Exposure to Phthalates and Alternative Plasticizers Is Associated with Methylation Changes of ESR1 and PGR in Uterine Leiomyoma: The ELENA Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Subjects
2.2. Data and Biospecimens (Urine and Tissues) Collection
2.3. Analysis of Urinary Phthalate and Alternative Plasticizer Metabolites
2.4. DNA Methylation Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Clinical Characteristics of Patients
3.2. Urinary Concentrations of Phthalate and Alternative Plasticizer Metabolites
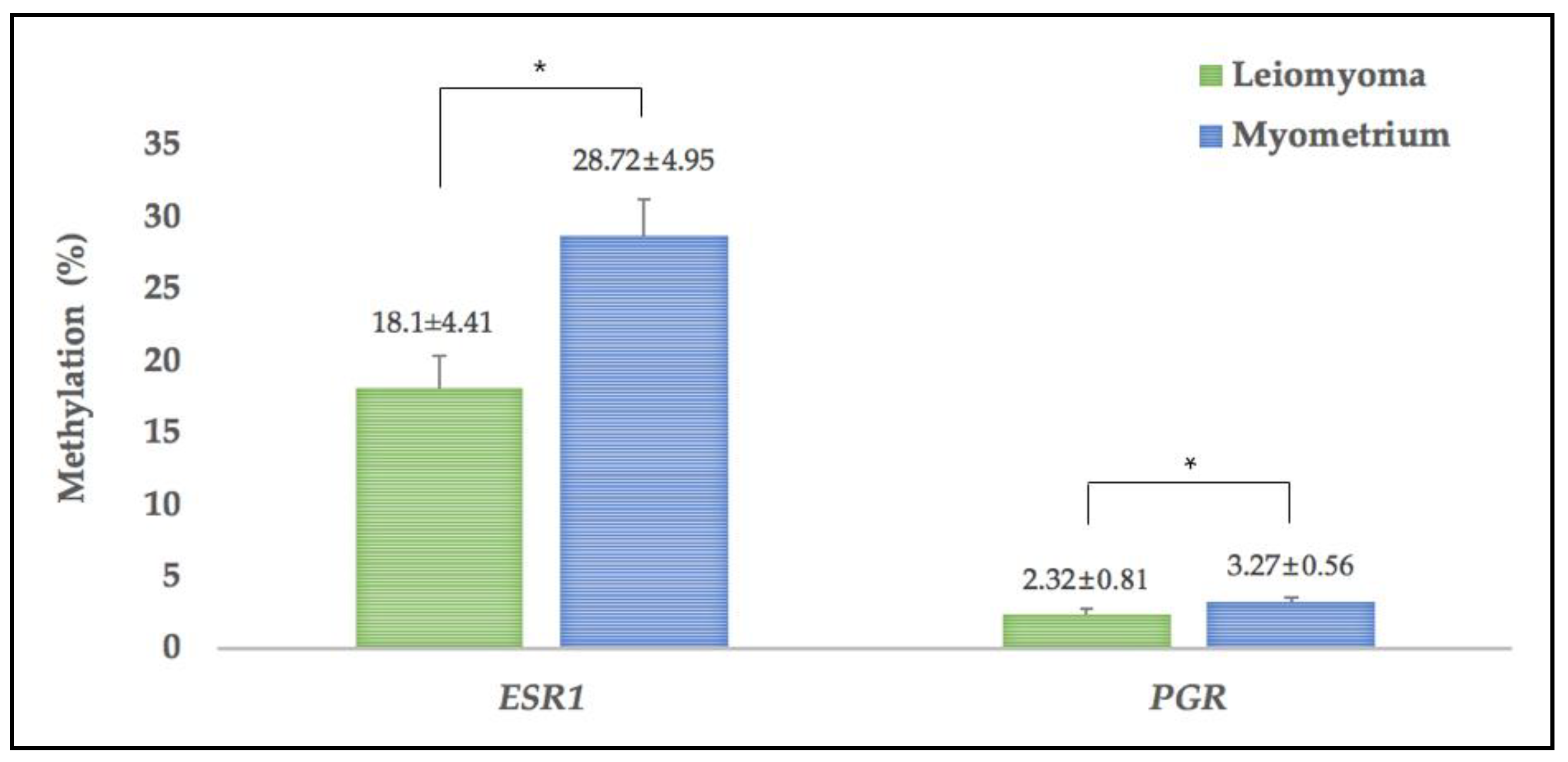
3.3. Methylation of ESR1 and PGR in Leiomyoma and MYOMETRIUM Tissues
3.4. Methylation Changes and Exposure to Phthalates and Alternative Plasticizers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kluwe, W.M. Overview of phthalate ester pharmacokinetics in mammalian species. Environ. Health Perspect. 1982, 45, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Latini, G.; De Felice, C.; Razzi, S.; Paris, I.; Ruggieri, F.; Mazzeo, P.; Petraglia, F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum. Reprod. 2003, 18, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rozati, R.; Reddy, B.V.; Raman, N.V. Association of phthalate esters with endometriosis in Indian women. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.Y.; Huang, P.C.; Lee, C.C.; Wu, M.H.; Lin, S.J. Phthalate exposure in girls during early puberty. J. Pediatr. Endocrinol. Metab. JPEM 2009, 22, 69–77. [Google Scholar] [CrossRef]
- Colón, I.; Caro, D.; Bourdony, C.J.; Rosario, O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ. Health Perspect. 2000, 108, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Durmaz, E.; Ozmert, E.N.; Erkekoglu, P.; Giray, B.; Derman, O.; Hincal, F.; Yurdakök, K. Plasma phthalate levels in pubertal gynecomastia. Pediatrics 2010, 125, e122–e129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Atek, D.; Belhaneche-Bensemra, N.; Turki, M. Migration of epoxidized sunflower oil and dioctyl phthalate from rigid and plasticized poly (vinyl chloride). Int. J. Polym. Mater. 2010, 59, 342–352. [Google Scholar] [CrossRef]
- Beach, E.S.; Weeks, B.R.; Stern, R.; Anastas, P.T. Plastics additives and green chemistry. Pure Appl. Chem. 2013, 85, 1611–1624. [Google Scholar] [CrossRef]
- Lioy, P.J.; Hauser, R.; Gennings, C.; Koch, H.M.; Mirkes, P.E.; Schwetz, B.A.; Kortenkamp, A. Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 343–353. [Google Scholar] [CrossRef]
