Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures
Abstract
:1. Introduction
2. Materials and Methods
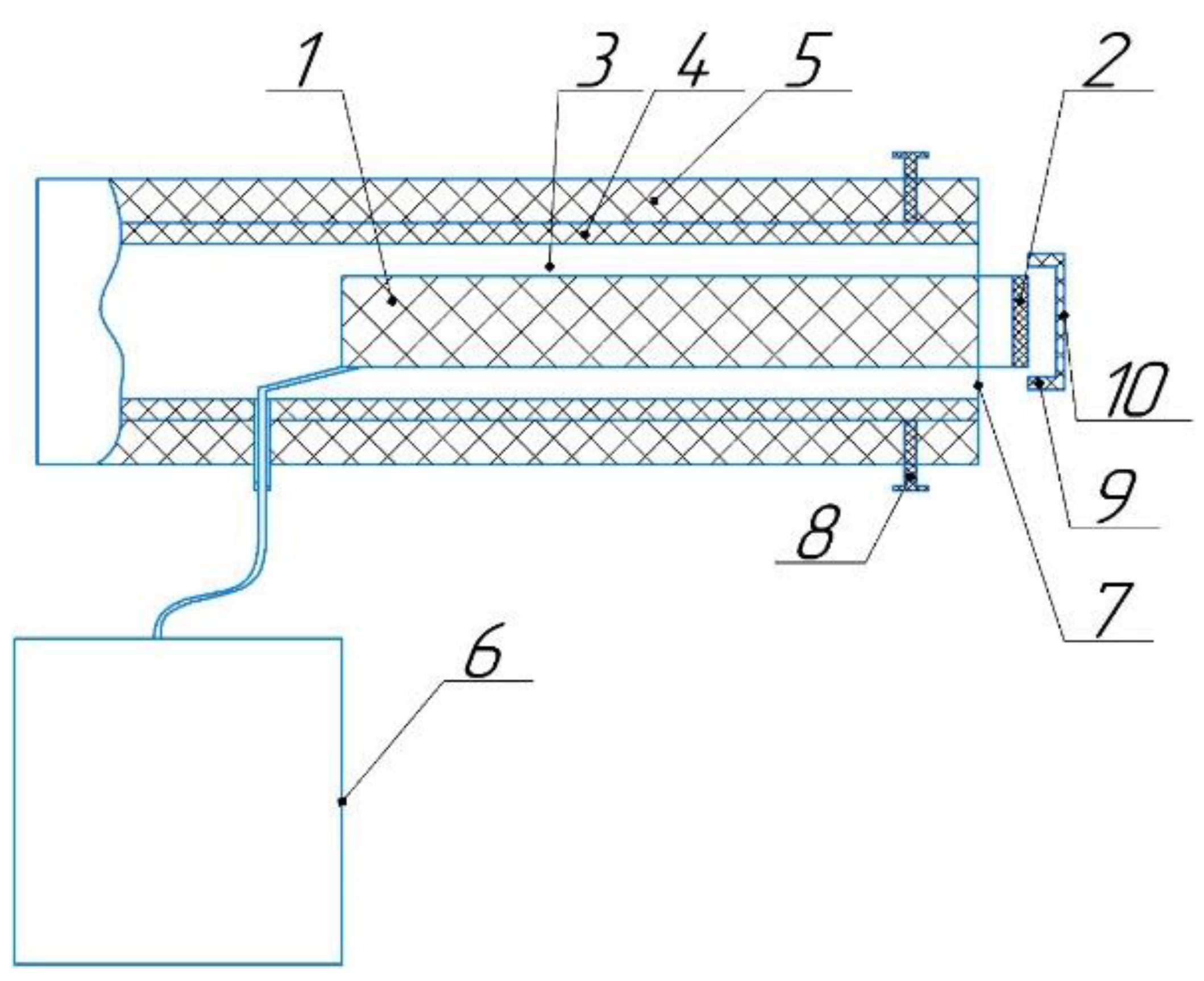
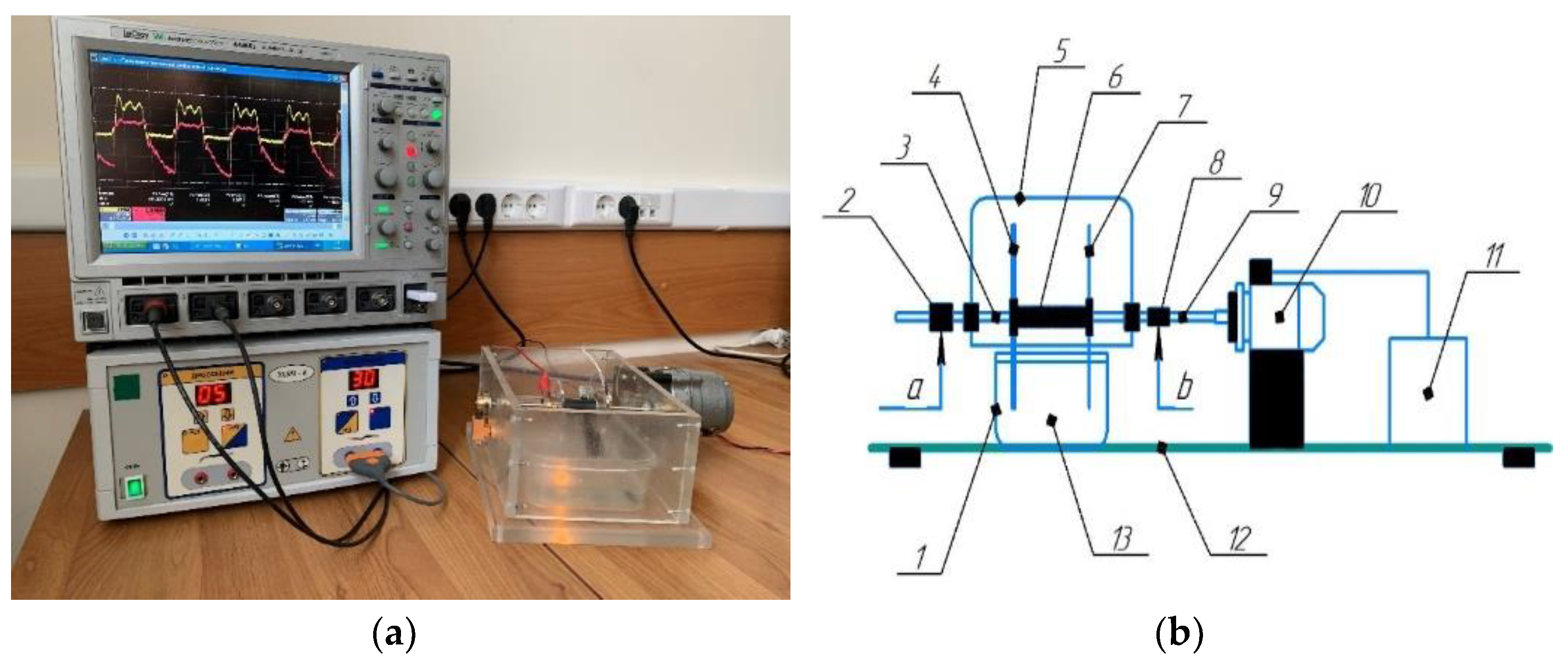
2.1. CAP Generation Method
2.2. PTS Generation Method
2.3. Physicochemical Properties of Aqueous Solutions
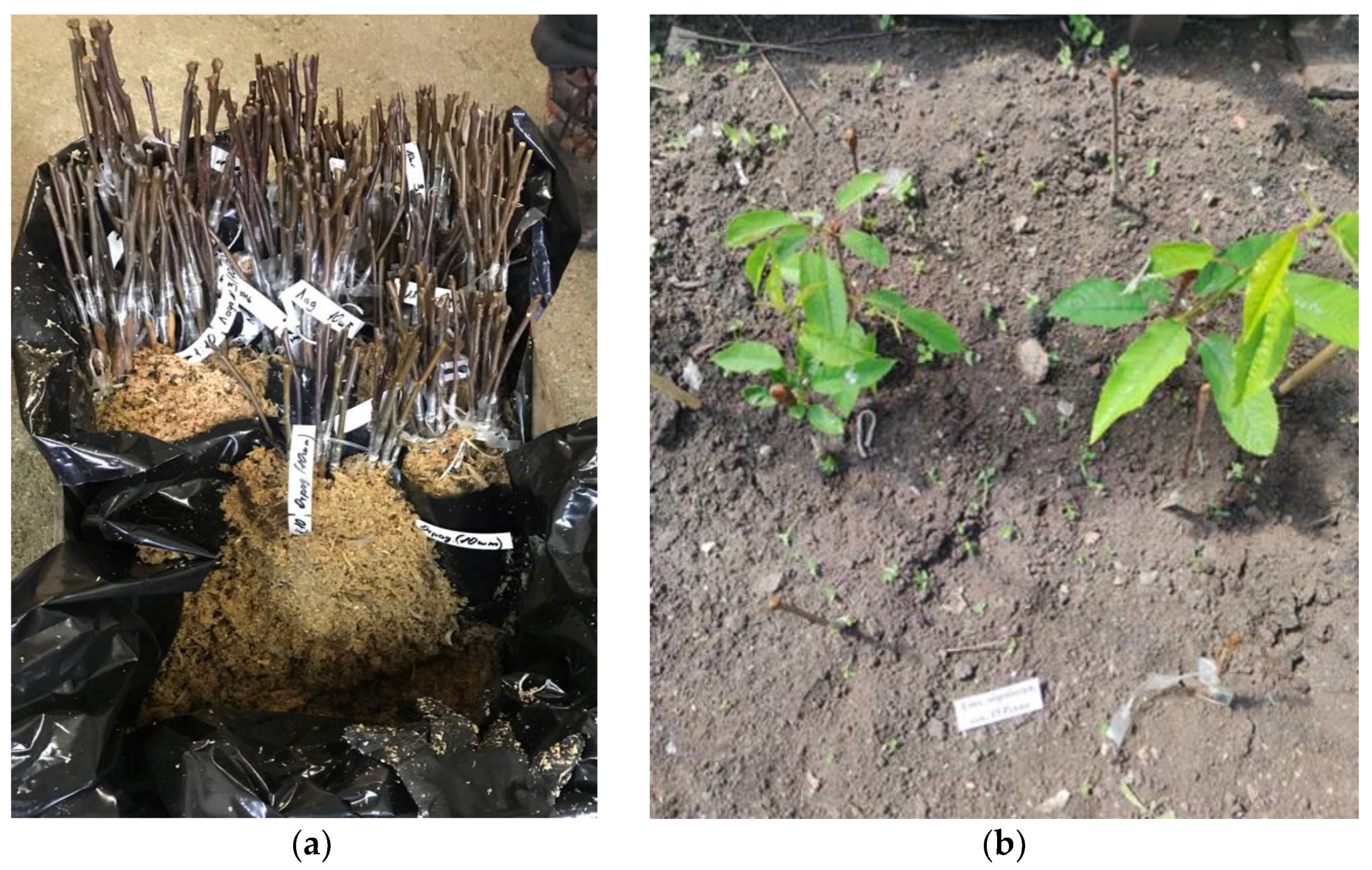
2.4. Plant Samples and Field Experiment
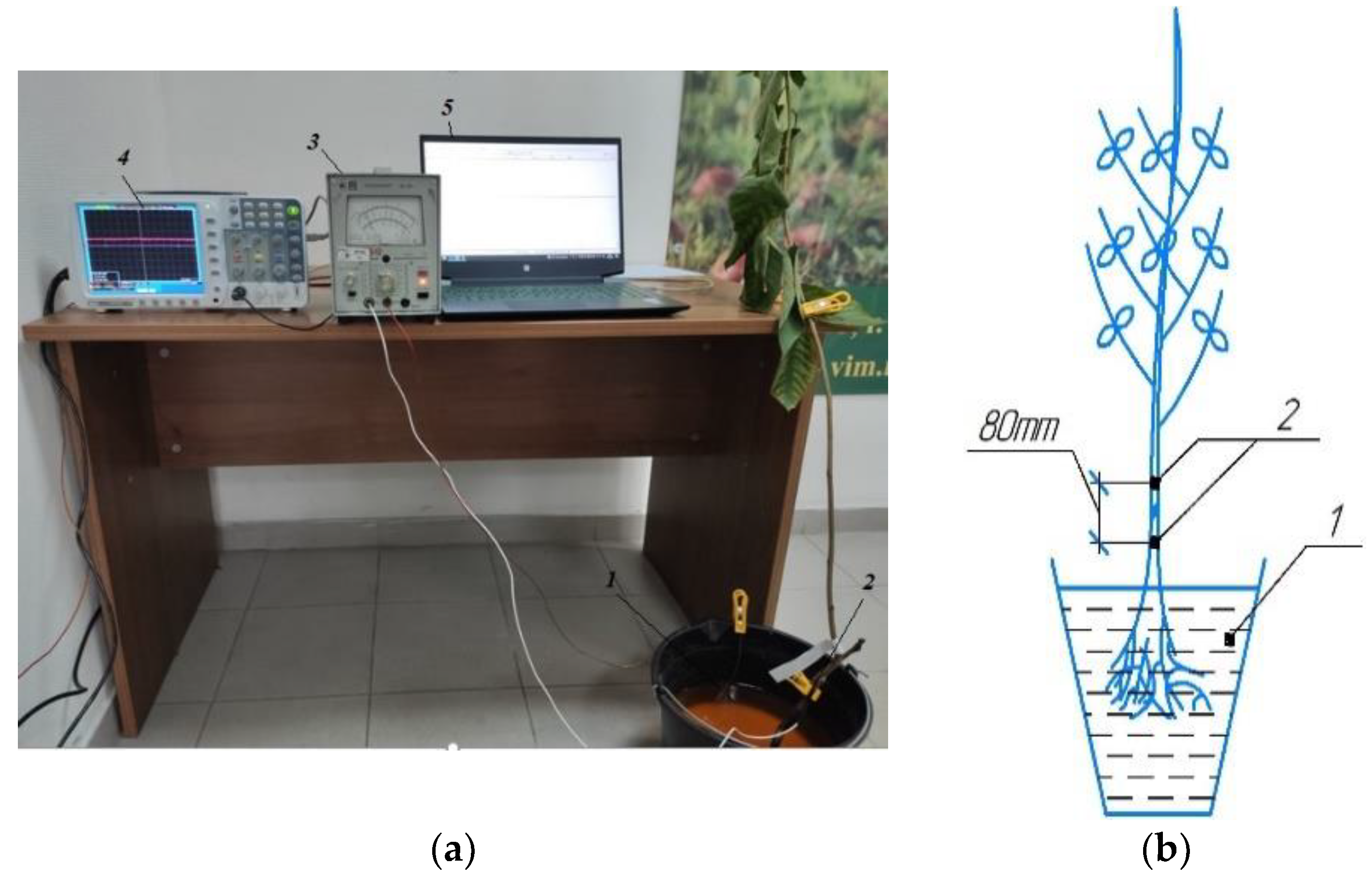
2.5. Graft Conductivity Measurements
2.6. Statistics
3. Results and Discussion
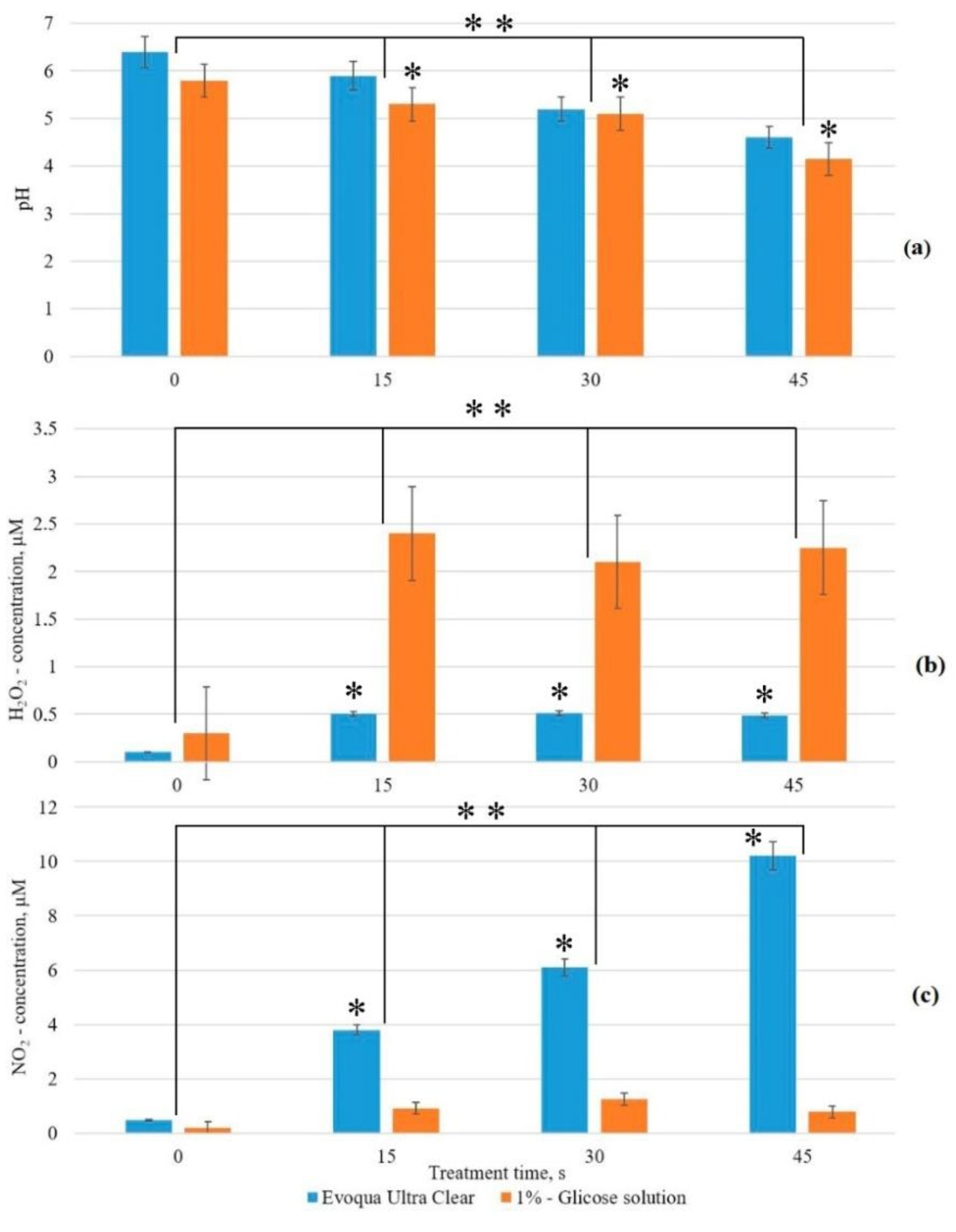
3.1. Physicochemical Properties of CAP
3.2. Physicochemical Properties of PTS
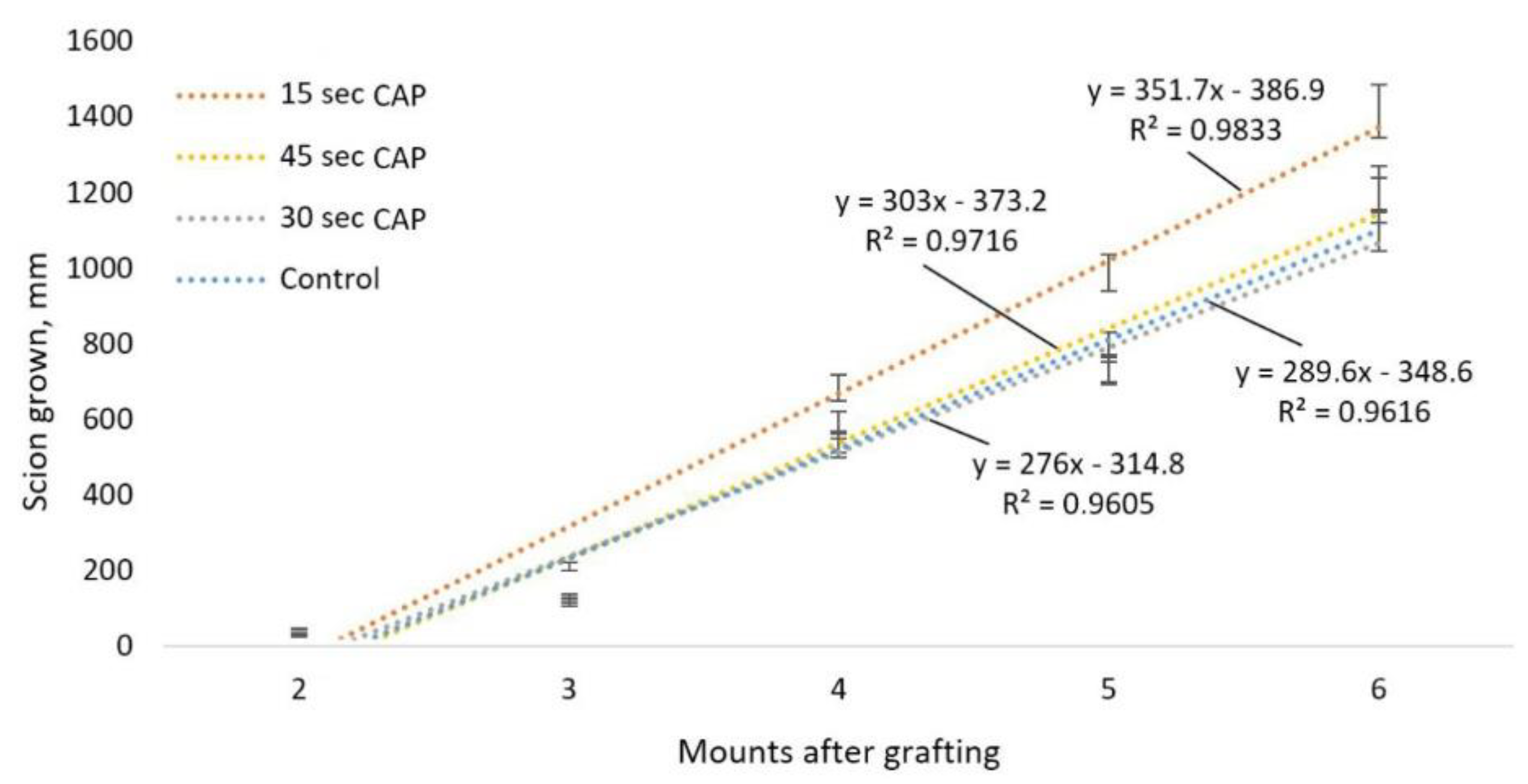
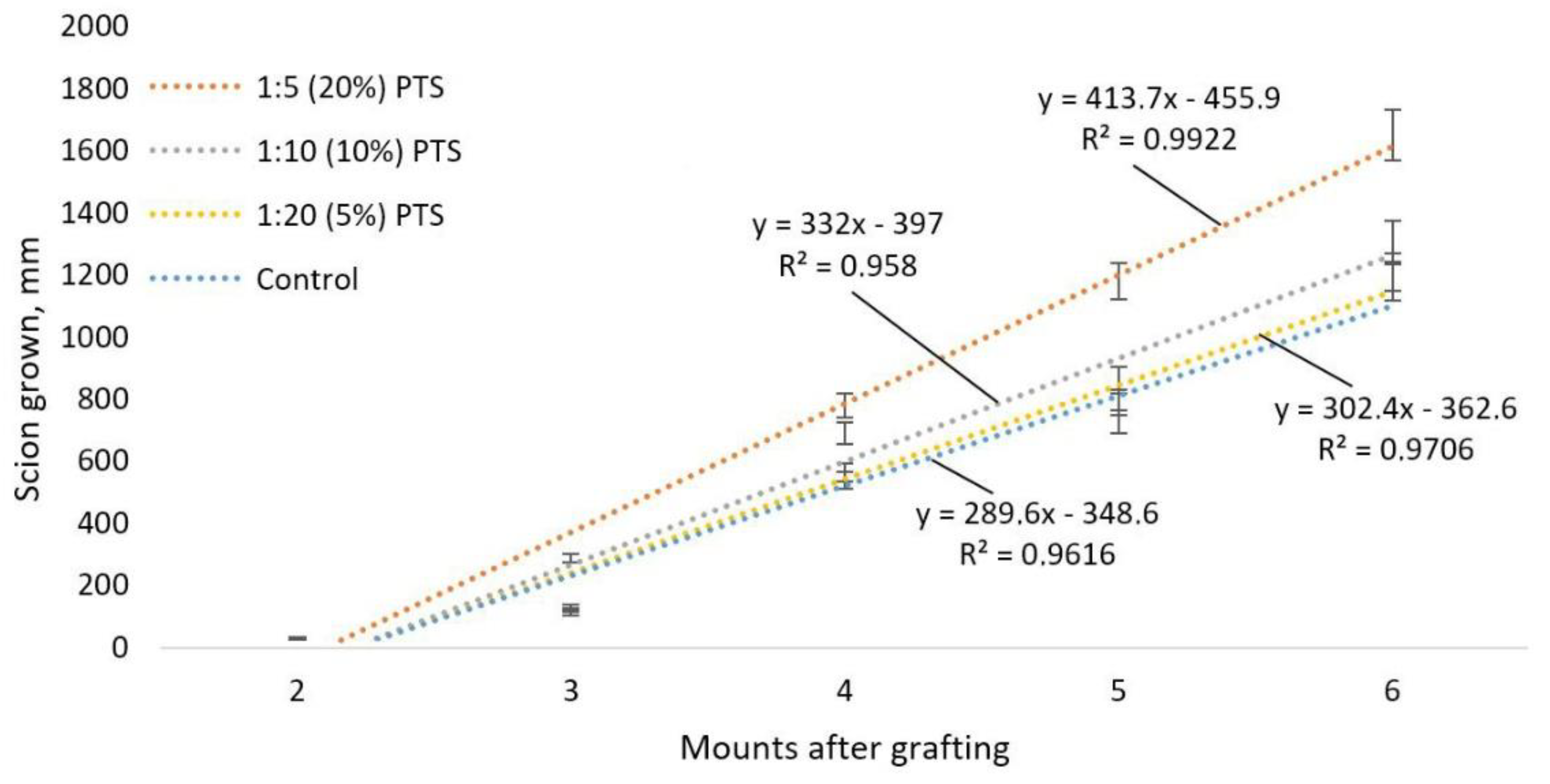
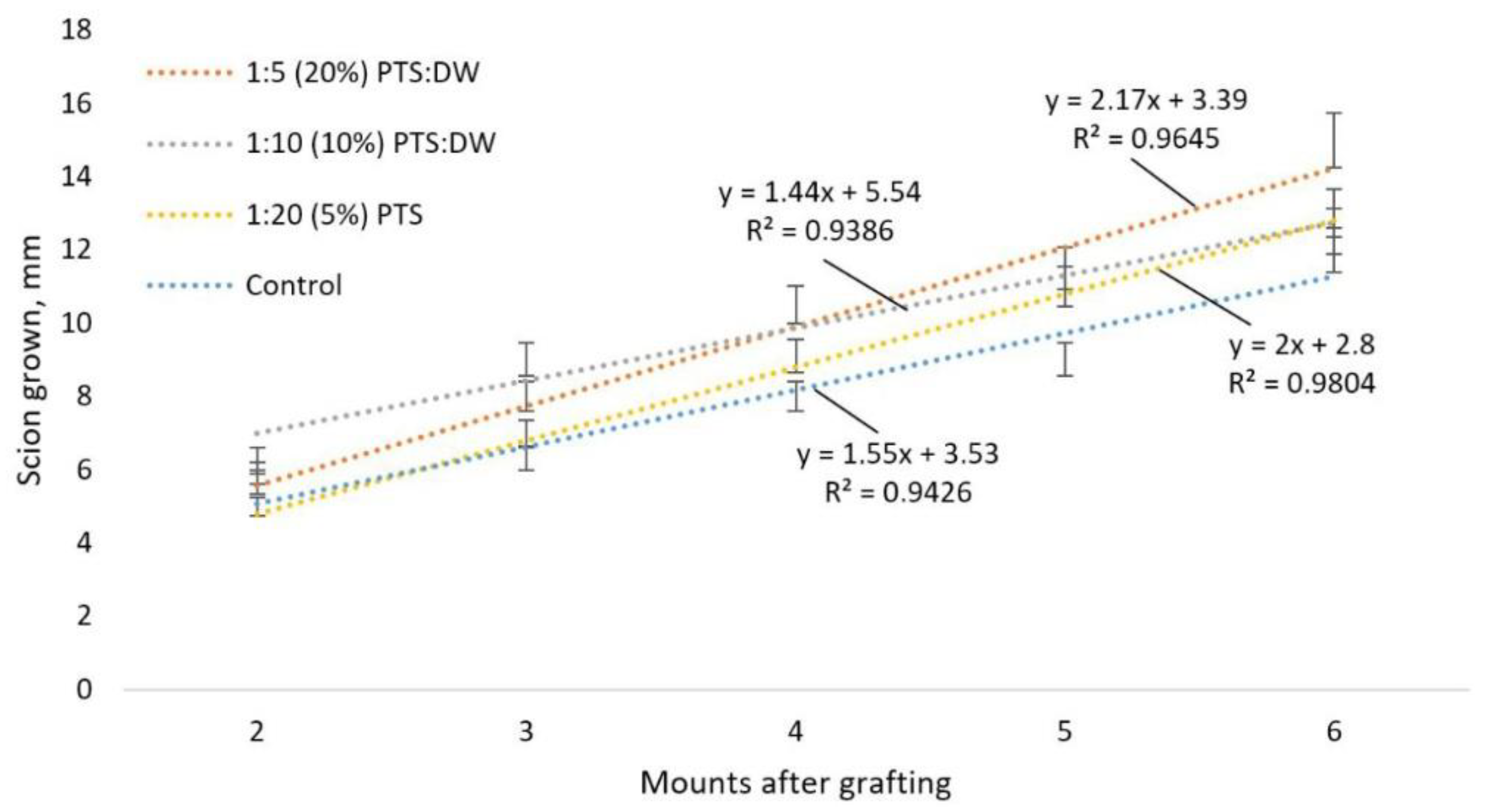
3.3. Study of the Effectiveness of the Action of CAP and PTS on the Grafts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakubowski, T. Transfer of microwave irradiation effects of seed potatoes (Solanum tuberosum L.) to the plants of next generations. Bulg. J. Agric. Sci. 2015, 21, 1185–1193. [Google Scholar]
