Abstract
In the past two decades, many studies reported the efficacy of upper limb robotic rehabilitation in patients after stroke, also in its chronic phase. Among the possible advantages of robotic therapy over conventional therapy are the objective measurements of kinematic and kinetic parameters during therapy, such as the spatial volume covered by the patient’s upper limb and the weight support provided by the robot. However, the clinical meaning and the usability of this information is still questioned. Forty patients with chronic stroke were enrolled in this study and assessed at the beginning of upper limb robotic therapy (Armeo® Power) and after two weeks (ten sessions) of therapy by recording the working volume and weight support provided by the robot and by administering six clinical scales to assess upper limb mobility, strength, spasticity, pain, neurological deficits, and independency. At baseline, the working volume significantly correlated with spasticity, whereas weight support significantly correlated with upper limb strength, pain, spasticity, and neurological deficits. After two weeks of robotic rehabilitation, all the clinical scores as well as the two parameters improved. However, the percentage changes in the working volume and weight support did not significantly correlate with any of the changes in clinical scores. These results suggest caution in using the robotic parameters as outcome measures because they could follow the general improvement of the patient, but complex relationships with clinical features are possible. Robotic parameters should be analyzed in combination with the clinical scores or other objective measures because they may be informative about therapy progression, and there is a need to combine their clinical, neuroscientific, and biomechanical results to avoid misleading interpretations.
1. Introduction
In the past two decades, the numbers and types of commercial robots used in rehabilitation have increased [1]. Robot-assisted rehabilitation may help to improve arm functions, arm muscle strength and activities of daily living. However, the results depend on intensity, duration, amount of training, type of treatment, type of device and patient characteristics [2].
Robots for neurorehabilitation should be divided into exoskeleton and end-effectors, with different devices providing different sets of rehabilitation exercises that might involve different neural mechanisms of plasticity-dependent recovery [1]. There are devices providing passive movements of the patient’s arm [3], assisting voluntary arm movements or providing resistance during training [4], and assisting active movements of an isolated joint, while other devices move multiple segments [5]. The progression of therapy with robotic devices has been continuously adapted by varying the guidance force, decreasing assistance, increasing resistance, expanding the movement amplitude, and increasing the quantity of volitional movement [2].
Another theoretical advantage of robots is the possibility they provide to measure kinematic (related to the description of movements) and kinetic (related to the forces and torques causing the movements) parameters associated with arm movements during therapy, a feature stated as key for a continuous objective assessment of motor functions [6]. For some of these parameters, the therapists selected the limits within which the patient and robot will cooperate, such as the maximum working volume of reachable space or the maximum weight support that the robot can provide [1,7]. In manual rehabilitation, the therapist could provide a semiquantitative evaluation of the patient’s participation at his/her own rehabilitation [8], but the robot can quantify the physical parameters, obtaining a more objective assessment. In fact, most robotic systems allow therapists to calibrate the working space according to the patient’s mobility, providing a quantitative assessment of specific movement parameters such as resistance, strength, range of motion and coordination, allowing proper adjustments of the level of difficulty for each patient during the entire recovery process [9]. In the field of robotic-assisted rehabilitation, parallel mechanisms guide the anatomic limb and the exoskeleton, two interconnected kinematic chains [10]. Robots not only could measure upper limb joint ranges of motion (ROM) but could also combine them to evaluate the volume of the working space, both in passive and active conditions. Although volume could be more interesting during active movements, the exploration of the space by the patient could be affected not only by motor deficits, but also by cognitive and sensory ones. For this reason, the working volume is often passively assessed under the guidance of a robot or therapist and used during therapy as a reference parameter [11,12,13]. Another important assistive aspect of robotic therapy is the weight support, usually implemented using powered or spring-based exoskeletons [9]. These last two parameters (working volume and weight support) could strictly be intertwined during the execution of tasks: increasing the amount of weight compensation has been shown to increase range of motion and decrease muscle activation, decoupling flexor synergies after stroke [9,14]. Both used during therapy as reference values, these parameters could theoretically provide important information about the progression of therapy, as proposed for the step length and body weight support in lower limb robotic rehabilitation [15].
However, the definition of working volume may depend on several factors. From a biomechanical point of view, the working volume depends on the joint ROMs. From a bioengineering point of view, the ROMs of the exoskeleton joints could define the limits of human movement. From a clinical point of view, the patient joint’s passive ROMs could depend on the presence of contractures, spasticity, or pain. These clinical features are also taken into account from a therapeutic point of view when the therapist sets the working volume, moving the upper limb according to the patient’s residual mobility. The working volume and weight support are set by the therapist during the initial calibration of the system and being strictly related to the clinical status of the patient, could be useful to quantify the deficits, as for passive ranges of motion [11,13] and the body weight support of lower limb in robotic gait rehabilitation [12].