- Campioli, E.; Lee, S.; Lau, M.; Marques, L.; Papadopoulos, V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Sci. Rep. 2017, 7, 11072. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kho, Y.; Chun, K.C.; Koh, J.W.; Park, J.W.; Bunderson-Schelvan, M.; Cho, Y.H. Increased urinary phthalate levels in women with uterine leiomyoma: A case-control study. Int. J. Environ. Res. Public Health 2016, 13, 1247. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.-C.; Li, W.-F.; Liao, P.-C.; Sun, C.-W.; Tsai, E.-M.; Wang, S.-L. Risk for estrogen-dependent diseases in relation to phthalate exposure and polymorphisms of CYP17A1 and estrogen receptor genes. Environ. Sci. Pollut. Res. 2014, 21, 13964–13973. [Google Scholar] [CrossRef]
- Huang, P.-C.; Tsai, E.-M.; Li, W.-F.; Liao, P.-C.; Chung, M.-C.; Wang, Y.-H.; Wang, S.-L. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum. Reprod. 2010, 25, 986–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luisi, S.; Latini, G.; De Felice, C.; Sanseverino, F.; Di Pasquale, D.; Mazzeo, P.; Petraglia, F. Low serum concentrations of di-(2-ethylhexyl) phthalate in women with uterine fibromatosis. Gynecol. Endocrinol. 2006, 22, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Reich, D.; Cozier, Y.C.; Rosenberg, L. Hair relaxer use and risk of uterine leiomyomata in African-American women. Am. J. Epidemiol. 2012, 175, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Fruh, V.; Henn, B.C.; Weuve, J.; Wesselink, A.K.; Orta, O.R.; Heeren, T.; Hauser, R.; Calafat, A.M.; Williams, P.L.; Baird, D.D. Incidence of uterine leiomyoma in relation to urinary concentrations of phthalate and phthalate alternative biomarkers: A prospective ultrasound study. Environ. Int. 2021, 147, 106218. [Google Scholar] [CrossRef]
- Kelly, T.K.; De Carvalho, D.D.; Jones, P.A. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 2010, 28, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Sadan, O.; Van Iddekinge, B.; Van Gelderen, C.; Savage, N.; Becker, P.; Van Der Walt, L.; Robinson, M. Oestrogen and progesterone receptor concentrations in leiomyoma and normal myometrium. Ann. Clin. Biochem. 1987, 24, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, H.; Yamagata, Y.; Taketani, T.; Matsuoka, A.; Tamura, H.; Hattori, N.; Ohgane, J.; Hattori, N.; Shiota, K.; Sugino, N. Potential link between estrogen receptor-α gene hypomethylation and uterine fibroid formation. Mol. Hum. Reprod. 2008, 14, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, R.; Sato, S.; Yamagata, Y.; Asada, H.; Tamura, I.; Lee, L.; Okada, M.; Tamura, H.; Takaki, E.; Nakai, A. Genome-wide DNA methylation analysis reveals a potential mechanism for the pathogenesis and development of uterine leiomyomas. PLoS ONE 2013, 8, e66632. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Sefton, E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2012, 358, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Schütze, A.; Kolossa-Gehring, M.; Apel, P.; Brüning, T.; Koch, H.M. Entering markets and bodies: Increasing levels of the novel plasticizer Hexamoll®® DINCH®® in 24 h urine samples from the German Environmental Specimen Bank. Int. J. Hyg. Environ. Health 2014, 217, 421–426. [Google Scholar] [CrossRef]
