Reasons for the High Electrical Conductivity of Bismuth Ferrite and Ways to Minimize It
Abstract
1. Introduction
2. Materials and Methods
3. Results
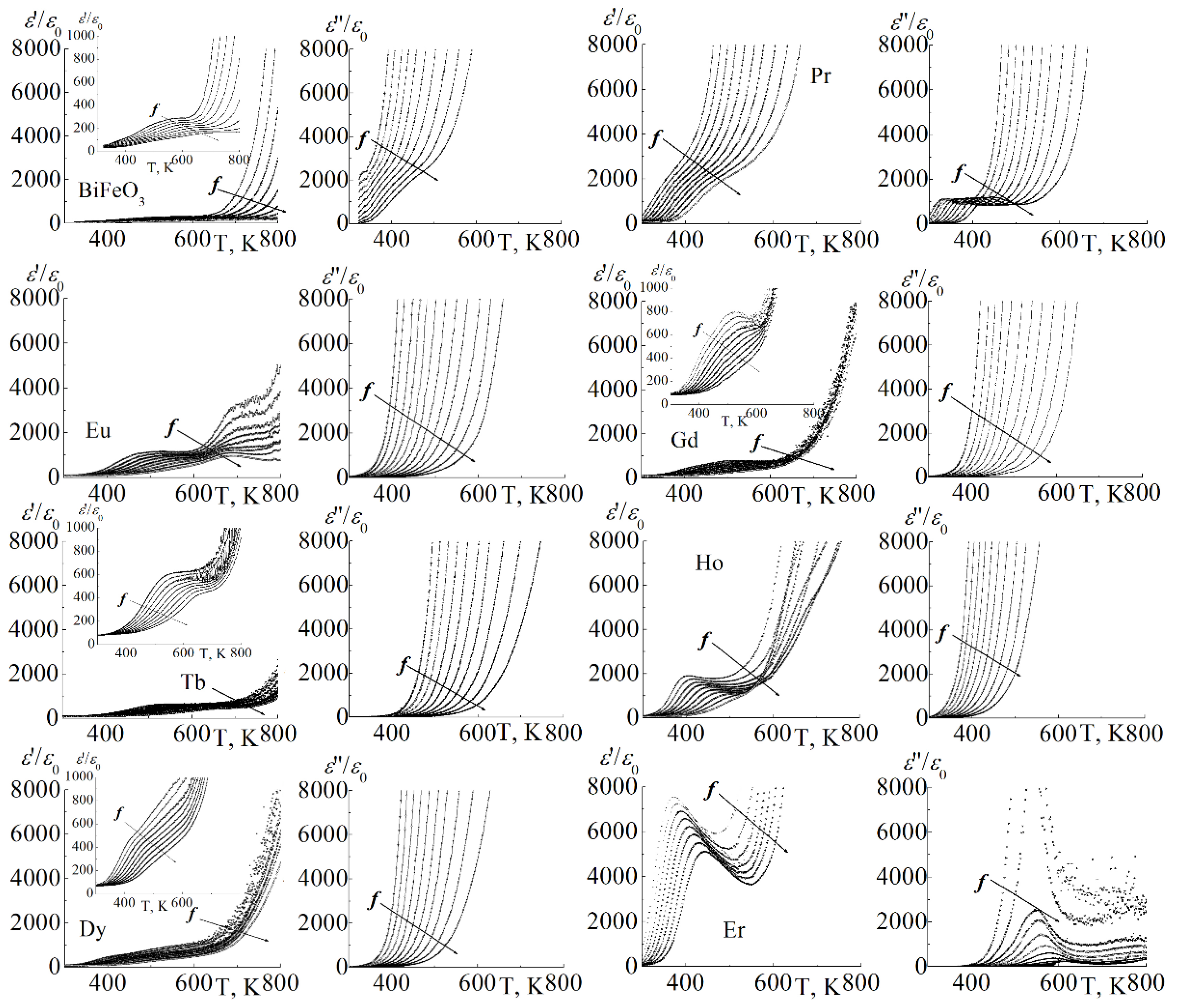
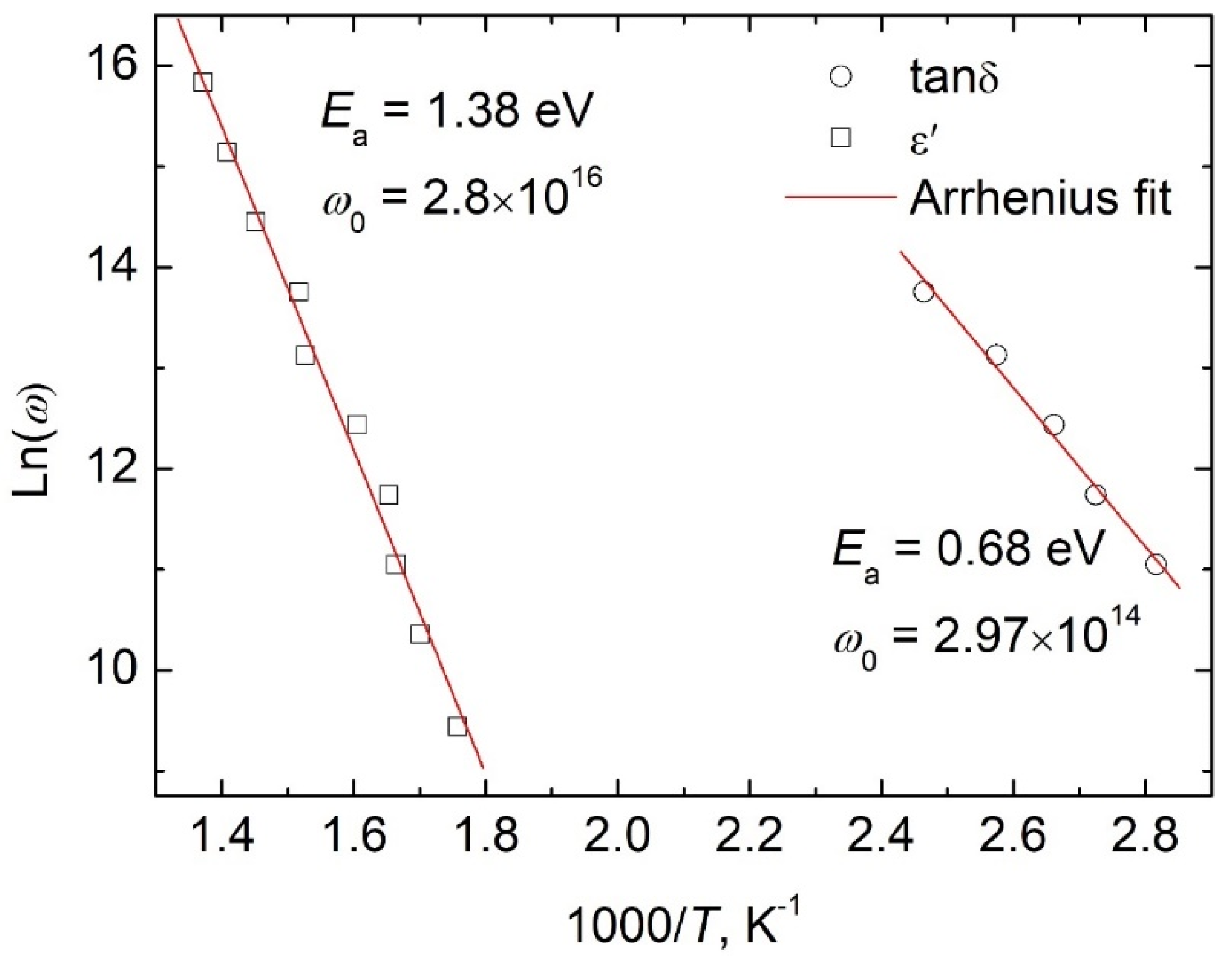
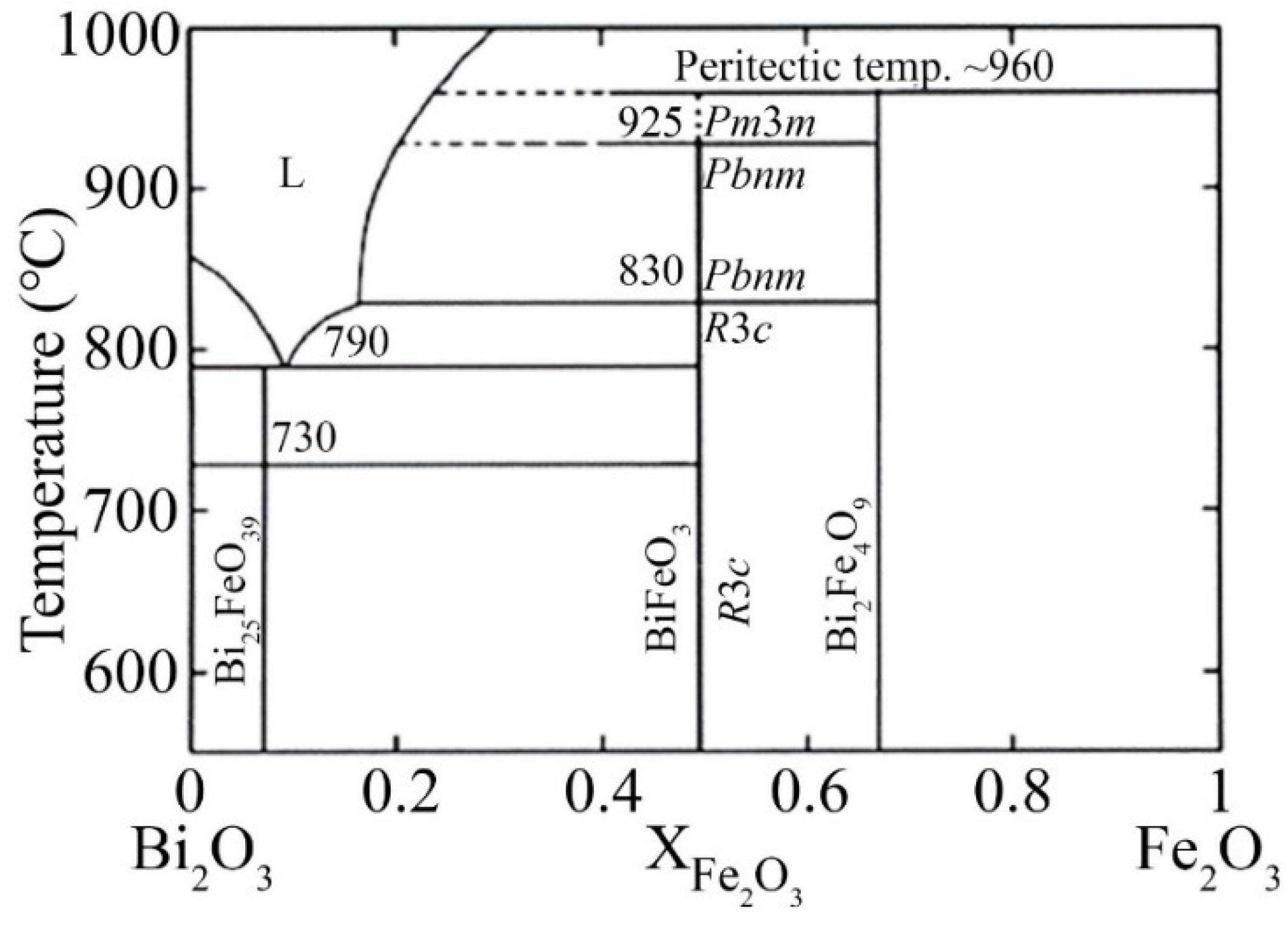
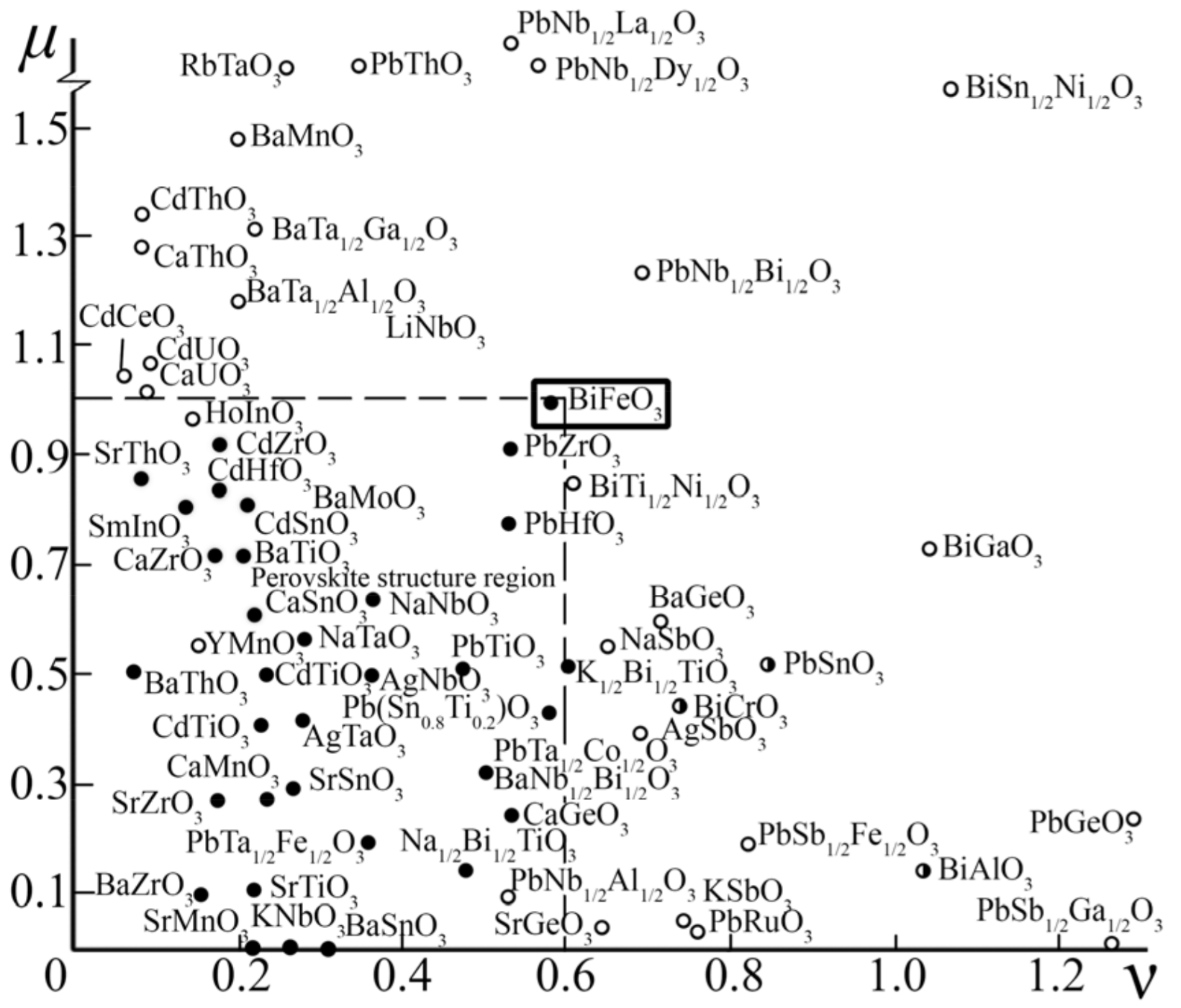
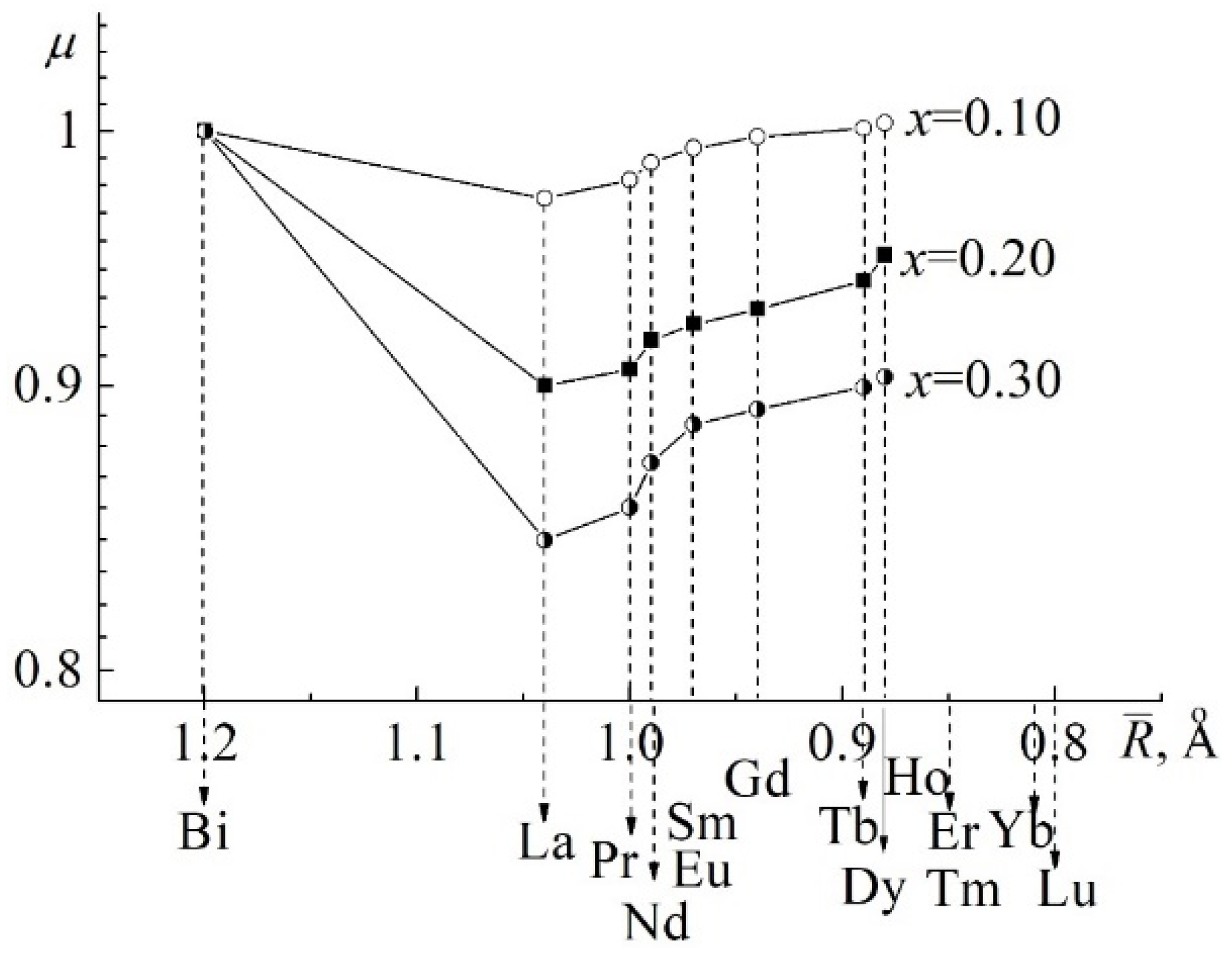
3.1. About the Causes of the High Conductivity of BiFeO3
3.2. About Ways to Minimize the Conductivity of BiFeO3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.F.; Liu, J.-M.; Ren, Z.F. Multiferroicity: The coupling between magnetic and polarization orders. Adv. Phys. 2009, 58, 321–448. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nat. Cell Biol. 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Seidel, J.; Maksymovych, P.; Batra, Y.; Katan, A.; Yang, S.Y.; He, Q.; Baddorf, A.P.; Kalinin, S.V.; Yang, C.-H.; Yang, J.-C.; et al. Domain Wall Conductivity in La-DopedBiFeO3. Phys. Rev. Lett. 2010, 105, 197603. [Google Scholar] [CrossRef] [PubMed]
- Rojac, T.; Bencan, A.; Malic, B.; Tutuncu, G.; Jones, J.L.; Daniels, J.E.; Damjanovic, D. BiFeO3 Ceramics: Processing, Electrical, and Electromechanical Properties. J. Am. Ceram. Soc. 2014, 97, 1993–2011. [Google Scholar] [CrossRef]
- Karpinsky, D.; Shilyaeva, Y.I.; Trukhanov, S.V.; Trukhanov, A.; Zhaludkevich, A.L.; Latushka, S.; Zhaludkevich, D.V.; Khomchenko, V.; Alikin, D.; Abramov, A.; et al. Peculiarities of the Crystal Structure Evolution of BiFeO3–BaTiO3 Ceramics across Structural Phase Transitions. Nanomaterials 2020, 10, 801. [Google Scholar] [CrossRef]
- Maître, A.; François, M.; Gachon, J.C. Experimental study of the Bi2O3-Fe2O3 pseudo-binary system. J. Phase Equilibria Diffus. 2004, 25, 59–67. [Google Scholar] [CrossRef]
- Carvalho, T.; Tavares, P.B. Synthesis and thermodynamic stability of multiferroic BiFeO3. Mater. Lett. 2008, 62, 3984–3986. [Google Scholar] [CrossRef]
- Phapale, S.; Mishra, R.; Das, D. Standard enthalpy of formation and heat capacity of compounds in the pseudo-binary Bi2O3–Fe2O3 system. J. Nucl. Mater. 2008, 373, 137–141. [Google Scholar] [CrossRef]