Despite the importance of working volume, related ROMs and weight support, an analysis of the relationship between these values and the clinical status of the patients is still lacking. In fact, little attention has been given to evaluate the vast variety of kinematic parameters used in current robot-assisted rehabilitation research, particularly to the suitability of the above parameters to significantly capture the condition and the changes in the patient’s upper limb functions [16]. Although these few parameters (volume, ROMs, and weight support) are not sufficient to allow anyone to capture clinical status in stroke and effectively personalize interventions for patients, also in relation to the heterogeneity of stroke severity and manifestations, they are the only parameters that the therapist could set in the device for tailoring the robotic intervention.
The recent Italian Consensus Conference on Robotics in Neurorehabilitation claimed that in most studies, the information on the technical aspects of the device was not exhaustive for explaining the complexity of the performed movements and poor attention was deserved to the level of robotic assistance, types of the movements performed, and in general to the training modalities [17]. This consensus conference also reported that a lot of studies were focused on chronic stages of stroke, implicitly suggesting that neuroplasticity induced by robotic interventions may occur also in this phase [17]. On the other hand, studies about robotic training and conventional rehabilitation could be affected by the so-called “effectiveness paradox” [17,18,19]. This paradox highlights the discrepancy between the clinical approach, claiming that therapy should be personalized to be more effective, and the scientific approach which needs standardized therapeutic protocols to prove the efficacy of a treatment. Because robots for neurorehabilitation are equipped with sensors for warranting their adaptability (without which they should be classified as simple electromechanical devices), the use of the measured parameters could help obtain objective information about the progression of therapy and, hence, of the recovery of a patient’s abilities [19].
In this study, we aimed to analyze the interconnection between the robotic parameters (working volume, weight support), the clinical status of patients (assessed by different clinical scales) and the changes that occurred during rehabilitation in a wide sample of patients with chronic stroke treated with one of the most used and most advanced robotic devices for upper limb rehabilitation, the Armeo® Power. This study was focused on patients with chronic stroke because their clinical conditions are more stable than in the subacute phase, so the robotic intervention could be less influenced by possible effects of spontaneous recovery, rehabilitative and pharmacological interventions, clinical complications, and other factors more frequent in the subacute phase.
2. Materials and Methods
2.1. Participants
We analyzed data of 40 patients after stroke in the chronic phase (mean age: 60.3 ± 15.6 years; 19 women and 21 men; 34 ischemic and 6 hemorrhagic strokes; affected brain hemisphere: 21 right and 19 left; median time from acute event: 34 months, 1st quartile: 23 months, 3rd quartile: 99 months). Data referred to baseline pre-robotic rehabilitation (T1) and after 2 weeks (T2) during which upper limb robotic therapy was administered to patients five days a week, with one session per day of 40 min, plus 20 min for preparation. Inclusion criteria were single stroke confirmed by brain imaging (CT or MRI); chronic stroke (time from acute event > 6 months); ability to understand and the instructions given by doctors and therapists (Mini Mental State Evaluation MMSE ≥ 24); Modified Ashworth Scale score on each joint < 3; and Fugl-Meyer score ≥ 3 (so that the upper limb was not completely paralyzed). Exclusion criteria were chronic paretic limb deformities; absence of voluntary movements at proximal part of upper limb (shoulder and elbow); severe unilateral spatial neglect; epilepsy; previous neurosurgical interventions; severe upper limb osteoporosis; and upper limb strength or joint movement limitation due to previous fractures or surgical interventions.
All the patients signed an informed consent agreement to participate in this study, which was previously approved by the Independent Ethical Committee of our hospital.
2.2. Clinical Assessment
A set of six clinical scales were used to assess the patients by expert physicians and physiotherapists at the beginning (T1) and after 2 weeks (T2) of robotic therapy. Some of these scales were administered to assess the deficits of the paretic upper limb: Medical Research Council scale (MRC), assessing muscle strength in the paretic upper limb; the domain of Upper Extremity of Fugl-Meyer Assessment scale (FMA-UE) that measures the motor functioning of upper limb; the Visual Analogue Scale (VAS) for pain felt in the paretic upper limb; and the Modified Ashworth scale (MAS) used to measure spasticity in the three upper limb joints, reported as the sum of the three scores. Two other scales were administered to assess the general clinical status of the patients: the NIH Stroke Scale (NIH-SS) that provides a quantitative measure of stroke-related neurologic deficit, and the Barthel Index (BI) that measures the functional independence of a patient in the activities of daily living. Higher scale scores are associated with the worst deficits for NIH-SS, VAS and MAS, and lower deficits for FMA-UE, BI and MRC. As shown in Table 1, the enrolled sample could be considered as mildly affected, the baseline means of FMA-UE being 28 ± 15 and the Barthel Index mean being 82 ± 12.

