Biogenic Calcium Phosphate from Fish Discards and By-Products
Abstract
1. Introduction
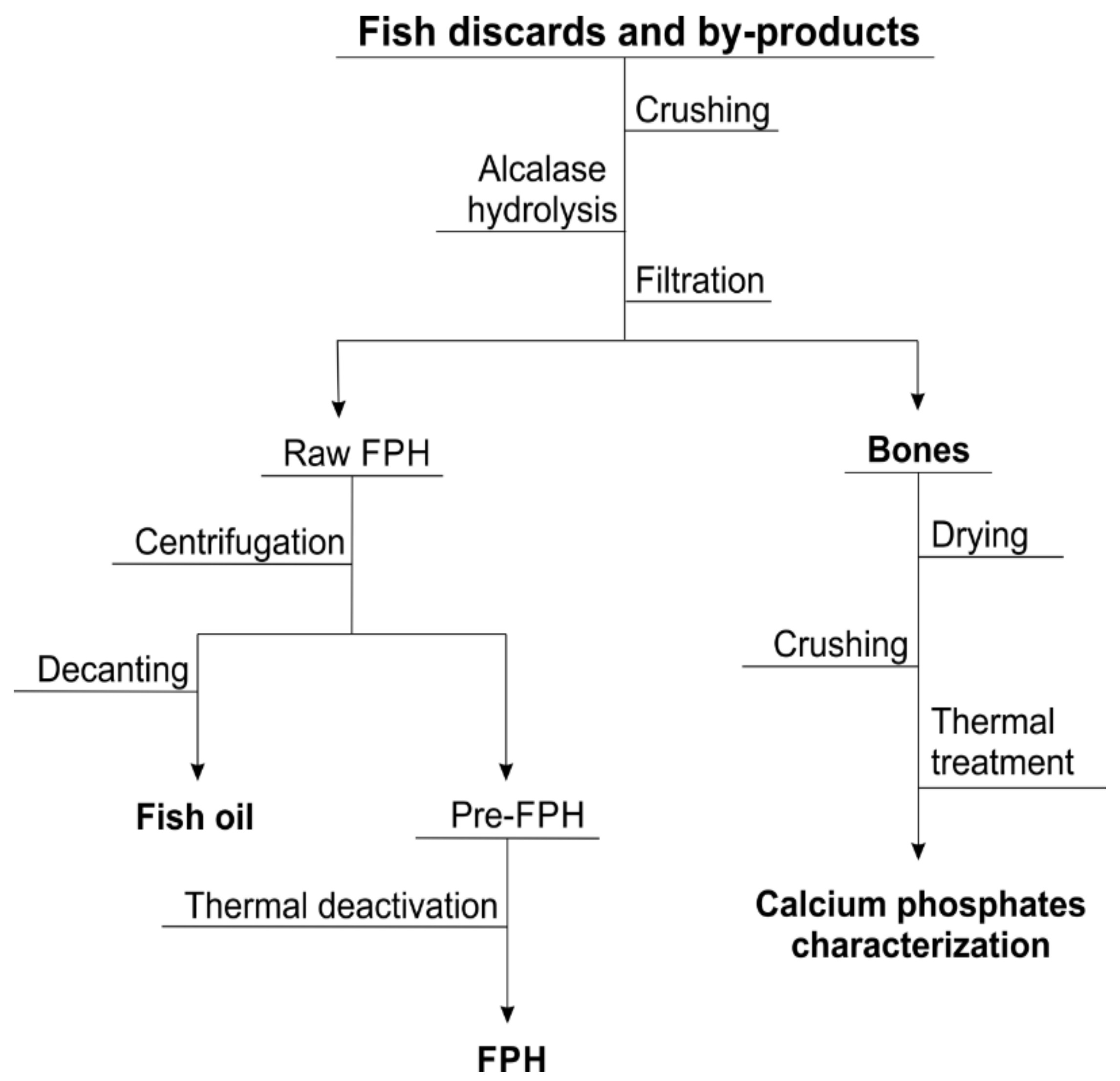
2. Materials and Methods
2.1. Fish Samples
2.2. Characterization Techniques
3. Results and Discussion
3.1. Raw Material and Thermal Treatment
3.2. Chemical Composition
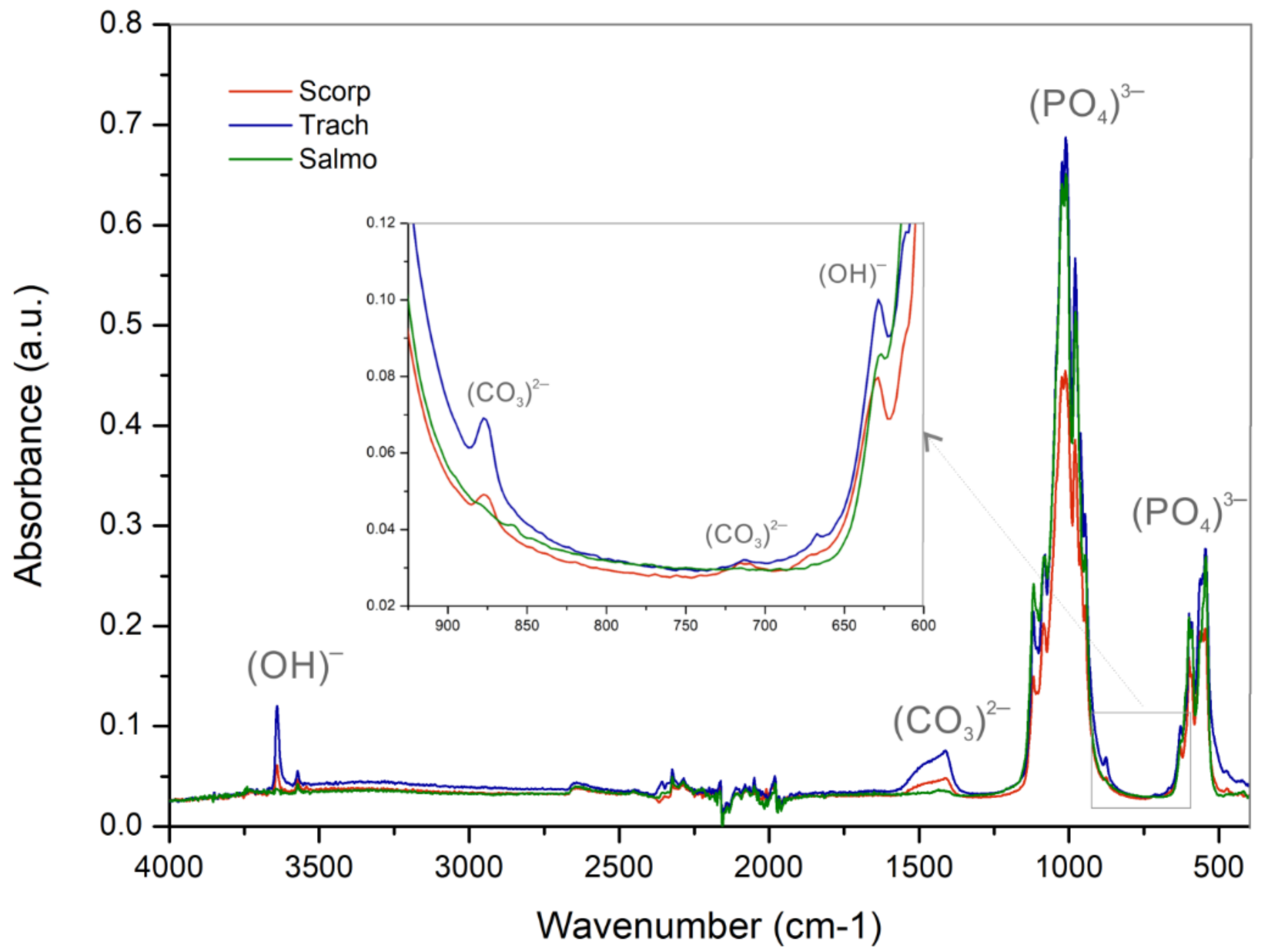
3.2.1. Functional Groups Analysis
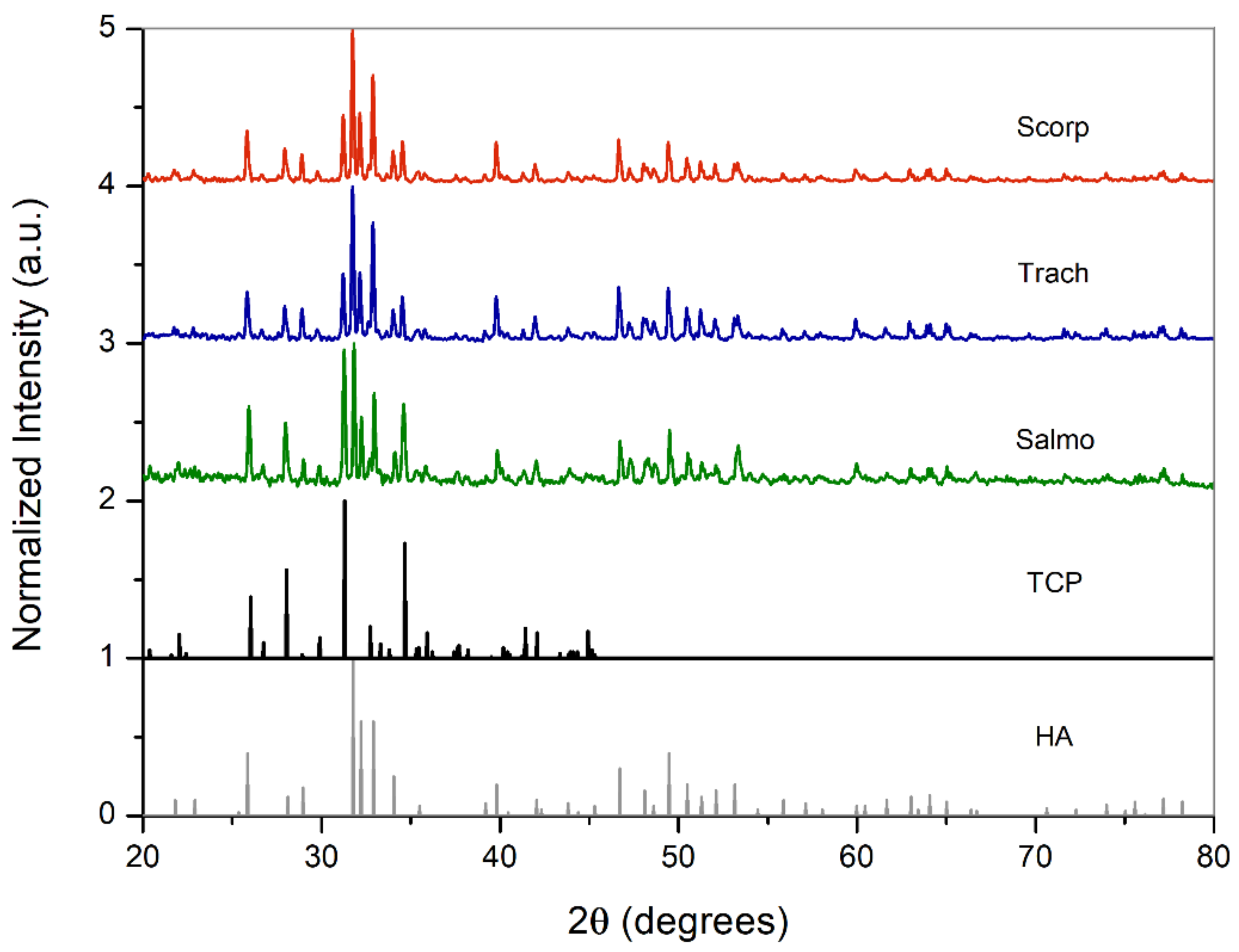
3.2.2. Crystalline Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roda, M.A.P.; Gilman, E.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P.A. A Third Assessment of Global Marine Fisheries Discards; FAO Fisheries and Aquaculture Technical Paper No. 633; FAO: Rome, Italy, 2019; p. 78. [Google Scholar]
- González, A.F.; Gracia, J.; Miniño, I.; Romón, J.; Larsson, C.; Maroto, J.; Requeira, M.; Pascual, S. Approach to reduce the zoonotic parasite load in fish stocks: When science meets technology. Fish. Res. 2018, 202, 140–148. [Google Scholar] [CrossRef]
- Aranda, M.; Ulrich, C.; Le Gallic, B.; Borges, L.; Metz, S.; Prellezo, R.; Santurtún, M. Research for PECH Committee—EU Fisheries Policy–Latest Developments and Future Challenges; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2019. [Google Scholar]
- Daouda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.L.; Vázquez, J.A.; González, M.P. Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod. Process. 2012, 90, 491–498. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Shaw, N.B.; Murphy, S.C.; van de Vis, J.W.; van Pelt-Heerschap, H.; Kerry, J.P. Extraction of collagen from fish skins and its use in the manufacture of biopolymer films. J. Aquat. Food Prod. Technol. 