- Shilkina, L.A.; Verbenko, I.A.; Abubakarov, A.G.; Reznichenko, L.A.; Razumovskaya, O.N.; Sorokun, T.N.; Aleshin, V.A. Features of phase formation in the preparation of bismuth ferrite. In Advanced Materials; Chang, S.-H., Parinov, I.A., Topolov, V.Y., Eds.; Springer Proceedings in Physics: Cham, Switzerland, 2016; Volume 175, pp. 79–86. [Google Scholar] [CrossRef]
- Andryushin, K. Technological regimes for the preparing of solid solutions Bi1−xREExFeO3 (REE—La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). Mendeley Data 2021, V1. [Google Scholar] [CrossRef]
- Andryushin, K. Densities of the solid solutions Bi1−xREExFeO3 (REE—La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). Mendeley Data 2021, V1. [Google Scholar] [CrossRef]
- Garnett, J.C.M. Colours in metal glasses and in metallic films. Philoso. Trans. R. Soc. A 1904, 203, 385–420. [Google Scholar] [CrossRef]
- Turik, A.V.; Pavlenko, A.V.; Reznichenko, L.A. Maxwell–Wagner relaxation and magnetodielectric properties of Bi0.5La0.5MnO3 ceramics. Phys. Solid State 2016, 58, 1549–1551. [Google Scholar] [CrossRef]
- Turik, A.V.; Radchenko, G.S. Maxwell-wagner relaxation of elastic constants of layered polar dielectrics. Phys. Solid State 2003, 45, 1060–1064. [Google Scholar] [CrossRef]
- Selbach, S.M.; Einarsrud, M.-A.; Grande, T. On the Thermodynamic Stability of BiFeO3. Chem. Mater. 2009, 21, 169–173. [Google Scholar] [CrossRef]
- Fedulov, S.A.; Venevtsev, Y.N.; Zhdanov, G.S.; Smazhevskaya, E.G. Vysokotemperaturnyye rentgenovskiye i termograficheskiye issledovaniya ferrita vismuta. [English translation: High-temperature X-ray and thermographic studies of bismuth ferrite. Crystallography]. Kristallografiya 1961, 6, 795–796. (In Russian) [Google Scholar]
- Mukherjee, J.L.; Wang, F.F.Y. Kinetics of Solid-State Reaction of Bi2O3 and Fe2O3. J. Am. Ceram. Soc. 1971, 54, 31–34. [Google Scholar] [CrossRef]
- Achenbach, G.D.; James, W.J.; Gerson, R. Preparation of Single-Phase Polycrystalline BiFeO3. J. Am. Ceram. Soc. 1967, 50, 437. [Google Scholar] [CrossRef]
- Valant, M.; Axelsson, A.A.-K.; Alford, N. Peculiarities of a Solid-State Synthesis of Multiferroic Polycrystalline BiFeO3. Chem. Mater. 2007, 19, 5431–5436. [Google Scholar] [CrossRef]
