Evaluation of α-Chitosan from Crab Shell and β-Chitosan from Squid Gladius Based on Biochemistry Performance
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
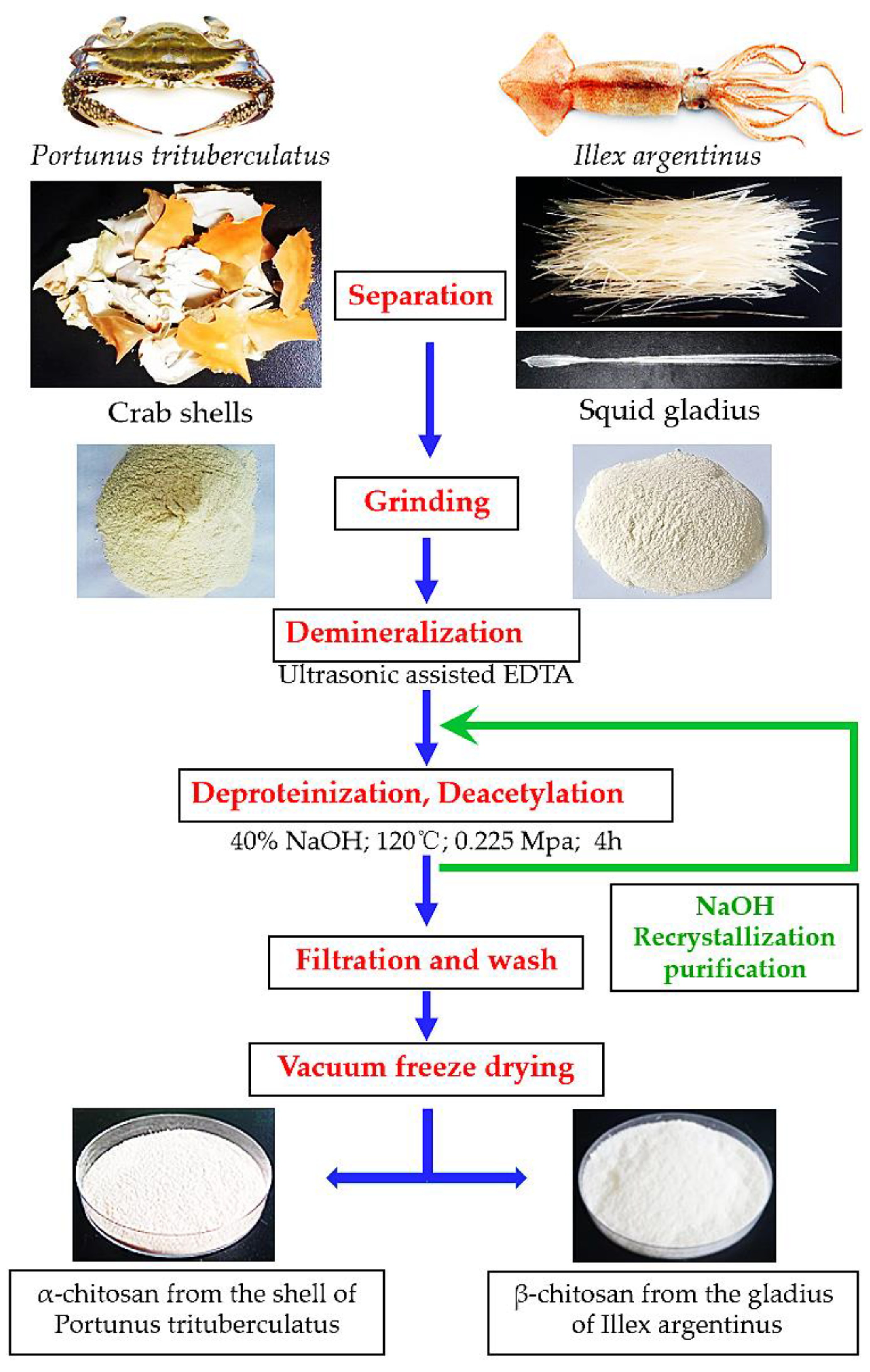
2.2. Preparation of Chitosan
2.3. Characterization of Chitosan
2.3.1. Determination of the Molecular Weight
2.3.2. Measurement of Deacetylation Degree
2.3.3. Water Binding Capacity
2.3.4. Fat Binding Capacity
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
2.3.8. Circular Dichroism Spectroscopy (CD)
2.3.9. Scanning Electron Microscopy (SEM)
2.3.10. Polarized Optical Microscopy (POM)
2.4. Weighted Composite Index Evaluation
2.4.1. Index Selection, Classification and Determination of Weight Coefficient
2.4.2. Data Standard Normalization Processing
2.4.3. Calculation and Evaluation of Weighted Composite Index
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition of Experimental Raw Materials
3.2. Extraction of Chitosan
3.3. Analysis of Molecular Structural Characteristics
3.3.1. Molecular Weight
3.3.2. Deacetylation Degree
3.4. Analysis of Physiochemical Characteristics
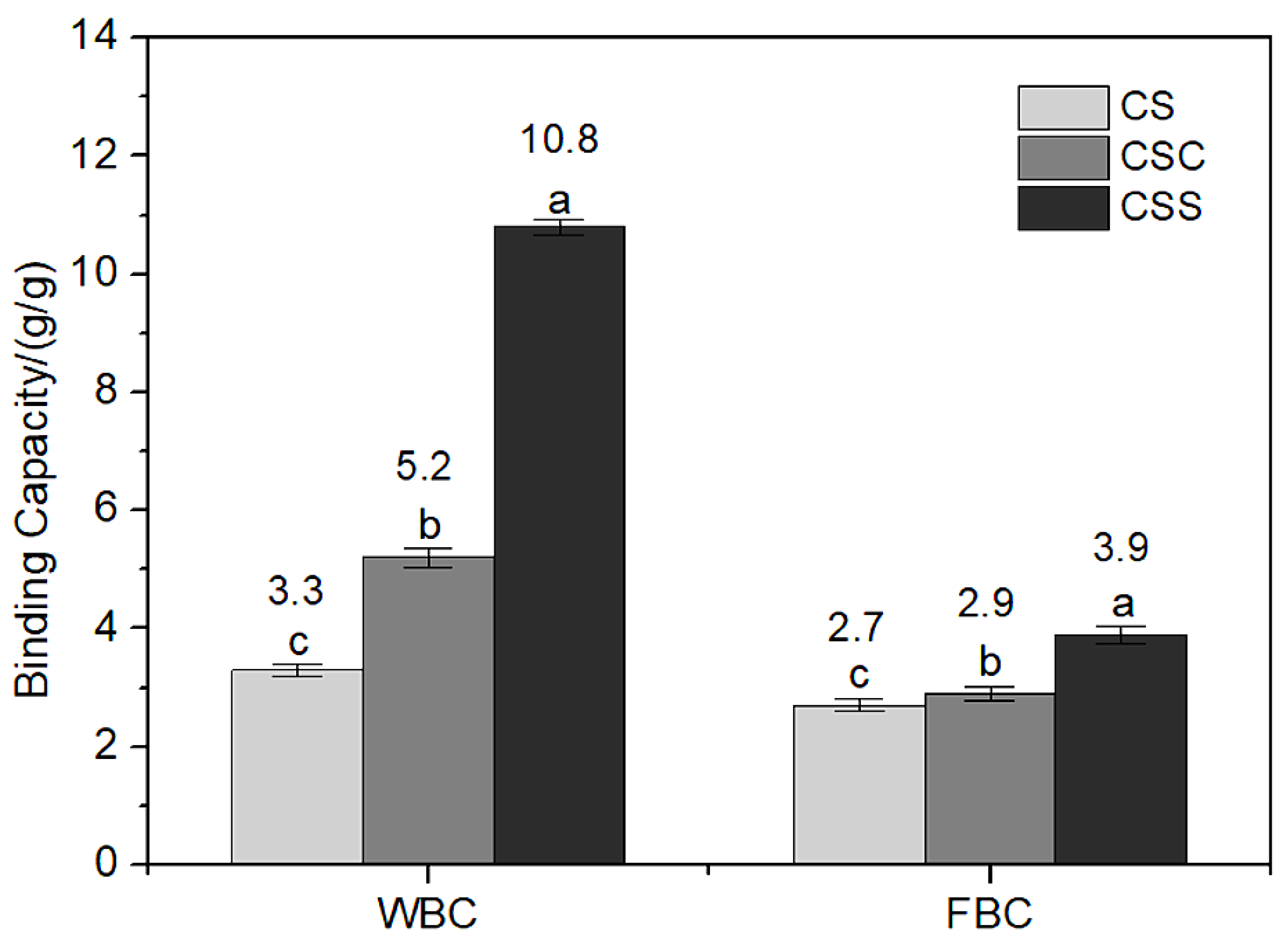
3.4.1. Water Binding Capacity
3.4.2. Fat Binding Capacity
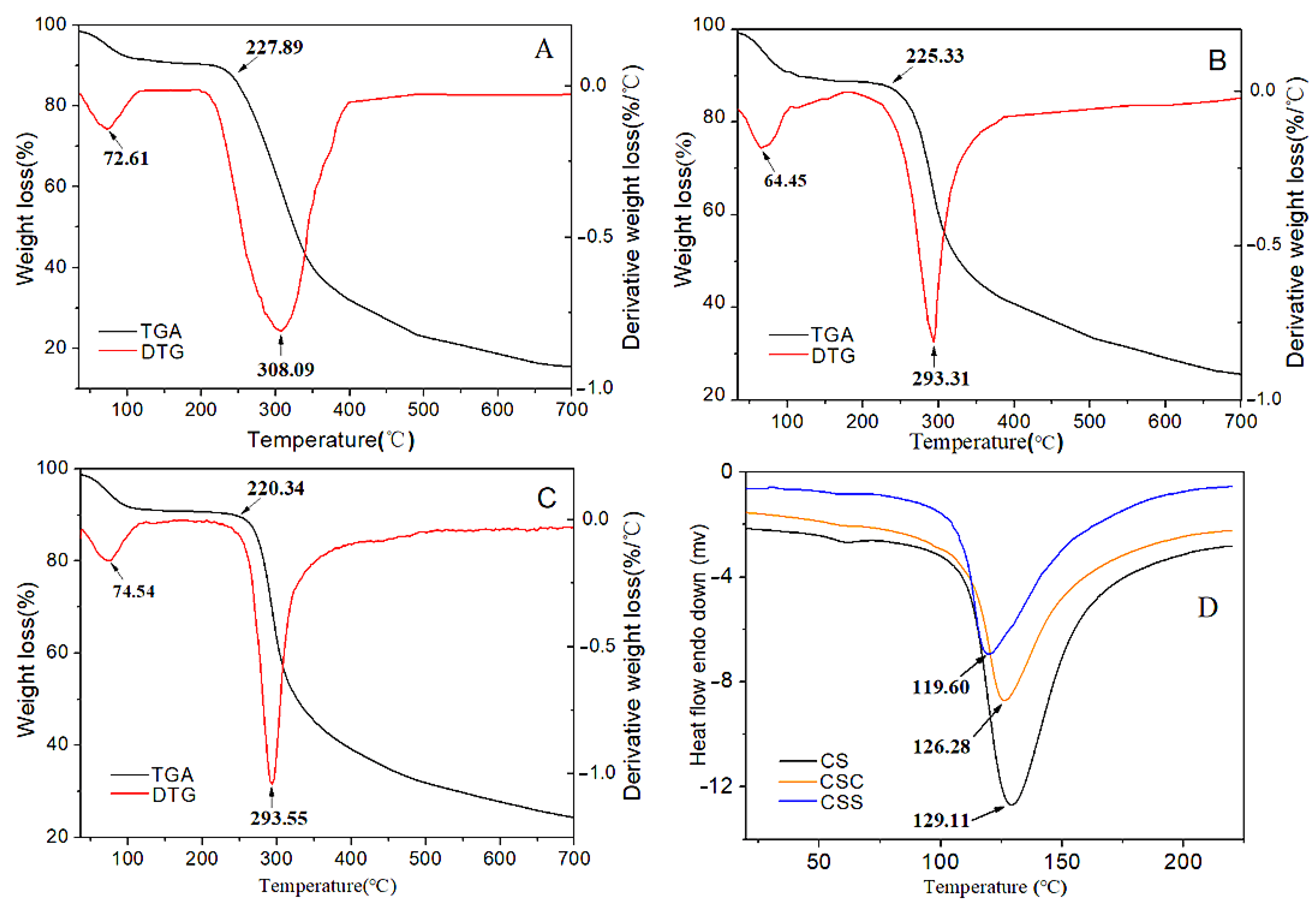
3.4.3. Thermal Stability Analysis
3.5. Analysis of Bio-Functional Characteristics
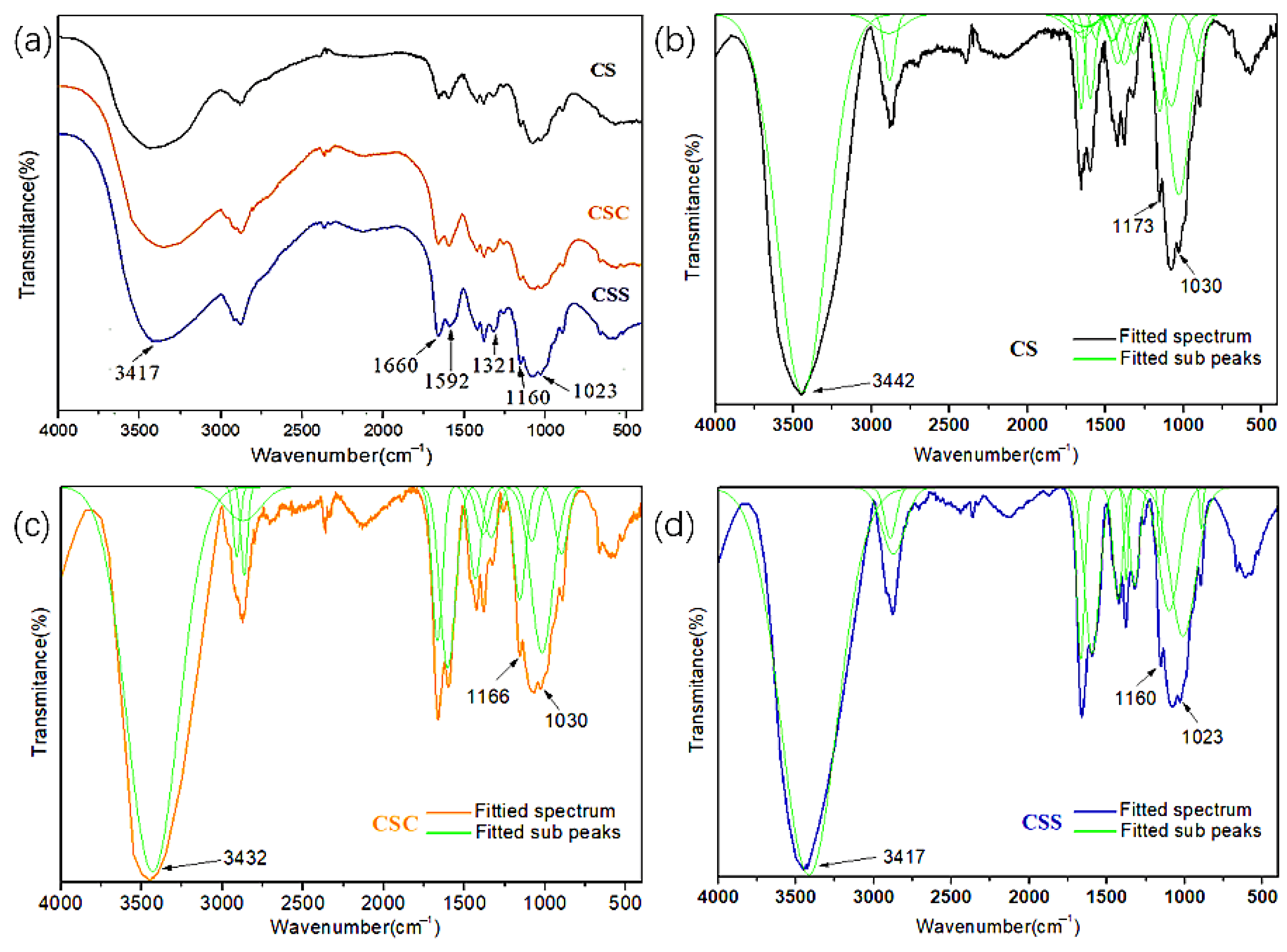
3.5.1. FT-IR Analysis
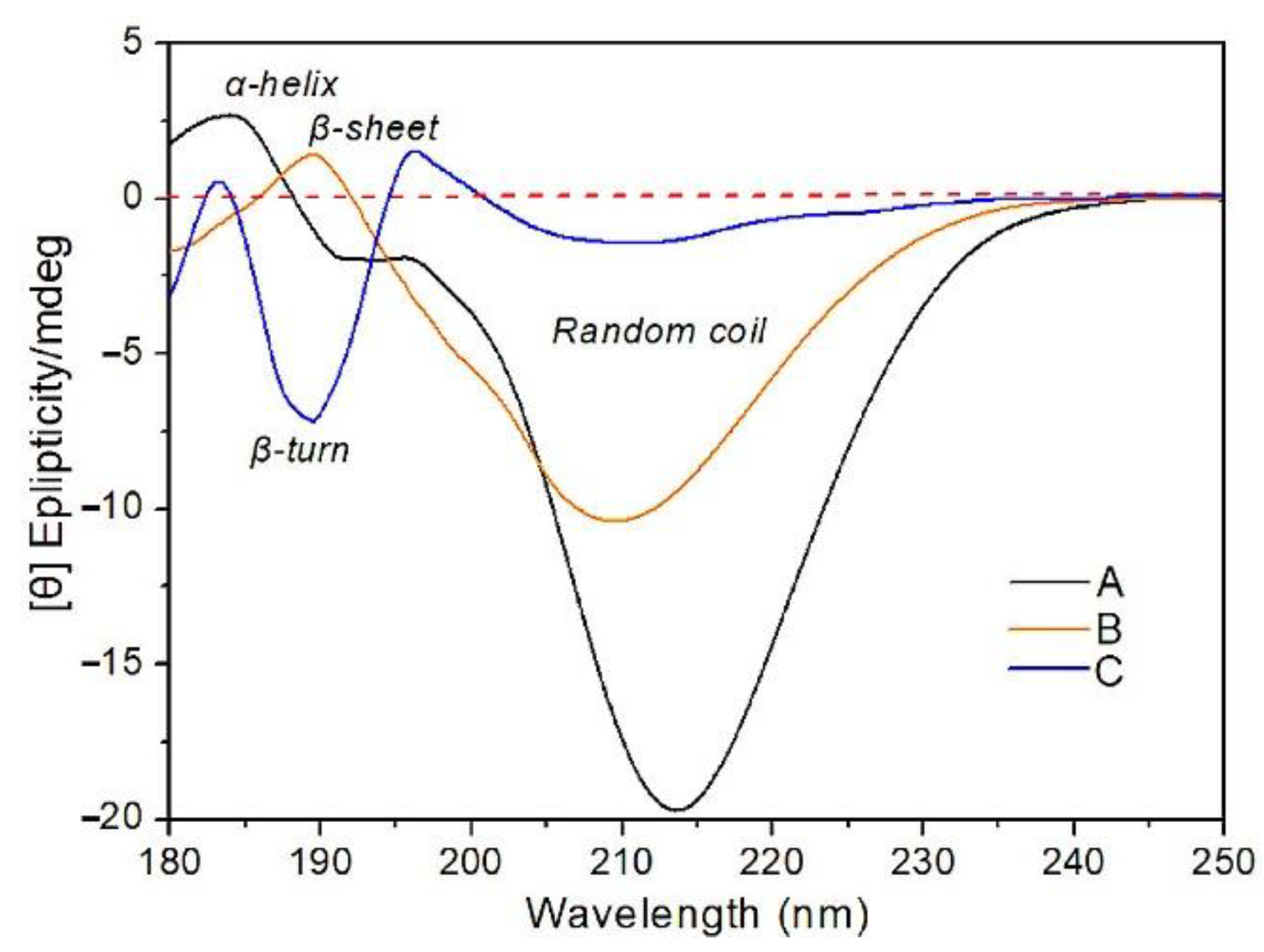
3.5.2. CD Analysis
3.6. Morphological Observation
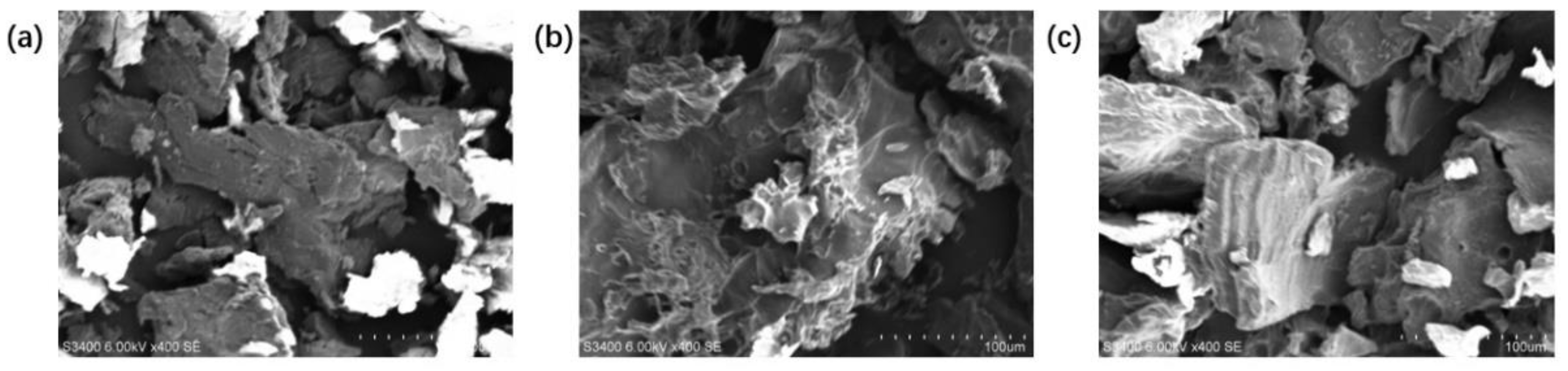
3.6.1. Scanning Electron Microscopy
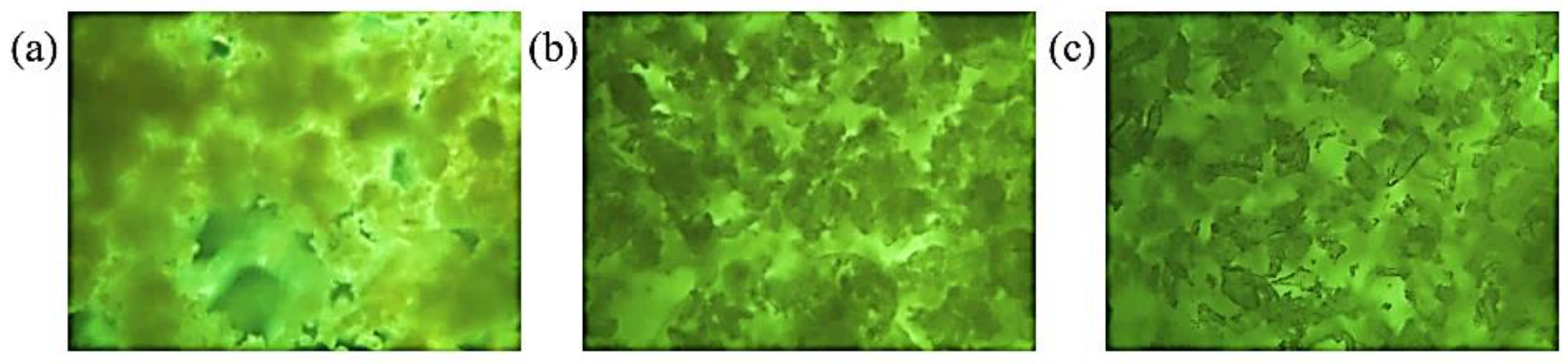
3.6.2. Polarized Optical Microscopy
3.7. Weighted Comprehensive Index Evaluation Method
3.7.1. Original Data
3.7.2. Determine the Weight Coefficient
3.7.3. Standardization Results