2006, 15, 21–32. [Google Scholar] [CrossRef]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020, 27, 73. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. Biomater. Sci. Polym. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Seixas, M.J.; Martins, E.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Extraction and characterization of collagen from elasmobranch byproducts for potential biomaterial use. Mar. Drugs 2020, 18, 617. [Google Scholar] [CrossRef]
- Herpandi, H.; Huda, N.; Adzitey, F. Fish bone and scale as a potential source of halal gelatin. J. Fish. Aquat. Sci. 2011, 6, 379–389. [Google Scholar]
- Lv, L.C.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Rafael, M.Y.B.; Rafael, R.R.; Landingin, E.P.; Rafael, R.B.; Tayag, G.G.; Santos, J.P.E.; Rafael, M.J.R. Gelatin from Milkfish Scales for Food Application. CLSU Int. J. Sci. Technol. 2021, 5, 1. [Google Scholar]
- Terzioğlu, P.; Öğüt, H.; Kalemtaş, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C 2018, 91, 899–911. [Google Scholar] [CrossRef]
- Venkatesan, J.; Qian, Z.J.; Ryu, B.; Thomas, N.V.; Kim, S.K. A comparative study of thermal calcination and an alkaline hydrolysis method in the isolation of hydroxyapatite from Thunnus obesus bone. Biomed. Mater. 2011, 6, 035003. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.; Pullar, R.; da Cruz, I.B.; Jorge, R.; Pintado, M.; Castro, P.M. Extraction and characterisation of apatite-and tricalcium phosphate-based materials from cod fish bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Sasaki, K. Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram. Int. 2014, 40, 10777–10785. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.C.; Tobaldi, D.M.; Castro, P.M.L.; Pintado, M.M.E. Hydroxyapatite and chloroapatite derived from sardine by-products. Ceram. Int. 2014, 40, 13231–13240. [Google Scholar] [CrossRef]
- Sunil, B.R.; Jagannatham, M. Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 2016, 185, 411–414. [Google Scholar] [CrossRef]
- Pal, A.; Paul, S.; Choudhury, A.R.; Balla, V.K.; Das, M.; Sinha, A. Synthesis of hydroxyapatite from Lates calcarifer fish bone for biomedical applications. Mater. Lett. 2017, 203, 89–92. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish discard. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of aquaculture by-products of salmonids to produce enzymatic hydrolysates: Process optimization, chemical characterization and evaluation of bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemistry. Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1997. [Google Scholar]
- Grossardt, C.; Ewald, A.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Passive and active in vitro resorption of calcium and magnesium phosphate cements by osteoclastic cells. Tissue Eng. A 2010, 16, 3687–3695. [Google Scholar] [CrossRef]
- Sunouchi, K.; Tsuru, K.; Maruta, M.; Kawachi, G.; Matsuya, S.; Terada, Y.; Ishikawa, K. Fabrication of solid and hollow carbonate apatite microspheres as bone substitutes using calcite microspheres as a precursor. Dent. Mater. J. 2012, 31, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Nosenko, V.; Strutynska, N.; Vorona, I.; Zatovsky, I.; Dzhagan, V.; Lemishko, S.; Epple, M.; Prymak, O.; Baran, N.; Ishchenko, S.; et al. Structure of biocompatible coatings produced from hydroxyapatite nanoparticles by detonation spraying. Nanoscale Res. Lett. 2015, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Pu’ad, N.A.S.M.