Corrosion-Resistance Analysis of HA Layer Deposited through Electrophoresis on Ti4Al4Zr Metallic Substrate
Abstract
1. Introduction
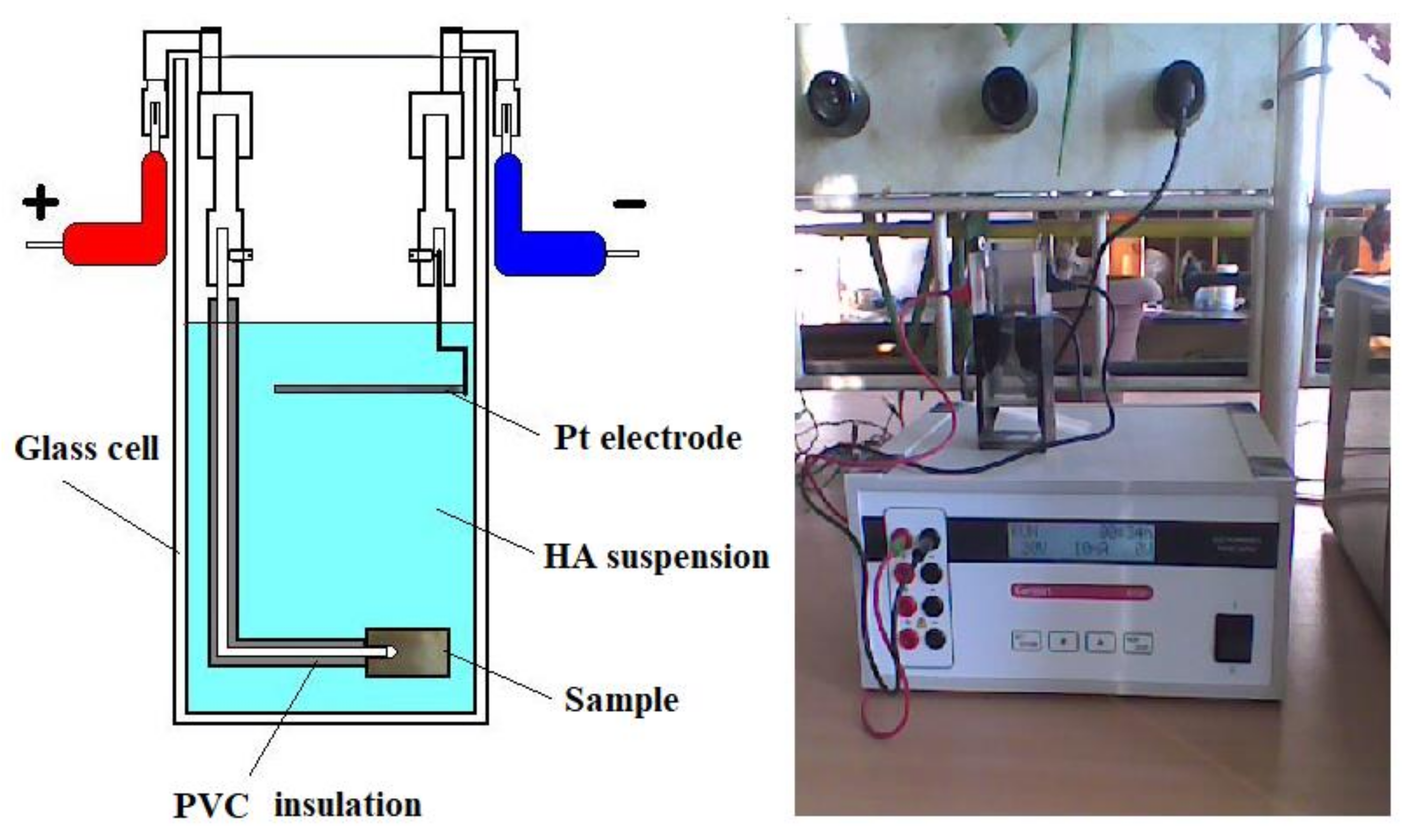
2. Materials and Methods
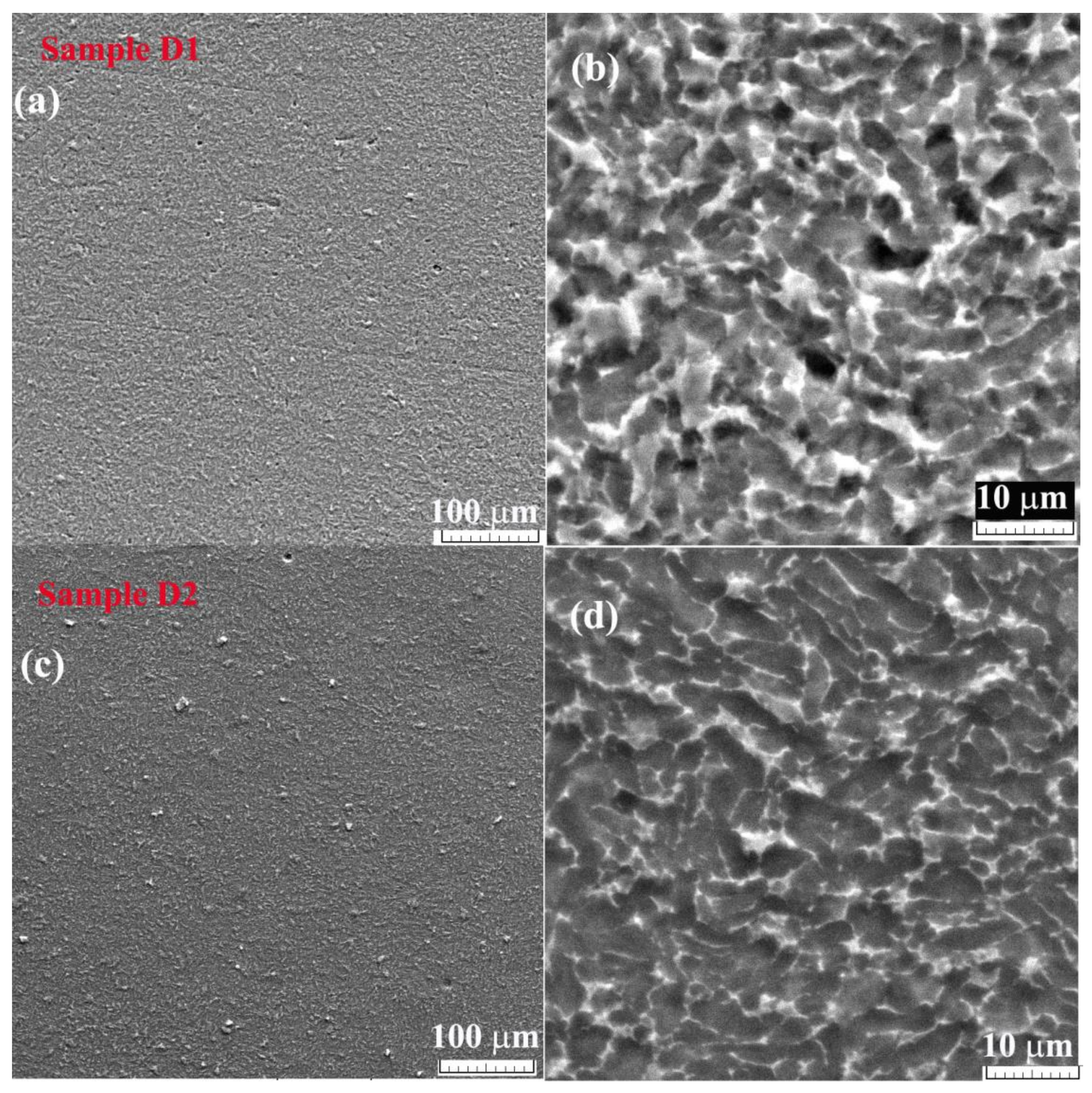
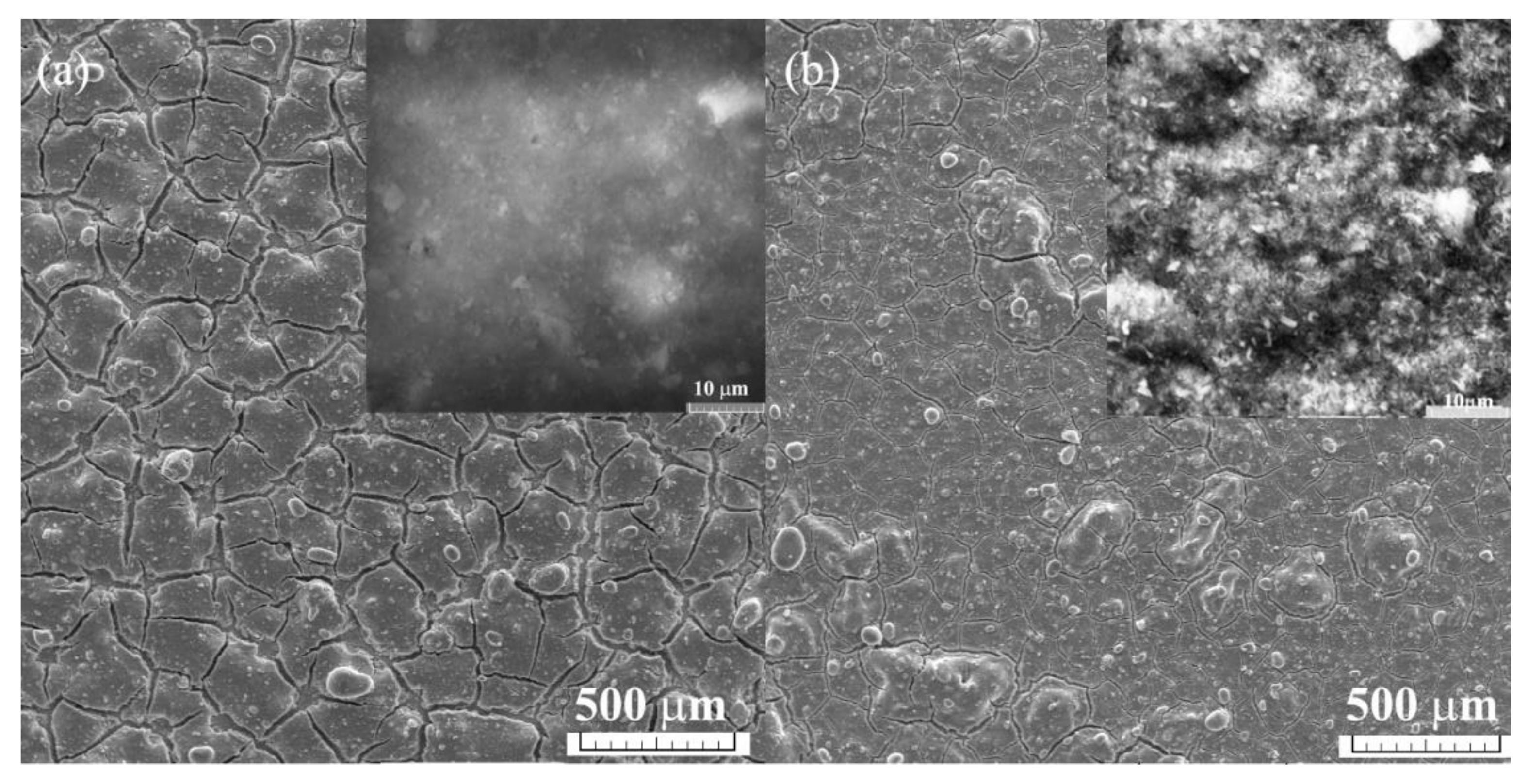
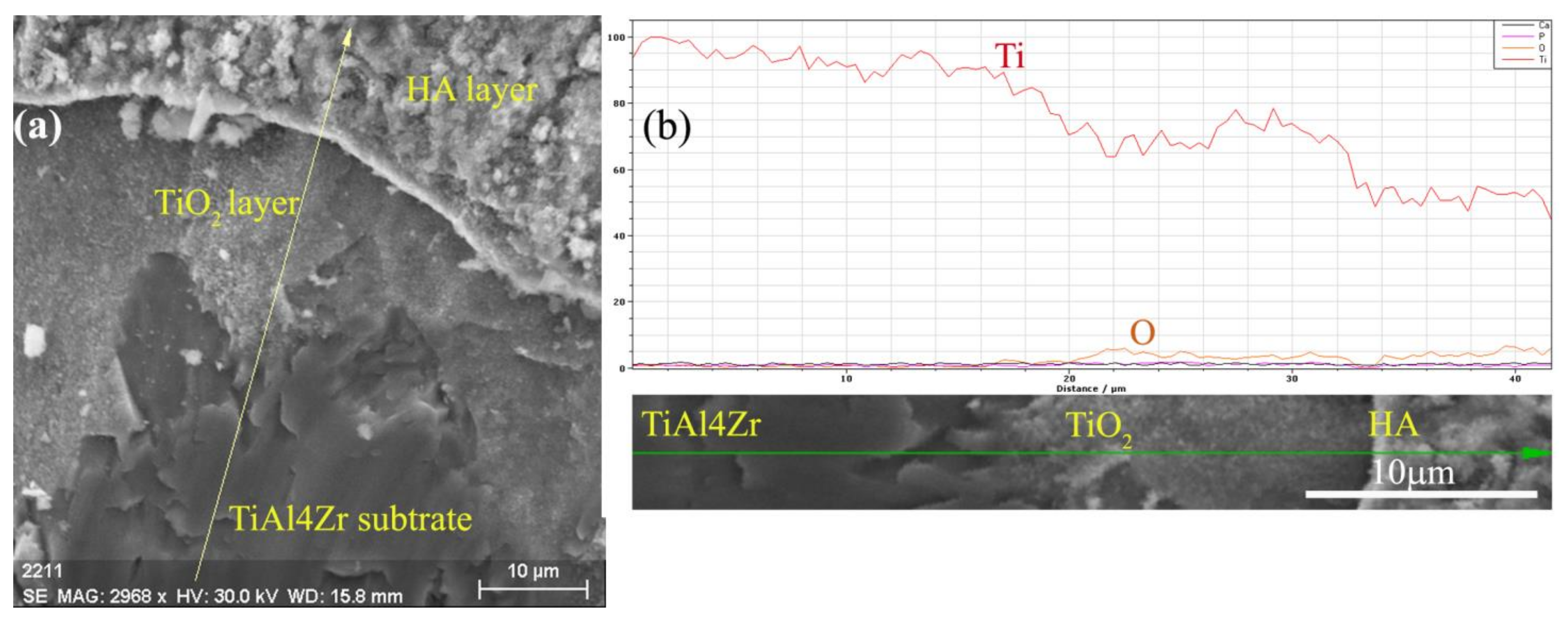
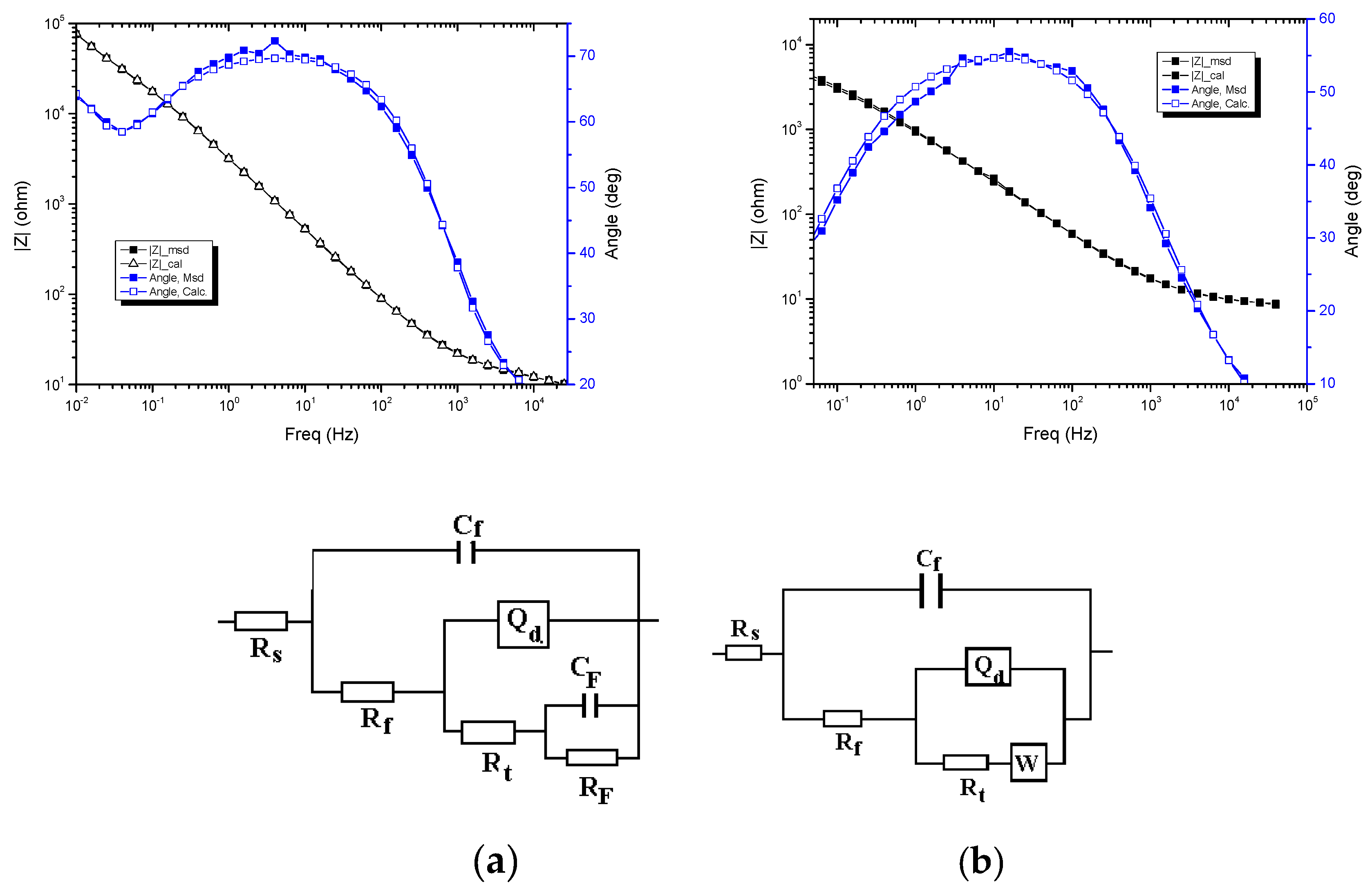
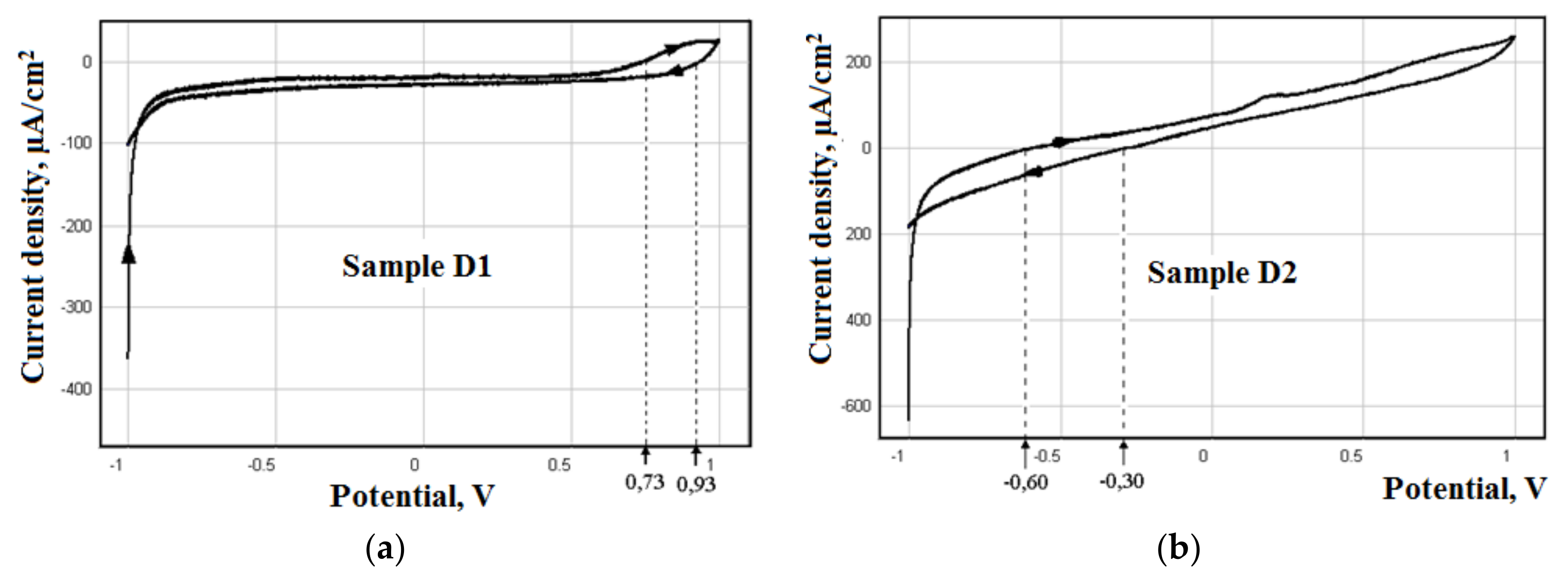
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on titanium and titanium-based alloys as biomaterials for orthopedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Luo, H.; Wu, Y.; Diao, X.; Shi, W.; Feng, F.; Qian, F.; Umeda, J.; Kondoh, K.; Xin, H.; Shen, J. Mechanical properties and biocompatibility of titanium with a high oxygen concentration for dental implants. Mater. Sci. Eng. C 2020, 117, 