Low Doses of Gamma Irradiation Stimulate Synthesis of Bioactive Compounds with Antioxidant Activity in Fomes fomentarius Living Mycelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Fungal Identification
2.3. Mycelial Biomass Generation
2.4. Irradiation of the Mycelium of Fomes fomentarius
2.5. Preparation of Samples and Obtaining the Extract
2.6. Evaluation of the Antioxidant Capacity of the Extract
2.6.1. Total Polyphenols Content (TPC)
2.6.2. Total Flavonoids Content (TFC)
2.6.3. The DPPH Method
2.7. Statistical Analysis
3. Results
3.1. Statistics
3.2. DPPH Method
3.3. Total Polyphenols Content (TPC)
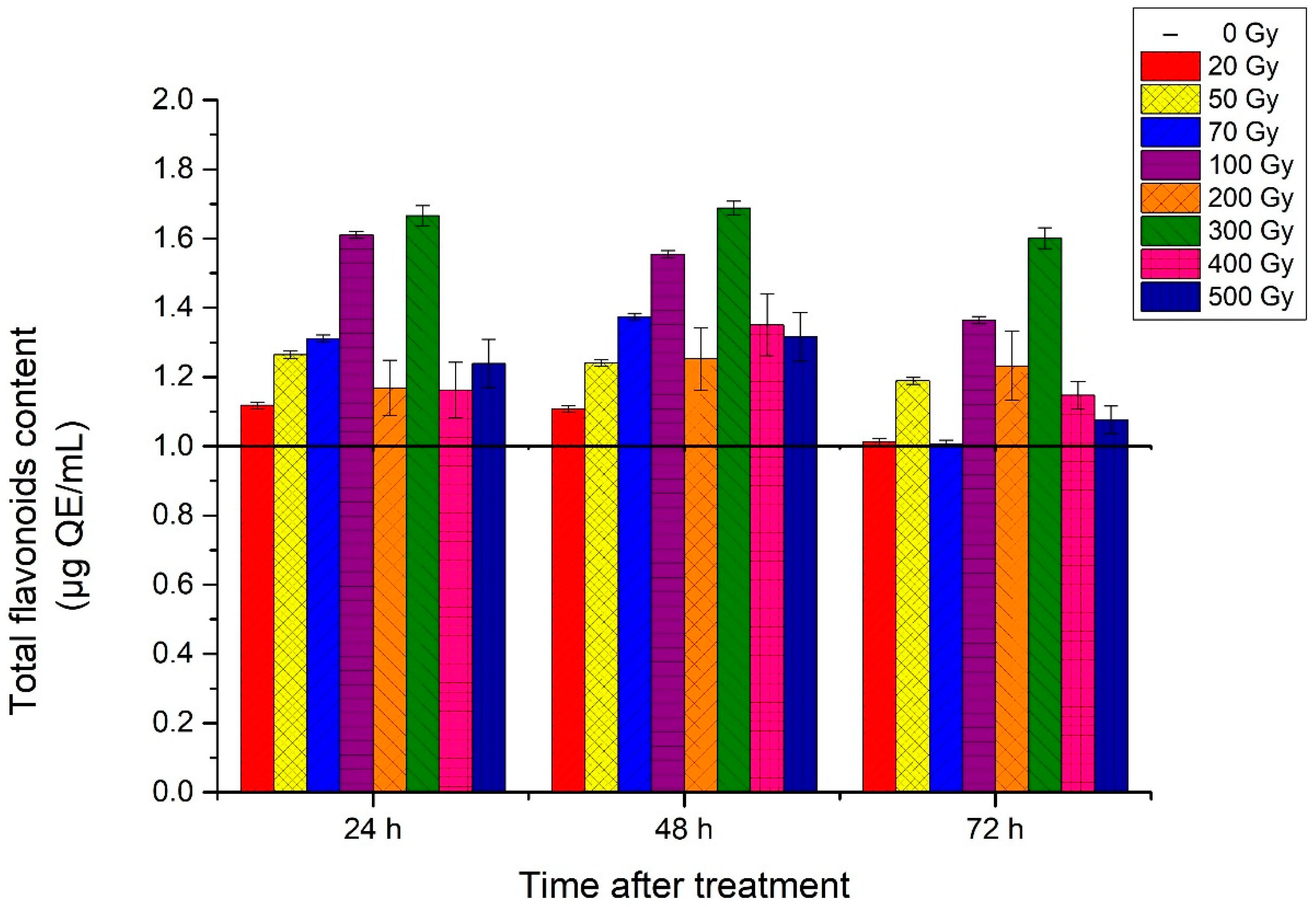
3.4. Total Flavonoids Content (TFC)
3.5. Response Time after Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Chatterjee, S.; Kataria, S.; Joshi, J.; Datta, S.; Vairale, M.; Veer, V. A Review on Responses of Plants to UV-B Radiation Related Stress. In UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth, 1st ed.; Singh, V.P., Singh, S., Prasad, S.M., Parihar, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 75–97. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F. Antioxidant Properties and Phenolic Profile of the Most Widely Appreciated Cultivated Mushrooms: A Comparative Study Between in Vivo and in Vitro Samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tepe, B.; Semiz, D.K.; Solak, M.H. Evaluation of Metal Concentration and Antioxidant Activity of Three Edible Mushrooms from Mugla, Turkey. Food Chem. Toxicol. 2010, 48, 1230–1233. [Google Scholar] [CrossRef]
- Patil, A.S.; Suryavanshi, P.; Fulzele, D. Evaluation of Effect of Gamma Radiation on Total Phenolic Content, Flavonoid and Antioxidant Activity of in Vitro Callus Culture of Artemisia Annua. Nat. Prod. Chem Res. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Charbaji, T.; Nabulsi, I. Effect of Low Doses of Gamma Irradiation on in Vitro Growth of Grapevine. Plant. Cell Tissue Organ. Cult. 1999, 57, 129–132. [Google Scholar] [CrossRef]
- Pradhan, B.; Baral, S.; Patra, S.; Behera, C.; Nayak, R.; MubarakAli, D.; Jena, M. Delineation of Gamma Irradiation (60Co) Induced Oxidative Stress by Decrypting Antioxidants and Biochemical Responses of Microalga, Chlorella sp. Biocatal. Agric. Biotechnol. 2020, 25, 101595. [Google Scholar] [CrossRef]
- Gáper, J.; Gáperová, S.; Pristas, P.; Naplavova, K. Medicinal Value and Taxonomy of the Tinder Polypore, Fomes Fomentarius (Agaricomycetes): A Review. Int. J. Med. Mushrooms 2016, 18, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Y.; Wang, P.; Liu, Q. Compositions and Anti-Tumor Activity of Pyropolyporus fomentarius Petroleum Ether Fraction In Vitro and In Vivo. PLoS ONE 2014, 9, 109599. [Google Scholar] [CrossRef]