Table 1.
Mean ± standard deviation (SD) of working volume and weight support (expressed in percentage of the upper limb weight) with respect to the clinical scale scores and their correlations (in terms of Spearman’s correlation coefficients R and the relevant p-values, in bold if statistically significant).
2.3. Robotic Therapy
Robotic therapy was administered using the commercial device Armeo® Power (Hocoma, Volketswil, Switzerland). This robot is an exoskeleton composed of an orthosis for the paretic upper limb with six degrees of freedom: three for the shoulder, one for the elbow flexion, one for the forearm supination and one for the hand closure. Each joint is powered by a motor and equipped with 2D sensors. The device can support the patient’s upper limb weight, providing a feeling of fluctuation. The selected support is related to the maximum force (in percentage of upper limb weight) that the robot should exert to assist the movements of patients. A support > 100% could also compensate for adjunctive forces directed downwards. The device also includes an interface used for the exergame, designed to obtain task-oriented gestures in a specifically developed video game environment with exergames specifically developed for rehabilitative purposes [20,21]. The computer–robot interface is designed to provide some exergames requiring specific upper limb movements consisting of single-joint movements around a single axis or combined movements of single joint around two or three axes, exercises for the opening and closing hand (grasping exercises) and multi-joint exercises along one, two or three spatial axes (coordination exercises). The training characteristics (difficulty level, duration, visual detail) were set conforming to the residual ability of the patient [21].
At T1 and T2, the data related to joint ROMs and the total working volume obtained with passive mobilization of the paretic upper limb performed by a therapist up to the articular limit in the three planes of the space were collected, together with the weight support selected for performing the proposed exergames and analyzed with Armeo® Control Software (Figure 1). According to previous studies [11,12,13,21,22], the assessment procedure was performed with the measure of the patient’s passive range of motion with the robotic device in a kinematic transparent mode, and the therapist moving the affected limb linked to the exoskeleton for exploring the entire reachable space to interact with the video game during therapy without experiencing resistance to the movement or elicit pain.
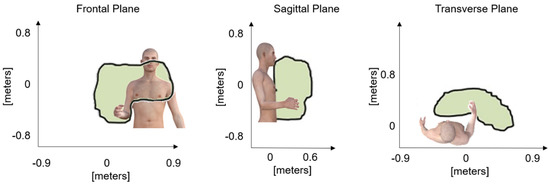
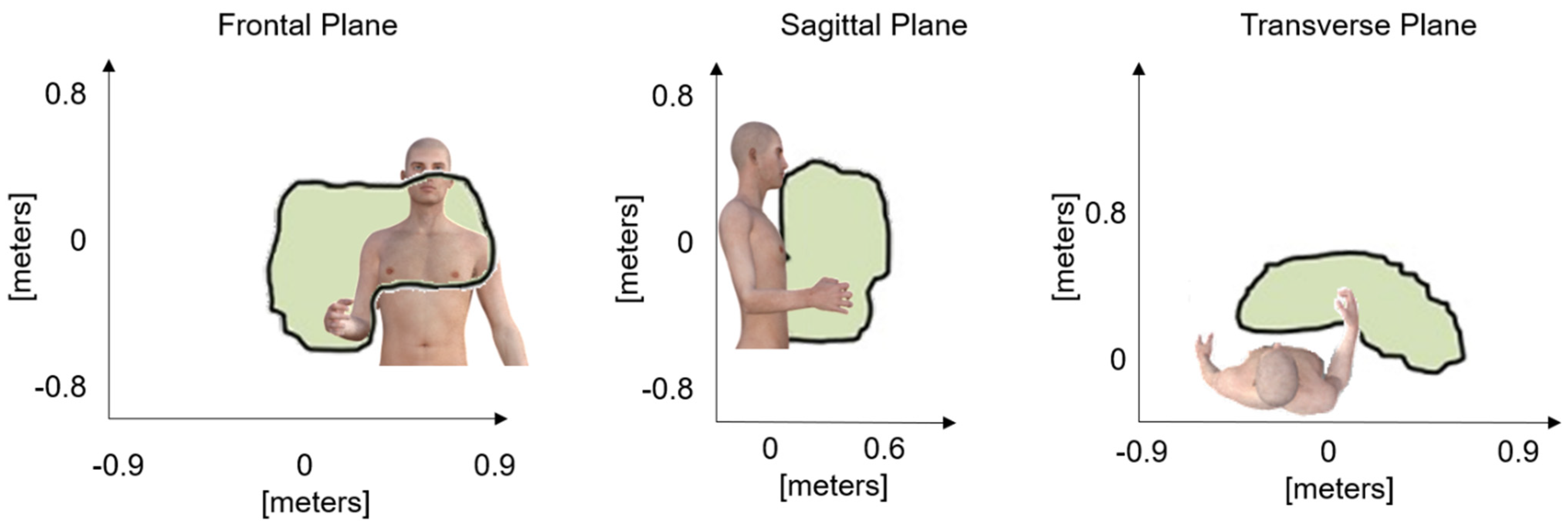
Figure 1.