111306. [Google Scholar] [CrossRef]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Modifying the TiAlZr biomaterial surface with coating, for a better anticorrosive and antibacterial performance. Appl. Surf. Sci. 2011, 257, 9164–9168. [Google Scholar] [CrossRef]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Antimicrobial activity of the surface coatings on TiAlZr implant biomaterial. J. Biosci. Bioeng. 2011, 112, 630–634. [Google Scholar] [CrossRef]
- Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Antibacterial polymeric coating based on polypyrrole and polyethylene glycol on a new alloy TiAlZr. Prog. Org. Coatings 2012, 75, 349–355. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K. Point defect model and corrosion of anodic oxide coatings on Ti–6Al–4V. Corros. Sci. 2008, 50, 1521–1529. [Google Scholar] [CrossRef]
- Demetrescu, I. Passive and Bioactive Films on Implant Materials and their Efficiency in Regenerative Medicine. Mol. Cryst. Liq. Cryst. 2008, 486, 110–119. [Google Scholar] [CrossRef]
- Wu, C.; Tang, Y.; Mao, B.; Zhao, K.; Cao, S.; Wu, Z. Rapid apatite induction of polarized hydrophilic HA/PVDF bio-piezoelectric coating on titanium surface. Surf. Coatings Technol. 2021, 405, 126510. [Google Scholar] [CrossRef]
- Izquierdo, J.; Bolat, G.; Cimpoesu, N.; Trinca, L.C.; Mareci, D.; Souto, R.M. Electrochemical characterization of pulsed layer deposited hydroxyapatite-zirconia layers on Ti-21Nb-15Ta-6Zr alloy for biomedical application. Appl. Surf. Sci. 2016, 385, 368–378. [Google Scholar] [CrossRef]