- Lee, S.O.; Lee, M.H.; Lee, K.R.; Lee, E.O.; Lee, H.J. Fomes Fomentarius Ethanol Extract Exerts Inhibition of Cell Growth and Motility Induction of Apoptosis via Targeting AKT in Human Breast Cancer MDA-MB-231 Cells. Int. J. Mol. Sci. 2019, 20, 1147. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.Z.; Ahmad, S. Antifungal, Anti-Inflammatory and Cytotoxicity Activities of Three Varieties of Labisia pumila Benth: From Microwave Obtained Extracts. BMC Complement. Altern Med. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.L.; Shi, C.; Sun, L.T.; Yang, H.X.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Chemical Constituents and Their Biological Activities from the Mushroom Pyropolyporus fomentarius. Phytochemistry 2021, 183, 112625. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.; Kars, M.; Özdemir, Ö.; Gunduz, U. Fomes fomentarius and Tricholoma anatolicum (Agaricomycetes) Extracts Exhibit Significant Multiple Drug Resistant Modulation Activity in Drug Resistant Breast Cancer Cells. Int. J. Med. Mushrooms 2020, 22, 105–114. [Google Scholar] [CrossRef]
- Sandle, T. Gamma Radiation. In Sterility, Sterilisation and Sterility Assurance for Pharmaceuticals, 1st ed.; Sandle, T., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 55–68. ISBN 9781907568381. [Google Scholar]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Effects of Gamma-Irradiation Mutagenesis for Induction of Seedlessness, on the Quality of Mandarin Fruit. Food Sci. Nutr. 2014, 5, 943–952. [Google Scholar] [CrossRef]
- Huang, Y.L.; Yuan, S.C.; Chang, K.W.; Chen, F.C. Gamma Irradiation Mutagenesis in Monstera Deliciosa. Acta Hortic. 2017, 1167, 213–216. [Google Scholar] [CrossRef]
- Petre, M.; Petre, V.; Teodorescu, A.; Pătrulescu, F. Nutritive Mushroom Biomass Producing Through Submerged Fermentation of Agricultural Organic Wastes. Studia UBB Ambient. 2013, 58, 79–86. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol Vitic. 1965, 16, 144–158. [Google Scholar]
- Stan, M.S.; Voicu, S.N.; Caruntu, S.; Nica, I.C.; Olah, N.K.; Burtescu, R.; Balta, C.; Rosu, M.; Herman, H.; Hermenean, A.; et al. Antioxidant and Anti-Inflammatory Properties of a Thuja occidentalis Mother Tincture for the Treatment of Ulcerative Colitis. Antioxidants 2019, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Korte, K.N.; Odamtten, G.T.; Obodai, M.; Appiah, V.; Akuamoa, F.; Adu-Bobi, A.K.; Annann, S.N.Y.; Armah, J.N.O.; Acquah, S.A. Evaluating the Effect of Gamma Radiation on the Total Phenolic Content, Flavonoids, and Antioxidant Activity of Dried Pleurotus ostreatus ((Jacq. ex. Fr) Kummer) Stored in Packaging Materials. Adv. Pharm. 2014, 1–8. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnet, A. Teneurs en Principaux Flavonoids des Fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret, D. C) en Fonction de la Vegetation. Pharm. Acta. Helv. 1990, 65, 315–320. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid Based Complement. Alternat Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella sativa Essential Oil. Phytother Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Bakir, T.; Karadeniz, M.; Unal, S. Investigation of Antioxidant Activities of Pleurotus ostreatus Stored at Different Temperatures. Food Sci. Nutr. 2018, 6, 1040–1044. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zong, S.; Ye, M. Optimization of Fermentation of Fomes fomentarius Extracellular Polysaccharide and Antioxidation of Derivatized Polysaccharides. Cell Mol. Biol. 2020, 66, 56–65. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as Developmental Regulators. Curr. Opin. Plant. Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Gayomba, S.R.; Muday, G.K. Flavonols Regulate Root Hair Development by Modulating Accumulation of Reactive Oxygen Species in the Root Epidermis. Development 2020, 147, dev185189. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Huang, S.J.; Lin, C.P.; Mau, J.L.; Li, Y.S.; Tsai, S.Y. Effect of UV-B Irradiation on Physiologically Active Substance Content and Antioxidant Properties of the Medicinal Caterpillar Fungus Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms. 2015, 17, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Abdullah, T.L.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves. Int. J. Mol. Sci. 2014, 15, 13077–13090. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, X.; Cai, L.; Lu, X.; Ying, T.; Jiang, Z. Postharvest UV-B Irradiation Maintains Sensory Qualities and Enhances Antioxidant Capacity in Tomato Fruiting During Storage. Postharvest Biol. Technol. 2011, 59, 232–237. [Google Scholar] [CrossRef]
- Du, W.X.; Avena-Bustillos, R.J.; Breksa, A.P., 3rd; McHugh, T.H. Effect of UV-B Light and Different Cutting Styles on Antioxidant Enhancement of Commercial Fresh-Cut Carrot Products. Food Chem. 2012, 134, 1862–1869. [Google Scholar] [CrossRef]
- Köhler, H.; Contreras, R.A.; Pizarro, M.; Cortés-Antíquera, R.; Zúñiga, G.E. Antioxidant Responses Induced by UVB Radiation in Deschampsia antarctica. Desv. Front. Plant. Sci. 2017, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Mau, J.L. Antioxidant Properties of Methanolic Extracts from Antrodia camphorata with Various Doses of γ-Irradiation. Food Chem. 2007, 105, 1702–1710. [Google Scholar] [CrossRef]