Representation of the working areas on the anatomical planes that formed the working volume and define the space for interacting with the video game-based exercises during the therapy sessions.
2.4. Statistical Analysis
The sample size was computed setting an alpha level of 5% and a power of 80%. Data were reported in terms of mean ± standard deviation. Percentage change (Δ) was computed as the difference in the value recorded at T2 and at T1, divided by the value at T1 and multiplied by 100. The Shapiro–Wilk test was used to verify the normality of data and showed that most of the parameters were not normally distributed, so nonparametric statistics were performed for inferential tests, such as the Wilcoxon rank test used for pairwise comparison of data within subjects between T1 and T2, and Spearman’s coefficient (R) used to assess correlation between couples of variables. Data analysis was performed using Jamovi software version 1.6.23.
3. Results
Table 1 reports mean ± standard deviations of working volume, weight support, clinical scale scores and the relevant correlations at baseline (T1). Correlation was also tested with respect to the upper limb ranges of motion, finding that the working volume significantly correlated with the shoulder ab-adduction (R = 0.594, p < 0.001), flexo-extension (R = 0.361, p = 0.023), internal/external rotation ROMs (R = 0.364, p = 0.021) and the elbow flexo-extension ROM (R = 0.491, p = 0.001), but not with the forearm pronosupination (R = 0.166, p = 0.304) or arm aperture (R = −0.120, p = 0.458). Weight support was found significantly correlated only with shoulder ab-adduction (R = −0.365, p = 0.021) and internal/external rotation ROMs (R = −0.506, p < 0.001).
At T2, the working volume was increased, and the weight support was reduced, and all the clinical scale scores significantly improved in terms of deficits reduction. Table 2 shows the percentage changes between T1 and T2 for the parameters also reported in Table 1. The p-values refer to the pairwise comparison of the values between the two assessments. Despite the common trends related to a general amelioration, neither the percentage changes in working volume nor weight support significantly correlated with the changes in clinical scale scores.