- Lee, I.; Alakeel, R.; Kim, S.; Al-Sheikh, Y.A.; Al-Mandeel, H.; Alyousef, A.A.; Kho, Y.; Choi, K. Urinary phthalate metabolites among children in Saudi Arabia: Occurrences, risks, and their association with oxidative stress markers. Sci. Total Environ. 2019, 654, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, S.; Kho, Y.; Kim, S.; Lee, S.; Choi, G.; Park, J.; Worakhunpiset, S.; Moon, H.-B.; Okanurak, K. Urinary levels of phthalates and DINCH metabolites in Korean and Thai pregnant women across three trimesters. Sci. Total Environ. 2020, 711, 134822. [Google Scholar] [CrossRef]
- Servaes, K.; Voorspoels, S.; Lievens, J.; Noten, B.; Allaerts, K.; Van De Weghe, H.; Vanermen, G. Direct analysis of phthalate ester biomarkers in urine without preconcentration: Method validation and monitoring. J. Chromatogr. A 2013, 1294, 25–32. [Google Scholar] [CrossRef]
- Brown, T.A.; Lee, J.W.; Holian, A.; Porter, V.; Fredriksen, H.; Kim, M.J.; Cho, Y.H. Alternation in DNA methylation corresponding with lung inflammation and as a biomarker for disease development after MWCNT exposure. Nanotoxicology 2016, 10, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cline, R.E.; Hill, R.H.; Phillips, D.L.; Needham, L.L. Pentachlorophenol measurements in body fluids of people in log homes and workplaces. Arch. Environ. Contam. Toxicol. 1989, 18, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Shealy, D.B.; Barr, J.R.; Ashley, D.L.; Patterson, D.G., Jr.; Camann, D.E.; Bond, A.E. Correlation of environmental carbaryl measurements with serum and urinary 1-naphthol measurements in a farmer applicator and his family. Environ. Health Perspect. 1997, 105, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammar, I.; Glynn, A.; Jönsson, B.A.; Lindh, C.H.; Darnerud, P.O.; Svensson, K.; Lignell, S. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ. Res. 2017, 153, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Lessmann, F.; Schütze, A.; Weiss, T.; Langsch, A.; Otter, R.; Brüning, T.; Koch, H.M. Metabolism and urinary excretion kinetics of di (2-ethylhexyl) terephthalate (DEHTP) in three male volunteers after oral dosage. Arch. Toxicol. 2016, 90, 1659–1667. [Google Scholar] [CrossRef]
