; Barbu, V.; Stănciuc, N. Tailoring the functional potential of red beet purées by inoculation with lactic acid bacteria and drying. Foods 2020, 9, 1611. [Google Scholar] [CrossRef]
- Devadiga, D.; Ahipa, T.N. Betanin: A Red-Violet Pigment-Chemistry and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR Spectroscopy Combined with Multivariate Regression Modeling as a Preliminary Approach for Carotenoids Determination in Cucurbita spp. Appl. Sci. 2020, 10, 3722. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Osorio-Revilla, G.I.; Gallardo-Velázquez, T.; Proal-Nájera, J. Uso de FTIR-HATR y análisis multivariable para el seguimiento de la degradación de compuestos bioactivos durante el secado de pimiento rojo. Rev. Mex. Ing. Química 2013, 12, 193–204. [Google Scholar]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197 Pt B, 1280–1285. [Google Scholar] [CrossRef]
- Coy-Barrera, E. Chapter 17-Analysis of betalains (betacyanins and betaxanthins). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of Microencapsulation by Spray Drying and Freeze Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Czyżowska, A.; Klewicka, E.; Libudzisz, Z. The influence of lactic acid fermentation process of red beet juice on the stability of biologically active colorants. Eur. Food Res. Technol. 2006, 223, 110–116. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.; Sporns, P. Carotenoids. In Handbook of Food Analytical Chemistry; John Wiley & Sons, Inc.: London, UK, 2005; pp. 71–119. [Google Scholar]
- Giusti, M.; Wrolstad, R. Characterization and measurement with UV-visible spectroscopy. Unit F2. 2, Ch. 2 In Current Protocols in Food Analytical Chemistry; King, S., Gates, M., Scalettar, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]

| Sample | Viscosity (mPas) | Dry Matter (g/g) | Total Acidity (g Lactic Acid/100 g Product) | Color Coefficients | ΔE | Number of Lactic Acid Bacteria log CFU/g d.m. | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| B_raw | 1.51 ± 0.09 ab | 0.054 ± 0.008 b | 0.82 ± 0.13 b | 6.33 ± 0.06 c | 14.46 ± 0.16 e | 1.91 ± 0.08 g | - | 2.06 ± 0.03 a |
| B_J_LF | 1.15 ± 0.10 a | 0.070 ± 0.006 c | 0.73 ± 0.02 b | 6.47 ± 0.21 c | 9.85 ± 0.44 d | 0.35 ± 0.06 f | 4.73 ± 0.40 a | 6.64 ± 0.16 c |
| B_J_LB | 1.63 ± 0.27 b | 0.072 ± 0.001 c | 0.68 ± 0.04 b | 5.68 ± 0.05 b | 5.37 ± 0.01 a | −0.31 ± 0.13 e | 9.23 ± 0.04 d | 7.94 ± 0.01 e |
| B_J_SF | 1.62 ± 0.07 b | 0.052 ± 0.002 b | 0.35 ± 0.03 a | 4.15 ± 0.05 a | 9.08 ± 0.16 c | −1.20 ± 0.12 d | 6.42 ± 0.10 b | 6.98 ± 0.06 d |