- Jakubowski, T. The effect of stimulation of seed potatoes (Solanum tuberosum L.) in the magnetic field on selected vegetation parameters of potato plants. Prz. Elektrotech 2020, 96, 166–169. [Google Scholar]
- Belov, S.V.; Lobachevsky, Y.P.; Danilejko, Y.K.; Egorov, A.B.; Simakin, A.B.; Maleki, A.; Temnov, A.A.; Dubinin, M.V.; Gudkov, S.V. The Role of Mitochondria in the Dual Effect of Low-Temperature Plasma on Human Bone Marrow Stem Cells: From Apoptosis to Activation of Cell Proliferation. Appl. Sci. 2020, 10, 8971. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. The selective effect of plasma activated medium in an in vitro co-culture of liver cancer and normal cell. J. Appl. Phys. 2017, 121, 013302. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishikawa, K.; Mizuno, M.; Toyokuni, S.; Kajiyama, H.; Kikkawa, F.; Metelmann, H.; Hori, M. State of the art in medical applications using non-thermal atmospheric pressure plasma. Rev. Modern Plasma Phys. 2017, 1, 89. [Google Scholar] [CrossRef]
- Sergeichev, K.F.; Lukina, N.A.; Sarimov, R.M.; Smirnov, I.G.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Physicochemical Properties of Pure Water Treated by Pure Argon Plasma Jet Generated by Microwave Discharge in Opened Atmosphere. Front. Phys. 2021, 8, 614684. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Piskarev, I.M. Features of the Impact of Pulsed Radiation of Hot Plasma on Water and Aqueous Solutions. Plasma Chem. Plasma Process. 2021, 41, 1347–1361. [Google Scholar] [CrossRef]
- Baymler, I.V.; Simakin, A.V.; Gudkov, S.V. Investigation of the laser-induced breakdown plasma, acoustic vibrations and dissociation processes of water molecules caused by laser breakdown of colloidal solutions containing Ni nanoparticles. Plasma Sources Sci. Technol. 2021, 30, 125015. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 1–26. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artem’ev, K.V.; Bogachev, N.N.; Gusein-zade, N.G.; Dolmatov, T.V.; Kolik, L.V.; Konchekov, E.M.; Andreev, S.E. Study of Characteristics of the Cold Atmospheric Plasma Source Based on a Piezo Transformer. Russ. Phys. J. 2020, 62, 2073–2080. [Google Scholar] [CrossRef]