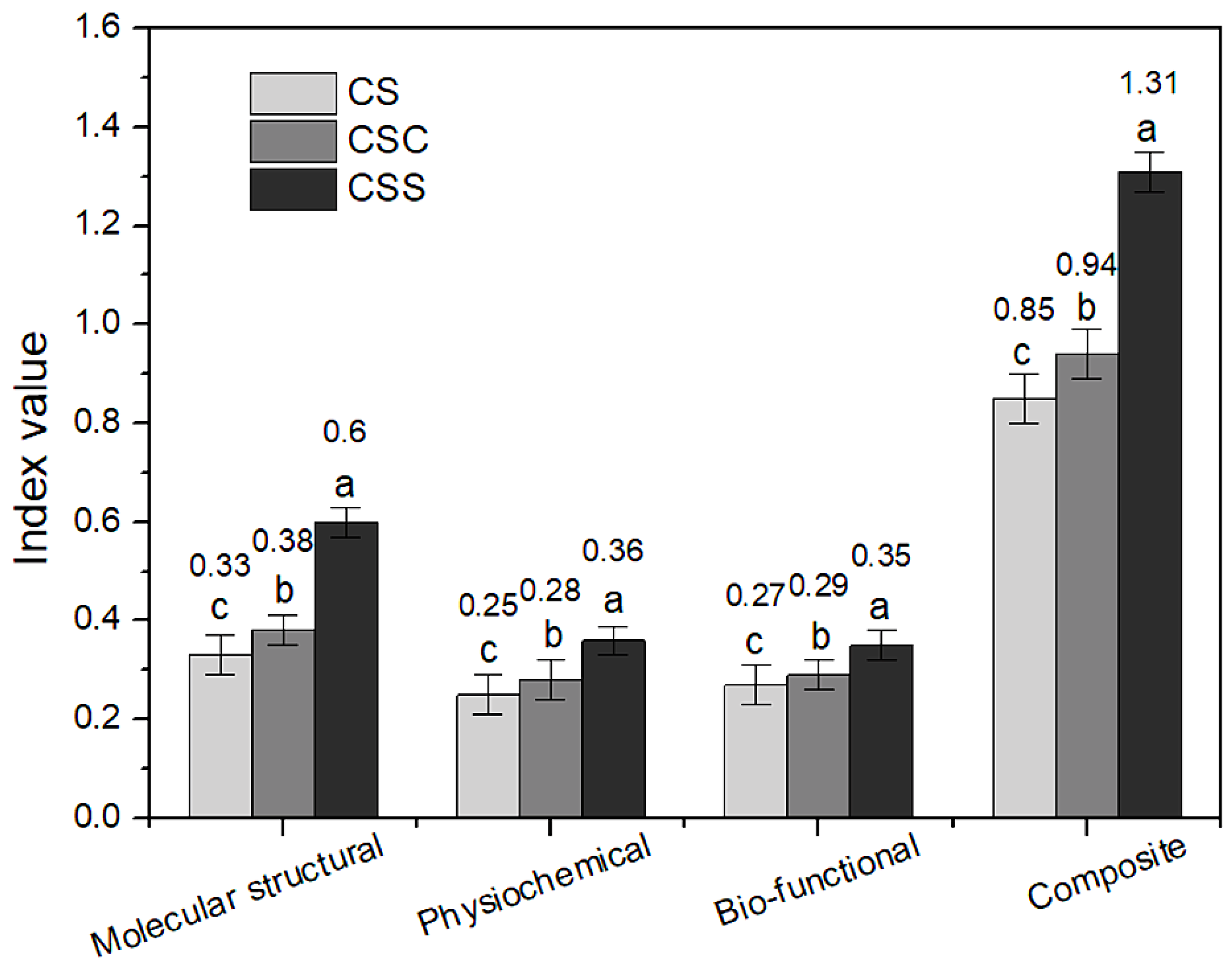
3.7.4. Calculation of Composite Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Fishery and Aquaculture Statistics; FAO Fisheries and Aquaculture Department: Rome, Italy, 2016. [Google Scholar]
- Ruchi, M.; Abhijeet, T.; Arun, G. Chapter 13—Chitin and Chitosan: Current Status and Future Opportunities; Sreerag, G., Sabu, T., Anitha, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 401–417. [Google Scholar]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Saad, M.A.; Mathai, T.; Kranthi, K.R.; Sujata, G.S.; Amit, A.; Ira, B. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar]
- Singh, J.; Dutta, P.K. Preparation, circular dichroism induced helical conformation and optical property of chitosan acid salt complexes for biomedical applications. Int. J. Biol. Macromol. 2009, 45, 384–392. [Google Scholar] [CrossRef]
- Jaworska, M.M. Kinetics of enzymatic deacetylation of chitosan. Cellulose 2012, 19, 363–369. [Google Scholar] [CrossRef][Green Version]
- Vázquez, J.; Rodríguez-Amado, I.; Montemayor, M.; Fraguas, J.; González, M.; Murado, M. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sampedro, R.; Revilla, E.; Villar, J.C.; Eugenio, M.E. Enhancement of enzymatic saccharification of Eucalyptus globulus: Steam explosion versus steam treatment. Bioresour. Technol. 2014, 167, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.S.; Chin, H.Y.; Tsai, M.L.; Liu, C.L. Structural alterations, pore generation, and deacetylation of α- and β-chitin submitted to steam explosion. Carbohydr. Polym. 2015, 122, 321–328. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lucas, A.J.; Oreste, E.O.; Costa, L.H.G.; López, H.M.; Saad, C.D.M.