- Adamo, M.; Capitani, D.; Mannina, L.; Cristinzio, M.; Ragni, P.; Tata, A.; Coppola, R. Truffles Decontamination Treatment by Ionizing Radiation. Radiat. Phys. Chem. 2004, 71, 167–170. [Google Scholar] [CrossRef]


| Assay | Compared Variables (Irradiation Doses) | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Statistical Significance of the Difference (p-Value) | ||||
| DPPH | 0 Gy/200 Gy | 0.0001 | 0.0014 | 0.0532 |
| 0 Gy/300 Gy | 0.0000 | 0.0000 | 0.0003 | |
| 0 Gy/400 Gy | 0.0000 | 0.0002 | 0.0007 | |
| 0 Gy/500 Gy | 0.0001 | 0.0003 | 0.0027 | |
| TPC | 0 Gy/200 Gy | 0.0000 | 0.0036 | 0.7250 |
| 0 Gy/300 Gy | 0.0000 | 0.0001 | 0.5286 | |
| 0 Gy/400 Gy | 0.0000 | 0.0086 | 0.0382 | |
| 0 Gy/500 Gy | 0.0000 | 0.0073 | 0.0006 | |
| TFC | 0 Gy/200 Gy | 0.0200 | 0.0006 | 0.6781 |
| 0 Gy/300 Gy | 0.0086 | 0.0254 | 0.0000 | |
| 0 Gy/400 Gy | 0.0010 | 0.0433 | 0.0204 | |
| 0 Gy/500 Gy | 0.1100 | 0.0879 | 0.0000 | |
| Assay | Compared Variables (Irradiation Doses) | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Statistical Significance of the Difference (p-Value) | ||||
| DPPH | 0 Gy/20 Gy | 0.0234 | 0.0012 | 0.0186 |
| 0 Gy/50 Gy | 0.1220 | 0.0003 | 0.0719 | |
| 0 Gy/70 Gy | 0.6468 | 0.0001 | 0.0045 | |
| 0 Gy/100 Gy | 0.0720 | 0.0000 | 0.0004 | |
| TPC | 0 Gy/20 Gy | 0.0250 | 0.0929 | 0.8858 |
| 0 Gy/50 Gy | 0.1468 | 0.0183 | 0.2233 | |
| 0 Gy/70 Gy | 0.0002 | 0.6792 | 0.1678 | |
| 0 Gy/100 Gy | 0.0006 | 0.0001 | 0.0002 | |
| TFC | 0 Gy/20 Gy | 0.2837 | 0.1741 | 0.5092 |
| 0 Gy/50 Gy | 0.0447 | 0.0162 | 0.0146 | |
| 0 Gy/70 Gy | 0.0304 | 0.0026 | 0.4435 | |
| 0 Gy/100 Gy | 0.0007 | 0.0029 | 0.0012 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelcaru, C.F.; Ene, M.; Petrache, A.-M.; Neguț, D.C. Low Doses of Gamma Irradiation Stimulate Synthesis of Bioactive Compounds with Antioxidant Activity in Fomes fomentarius Living Mycelium. Appl. Sci. 2021, 11, 4236. https://doi.org/10.3390/app11094236
Pelcaru CF, Ene M, Petrache A-M, Neguț DC. Low Doses of Gamma Irradiation Stimulate Synthesis of Bioactive Compounds with Antioxidant Activity in Fomes fomentarius Living Mycelium. Applied Sciences. 2021; 11(9):4236. https://doi.org/10.3390/app11094236
Chicago/Turabian StylePelcaru, Cristina Florentina, Mihaela Ene, Alina-Maria Petrache, and Daniel Constantin Neguț. 2021. "Low Doses of Gamma Irradiation Stimulate Synthesis of Bioactive Compounds with Antioxidant Activity in Fomes fomentarius Living Mycelium" Applied Sciences 11, no. 9: 4236. https://doi.org/10.3390/app11094236
APA StylePelcaru, C. F., Ene, M., Petrache, A.-M., & Neguț, D. C. (2021). Low Doses of Gamma Irradiation Stimulate Synthesis of Bioactive Compounds with Antioxidant Activity in Fomes fomentarius Living Mycelium. Applied Sciences, 11(9), 4236. https://doi.org/10.3390/app11094236