- Baldanov, B.B.; Ranzhurov, T.V.; Semenov, A.P.; Gomboeva, S.V. Cold atmospheric argon plasma jet source and its application for bacterial inactivation. J. Theor. Appl. Phys. 2019, 13, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.-L.; Jawaid, P.; Mitsuhashi, Y.; Imaue, S.; Fujiwara, K.; Ogawa, R.; Tomihara, K.; Saitoh, J.; et al. Roles of intracellular and extracellular ROS formation in apoptosis induced by cold atmospheric helium plasma and X-irradiation in the presence of sulfasalazine. Free Radic. Biol. Med. 2018, 129, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z. Hydroperoxide Reducing Enzymes in the Regulation of Free Radical Processes. Biochemistry 2021, 86, 1256–1274. [Google Scholar] [CrossRef] [PubMed]
- Shaparov, M.G.; Gudkov, S.V.; Lankin, V.Z.; Novoselov, V.I. Role of Glutathione Peroxidases and Peroxiredoxins in Free Radical Induced Pathologies. Biochemistry 2021, 86, 1418–1433. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wu, R.A.; Liao, X.; Liu, D.; Cullen, P.J.; Zhou, R.V.; Ding, T. Diagnostic analysis of reactive species in plasma-activated water (PAW): Current advances and outlooks. J. Phys. D Appl. Phys. 2021, 55, 023002. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Tech. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Christos, A. Aggelopoulos, Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Glinushkin, A.P.; Kalinitchenko, V.P.; Artem’ev, K.V.; Burmistrov, D.E.; Kozlov, V.A.; Kolik, L.V. Properties and Use of Water Activated by Plasma of Piezoelectric Direct Discharge. Front. Phys. 2021, 8, 616385. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Shcherbakov, I.A. Vibration–Vortex Mechanism of Radical-Reaction Activation in an Aqueous Solution: Physical Analogies. Phys. Wave Phenom. 2021, 29, 108–113. [Google Scholar] [CrossRef]
- Shcherbakov, I.A.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 9–11. [Google Scholar] [CrossRef]
- Baymler, I.V.; Gudkov, S.V.; Sarimov, R.M.; Simakin, A.V.; Shcherbakov, I.A. Concentration Dependences of Molecular Oxygen and Hydrogen in Aqueous Solutions. Dokl. Phys. 2020, 65, 5–7. [Google Scholar] [CrossRef]
- Penkov, N.V.; Baimler, I.V.; Lyakhov, G.A.; Pustovoy, V.I.; Simakin, A.V.; Sarimov, R.M.; Scherbakov, I.A. Effect of Mechanical Shaking on the Physicochemical Properties of Aqueous Solutions. Int. J. Mol. Sci. 2020, 21, 8033. [Google Scholar] [CrossRef]
- Simakin, A.V.; Sarimov, R.M.; Kurilov, A.D.; Chausov, D.N. Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties. Nanomaterials 2021, 11, 2804. [Google Scholar] [CrossRef]
- Baimler, I.V.; Lisitsyn, A.B.; Gudkov, S.V. Water decomposition occurring during laser breakdown of aqueous solutions containing individual gold, zirconium, molybdenum, iron or nickel nanoparticles. Front. Phys. 2020, 8, 620938. [Google Scholar] [CrossRef]
- Batukaev, A.; Malykh, G.P.; Magomadov, A.S.; Seget, O.L. New technological solutions for the production of planting material of grapes. J. Environ. Treat. Tech. 2019, 7, 581–587. [Google Scholar]
- Zhang, J.; Pizzi, A.; Lagel, M.C.; Du, G.; Zhou, X.; Wang, H. Dielectric barrier discharge plasma at atmospheric pressure to enhance pine wood surfaces hydrophilic character and adhesion properties. Wood Res. 2015, 60, 773–782. [Google Scholar]