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Anand, M.A.V.; Wang, M.H. pH-controlled nucleolin targeted release of dual drug from chitosan-gold based aptamer functionalized nano drug delivery system for improved glioblastoma treatment. Carbohydr. Polym. 2021, 262, 117907. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.A.; Bekhit, A.E.A.; Sun, Z.F.; Ali, M.A. Preparation and characterisation of irradiated crab chitosan and New Zealand Arrow squid pen chitosan. Mater. Chem. Phys. 2015, 167, 295–302. [Google Scholar] [CrossRef]
- Subhapradha, N.; Ramasamy, P.; Shanmugam, V.; Madeswaran, P.; Srinivasan, A.; Shanmugam, A. Physicochemical characterisation of b-chitosan from Sepioteuthis lessoniana gladius. Food Chem. 2013, 141, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Guha, A.K.; Chatterjee, B.P. Evaluation of quantity and quality of chitosan produce from Rhizopus oryzae by utilizing food product processing waste whey and molasses. J. Environ. Manag. 2019, 251, 109565. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.C.; Wang, M.Y.; Huang, L.; Qin, D.; Cheng, X.J.; Chen, X.G. Evaluation of structure transformation and biocompatibility of chitosan in alkali/urea dissolution system for its large-scale application. Int. J. Biol. Macromol. 2020, 154, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Tsai, Y.H. Extraction of chitosan from squid pen waste by high hydrostatic pressure: Effects on physiochemical properties and antioxidant activities of chitosan. Int. J. Biol. Macromol. 2020, 160, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Noriega, D.; Ramos, P.; Valcarcel, J.; Novoa-Carballal, R.; Pastrana, L.; Reis, R.L.; Perez-Martin, R.I. Optimization of high purity chitin and chitosan production from Illex argentinus pens by a combination of enzymatic and chemical processes. Carbohydr. Polym. 2017, 174, 262–272. [Google Scholar] [CrossRef]