- Shamir, N.; Gurewitz, E.; Shaked, H. The magnetic structure of Bi2Fe4O9—Analysis of neutron diffraction measurements. Acta Crystallogr. 1978, 34, 662–666. [Google Scholar] [CrossRef]
- Pavelko, A.A.; Khasbulatov, S.V.; Reznichenko, L.A.; Shilkina, L.; Gadjiev, G.G.; Bakmaev, A.; Omarov, Z.; Verbenko, I.; Alyoshin, V.; Parinov, I.A.; et al. Features of the Formation of the Crystal Structure, Grain Structure, Dielectric and Thermophysical Properties of Bismuth Ferrite Doped with Erbium. Appl. Sci. 2018, 8, 2183. [Google Scholar] [CrossRef]
- Verbenko, I.A.; Gufan, Y.M.; Kubrin, S.P.; Amirov, A.A.; Pavelko, A.; Aleshin, V.A.; Shilkina, L.A.; Razumovskaya, O.N.; Reznichenko, L.A.; Osipenko, I.A.; et al. The crystal and grain structure and physical properties of Bi1−xAxFeO3 (A = La, Nd) solid solutions. Bull. Russ. Acad. Sci. Phys. 2010, 74, 1141–1143. [Google Scholar] [CrossRef]
- Andryushin, K.P.; Pavelko, A.; Verbenko, I.A.; Razumovskaya, O.N.; Shilkina, L.A.; Aleshin, V.A.; Reznichenko, L.A. Thermal stability and electrical conductivity of multiferroics BiFeO3/REEs. Bull. Russ. Acad. Sci. Phys. 2011, 75, 1082–1084. [Google Scholar] [CrossRef]
- Khasbulatov, S.V.; Pavelko, A.A.; Shilkina, L.A.; Reznichenko, L.A.; Gadjiev, G.G.; Bakmaev, A.G.; Magomedov, M.-R.M.; Omarov, Z.M.; Aleshin, V.A. Phase composition, microstructure, and thermophysical and dielectric properties of multiferroic Bi1−xDyxFeO3. Thermophys. Aeromech. 2016, 23, 445–450. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics; John Wiley and Sons: New York, NY, USA, 1953; 396p. [Google Scholar]
- Koops, C.G. On the Dispersion of Resistivity and Dielectric Constant of Some Semiconductors at Audiofrequencies. Phys. Rev. 1951, 83, 121–124. [Google Scholar] [CrossRef]
- Von Hippel, A.R.; Morgan, S.O. Dielectrics and Waves. J. Electrochem. Soc. 1955, 102, 68C. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.-G.; Yin, W.-G.; Mei, W.N.; Smith, R.W.; Hardy, J.R. Large dielectric constant and Maxwell-Wagner relaxation inBi2∕3Cu3Ti4O12. Phys. Rev. B 2004, 70, 144106. [Google Scholar] [CrossRef]
- Lemanov, V.V.; Sotnikov, A.V.; Smirnova, E.P.; Weihnacht, M. Giant dielectric relaxation in SrTiO3-SrMg1/3Nb2/3O3 and SrTiO3-SrSc1/2Ta1/2O3 solid solutions. Phys. Solid State 2002, 44, 2039–2049. [Google Scholar] [CrossRef]