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Ionic substitutions in biphasic hydroxyapatite and β-Tricalcium phosphate mixtures: Structural analysis by Rietveld refinement. J. Am. Ceram. Soc. 2008, 91, 1–12. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. Biology 2015, 32, 86–106. [Google Scholar] [CrossRef]
- Baier, M.; Staudt, P.; Klein, R.; Sommer, U.; Wenz, R.; Grafe, I.; Meeder, P.J.; Nawroth, P.P.; Kasperk, C. Strontium enhances osseointegration of calcium phosphate cement: A histomorphometric pilot study in ovariectomized rats. J. Orthop. Surg. Res. 2013, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, E.; Davarpanah, M.; Nemati, N.H.; Tavakoli, S.A. Fabrication of a hard tissue replacement using natural hydroxyapatite derived from bovine bones by thermal decomposition method. Int. J. Organ Transplant. Med. 2014, 5, 23–31. [Google Scholar]
- Arias, J.L.; Mayor, M.B.; García-Sanz, F.J.; Pou, J.; León, B.; Pérez-Amor, M. Structural analysis of calcium phosphate coatings produced by pulsed laser deposition at different water-vapour pressures. J. Mater. Sci. Mater. Med. 1997, 8, 873–876. [Google Scholar] [CrossRef]
- Barinov, S.M.; Rau, J.V.; Cesaro, S.N.; Durisin, J.; Fadeeva, I.V.; Ferro, D.; Medvecky, L.; Trionfetti, G. Carbonate release from carbonated hydroxyapatite in the wide temperature rage. J. Mater. Sci. Mater. Med. 2006, 17, 597–604. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Siddharthan, A.; Seshadri, S.K.; Kumar, T.