- Yang, J.F.; Wu, W.H.; Gao, K.L.; Bao, B. A Method of Preparing β-Chitin from Squid Gladius; CN 105348411 A; State Intellectual Property Office Intellectual Property Press: Beijing, China, 2016. [Google Scholar]
- Wang, W.; Bo, S.Q.; Li, S.Q.; Qin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S. Determination of the degree of acetylation (DA) of chitin and chitosan by an improved first derivative UV method. Carbohydr. Polym. 2008, 73, 248–253. [Google Scholar] [CrossRef]
- Wang, J.C.; Kinsella, J.E. Functional properties of novel proteins: Alfalfa leaf protein. J. Food Sci. 1976, 41, 286–292. [Google Scholar] [CrossRef]
- Chen, C.; Li, Q.; Gu, H.N.; Zhang, Q. Application of the general stability index method to establish lettuce shelf life prediction model. J. Henan Univ. Technol. 2013, 6, 86–90. [Google Scholar]
- Li, Y.M.; Wu, J.X. Application of Weighted Comprehensive Index Method in Medical Quality Evaluation of Clinical Departments. Chin. Med Record 2017, 18, 11–16. [Google Scholar]
- Dolge, K.; Kubule, A.; Blumberga, D. Composite index for energy efficiency evaluation of industrial sector: Sub-sectoral comparison. Environ. Sustain. Indic. 2020, 8, 100062. [Google Scholar] [CrossRef]
- Subhapradha, N.; Ramasamy, P.; Sudharsan, S.; Seedevi, P.; Moovendhan, M.; Srinivasan, A.; Shanmugam, V.; Shanmugam, A. Preparation of phosphorylated chitosan from gladius of the squid Sepioteuthis lessoniana (Lesson, 1830) and its in vitro antioxidant activity. Bioact. Carbohydr. Diet. Fibre 2013, 1, 148–155. [Google Scholar] [CrossRef]