- Tuncer, E.; Nettelblad, B.; Gubañski, S.M. Non-Debye dielectric relaxation in binary dielectric mixtures (50-50): Randomness and regularity in mixture topology. J. Appl. Phys. 2002, 92, 4612–4624. [Google Scholar] [CrossRef]
- Tuncer, E.; Gubański, S.M.; Nettelblad, B. Dielectric relaxation in dielectric mixtures: Application of the finite element method and its comparison with dielectric mixture formulas. J. Appl. Phys. 2001, 89, 8092–8100. [Google Scholar] [CrossRef]
- Tuncer, E.; Serdyuk, Y.V.; Gubanski, S.M. Comparing dielectric properties of binary composite structures obtained with different calculation tools and methods. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Kitchener, ON, Canada, 14–17 October 2001; pp. 665–668. [Google Scholar] [CrossRef]
- Tuncer, E.; Gubański, S.M.; Nettelblad, B. Electrical properties of 4 × 4 binary dielectric mixtures. J. Electrost. 2002, 56, 449–463. [Google Scholar] [CrossRef]
- Bogatin, A.S.; Turik, A.V. Protsessy Relaksatsionnoy Polyarizatsii v Dielektrikakh s Bol’shoy Skvoznoy Provodimost’yu; [English translation: Relaxation Polarization Processes in Dielectrics with High through Conduction]; Feniks: Rostov-on-Don, Russia, 2013; 256p. (In Russian) [Google Scholar]
- Emets, Y.P. Dispersion of permittivity of two-component media. J. Exp. Theor. Phys. 2002, 94, 1149–1159. [Google Scholar] [CrossRef]
- Turik, A.V.; Radchenko, G.S.; Chernobabov, A.I.; Turik, S.A.; Suprunov, V.V. Dielectric spectra of disordered ferroelectric systems: Polycrystals and composites. Phys. Solid State 2006, 48, 1157–1159. [Google Scholar] [CrossRef]
- Haumont, R.; Kornev, I.A.; Lisenkov, S.; Bellaiche, L.; Kreisel, J.; Dkhil, B. Phase stability and structural temperature dependence in powdered multiferroic BiFeO3. Phys. Rev. B 2008, 78, 134108. [Google Scholar] [CrossRef]
- Abubakarov, A.G.; Shilkina, L.A.; Verbenko, I.A.; Reznichenko, L.A.; Dudkina, S.I. Effect of nonstoichiometry on the structure and dielectric properties of bismuth ferrite. Bull. Russ. Acad. Sci. Phys. 2014, 78, 713–715. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, J.; Banks, E. New Directions in Solid State Chemistry: Structures, Synthesis, Properties, Reactivity and Materials Design. Phys. Today 1988, 41, 112. [Google Scholar] [CrossRef]