- Mareci, D.; Cimpoeşu, N.; Popa, M.I. Electrochemical and SEM characterization of NiTi alloy coated with chitosan by PLD technique. Mater. Corros. 2012, 63, 985–991. [Google Scholar] [CrossRef]
- Istrate, B.; Rau, J.V.; Munteanu, C.; Antoniac, I.V.; Saceleanu, V. Properties and in vitro assessment of ZrO2-based coatings obtained by atmospheric plasma jet spraying on biodegradable Mg-Ca and Mg-Ca-Zr alloys. Ceram. Int. 2020, 46, 15897–15906. [Google Scholar] [CrossRef]
- Vargas-Becerril, N.; Sánchez-Téllez, D.; Zarazúa-Villalobos, L.; González-García, D.; Álvarez-Pérez, M.; de León-Escobedo, C.; Téllez-Jurado, L. Structure of biomimetic apatite grown on hydroxyapatite (HA). Ceram. Int. 2020, 46, 28806–28813. [Google Scholar] [CrossRef]
- Zu, X.T.; Liu, Y.Z.; Lian, J.; Liu, H.; Wang, Y.; Wang, Y.H.; Wang, L.M.; Ewing, R.C. Surface modification of a Ti–Al–Zr alloy by niobium ion implantation. Surf. Coat. Tech. 2006, 201, 3756–3760. [Google Scholar] [CrossRef]