- Cuong, H.N.; Minh, N.C.; Hoa, N.V.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Gao, Y.; Zhao, B.N. Determination of Calcium Carbonate in Crab Shell. Food Drug 2013, 15, 51–52. [Google Scholar]
- Cortizo, M.S.; Berghoff, C.F.; Alessandrini, J.L. Characterization of chitin from Illex argentinus squid pen. Carbohydr. Polym. 2008, 74, 10–15. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Rinaudo, M.; Jellouli, K.; Nasri, M. Characterization and in Vitro Evaluation of Cytotoxicity, Antimicrobial and Antioxidant Activities of Chitosans Extracted from Three Different Marine Sources. Appl. Biochem. Biotechnol. 2015, 177, 18–35. [Google Scholar] [CrossRef]
- Panith, N.; Wichaphon, J.; Lertsiri, S.; Niamsiri, N. Effect of physical and physicochemical characteristics of chitosan on fat-binding capacities under in vitro gastrointestinal conditions. LWT-Food Sci. Technol. 2016, 71, 25–32. [Google Scholar] [CrossRef]
- Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Wang, A.Q.; Yu, X.D. The preparation of β-chitosan and its characteristic properties. Chin. J. Mar. Drugs. 2000, 2, 21–23. [Google Scholar]
- Zhu, C.L.; Sun, D.F.; Zhang, J.; Qi, S.L.; Zhang, W.M. Study on Preparation of High Deacetylation Degree of Chitosan. Chin. Wild Plant Resour. 2011, 30, 44–47. [Google Scholar]
- Chen, W.; Bai, Z.W. Design on comprehensive experiment of preparation and characterization of chitosan with super-high degree of deacetylation. Exp. Technol. Manag. 2018, 35, 42–45. [Google Scholar]
- Tian, Y.; Jiao, Y.P.; Chen, Y.K.; Li, L.H.; Zhou, C.R. Effect of Deacetylation Degree on the Capability of Surface Physics and Adsorption of Chitosan. Guangzhou Chem. 2005, 30, 20–25. [Google Scholar]
- Ocloo, F.C.K.; Quayson, E.T.; Adu-Gyamfi, A.; Quarcoo, E.A.; Asare, D.; Serfor-Armah, Y.; Woode, B.K. Physicochemical and functional characteristics of radiation-processed shrimp chitosan. Radiat. Phys. Chem. 2011, 80, 837–841. [Google Scholar]