- Reznichenko, L.A.; Shilkina, L.A.; Gagarina, E.S.; Yuzyuk, Y.I.; Razumovskaya, O.N.; Kozinkin, A.V. Crystallographic shear in niobium oxides of different compositions. Crystallogr. Rep. 2004, 49, 820–827. [Google Scholar] [CrossRef]
- Kravchenko, O.Y.; Gadzhiev, G.G.; Omarov, Z.; Reznichenko, L.A.; Abdullaev, K.K.; Razumovskaya, O.N.; Shilkina, L.A.; Komarov, V.D.; Verbenko, I.A. Phase composition, microstructure, and properties of Na1−yNbO3−y/2 ceramics. Inorg. Mater. 2011, 47, 679–685. [Google Scholar] [CrossRef]
- Kravchenko, O.Y.; Gadzhiev, G.G.; Omarov, Z.; Reznichenko, L.A.; Abdullaev, K.K.; Razumovskaya, O.N.; Shilkina, L.A.; Komarov, V.D.; Verbenko, I.A. Phase transformations and properties of Ag1−yNbO3−y/2 (0 ≤ y ≤ 0.20) ceramics. Inorg. Mater. 2011, 47, 919–925. [Google Scholar] [CrossRef]
- Bokiy, G.B. Vvedeniye v kristallokhimiyu; [English translation: Introduction to Crystal Chemistry]; Moscow Government University Press: Moscow, Russia, 1954. (In Russian) [Google Scholar]
- Letwein, F.; Zomer-Kulachevsky, S. Kristallografiya; [English translation: Crystallography]; Higher School: Moscow, Russia, 1968; 378p. (In Russian) [Google Scholar]
- Reznichenko, L.A.; Shilkina, L.A.; Razumovskaya, O.N.; Dudkina, S.I.; Gagarina, E.S.; Borodin, A.V. Dielectric and Piezoelectric Properties of NaNbO3-Based Solid Solutions. Inorg. Mater. 2003, 39, 139–151. [Google Scholar] [CrossRef]
- Fesenko, E.G. Semejstvo Perovskita i Segnetoehlektrichestvo; [English translation: The Perovskite Family and Ferroelectricity]; Atomizdat: Moscow, Russia, 1972; 248p. (In Russian) [Google Scholar]
- Kartavtseva, M.S.; Gorbenko, O.Y.; Kaul’, A.R.; Savinov, S.A. Investigation of BiFeO3 Multiferroic thin films prepared by metalloorganic chemical vapor deposition. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2008, 2, 1–11. [Google Scholar] [CrossRef]
- Shuvaev, A.T. Issledovaniye soyedineniy so strukturoy perovskita po rentgenovskim spektram. [English translation: Investigation of compounds with a perovskite structure using X-ray spectra]. Proc. USSR Acad. Sci. Phys. Ser. 1959, 23, 569–572. [Google Scholar]