Table 2.
Mean ± standard deviation (SD) of percentage changes in working volume and weight support with respect to the clinical scale scores and their correlations (in terms of Spearman’s correlation coefficients R and the relevant p-values). The column p-values report the pairwise comparison of the values between T1 and T2 with the relevant effect sizes. p-Values are in bold if statistically significant.
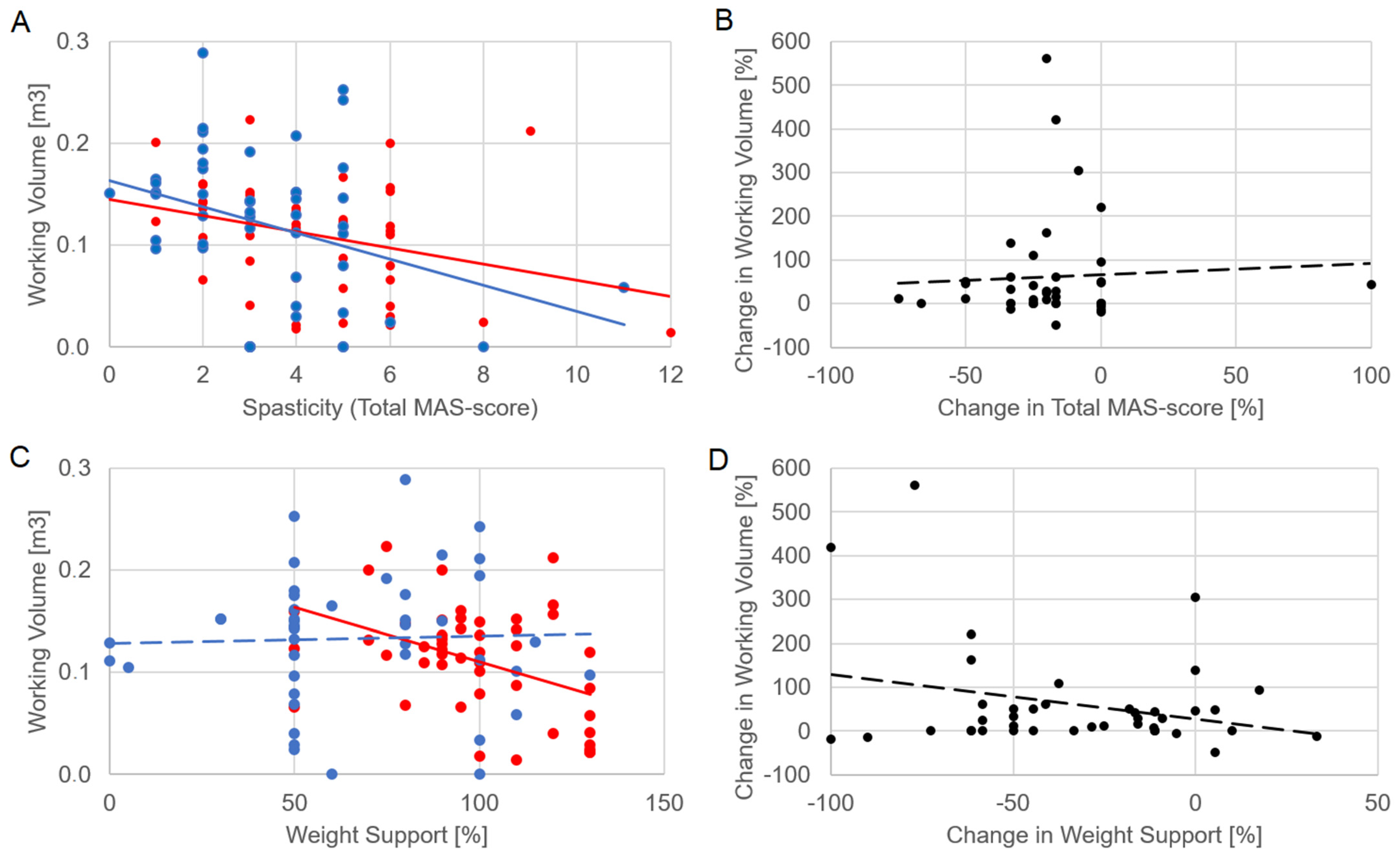
Figure 2 shows the significant relationships of the working volume with spasticity at T1 (R = −0.0395, p = 0.012) and T2 (R = −0.319, p = 0.04). Despite these correlations, the change in volume was not significantly correlated with the change in spasticity (R = −0.094, p = 0.564). The correlations between working volume and weight support were statistically significant at T1, as reported in Table 1, but neither at T2 (R = 0.118, p = 0.469) nor between their percentage changes (R = −0.046, p = 0.779).
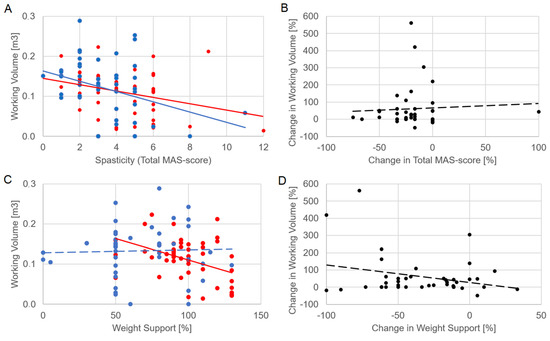
Figure 2.
The relationship of working volume with spasticity (panel (A), assessed as total MAS score computed on the three upper limb joints) and weight support (C). The relationships of the percentage change in working volume with percentage change in spasticity (B) and that of weight support (D). Each dot represents the performance of a patient: red dots, T1; blue dots, T2; black dots, percentage changes between T1 and T2. Lines represent linear fits and follow the colors of relevant dots (solid if statistically significant, dotted if not).
All the ranges of motion significantly increased from T1 to T2 (Table 3), but of angles ranging from 1.5° (for the shoulder flexo-extension) to 7° (for the shoulder ab-adduction), with small effect sizes. A statistically significant correlation related to improvements in ROM and in clinical scale score was found only for the percentage change in forearm prono-supination and that of the FMA-UE score (R = 0.455, p = 0.003).

Table 3.
Mean ± standard deviation (SD) of ranges of motion (ROM) at T0 and T1, compared by Wilcoxon test (p-values are reported with effect size).