- Jin, Q. Study on the anti-obesity effect and lipid-lowering activity of chitosan with different average molecular weight and different molecular configuration (α,β). Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2017. [Google Scholar]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Wang, X.K.; Wang, J.G.; Guo, P.Q.; Guo, W.L.; Li, G.L. Chemical effect of swirling jet-induced cavitation: Degradation of rhodamine B in aqueous solution. Ultrason. Sonochem. 2008, 15, 357–363. [Google Scholar] [CrossRef]
- Domard, A. Determination of N-aeetyl content in chitosan samples by c.d. nreasurements. Int. J. Biol. Macromol. 1987, 9, 333–336. [Google Scholar] [CrossRef]
- Farooqahamed, S.K.; Acharya, B.; Rudrapatnam, N.T. Low molecular weight chitosans-preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res. 2003, 338, 1283–1290. [Google Scholar]
- Chen, Y.H.; Yang, J.T.; Chau, K.H. Determination of the helix and β form of proteins in aqueous solution by circular dichroism. Biochemistry 1974, 13, 3350–3359. [Google Scholar] [CrossRef]
- Kucukgulmez, A.; Celik, M.; Yanar, Y.; Sen, D.; Polat, H.; Eslem Kadak, A. Physicochemical characterisation of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 2011, 126, 144–1148. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Erratum to “Thermogravimetric and FTIR studies of chitosan blends”. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhang, H.L. Physicochemical Properties of Chitosan: Determination of Viscosity Average Molecular Weight, Degree of Deacetylation and Crystallinity. Guangdong Chem. Ind. 2019, 22, 22–25. [Google Scholar]