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Goyenvalle, E.; Aguado, E.; Nguyen, J.M.; Passuit, N.; Le Guenennec, L.; Layrolle, P.; Daculsi, G. Osteointegration of femoral stem prostheses with a bilayered calcium phosphate coating. Biomaterials 2006, 27, 1119–1128. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Myers, H. (Eds.) Calcium Phosphates in Oral Biology and Medicine. In Monographs in Oral Sciences; Karger: Basel, Switzerland, 1991; Volume 15, pp. 82–107. [Google Scholar]
- Stock, S.R. The Mineral–Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamaru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J. Mater. Sci. Mater. Med. 2015, 26, 99. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. An Introduction to Bioceramics; World Scientifi: Singapore, 1993; Volume 1. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Lusquiños, F.; Riveiro, A.; Comesaña, R.; Pou, J. Hydroxylapatite nanoparticles obtained by fiber laser-induced fracture. Appl. Surf. Sci. 2009, 255, 5382–5385. [Google Scholar] [CrossRef]
- Da Silva, E.A.B.; Costa, C.A.E.; Vilar, V.J.P.; Botelho, C.M.S.; Larosi, M.B.; Saracho, J.M.P.; Boaventura, R.A.R. Water remediation using calcium phosphate derived from marine residues. Water Air Soil Pollut. 2012, 223, 989–1003. [Google Scholar] [CrossRef]
- Piccirillo, C.; Fernández-Arias, M.; Boutinguiza, M.; Tobaldi, D.M.; Del Val, J.; Pintado, M.M.; Pou, J. Increased UV absorption properties of natural hydroxyapatite-based sunscreen through laser ablation in liquid. J. Amer. Ceram. Soc. 2019, 102, 3163–3174. [Google Scholar] [CrossRef]