4. Discussion
At baseline, the working volume significantly correlated only with the MAS total score, revealing that spasticity is the main factor that could reduce the volume of movements selected for working with the robots. Although this correlation could be expected because the volume was computed on passive ROMs, the fact that spasticity was the only factor affecting this volume was quite unexpected, as this parameter was neither influenced by pain nor by residual mobility. This result may support the purpose of administering specific treatments for spasticity before the beginning of robotic therapy. This approach was tested in a recent case report of a patient with chronic stroke treated with Armeo® Power after Botulinum (BoNT-A) injection [21]. The patient obtained a favorable outcome in terms of upper limb functional recovery [21]. In terms of kinematics, the working volume correlated with all the ranges of shoulder movement around the three anatomical axes, and with the flexo-extension of the elbow, as expected. From a kinetic point of view, the working volume was inversely correlated with the weight support at T1. However, our results showed an important difference between these two parameters: if the working volume was mainly related just to spasticity, the weight support correlated with MRS, VAS, MAS and NIH-SS scores, and a trend was observed even with the BI score, suggesting that this parameter was set by the therapist reflecting the entire clinical condition of the patients.
After two weeks of treatment, a general, statistically significant improvement was observed in the patients in terms of increment in working volume and upper limb functions, as well as of decrement in needed weight support, spasticity, pain, and neurological deficits. The intervention was only two weeks, with ten sessions of treatment—while intensive, it is a short period to provide a clear conclusion about the clinical stability of these results, and further studies are needed including follow-up observations for assessing the long-term efficacy of short, intensive robotic therapies. However, it is important to highlight that neuroplasticity can be elicited also in chronic stages of stroke by a short, intensive intervention based on robotic mobilization of the upper limb kinematic chain combined with exergames, providing proprioceptive and visual feedback. Despite these general improvements, the percentage changes in robotic parameters did not correlate with those of clinical scores. Only the improvements in forearm prono-supination were found correlated with the improvements in the FMA-UE score, but also the change in this ROM referred to few degrees. Two weeks of robotic rehabilitation likely induced some changes, but not enough and not for all the patients to show clear correlations among the clinical status of heterogeneous patients and very specific biomechanical parameters of the robot. It limited the possibility of using the parameters used by the robot to assess the rehabilitation outcome, despite providing interesting information about the adaptation that occurred during therapy.
The relationship between working volume and spasticity shown in Figure 2 is meaningful. The working volume was significantly correlated with total MAS score both at T1 and T2. Furthermore, a clinical improvement was observed for both parameters, with spasticity significantly reduced in T2 by about 20% despite a high variability among subjects, and working volume increased by about 45% on average. However, their percentage changes were not significantly correlated. These results highlight a clinical meaning of the working volume, but a not clear interpretability of its change, as well as for the weight support. On one hand, these results should be interpreted considering the limits having computed a total score of MAS for obtaining a general level of spasticity of the upper limb, and having not measured the speed of movements, a key factor of which could be spasticity, a velocity-dependent phenomenon [23]. On the other hand, a similar trend was observed for weight support also in relation with other clinical scores. In general, we choose generic clinical scales commonly used for describing the patients’ health status before and after the therapy, but more specific clinical scores could be used to link specific arm functions to the robotic parameters. Another limit of our study was that the patients followed personalized, and hence different, therapies over the two-week period. Despite the above limits, the results showed significant relationships between robot parameters and clinical scores, but these were more complex than expected, probably because they depend on many factors that are not mutually exclusive. The patients probably improved their abilities from a clinical point of view, but also in terms of the specific ability to manage the upper limb movements during robotic training and assessment. Thus, as previously stated [19], there is a need to consider the therapist within the patient–robot loop, defining three elements in this complex relationship. To solve the effectiveness paradox, personalization of therapy should be based on a unique, clear neuroscientific framework. It could also define the best set-up in terms of kinematic and kinetic robotic parameters for each clinical condition, for example, by defining when it is better to train the patient on a large volume with a high support, and when it is better to have a small volume with a lower support. However, although the possibility to set up the working volume, the single joint ROMs and the weight support allows the therapist to tailor the robotic therapy, these parameters seem too few to highly personalize the therapy with respect to the heterogeneity of stroke. Nonetheless, some improvements were observed, and it could also depend on the possibility given to the therapist to select the exergame that the patient should perform during the robotic therapy. Further studies should investigate the cognitive and motor aspects involved in these exergames, that probably introduced many more variables to adapt than the biomechanical robotic parameters alone. Future robots should also provide kinematic and kinetic information extracted by arm and hand trajectories and torques, respectively. The technology for embedding sensors with this scope in the robots already exist, such as wearable devices for motion capture [24] and for interacting with virtual reality [25].