- Chen, M.M.; Huang, Y.Q.; Cao, H.; Liu, Y.; Guo, H.; Chen, L.S.; Zhang, Q.Q. Collagen/chitosan film containing biotinylated glycol chitosan nanoparticles for localized drug delivery. Colloids Surf. Biointerfaces 2015, 128, 339–346. [Google Scholar] [CrossRef] [PubMed]

| Name | Protein (%) | Fat (%) | Ash (%) | Moisture (%) | Yield (%) |
|---|---|---|---|---|---|
| Shell | 18.36 | 3.22 | 58.68 | 2.32 | NA a |
| Gladius | 59.34 | 2.06 | 0.86 | 1.13 | NA a |
| CS | ND a | ND a | 0.18 | 0.35 | NA a |
| CSC | ND a | ND a | ND a | 0.32 | 11.81 b |
| CSS | ND a | ND a | ND a | 0.26 | 26.15 b |
| C(g/100 mL) | CS | CSC | CSS | |||
|---|---|---|---|---|---|---|
| ηsp | ηsp/C | ηsp | ηsp/C | ηsp | ηsp/C | |
| 0.20 | 0.93 | 463.31 | 1.43 | 713.03 | 0.41 | 204.58 |
| 0.13 | 0.55 | 413.22 | 0.82 | 616.22 | 0.21 | 153.86 |
| 0.10 | 0.39 | 389.10 | 0.55 | 546.07 | 0.13 | 130.44 |
| 0.07 | 0.24 | 367.25 | 0.33 | 490.28 | 0.07 | 105.21 |
| 0.05 | 0.18 | 359.34 | 0.22 | 449.31 | 0.05 | 94.01 |
| Molecular Structural | Physiochemical | Bio-Functional | |||||
|---|---|---|---|---|---|---|---|
| Mw (kDa) | DD (%) | WBC (g/g) | FBC (g/g) | TS | PS | SS | |
| CS | 377.1 | 83.4 | 3.3 | 2.7 | 5.25 | 0.28 | 1.03 |
| CSC | 249.8 | 92.7 | 5.2 | 2.9 | 4.70 | 0.30 | 1.07 |
| CSS | 22.5 | 97.8 | 10.8 | 3.9 | 4.56 | 0.31 | 1.46 |
| -OH&-NH2 | All Groups | PS | |
|---|---|---|---|
| CS | 1225..89 | 4360.71 | 0.28 |
| CSC | 1291.36 | 4306.67 | 0.30 |
| CSS | 1306.55 | 4216.13 | 0.31 |
| α-Helix | β-Sheet | β-Turn | Random Coil | |
|---|---|---|---|---|
| CS | 8.5 | 35.1 | 7.1 | 49.3 |
| CSC | 8.3 | 36.6 | 6.9 | 48.2 |
| CSS | 7.7 | 39.5 | 11.5 | 40.3 |
| Dimension | Indicator | Impact on GQI | Weight |
|---|---|---|---|
| Molecular structural (0.4) | MW | – | 0.20 |
| DD | + | 0.20 | |