- Fesenko, E.G.; Shuvaev, A.T. Usloviya sushchestvovaniya struktury tipa perovskita i segnetoelektricheskogo sostoyaniya s uchetom khraktera khimicheskoy svyazi. [English translation: Conditions for the existence of a perovskite-type structure and a ferroelectric state, taking into account the nature of the chemical bond]. Proc. USSR Acad. Sci. Phys. Ser. 1969, 33, 1133–1138. (In Russian) [Google Scholar]
- Speranskaya, E.I.; Skorikov, V.M.; Rode, E.Y.; Terekhova, V.A. The phase diagram of the system bismuth oxide-ferric oxide. Russ. Chem. Bull. 1965, 14, 873–874. [Google Scholar] [CrossRef]
- Miller, A.; Gusev, A.A.; Verbenko, I.A.; Shilkina, L.A.; Reznichenko, L.A. Properties of mechanically activated bismuth ferrite. Bull. Russ. Acad. Sci. Phys. 2012, 76, 798–800. [Google Scholar] [CrossRef]
- Palai, R.; Katiyar, R.S.; Schmid, H.; Tissot, P.; Clark, S.J.; Robertson, J.; Redfern, S.A.T.; Catalan, G.; Scott, J.F. βphase andγ−βmetal-insulator transition in multiferroic BiFeO3. Phys. Rev. B 2008, 77, 014110. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, K.; Varma, G. Large magnetization and weak polarization in sol–gel derived BiFeO3 ceramics. Mater. Lett. 2008, 62, 1159–1161. [Google Scholar] [CrossRef]
- Mazumder, R.; Chakravarty, D.; Bhattacharya, D.; Sen, A. Spark plasma sintering of BiFeO3. Mater. Res. Bull. 2009, 44, 555–559. [Google Scholar] [CrossRef]
- Xu, J.-H.; Ke, H.; Jia, D.; Wang, W.; Zhou, Y. Low-temperature synthesis of BiFeO3 nanopowders via a sol–gel method. J. Alloys Compd. 2009, 472, 473–477. [Google Scholar] [CrossRef]
- Farhadi, S.; Zaidi, M. Bismuth ferrite (BiFeO3) nanopowder prepared by sucrose-assisted combustion method: A novel and reusable heterogeneous catalyst for acetylation of amines, alcohols and phenols under solvent-free conditions. J. Mol. Catal. A Chem. 2009, 299, 18–25. [Google Scholar] [CrossRef]
- He, X.; Gao, L. Synthesis of pure phase BiFeO3 powders in molten alkali metal nitrates. Ceram. Int. 2009, 35, 975–978. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.; Zhang, M.; Chen, X.; Liu, J.-M.; Liu, Z.G. Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl. Phys. Lett. 2004, 84, 1731–1733. [Google Scholar] [CrossRef]