Another interesting aspect is that, despite the robot allowing tridimensional movements, the tasks proposed during robotic rehabilitation are displayed on a 2D monitor. This monitor may limit the movements along antero-posterior axis, as shown in the example of Figure 1, flatting them mainly on the frontal plane. A possible solution is to combine robotic therapy with virtual reality [26,27], which is more immersive, interactive, and imaginative than video games confined to a bidimensional plane [28]. Interestingly, cognitive functions, and in particular, executive functions, are widely involved in technologically supported neurorehabilitation thanks to top-down stimulations [29,30] and could also benefit from the combination of robotic therapy with virtual reality [31].
In light of our findings and the above discussion, the clinical interpretation of working volume and weight support, despite being two fundamental parameters to set an effective therapy, need caution. They should be accompanied by validated outcome measures for assessing the efficacy of robotic treatments. It should be considered that the absence of correlation refers to linear correlation, and other types of more complex relationships are possible. For these reasons, some researchers likely proposed complex assessment protocols based on evaluating the ROM or the volume at different support conditions [14,30]. However, their application in clinical routine seems to be time-consuming and does not completely solve all the criticisms highlighted in our study. Further studies could investigate the relationship between robotic parameters and clinical conditions also in other pathologies, according to the observation that robotic-assisted therapy could be effective both in patients with stroke [32] and also in people with other chronical diseases [33].
5. Conclusions
The parameters set for robotic therapy are strictly intertwined and the effects of their combined selection could have an unclear impact on the efficacy of therapy. Although it is fundamental to monitor the progressive adaptation of these parameters following the improvements seen in the patient, the changes in these parameters do not univocally reflect the reduction of deficits. There is a need for stricter collaborations between clinicians, neuropsychologists, and bioengineers to develop transversal know-how about the clinical–biomechanical–neuroscientific interconnection between different robotic parameters and their effects on patients’ neuroplasticity. As claimed by the Italian Consensus Conference on Robotics in Neurorehabilitation, there is a limited understanding of the clinical relevance of robots’ main features and their rehabilitative contents, and thus, the neurological underpinnings of robot-assisted recovery are not entirely understood [17].
Author Contributions
Conceptualization, M.I. and G.M.; methodology, all authors; software, M.I. and A.M.C.; validation, G.M., F.C., A.C. and M.P.; formal analysis, M.I.; investigation, M.I.; resources, S.P. and V.D.L.; data curation, M.I.; writing—original draft preparation, M.I.; writing—review and editing, G.M., F.C., A.C. and M.P.; supervision, S.P. and V.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Local Independent Ethical Committee of the Santa Lucia Foundation.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morone, G.; Cocchi, I.; Paolucci, S.; Iosa, M. Robot-assisted therapy for arm recovery for stroke patients: State of the art and clinical implication. Expert Rev. Med. Devices 2020, 17, 223–233. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef]
- Fazekas, G.; Horvath, M.; Troznai, T.; Toth, A. Robot-mediated upper limb physiotherapy for patients with spastic hemiparesis: A preliminary study. J. Rehabil. Med. 2007, 39, 580–582. [Google Scholar] [CrossRef]
- Rabadi, M.; Galgano, M.; Lynch, D.; Akerman, M.; Lesser, M.; Volpe, B. A pilot study of activity based therapy in the arm motor recovery post stroke: A randomized controlled trial. Clin. Rehabil. 2008, 22, 1071–1082. [Google Scholar] [CrossRef]