- Kastner, J.; Cooper, D.G.; Marić, M.; Dodd, P.; Yargeau, V. Aqueous leaching of di-2-ethylhexyl phthalate and “green” plasticizers from poly (vinyl chloride). Sci. Total Environ. 2012, 432, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, H.M.; Schütze, A.; Pälmke, C.; Angerer, J.; Brüning, T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH®) in humans after single oral doses. Arch. Toxicol. 2013, 87, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Karamfilova, E. Food Contact Materials-Regulation (EC) 1935/2004. Eurpean Implementation Assessment. EPRS|European Parliamentary Research Service. 2016, PE 581.411. Available online: https://www.europarl.europa.eu/thinktank (accessed on 3 May 2021).
- Rodríguez-Carmona, Y.; Ashrap, P.; Calafat, A.M.; Ye, X.; Rosario, Z.; Bedrosian, L.D.; Huerta-Montanez, G.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Maekawa, R.; Asada, H.; Taketani, T.; Tamura, I.; Tamura, H.; Ogane, J.; Hattori, N.; Shiota, K.; Sugino, N. Aberrant DNA methylation status in human uterine leiomyoma. MHR Basic Sci. Reprod. Med. 2009, 15, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, R.; Yagi, S.; Ohgane, J.; Yamagata, Y.; Asada, H.; Tamura, I.; Sugino, N.; Shiota, K. Disease-dependent differently methylated regions (D-DMRs) of DNA are enriched on the X chromosome in uterine leiomyoma. J. Reprod. Dev. 2011, 57, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.-M.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Yin, P.; Monsivais, D.; Lin, S.M.; Du, P.; Wei, J.-J.; Bulun, S.E. Genome-wide DNA methylation indicates silencing of tumor suppressor genes in uterine leiomyoma. PLoS ONE 2012, 7, e33284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Yin, P.; Kujawa, S.A.; Coon, J.S.; Okeigwe, I.; Bulun, S.E. Progesterone receptor integrates the effects of mutated MED12 and altered DNA methylation to stimulate RANKL expression and stem cell proliferation in uterine leiomyoma. Oncogene 2019, 38, 2722–2735. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhu, J.; Li, Y.; Lin, T.; Gan, L.; Yuan, X.; Xu, M.; Wei, G. Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: Epigenetic changes and their impact on gene expression. Int. J. Toxicol. 2010, 29, 193–200. [Google Scholar] [CrossRef]
- Scarano, W.R.; Bedrat, A.; Alonso-Costa, L.G.; Aquino, A.M.; Fantinatti, B.E.; Justulin, L.A.; Barbisan, L.F.; Freire, P.P.; Flaws, J.A.; Lemos, B. Exposure to an environmentally relevant phthalate mixture during prostate development induces microRNA upregulation and transcriptome modulation in rats. Toxicol. Sci. 2019, 171, 84–97. [Google Scholar] [CrossRef]