- Murashov, V.A.; Rakov, D.N.; Economov, N.A.; Zvezdin, A.K.; Dubenko, I.S. Quadratic magnetoelectric effect in single crystals (Bi, La) FeO3. Solid State Phys. 1990, 32, 2156–2159. [Google Scholar]
- Uniyal, P.; Yadav, K. Study of dielectric, magnetic and ferroelectric properties in Bi1−xGdxFeO3. Mater. Lett. 2008, 62, 2858–2861. [Google Scholar] [CrossRef]
- Nalwa, K.; Garg, A.; Upadhyaya, A. Effect of samarium doping on the properties of solid-state synthesized multiferroic bismuth ferrite. Mater. Lett. 2008, 62, 878–881. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Wang, H.; Ding, D.; He, Y. Synthesis and weak ferromagnetism of Dy-doped BiFeO3 powders. Mater. Lett. 2009, 63, 855–857. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Geochemistry; Muir, A., Ed.; Clarendon Press: Oxford, UK, 1954; 730p. [Google Scholar]
- Sakhnenko, V.P.; Dergunova, N.V.; Reznichenko, L.A. Energeticheskaya Kristallokhimiya Tverdykh Rastvorov Soyedineniy Kislorodno-Oktaedricheskogo Tipa i Modelirovaniye P’yezokeramicheskikh Materialov.; [English translation: Energy crystal chemistry of solid solutions of compounds of the oxygen-octahedral type and modeling of piezoceramic materials]; Publisher of Rostov State Pedagogical University: Rostov-on-Don, Russia, 1999; 321p. (In Russian) [Google Scholar]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Eur. Phys. J. A 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Sakhnenko, V.P.; Fesenko, E.G.; Shuvaev, A.T.; Shuvaeva, E.T.; Geguzina, G.A. Mezhatomnyye rasstoyaniya v okislakh so strukturoy tipa perovskita. [English translation: The interatomic bond distances calculation in the complex oxides with perovskite structure]. Crystallography 1972, 17, 316–322. (In Russian) [Google Scholar]
- Dergunova, N.V.; Sakhnenko, V.P.; Fesenko, E.G. Raschet parametrov kristallicheskoy reshetki tverdykh rastvorov okislov so strukturoy perovskita. [English translation: Calculation of the Lattice Parameters of Oxide Solid Solutions with the Perovskite Structure]. Crystallography 1978, 23, 94–98. (In Russian) [Google Scholar]
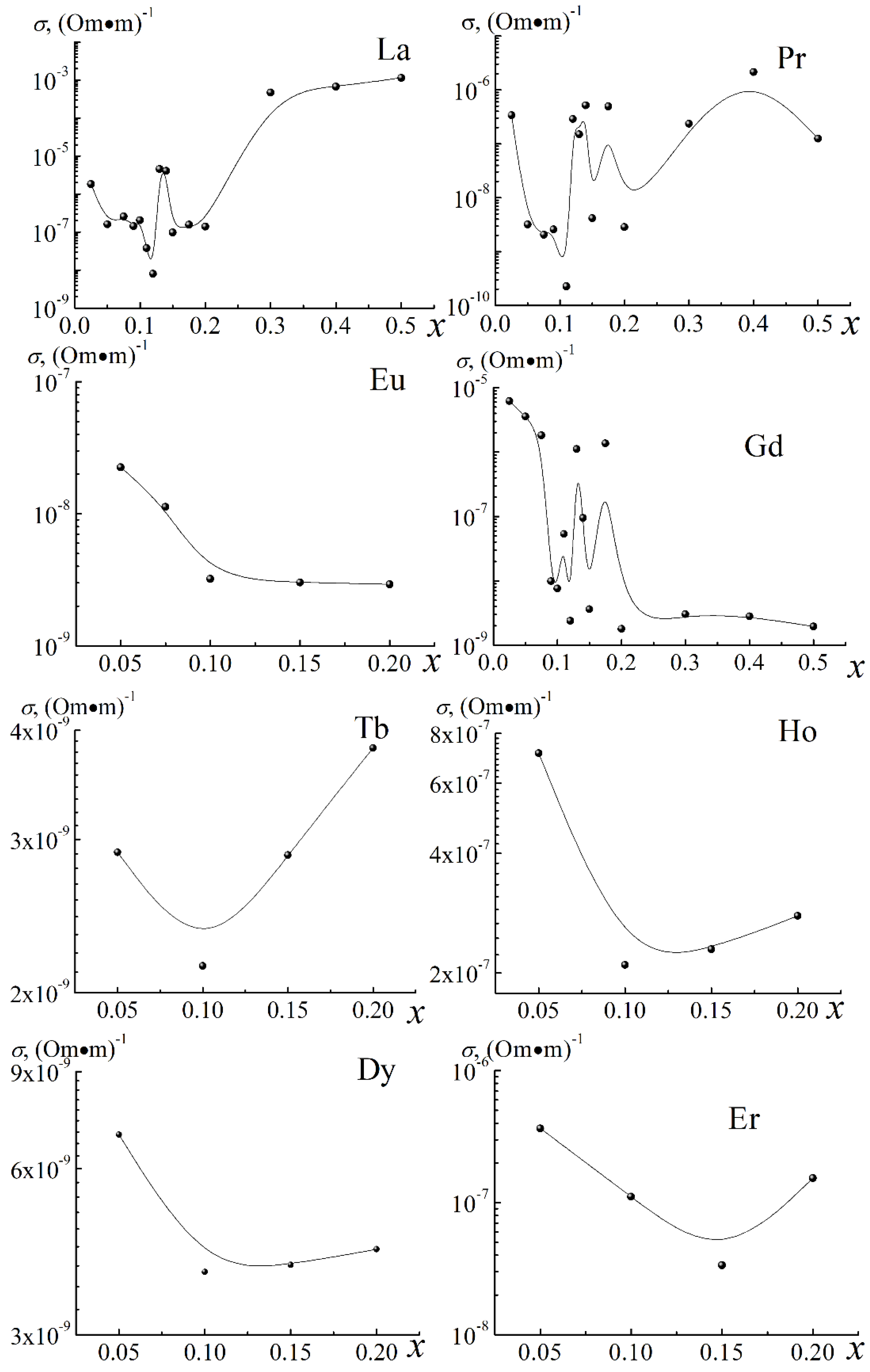
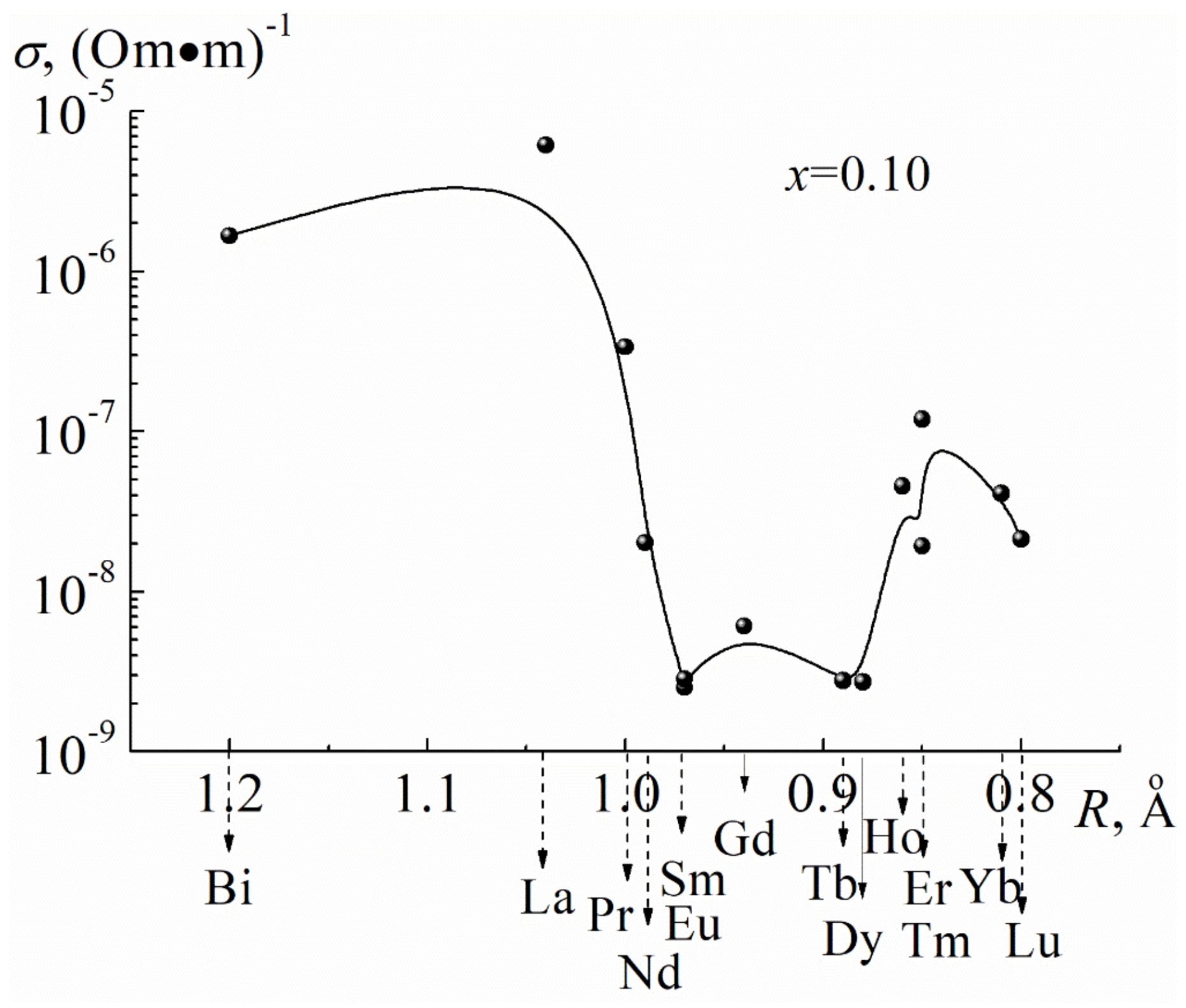