| B_PJ+S_LF | 1.21 ± 0.11 a | 0.033 ± 0.000 a | 0.61 ± 0.01 b | 6.84 ± 0.05 d | 4.91 ± 0.08 a | −2.57 ± 0.08 b | 10.42 ± 0.08 e | 6.27 ± 0.05 b |
| B_PJ+S_LB | 1.22 ± 0.11 a | 0.025 ± 0.001 a | 0.70 ± 0.00 b | 10.23 ± 0.05 f | 5.02 ± 0.07 a | −1.72 ± 0.06 c | 10.73 ± 0.06 e | 8.08 ± 0.09 e |
| B_PJ+S_SF | 1.40 ± 0.13 ab | 0.032 ± 0.001 a | 0.70 ± 0.01 b | 9.68 ± 0.06 e | 8.25 ± 0.11 b | −2.81 ± 0.02 a | 8.38 ± 0.06 c | 6.62 ± 0.26 c |
| RBP_raw | 1.25 ± 0.05 A | 0.018 ± 0.003 A | 0.73 ± 0.03 AB | 14.96 ± 0.03 A | 14.21 ± 0.05 C | 14.16 ± 0.06 C | - | 2.21 ± 0.09 A |
| RBP_J_LF | 1.51 ± 0.14 C | 0.047 ± 0.006 C | 1.96 ± 0.08 D | 24.39 ± 0.01 F | 18.98 ± 0.02 D | 31.40 ± 0.09 E | 20.19 ± 0.07 C | 7.91 ± 0.04 D |
| RBP_J_LB | 1.31 ± 0.08 AB | 0.035 ± 0.000 B | 1.60 ± 0.16 C | 24.00 ± 0.01 F | 19.17 ± 0.01 E | 28.28 ± 0.04 D | 17.45 ± 0.03 B | 7.06 ± 0.03 B |
| RBP_J_SF | 1.46 ± 0.01 B | 0.038 ± 0.002 BC | 1.33 ± 0.10 C | 21.96 ± 0.01 E | 15.38 ± 0.02 D | 31.38 ± 0.12 E | 20.14 ± 0.09 C | 8.37 ± 0.09 E |
| RBP_PJ+S_LF | 1.27 ± 0.09 A | 0.013 ± 0.002 A | 0.94 ± 0.04 B | 17.18 ± 0.03 D | 8.26 ± 0.07 A | 10.74 ± 0.04 A | 7.26 ± 0.07 A | 7.71 ± 0.07 C |
| RBP_PJ+S_LB | 1.36 ± 0.06 AB | 0.015 ± 0.000 A | 0.77 ± 0.04 AB | 16.97 ± 0.01 B | 8.45 ± 0.04 B | 11.05 ± 0.15 B | 6.65 ± 0.07 A | 7.15 ± 0.06 B |
| RBP_PJ+S_SF | 1.33 ± 0.01 AB | 0.076 ± 0.001 D | 0.63 ± 0.04 A | 17.06 ± 0.00 C | 8.26 ± 0.08 A | 10.66 ± 0.04 A | 7.26 ± 0.09 A | 8.33 ± 0.06 E |
| Sample | Dry Matter (g/g) | Total Acidity (g Lactic Acid/100 g Product) | Color Coefficients | Tg (°C) | Number of Lactic Acid Bacteria log CFU/g d.m. | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| B_J_LF | 0.840 ± 0.201 a | 2.78 ± 0.21 a | 37.92 ± 0.35 cd | 25.51 ± 0.26 ab | −10.38 ± 0.17 b | 61.51 ± 0.49 b | 5.80 ± 0.01 d |
| B_J_LB | 0.947 ± 0.006 a | 2.86 ± 0.09 a | 36.09 ± 1.41 bc | 26.81 ± 3.24 bc | −10.83 ± 1.17 b | 58.68 ± 0.86 a | 5.89 ± 0.01 d |
| B_J_SF | 0.982 ± 0.000 a | 2.96 ± 0.39 a | 35.31 ± 1.35 b | 21.82 ± 0.70 a | −9.62 ± 0.66 b | 59.60 ± 0.23 a | 5.37 ± 0.05 bc |
| B_PJ+S_LF | 0.940 ± 0.003 a | 2.72 ± 0.00 a | 36.05 ± 0.01 bc | 36.53 ± 0.01 e | −13.31 ± 0.02 a | 66.00 ± 0.49 d | 5.25 ± 0.01 b |
| B_PJ+S_LB | 0.931 ± 0.038 a | 2.57 ± 0.06 a | 32.85 ± 0.00 a | 33.03 ± 0.01 de | −0.29 ± 0.01 c | 62.55 ± 0.49 b | 5.15 ± 0.10 b |
| B_PJ+S_SF | 0.960 ± 0.000 a | 2.57 ± 0.06 a | 39.37 ± 0.01 d | 29.60 ± 0.04 cd | −10.70 ± 0.02 b | 64.12 ± 0.33 c | 4.53 ± 0.16 a |