- Kang, S.C.; Lee, B.M. DNA methylation of estrogen receptor alpha gene by phthalates. J. Toxicol. Environ. Health Part A 2005, 68, 1995–2003. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon, J.S.T.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.J.; et al. Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic implications. Hum. Reprod. Update 2015, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zota, A.R.; Geller, R.J.; VanNoy, B.N.; Marfori, C.Q.; Tabbara, S.; Hu, L.Y.; Baccarelli, A.A.; Moawad, G.N. Phthalate Exposures and MicroRNA Expression in Uterine Fibroids: The FORGE Study. Epigenet. Insights 2020, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.; Dale, B.; Elder, K. The negative impact of the environment on methylation/epigenetic marking in gametes and embryos: A plea for action to protect the fertility of future generations. Mol. Reprod. Dev. 2019, 86, 1273–1282. [Google Scholar] [CrossRef] [Green Version]
- Romani, F.; Tropea, A.; Scarinci, E.; Federico, A.; Dello Russo, C.; Lisi, L.; Catino, S.; Lanzone, A.; Apa, R. Endocrine disruptors and human reproductive failure: The in vitro effect of phthalates on human luteal cells. Fertil. Steril. 2014, 102, 831–837. [Google Scholar] [CrossRef]
- Li, N.; Liu, T.; Zhou, L.; He, J.; Ye, L. Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ. Toxicol. Pharm. 2012, 34, 869–875. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Abu-Elmagd, M.; Turki, R.F.; Damanhouri, G.A.; Beg, M.A.; Al-Qahtani, M. Endocrine disruption: In silico perspectives of interactions of di-(2-ethylhexyl)phthalate and its five major metabolites with progesterone receptor. BMC Struct. Biol. 2016, 16, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte-Guterman, P.; Navarro-Martín, L.; Trudeau, V.L. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen. Comp. Endocrinol. 2014, 203, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Flood, D.E.; Fernandino, J.I.; Langlois, V.S. Thyroid hormones in male reproductive development: Evidence for direct crosstalk between the androgen and thyroid hormone axes. Gen. Comp. Endocrinol. 2013, 192, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Buhrke, T.; Kasper, S.; Behr, A.C.; Braeuning, A.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. The urinary metabolites of DINCH(®®) have an impact on the activities of the human nuclear receptors ERα, ERβ, AR, PPARα and PPARγ. Toxicol. Lett. 2018, 287, 83–91. [Google Scholar] [CrossRef]
| Variable | Number (%) (n = 30) | Mean ± SD (Range) |
|---|---|---|
| Age (years) | 44.67 ± 4.99 (29–54) | |
| BMI (kg/m2) | 24.25 ± 4.43 (17.7–35.9) | |
| Waist circumference (cm) | 73.95 ± 6.54 (61–86.4) | |
| Diameter of the largest leiomyoma (mm) | 6.79 ± 2.42 (12–108) | |
| Number of leiomyoma | 3.93 ± 3.16 (1–12) | |
| Operation status | ||
| Myomectomy | 12 (40) | |
| Hysterectomy | 18 (60) | |
| Disease status | ||
| Leiomyoma only | 14 (46.7) | |
| Leiomyoma + Endometriosis (E) | 3 (10) | |
| Leiomyoma + Adenomyosis (A) | 9 (30) | |
| Leiomyoma + E + A | 4 (13.3) | |
| Parity | ||