- Liu, Y.; Zu, X.; Qiu, S.; Wang, L.; Ma, W.; Wei, C. Surface characterization and corrosion resistance of Ti–Al–Zr alloy by niobium ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 244, 397–402. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, G.; Sidhu, H.S. Improvement of surface properties and corrosion resistance of Ti13Nb13Zr titanium alloy by plasma-sprayed HA/ZnO coatings for biomedical applications. Mater. Chem. Phys. 2021, 257, 123738. [Google Scholar] [CrossRef]
- Gradinariu, I.; Stirbu, I.; Gheorghe, C.A.; Cimpoesu, N.; Agop, M.; Cimpoeşu, R.; Popa, C. Chemical properties of hydroxyapatite deposited through electrophoretic process on different sandblasted samples. Mater. Sci. 2014, 32, 578–582. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Kimura, K.; Ioku, K. Synthesis of nanosized porous hydroxyapatite granules in hydrogel by electrophoresis. Colloids Surf. B Biointerfaces 2012, 97, 236–239. [Google Scholar] [CrossRef]
- John, A.S.; Sidek, M.M.; Thang, L.Y.; Sami, S.; Tey, H.Y.; See, H.H. Online sample preconcentration techniques in nonaqueous capillary and microchip electrophoresis. J. Chromatogr. A 2021, 1638, 461868. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.R.; Ahangari, M.; Johar, M.H.; Allahkaram, S.R. Optimization of nano HA-SiC coating on AISI 316L medical grade stainless steel via electrophoretic deposition. Mater. Lett. 2021, 285, 129097. [Google Scholar] [CrossRef]
- Zirom-Titanium. Available online: https://www.zirom-titanium.com/ (accessed on 10 October 2020).