| Physiochemical (0.3) | WBC | + | 0.12 |
| FBC | + | 0.12 | |
| TS | + | 0.06 | |
| Bio-functional (0.3) | PS | + | 0.12 |
| SS | + | 0.18 |
| Molecular Structural | Physiochemical | Bio-Functional | |||||
|---|---|---|---|---|---|---|---|
| Mw | DD | WBC | FBC | TS | PS | SS | |
| CS | 7.2 | 0.83 | 1.8 | 2.7 | 5.25 | 0.28 | 1.03 |
| CSC | 6.2 | 0.93 | 2.3 | 2.9 | 4.70 | 0.30 | 1.07 |
| CSS | 2.8 | 0.98 | 3.3 | 3.9 | 4.56 | 0.31 | 1.46 |
| M | 5.4 | 0.91 | 2.5 | 3.2 | 4.84 | 0.30 | 1.19 |
| Molecular Structural | Physiochemical | Bio-Functional | |||||
|---|---|---|---|---|---|---|---|
| Mw | DD | WBC | FBC | TS | PS | SS | |
| CS | 0.75 | 0.91 | 0.72 | 0.84 | 1.09 | 0.94 | 0.87 |
| CSC | 0.87 | 1.02 | 0.92 | 0.91 | 0.97 | 1.01 | 0.93 |
| CSS | 1.93 | 1.08 | 1.32 | 1.22 | 0.94 | 1.04 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wu, X.; Bao, B.; Guo, R.; Wu, W. Evaluation of α-Chitosan from Crab Shell and β-Chitosan from Squid Gladius Based on Biochemistry Performance. Appl. Sci. 2021, 11, 3183. https://doi.org/10.3390/app11073183
Li B, Wu X, Bao B, Guo R, Wu W. Evaluation of α-Chitosan from Crab Shell and β-Chitosan from Squid Gladius Based on Biochemistry Performance. Applied Sciences. 2021; 11(7):3183. https://doi.org/10.3390/app11073183
Chicago/Turabian StyleLi, Bailei, Xue Wu, Bin Bao, Ruihua Guo, and Wenhui Wu. 2021. "Evaluation of α-Chitosan from Crab Shell and β-Chitosan from Squid Gladius Based on Biochemistry Performance" Applied Sciences 11, no. 7: 3183. https://doi.org/10.3390/app11073183
APA StyleLi, B., Wu, X., Bao, B., Guo, R., & Wu, W. (2021). Evaluation of α-Chitosan from Crab Shell and β-Chitosan from Squid Gladius Based on Biochemistry Performance. Applied Sciences, 11(7), 3183. https://doi.org/10.3390/app11073183