| Primipara | 13 (43.7) | |
| Multipara | 17 (56.7) | |
| Menstrual phase | ||
| Proliferative | 14 (46.7) | |
| Secretory phase | 13 (43.3) | |
| Atrophy | 3 (10) | |
| Menstrual cycle | ||
| Regular | 22 (73.3) | |
| Irregular | 8 (26.7) | |
| Dysmenorrhea | ||
| No | 13 (43.3) | |
| Yes | 17 (56.7) | |
| Smoking status | ||
| No | 21 (75) | |
| Yes | 7 (25) | |
| Alcohol drinking status | ||
| No | 1 (3.6) | |
| Yes | 27 (96.4) |
| Phthalates and Alternatives | Measured >LOD n (%) | GM ± GSD (µg/g Creatinine) | Median (IQR) (µg/g Creatinine) |
|---|---|---|---|
| MEHP | 27/30 (90) | 14.91 ±13.19 | 14.39 (8.31–22.34) |
| MEOHP | 30/30 (100) | 18.53 ±14.92 | 17.41 (11.45–28.22) |
| MEHHP | 30/30 (100) | 32.28 ±23.40 | 31.07 (18.22–54.36) |
| 2cx-MMHP | 30/30 (100) | 20.84 ±15.87 | 19.29 (13.86–26.90) |
| 5cx-MEPP | 30/30 (100) | 35.47 ±33.24 | 28.48 (22.15–55.80) |
| MBzP | 29/30 (96.7) | 10.15 ±12.52 | 8.73 (4.25–25.63) |
| MiBP | 30/30 (100) | 19.87 ± 26.80 | 41.20 (7.73–47.63) |
| MEP | 27/30 (90) | 11.23 ± 13.99 | 11.41 (7.06–29.87) |
| MnBP | 29/30 (96.7) | 32.54 ± 27.28 | 37.76 (17.20–55.22) |
| MMP | 30/30 (100) | 9.73 ± 11.61 | 6.79 (3.96–30.11) |
| MCPP | 22/30 (73.3) | 2.91 ± 1.92 | 2.77 (1.94–3.96) |
| MiNP | 6/30 (20) | 1.16 ± 1.36 | 1.17 (0.70–2.06) |
| OH-MiNP | 21/21 (100) | 4.35 ± 2.89 | 4.24 (3.21–6.00) |
| MnOP | 0/30 (0) | NA | NA |
| MCHP | 0/30 (0) | NA | NA |
| MiDP | 0/30 (0) | NA | NA |
| cx-MiDP | 6/21 (28.6) | 1.42 ± 1.20 | 1.56 (1.38–1.79) |
| MnPP | 1/30 (3.3) | 0.77 | 0.77 |
| MiPrP | 2/21 (9.5) | 0.23 ± 0.55 | 0.23 (0.20–0.27) |
| MHxP | 4/21 (19) | 0.48 ± 2.50 | 0.30 (0.19–2.20) |
| MEHTP | 4/21 (19) | 1.15 ± 0.31 | 1.20 (1.03–1.29) |
| 5cxMEHTP | 21/21 (100) | 28.78 ± 16.31 | 26.43 (22.77–44.47) |
| 5OH-MEHTP | 20/21 (95.2) | 4.07 ± 2.24 | 3.69 (2.71–6.54) |
| 5OXO-MEHTP | 19/20 (95) | 2.35± 1.62 | 2.12 (1.52–4.36) |
| MINCH | 1/21 (4.8) | 1.45 | 1.45 |
| OH-MINCH | 21/21 (100) | 0.66± 0.94 | 0.58 (0.41–0.84) |
| cx-MINCH | 16/21 (76.2) | 0.78± 1.21 | 0.82 (0.43–1.05) |
| ESR1 | PGR | |||
|---|---|---|---|---|
| Phthalates and Alternatives a | Leiomyoma b | Myometrium | Leiomyoma | Myometrium |
| MEHP | −4.267 (−9.432, 0.898) | 2.773 (−3.1171, 8.663) | −0.330 (−1.381, 0.722) | 0.566 (−0.080, 1.213) |
| MEOHP | −4.797 (−11.150, 1.574) | 2.932 (−3.678, 9.541) | 0.583 (−0.612, 1.777) | 0.003 (−0.805, 0.812) |
| MEHHP | −4.797 (−12.060, 2.466) | 2.010 (−5.541, 9.561) | 0.726 (−0.612, 2.063) | −0.644 (− 0.975, 0.846) |
| 2cx-MMHP | −9.389 (−15.425, −3.353) * | 2.086 (−5.374, 9.545) | −0.066 (−1.432,1.301) | −0.222 (−1.117, 0.672) |
| 5cx-MEPP | −3.772 (−9.280,1.735) | 2.231 (−3.461, 7.923) | 0.569 (−0.446, 1.585) | 0.068 (−0.624, 0.761) |