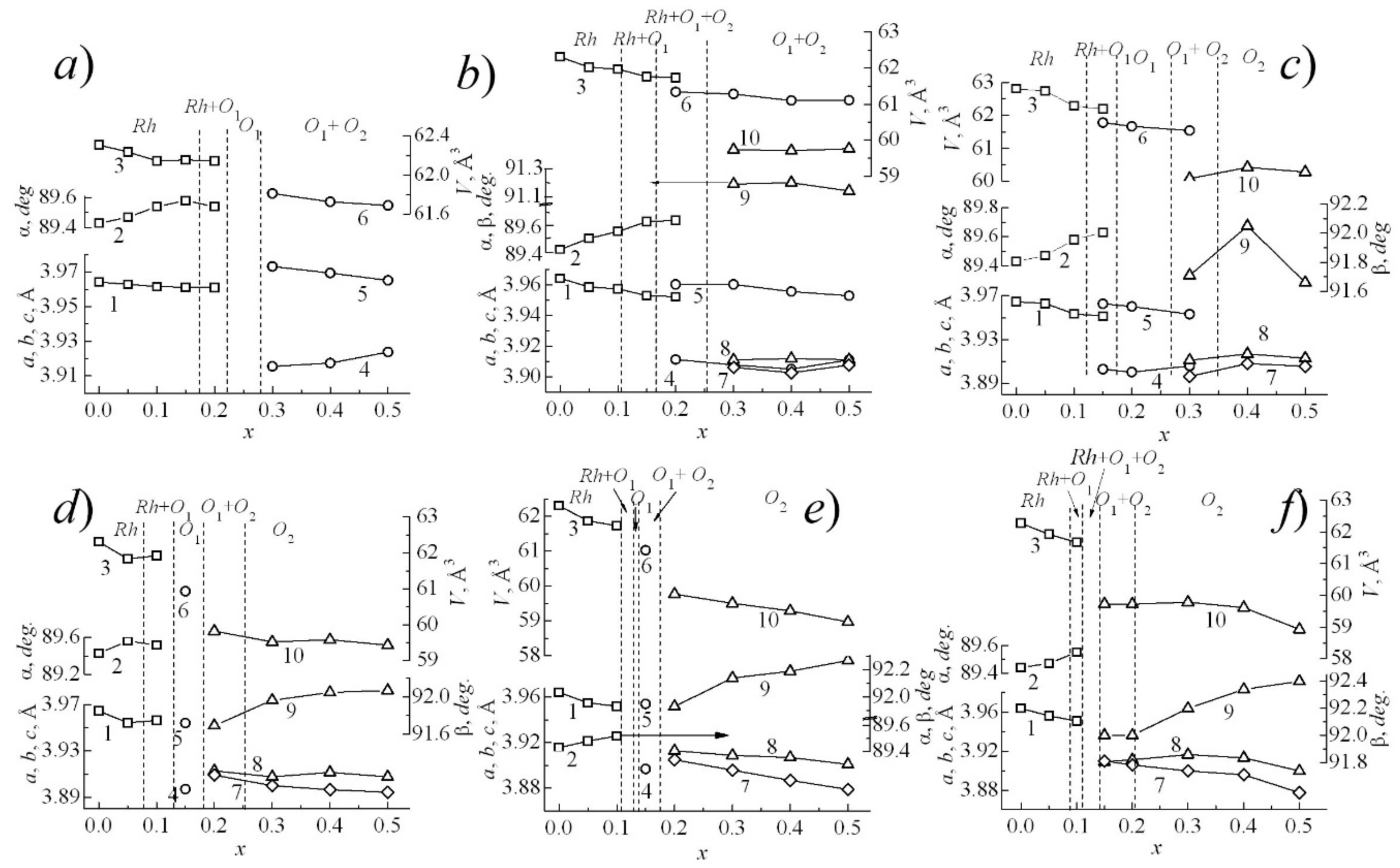
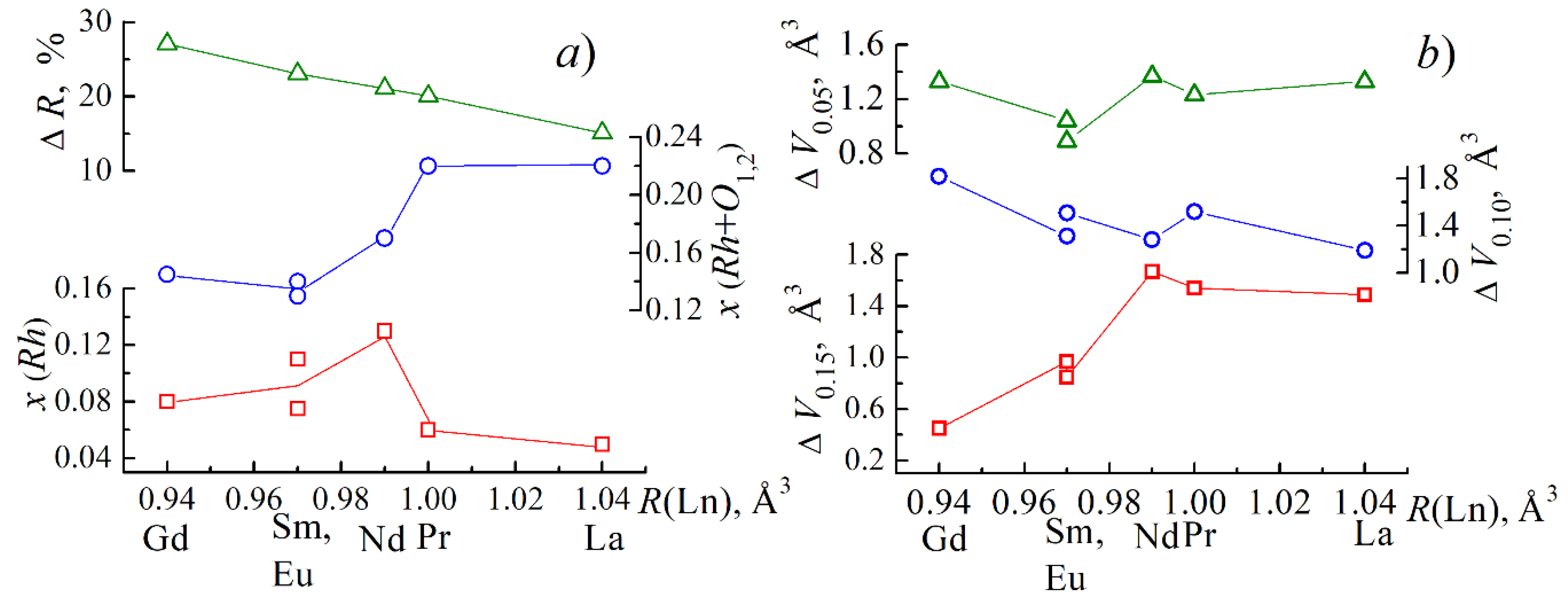
| A-Element | R, Å | μ, rel. Units | LA-O, Å | a, Å | ∆A-O, Å | ∆A-O/LA-O, rel. Units | Electronic Configurations of Outer Layers |
|---|---|---|---|---|---|---|---|
| Bi | 1.20 | 1.04 | 2.73 | 3.95 | 0.070 | 0.030 | (S2)p3 |
| La | 1.04 | 0.36 | 2.69 | 3.93 | 0.110 | 0.040 | (f0)ds2 |
| Pr | 1.00 | 0.39 | 0.65 | 3.90 | 0.116 | 0.044 | (f2)ds2 |
| Nd | 0.99 | 0.40 | 0.63 | 3.88 | 0.120 | 0.045 | (f3)ds2 |
| Sm | 0.97 | 0.44 | 2.62 | 3.88 | 0.130 | 0.050 | (f5)ds2 |
| Eu | 0.97 | 0.47 | 2.60 | 3.86 | 0.140 | 0.054 | (f6)ds2 |
| Gd | 0.94 | 0.50 | 2.59 | 3.86 | 0.148 | 0.057 | (f7)ds2 |
| Ho | 0.86 | 0.52 | 2.55 | 3.83 | 0.150 | 0.059 | (f10)ds2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andryushin, K.P.; Sakhnenko, V.P.; Turik, A.V.; Shilkina, L.A.; Pavelko, A.A.; Dudkina, S.I.; Rudskaya, A.G.; Rudskiy, D.D.; Verbenko, I.A.; Hasbulatov, S.V.; et al. Reasons for the High Electrical Conductivity of Bismuth Ferrite and Ways to Minimize It. Appl. Sci. 2021, 11, 1025. https://doi.org/10.3390/app11031025
Andryushin KP, Sakhnenko VP, Turik AV, Shilkina LA, Pavelko AA, Dudkina SI, Rudskaya AG, Rudskiy DD, Verbenko IA, Hasbulatov SV, et al. Reasons for the High Electrical Conductivity of Bismuth Ferrite and Ways to Minimize It. Applied Sciences. 2021; 11(3):1025. https://doi.org/10.3390/app11031025
Chicago/Turabian StyleAndryushin, Konstantin P., Vladimir P. Sakhnenko, Anatoliy V. Turik, Lidiya A. Shilkina, Alexey A. Pavelko, Svetlana I. Dudkina, Angela G. Rudskaya, Daniil D. Rudskiy, Iliya A. Verbenko, Sidek V. Hasbulatov, and et al. 2021. "Reasons for the High Electrical Conductivity of Bismuth Ferrite and Ways to Minimize It" Applied Sciences 11, no. 3: 1025. https://doi.org/10.3390/app11031025
APA StyleAndryushin, K. P., Sakhnenko, V. P., Turik, A. V., Shilkina, L. A., Pavelko, A. A., Dudkina, S. I., Rudskaya, A. G., Rudskiy, D. D., Verbenko, I. A., Hasbulatov, S. V., Reznichenko, L. A., Parinov, I. A., Chang, S.-H., & Wang, H.-Y. (2021). Reasons for the High Electrical Conductivity of Bismuth Ferrite and Ways to Minimize It. Applied Sciences, 11(3), 1025. https://doi.org/10.3390/app11031025