- Stirbu, I.; Vizureanu, P.; Cimpoesu, R.; Dascălu, G.; Gurlui, S.O.; Bernevig, M.; Benchea, M.; Cimpoeşu, N.; Postolache, P. Advanced metallic materials response at laser excitation for medical applications. J. Optoelectron. Adv. M. 2015, 17, 1179–1185. [Google Scholar]
- Afiq Harun, M.; Zamree Abd Rahim, S.; Nasir Mat Saad, M.; Fathullah Ghazali, M. Warpage analysis on front panel housing using response surface methodology (RSM). Eur. J Mater. Sci. Eng. 2016, 1, 9–18. [Google Scholar]
- Cimpoeşu, N.; Săndulache, F.; Istrate, B.; Cimpoeşu, R.; Zegan, G. Electrochemical Behavior of Biodegradable FeMnSi–MgCa Alloy. Metals 2018, 8, 541. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Lupescu, S.; Chelariu, R.; Vlad, M.D.; Vizureanu, P. Electrochemical Analysis and In Vitro Assay of Mg-0.5Ca-xY Biodegradable Alloys. Materials 2020, 13, 3082. [Google Scholar] [CrossRef] [PubMed]
- Cimpoeşu, R.H.; Pompilian, G.O.; Baciu, C.; Cimpoeşu, N.; Nejneru, C.; Agop, M.; Gurlui, S.; Focşa, C. Pulsed laser deposition of poly (L-Lactide) acid on nitinol substrate. Optoelectron. Adv. Mat. 2010, 4, 2148–2153. [Google Scholar]
| Sample/Dimensions (from 2D images) | Radius (μm) | Area (μm2) | ||||||
|---|---|---|---|---|---|---|---|---|
| min | med | max | StDev | min | med | max | StDev | |
| D1 | 1.86 | 3.19 | 4.79 | 0.74 | 10.83 | 33.70 | 72.18 | 15.87 |
| D2 | 1.24 | 2.41 | 5.44 | 0.71 | 4.87 | 19.91 | 92.97 | 13.87 |
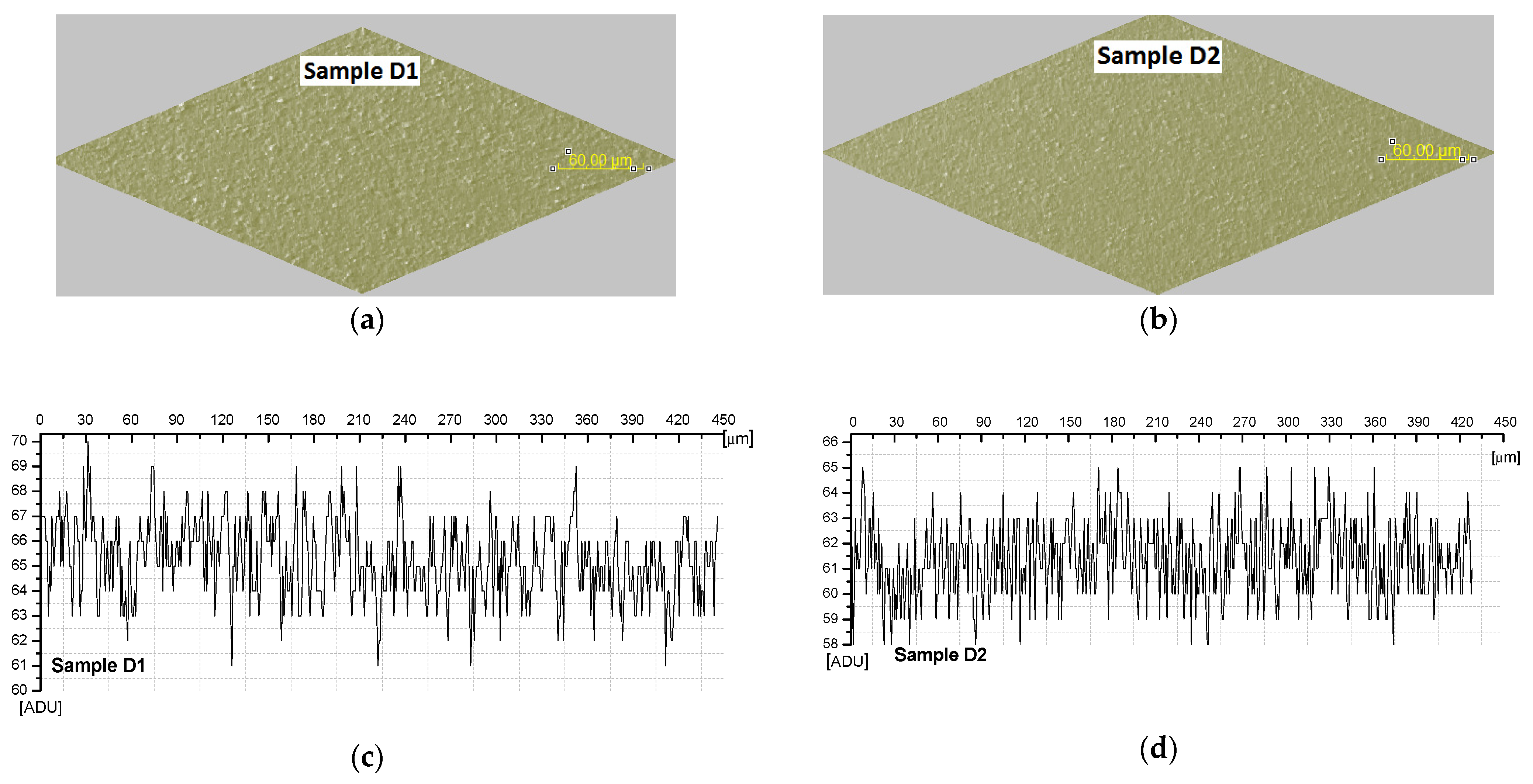
| Sample/Dimensions (from 3D images) | Hmin (μm) | Hmed (μm) | Hmax (μm) | StDev (μm) | Minimum variation (ADU) | Maximum variation (ADU) | Differences (ADU) | |
| D1 | 2.38 | 3.90 | 6.33 | 1.01 | 60 | 68.5 | 8.5 | |
| D2 | 1.08 | 1.33 | 1.87 | 0.21 | 59.6 | 63.4 | 3.8 | |