| RBP_J_LF | 0.840 ± 0.016 A | 6.18 ± 0.76 BC | 66.42 ± 0.00 B | 27.07 ± 0.01 E | 37.28 ± 0.01 D | 48.56 ± 0.23 C | 5.81 ± 0.01 C |
| RBP_J_LB | 0.971 ± 0.001 C | 7.50 ± 1.41 C | 62.48 ± 0.01 A | 28.99 ± 0.01 F | 37.88 ± 0.01 E | 61.17 ± 0.35 E | 5.92 ± 0.06 D |
| RBP_J_SF | 0.923 ± 0.004 B | 3.49 ± 0.04 AB | 75.78 ± 0.15 E | 14.47 ± 0.03 B | 25.81 ± 0.03 B | 56.43 ± 0.15 D | 5.54 ± 0.11 B |
| RBP_PJ+S_LF | 0.964 ± 0.010 C | 4.17 ± 0.73 AB | 71.58 ± 0.01 C | 22.51 ± 0.01 D | 31.21 ± 0.01 C | 52.86 ± 0.41 D | 5.61 ± 0.03 B |
| RBP_PJ+S_LB | 0.950 ± 0.013 BC | 3.89 ± 0.05 AB | 73.56 ± 0.01 D | 18.44 ± 0.01 C | 26.17 ± 0.01 B | 49.55 ± 0.41 B | 5.25 ± 0.20 A |
| RBP_PJ+S_SF | 0.824 ± 0.000 A | 2.20 ± 0.01 A | 83.58 ± 0.38 F | 8.50 ± 0.36 A | 16.48 ± 0.50 A | 46.52 ± 0.39 A | 5.11 ± 0.27 A |
| Step 1 | Step 2 | Step 3 | Sum [%] | Decomposition Temperature [°C] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. Range [°C] | Mass Loss [%] | Temp. Range [°C] | Mass Loss [%] | Temp. Range [°C] | Mass Loss [%] | 1 | 2 | ||||
| B_J_LF | 30–140 | 2.0 | 140–420 | 41.8 | 420–600 | 8.7 | 52.5 | 78.1 | 162.0 | 197.7 | 268.2 |
| B_J_LB | 30–140 | 2.2 | 140–420 | 45.3 | 420–600 | 5.2 | 52.8 | 79.3 | 161.3 | 195.7 | 267.6 |
| B_J_SF | 30–120 | 2.4 | 120–420 | 52.3 | 420–600 | 2.2 | 56.9 | 73.3 | 152.0 | 286.2 | |
| B_PJ+S_LF | 30–140 | 0.4 | 140–420 | 44.1 | 420–600 | 3.8 | 48.3 | 84.5 | 173.7 | 289.3 | |
| B_PJ+S_LB | 30–140 | 7.2 | 140–420 | 46.4 | 420–600 | 3.4 | 57.0 | 170.0 | 290.6 | ||
| B_PJ+S_SF | 30–140 | 5.4 | 140–420 | 55.5 | 420–600 | 2.2 | 63.2 | 169.4 | 291.8 | ||
| RBP_J_LF | 30–130 | 4.4 | 130–420 | 43.4 | 420–600 | 2.7 | 50.5 | 85.3 | 155.0 | 284.3 | |
| RBP_J_LB | 30–130 | 3.7 | 130–420 | 46.2 | 420–600 | 3.5 | 53.5 | 80.3 | 154.0 | 285.9 | |
| RBP_J_SF | 30–120 | 1.6 | 120–420 | 53.4 | 420–600 | 6.6 | 61.6 | 153.2 | 287.2 | ||
| RBP_PJ+S_LF | 30–140 | 2.6 | 140–420 | 47.8 | 420–600 | 3.9 | 54.3 | 92.2 | 168.6 | 293.3 | |
| RBP_PJ+S_LB | 30–140 | 0.6 | 140–420 | 47.1 | 420–600 | 6.8 | 54.5 | 75.9 | 293.0 | ||
| RBP_PJ+S_SF | 30–140 | 1.4 | 140–420 | 75.1 | 420–600 | 0.5 | 77.0 | 67.0 | 290.5 | ||
| Sample | Betaxanthin (mg/100 g dm) | Betacyanin (mg/100 g dm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Vulgaxanthin I | A | Betanin | B | Isobetanin | Betanidin | C | Neobetanin | |
| B_J_LF | 1.22 ± 0.08 b | 0 a | 60.22 ± 1.27 d | 0.29 ± 0.04 a | 5.77 ± 0.25 b | 6.38 ± 0.38 c | 0 a | 0 a |