- Busnel, F.; Blanchard, V.; Prégent, J.; Stafford, L.; Riedl, B.; Blanchet, P.; Sarkissian, A. Modification of Sugar Maple (Acer saccharum) and Black Spruce (Picea mariana) Wood Surfaces in a Dielectric Barrier Discharge (DBD) at Atmospheric Pressure. J. Adhes. Sci. Technol. 2010, 24, 1401–1413. [Google Scholar] [CrossRef]
- Westermark, U. Calcium promoted phenolic coupling by superoxide radical—A possible lignification reaction in wood. Wood Sci. Technol. 1982, 16, 71–78. [Google Scholar] [CrossRef]
- Reyt, G.; Chao, Z.; Flis, P.; Salas-González, I.; Castrillo, G.; Chao, D.-Y.; Salt, D.E. Uclacyanin Proteins Are Required for Lignified Nanodomain Formation within Casparian Strips. Curr. Biol. 2020, 30, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.I.; Choi, K.Y. Surface Characterization and morphology in Ar-plasma-treated polypropylene blend. Macromol. Res. 2009, 17, 886–893. [Google Scholar] [CrossRef]




| Parameters | |||||
|---|---|---|---|---|---|
| Specific Conductivity,mS/cm | [O2], µM | pH | Redox, mV | NO3−, mM | H2O2, mM |
| 25.1 ± 1.2 | 259 ± 8 | 8.1 ± 0.2 | 599 ± 26 | 21.97 ± 0.98 | 6.95 ± 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izmailov, A.; Khort, D.; Filippov, R.; Pishchalnikov, R.Y.; Simakin, A.V.; Shogenov, Y. Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures. Appl. Sci. 2022, 12, 4953. https://doi.org/10.3390/app12104953
Izmailov A, Khort D, Filippov R, Pishchalnikov RY, Simakin AV, Shogenov Y. Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures. Applied Sciences. 2022; 12(10):4953. https://doi.org/10.3390/app12104953
Chicago/Turabian StyleIzmailov, Andrey, Dmitry Khort, Rostislav Filippov, Roman Yu. Pishchalnikov, Alexander V. Simakin, and Yuri Shogenov. 2022. "Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures" Applied Sciences 12, no. 10: 4953. https://doi.org/10.3390/app12104953
APA StyleIzmailov, A., Khort, D., Filippov, R., Pishchalnikov, R. Y., Simakin, A. V., & Shogenov, Y. (2022). Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures. Applied Sciences, 12(10), 4953. https://doi.org/10.3390/app12104953