- Peraire, C.; Arias, J.L.; Bernal, D.; Pou, J.; Leon, B.; Arano, A.; Roth, W. Biological stability and osteoconductivity in rabbit tibia of pulsed laser deposited hydroxylapatite coatings. J. Biomed. Mater. Res. A 2006, 77, 370–379. [Google Scholar] [CrossRef] [PubMed]



| Yw (%, ww/w) | Yd (%, dw/w) | Hu (%) | Ash (%) | OM (%) | |
|---|---|---|---|---|---|
| Scorp | 11.2 ± 0.4 | 4.7 ± 0.1 | 1.70 ± 0.32 | 67.02 ± 0.46 | 31.29 ± 0.78 |
| Trach | 8.5 ± 0.7 | 3.7 ± 0.1 | 4.03 ± 0.89 | 62.33 ± 0.92 | 33.64 ± 0.03 |
| Salmo | 11.6 ± 0.4 | 6.0 ± 0.5 | 2.80 ± 0.29 | 59.24 ± 0.82 | 37.96 ± 1.11 |
| Fish Sample | Temperature of Thermal Treatment (°C) | Mass Loss (%) |
|---|---|---|
| Scorpaena scrofa | 750 | 35.7 |
| Trachurus trachurus | 750 | 39.9 |
| Salmo salar | 750 | 45.3 |
| Scorpaena scrofa | 950 | 35.9 |
| Trachurus trachurus | 950 | 40.4 |
| Salmo salar | 950 | 45.5 |
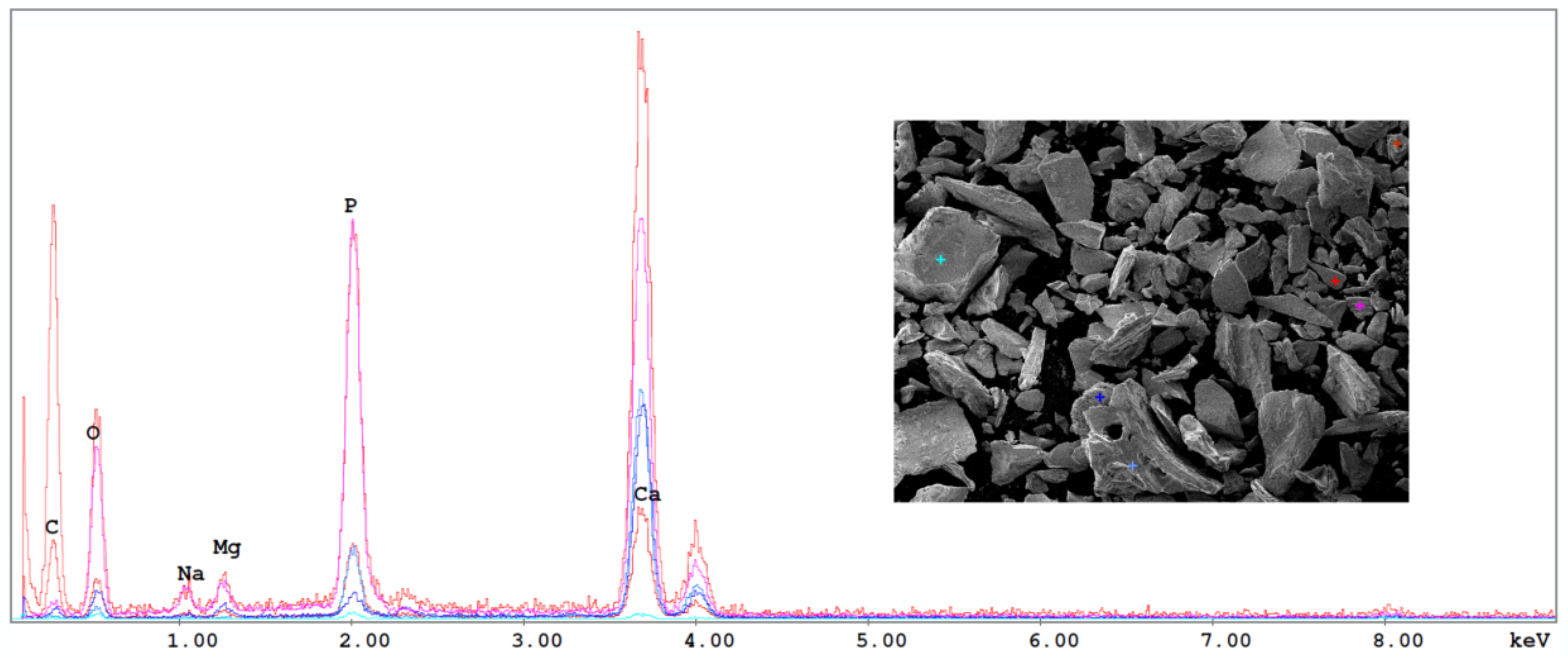
| Samples | Calcination Temperature (°C) | Ca | P | Mg | K | Sr | Ca/P (Molar) |
|---|---|---|---|---|---|---|---|
| Scorp | 750 | 35.88 | 20.23 | 1.04 | 0.22 | 0.19 | 1.77 ± 0.02 |
| Trach | 750 | 37.81 | 20.37 | 1.22 | 0.24 | 0.23 | 1.86 ± 0.02 |
| Salmo | 750 | 41.14 | 22.74 | 0.97 | 0.28 | 0.18 | 1.81 ± 0.02 |
| Scorp | 950 | 23.65 | 13.53 | 1.20 | 0.17 | 0.24 | 1.75 ± 0.02 |
| Trach | 950 | 39.26 | 21.61 | 1.25 | 0.20 | 0.24 | 1.82 ± 0.02 |
| Salmo | 950 | 31.20 | 18.43 | 1.02 | 0.23 | 0.19 | 1.69 ± 0.02 |
| Element | Maximum Concentration in Samples (ppm) | Maximum Legal Concentration (ppm) |
|---|---|---|
| Cd | 0.68 | 5 |
| Pb | 1.6 | 30 |
| Hg | 0.04 | 5 |
| Samples | Calcination Temperature (°C) | a (Å) | c (Å) | c/a | Unit Cell Volume (Å 3) | Crystallite Size (nm) | %CO3 |