| MBzP | −1.588 (−6.032, 2.856) | −0.419 (−4.858, 4.019) | 0.047(−0.748, 0.842) | −0.315 (−0.832, 0.201) |
| MiBP | 1.482 (−2.487, 5.451) | 0.308 (−3.718,4.334) | 0.630(−0.036,1.296) | −0.254 (−0.720, 0.213) |
| MEP | 0.015 (−5.046, 5.076) | 0.541(−4.900,5.982) | 0.557(−0.353,1.467) | 0.637 (0.093, 1.181) * |
| MnBP | 2.199 (−4.481, 8.879) | 0.917 (−5.785,7.619) | 1.128 (0.055, 2.201) * | −0.253 (−1.056, 0.550) |
| MMP | −2.107 (−6.888, 2.674) | 0.568 (−4.306, 5.443) | −0.569 (−1.412,0.275) | 0.421 (−.127, 0.969) |
| MCPP | −10.670 (−20.571, 0.769) * | 8.301 (−1.153, 17.753) | −0.836 (−2.979, 1.308) | 1.259 (0.250, 2.268) * |
| OH-MiNP | −1.453 (−6.580, 3.675) | 2.794(−1.775,7.364) | −0.114(−1.016,0.789) | −0.131(−0.683, 0.421) |
| 5cxMEHTP | 7.7836 (−5.812, 21.380) | 1.635 (−12.068, 15.339) | 1.498 (−0.823, 3.819) | −0.830 (−2.288, 0.627) |
| 5OH-MEHTP | 4.457 (−9.944, 18.857) | −5.732 (−18.016, 6.551) | 1.426 (−0.972, 3.824) | −1.304 (−2.641,0.032) |
| 5OXO-MEHTP | 17.928 (6.420, 29.437) * | −11.974 (−25.960, 2.011) | 1.702 (1–1.645, 5.048) | −1.424 (−3.304,0.457) |
| OH-MINCH | −1.649 (−8.029, 4.731) | 1.776 (−3.531, 7.083) | −0.868 (−1.809, 0.073) | 0.339 (−0.328, 1.006) |
| cx-MINCH | 1.606 (−8.18, 11.393) | −1.932 (−10.033, 6.169) | −1.220 (−2.210, −0.230) * | 0.128 (−1.033, 1.290) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.H.; Yoon, Y.S.; Koo, M.S.; Kim, W.; Kho, Y.; Kim, S.; Kim, Y.J.; Choi, H.; Choi, E.J.; Koh, J.W.; et al. Exposure to Phthalates and Alternative Plasticizers Is Associated with Methylation Changes of ESR1 and PGR in Uterine Leiomyoma: The ELENA Study. Appl. Sci. 2021, 11, 4234. https://doi.org/10.3390/app11094234
Cho YH, Yoon YS, Koo MS, Kim W, Kho Y, Kim S, Kim YJ, Choi H, Choi EJ, Koh JW, et al. Exposure to Phthalates and Alternative Plasticizers Is Associated with Methylation Changes of ESR1 and PGR in Uterine Leiomyoma: The ELENA Study. Applied Sciences. 2021; 11(9):4234. https://doi.org/10.3390/app11094234
Chicago/Turabian StyleCho, Yoon Hee, Yeong Sook Yoon, Min Sun Koo, Wanseo Kim, Younglim Kho, Sunmi Kim, Yang Jee Kim, Haewon Choi, Eun Jeong Choi, Jae Whoan Koh, and et al. 2021. "Exposure to Phthalates and Alternative Plasticizers Is Associated with Methylation Changes of ESR1 and PGR in Uterine Leiomyoma: The ELENA Study" Applied Sciences 11, no. 9: 4234. https://doi.org/10.3390/app11094234
APA StyleCho, Y. H., Yoon, Y. S., Koo, M. S., Kim, W., Kho, Y., Kim, S., Kim, Y. J., Choi, H., Choi, E. J., Koh, J. W., Chun, K. C., & Kim, Y. A. (2021). Exposure to Phthalates and Alternative Plasticizers Is Associated with Methylation Changes of ESR1 and PGR in Uterine Leiomyoma: The ELENA Study. Applied Sciences, 11(9), 4234. https://doi.org/10.3390/app11094234