| B_J_LB | 1.70 ± 0.08 c | 0.21 ± 0.05 b | 72.83 ± 0.77 e | 0.17 ± 0.02 a | 7.93 ± 0.32 c | 6.80 ± 0.08 c | 0.43 ± 0.04 b | 0 a |
| B_J_SF | 2.19 ± 0.00 d | 0 a | 41.96 ± 0.69 ab | 0.51 ± 0.03 b | 3.67 ± 0.32 a | 16.35 ± 0.90 d | 0.88 ± 0.08 c | 0 a |
| B_PJ+S_LF | 0.15 ± 0.00 a | 0.85 ± 0.06 c | 48.36 ± 1.87 c | 0.80 ± 0.08 c | 14.69 ± 0.40 d | 0.38 ± 0.07 a | 0 a | 0.54 ± 0.07 b |
| B_PJ+S_LB | 0.09 ± 0.00 a | 1.24 ± 0.01 d | 60.40 ± 4.73 d | 1.09 ± 0.04 d | 16.41 ± 0.32 e | 0 a | 0 a | 0.83 ± 0.03 c |
| B_PJ+S_SF | 1.71 ± 0.11 c | 0 ac | 36.90 ± 0.01 a | 0.54 ± 0.08 b | 7.08 ± 0.11 c | 4.15 ± 0.08 b | 0.73 ± 0.04 c | 0.60 ± 0.04 b |
| Sample | Betalain Results from HPLC Method | Betalain Results from the Spectrophotometric Method | ||
|---|---|---|---|---|
| Betacyanin (mg/100 g dm) | Betaxanthin (mg/100 g dm) | Betacyanin (mg/100 g dm) | Betaxanthin (mg/100 g dm) | |
| B_J_LF | 72.67 ± 1.94 bc | 1.22 ± 0.08 b | 94.89 ± 1.96 a | 50.72 ± 2.00 c |
| B_J_LB | 88.38 ± 1.28 d | 1.70 ± 0.08 c | 99.85 ± 0.14 ab | 54.82 ± 0.28 cd |
| B_J_SF | 63.37 ± 2.02 ab | 2.19 ± 0.00 d | 104.61 ± 7.55 ab | 56.14 ± 1.66 d |
| B_PJ+S_LF | 65.62 ± 2.54 bc | 0.15 ± 0.00 a | 98.39 ± 2.09 ab | 7.96 ± 0.59 a |
| B_PJ+S_LB | 79.96 ± 5.14 cd | 0.09 ± 0.00 a | 122.50 ± 0.79 c | 17.02 ± 1.88 b |
| B_PJ+S_SF | 49.99 ± 0.37 a | 1.71 ± 0.11 c | 111.53 ± 2.79 bc | 15.52 ± 0.24 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Turak, E.; Tracz, K.; Bielińska, P.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Gramza-Michałowska, A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Appl. Sci. 2022, 12, 5766. https://doi.org/10.3390/app12125766
Janiszewska-Turak E, Tracz K, Bielińska P, Rybak K, Pobiega K, Gniewosz M, Woźniak Ł, Gramza-Michałowska A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Applied Sciences. 2022; 12(12):5766. https://doi.org/10.3390/app12125766
Chicago/Turabian StyleJaniszewska-Turak, Emilia, Kacper Tracz, Patrycja Bielińska, Katarzyna Rybak, Katarzyna Pobiega, Małgorzata Gniewosz, Łukasz Woźniak, and Anna Gramza-Michałowska. 2022. "The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders" Applied Sciences 12, no. 12: 5766. https://doi.org/10.3390/app12125766
APA StyleJaniszewska-Turak, E., Tracz, K., Bielińska, P., Rybak, K., Pobiega, K., Gniewosz, M., Woźniak, Ł., & Gramza-Michałowska, A. (2022). The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Applied Sciences, 12(12), 5766. https://doi.org/10.3390/app12125766