|---|---|---|---|---|---|---|---|
| Scorp | 750 | 9.427 | 6.899 | 0.732 | 530.95 | 50 ± 8 | 2.37 |
| Trach | 750 | 9.427 | 6.899 | 0.732 | 530.95 | 50 ± 8 | 2.37 |
| Salmo | 750 | 9.420 | 6.892 | 0.732 | 529.63 | 35 ± 4 | 2.08 |
| Scorp | 950 | 9.427 | 6.899 | 0.732 | 530.95 | 59 ± 10 | 2.37 |
| Trach | 950 | 9.427 | 6.899 | 0.732 | 530.95 | 59 ± 10 | 2.37 |
| Salmo | 950 | 9.405 | 6.879 | 0.731 | 527.02 | 59 ± 10 | 1.49 |
| Synthetic HA | - | 9.418 | 6.884 | 0.731 | 527.80 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Arias, M.; Álvarez-Olcina, I.; Malvido-Fresnillo, P.; Vázquez, J.A.; Boutinguiza, M.; Comesaña, R.; Pou, J. Biogenic Calcium Phosphate from Fish Discards and By-Products. Appl. Sci. 2021, 11, 3387. https://doi.org/10.3390/app11083387
Fernández-Arias M, Álvarez-Olcina I, Malvido-Fresnillo P, Vázquez JA, Boutinguiza M, Comesaña R, Pou J. Biogenic Calcium Phosphate from Fish Discards and By-Products. Applied Sciences. 2021; 11(8):3387. https://doi.org/10.3390/app11083387
Chicago/Turabian StyleFernández-Arias, Mónica, Iago Álvarez-Olcina, Pablo Malvido-Fresnillo, José Antonio Vázquez, Mohamed Boutinguiza, Rafael Comesaña, and Juan Pou. 2021. "Biogenic Calcium Phosphate from Fish Discards and By-Products" Applied Sciences 11, no. 8: 3387. https://doi.org/10.3390/app11083387
APA StyleFernández-Arias, M., Álvarez-Olcina, I., Malvido-Fresnillo, P., Vázquez, J. A., Boutinguiza, M., Comesaña, R., & Pou, J. (2021). Biogenic Calcium Phosphate from Fish Discards and By-Products. Applied Sciences, 11(8), 3387. https://doi.org/10.3390/app11083387