| Sample | RS (Ω cm2) | Cf (F/cm2) | Rf (Ω cm2) | Qd (S sn cm−2) | n | Rt (Ω cm2) | CF (F/cm2) | Rf (Ω cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| D1 | 10.01 | 3.51 × 10−6 | 8.91 | 7.1 × 10−5 | 0.77 | 9.8 × 104 | 8.51 × 10−5 | 9.81 × 105 | 3.96 × 10−4 |
| Sample | RS (Ω cm2) | Cf (F/cm2) | Rf (Ω cm2) | Qd (S sn cm−2) | n | Rt (Ω cm2) | CF (F/cm2) | Rf (Ω cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| D2 | 8.49 | 4.22 ×10−6 | 4.57 | 3.01 ×10−5 | 0.6 | 1.0 ×10−7 | 3.81×10−6 | 6.92×103 | 9.41 ×10−4 |
| Sample | RS (Ω cm2) | Cf (F/cm2) | Rf (Ω cm2) | Qd (S sn cm−2) | n | Rt (Ω cm2) | W (S s1/2 cm−2) | χ2 |
|---|---|---|---|---|---|---|---|---|
| D2 | 8.71 | 4.28 × 10−6 | 3.37 | 2.94 × 10−4 | 0.63 | 6.09 × 103 | 2.25 × 10−3 | 5.54 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimpoeșu, R.; Vizureanu, P.; Știrbu, I.; Sodor, A.; Zegan, G.; Prelipceanu, M.; Cimpoeșu, N.; Ioanid, N. Corrosion-Resistance Analysis of HA Layer Deposited through Electrophoresis on Ti4Al4Zr Metallic Substrate. Appl. Sci. 2021, 11, 4198. https://doi.org/10.3390/app11094198
Cimpoeșu R, Vizureanu P, Știrbu I, Sodor A, Zegan G, Prelipceanu M, Cimpoeșu N, Ioanid N. Corrosion-Resistance Analysis of HA Layer Deposited through Electrophoresis on Ti4Al4Zr Metallic Substrate. Applied Sciences. 2021; 11(9):4198. https://doi.org/10.3390/app11094198
Chicago/Turabian StyleCimpoeșu, Ramona, Petrică Vizureanu, Ioan Știrbu, Alina Sodor, Georgeta Zegan, Marius Prelipceanu, Nicanor Cimpoeșu, and Nicoleta Ioanid. 2021. "Corrosion-Resistance Analysis of HA Layer Deposited through Electrophoresis on Ti4Al4Zr Metallic Substrate" Applied Sciences 11, no. 9: 4198. https://doi.org/10.3390/app11094198
APA StyleCimpoeșu, R., Vizureanu, P., Știrbu, I., Sodor, A., Zegan, G., Prelipceanu, M., Cimpoeșu, N., & Ioanid, N. (2021). Corrosion-Resistance Analysis of HA Layer Deposited through Electrophoresis on Ti4Al4Zr Metallic Substrate. Applied Sciences, 11(9), 4198. https://doi.org/10.3390/app11094198