- Wolf, S.L.; Sahu, K.; Bay, R.C.; Buchanan, S.; Reiss, A.; Linder, S.; Rosenfeldt, A.; Alberts, J. The HAAPI (Home Arm Assistance Progression Initiative) trial: A novel robotics delivery approach in stroke rehabilitation. Neurorehabil. Neural Repair 2015, 29, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Morone, G.; Fusco, A.; Bragoni, M.; Coiro, P.; Multari, M.; Venturiero, V.; De Angelis, D.; Pratesi, L.; Paolucci, S. Seven capital devices for the future of stroke rehabilitation. Stroke Res. Treat. 2012, 2012, 187965. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Grosmaire, A.G.; Krebs, H.I. Robot-assisted therapy in upper extremity hemiparesis: Overview of an evidence-based approach. Front. Neurol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Iosa, M.; Galeoto, G.; De Bartolo, D.; Russo, V.; Ruotolo, I.; Spitoni, G.F.; Ciancarelli, I.; Tramontano, M.; Antonucci, G.; Paolucci, S.; et al. Italian Version of the Pittsburgh Rehabilitation Participation Scale: Psychometric Analysis of Validity and Reliability. Brain Sci. 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.E.; Evans, E.K.; Stokic, D.S. Weight compensation characteristics of ArmeoSpring exoskeleton: Implications for clinical practice and research. J. Neuroeng. Rehabil. 2017, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Husty, M.; Birlescu, I.; Tucan, P.; Vaida, C.; Pisla, D. An algebraic parameterization approach for parallel robots analysis. Mech. Mach. Theory 2019, 140, 245–257. [Google Scholar] [CrossRef]
- van der Krogt, H.; Klomp, A.; de Groot, J.H.; de Vlugt, E.; van der Helm, F.C.; Meskers, C.G.; Arendzen, J.H. Comprehensive neuromechanical assessment in stroke patients: Reliability and responsiveness of a protocol to measure neural and non-neural wrist properties. J. Neuroeng. Rehabil. 2015, 12, 28. [Google Scholar] [CrossRef]
- de Jong, L.D.; Dijkstra, P.U.; Stewart, R.E.; Postema, K. Repeated measurements of arm joint passive range of motion after stroke: Interobserver reliability and sources of variation. Phys. Ther. 2012, 92, 1027–1035. [Google Scholar] [CrossRef]
- Waldman, G.; Yang, C.Y.; Ren, Y.; Liu, L.; Guo, X.; Harvey, R.L.; Roth, E.J.; Zhang, L.Q. Effects of robot-guided passive stretching and active movement training of ankle and mobility impairments in stroke. NeuroRehabilitation 2013, 32, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.D.; Sukal, T.; DeMott, T.; Dewald, J.P. Augmenting clinical evaluation of hemiparetic arm movement with a laboratory-based quantitative measurement of kinematics as a function of limb loading. Neurorehabil. Neural. Repair 2008, 22, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Morone, G.; Bragoni, M.; De Angelis, D.; Venturiero, V.; Coiro, P.; Pratesi, L.; Paolucci, S. Driving electromechanically assisted Gait Trainer for people with stroke. J. Rehabil. Res. Dev. 2011, 48, 135–146. [Google Scholar] [CrossRef]
- Nordin, N.; Xie, S.Q.; Wünsche, B. Assessment of movement quality in robot- assisted upper limb rehabilitation after stroke: A review. J. Neuroeng. Rehabil. 2014, 11, 137. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Posteraro, F.; Morone, G.; Dell’orco, A.; Botticelli, A. State of the art and challenges for the classification of studies on electromechanical and robotic devices in neurorehabilitation: A scoping review. Eur. J. Phys. Rehabil. Med. 2021, 57, 831–840. [Google Scholar] [CrossRef]
- Morasso, P.; Casadio, M.; Giannoni, P.; Masia, L.; Sanguineti, V.; Squeri, V.; Vergaro, E. Desirable features of a “humanoid” robot-therapist. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2418–2421. [Google Scholar]
- Iosa, M.; Morone, G.; Cherubini, A.; Paolucci, S. The Three Laws of Neurorobotics: A Review on What Neurorehabilitation Robots Should Do for Patients and Clinicians. J. Med. Biol. Eng. 2016, 36, 1–11. [Google Scholar] [CrossRef]
- Palermo, E.; Hayes, D.R.; Russo, E.F.; Calabrò, R.S.; Pacilli, A.; Filoni, S. Translational effects of robot-mediated therapy in subacute stroke patients: An experimental evaluation of upper limb motor recovery. PeerJ 2018, 6, e5544. [Google Scholar] [CrossRef] [PubMed]
- Martino Cinnera, A.; Pucello, A.; Lupo, A.; Gimigliano, F.; Mammucari, E.; Cicero, D.L.; Iosa, M.; Paolucci, S.; Morone, G. Upper limb motor improvement in chronic stroke after combining botulinum toxin A injection and multi-joints robot-assisted therapy: A case report. Oxf. Med. Case Rep. 2019, 2019, omz097. [Google Scholar] [CrossRef]
- Pilla, A.; Trigili, E.; McKinney, Z.; Fanciullacci, C.; Malasoma, C.; Posteraro, F.; Crea, S.; Vitiello, N. Robotic Rehabilitation and Multimodal Instrumented Assessment of Post-stroke Elbow Motor Functions-A Randomized Controlled Trial Protocol. Front. Neurol. 2020, 11, 587293. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.W. Symposium Synopsis; Feldman, R.G., Young, R.R., Koella, W.P., Eds.; Yearbook Medical: Chicago, IL, USA, 1980; pp. 485–494. [Google Scholar]
- Picerno, P.; Iosa, M.; D’Souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis: A five-year update. Expert Rev. Med. Devices 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Iosa, M.; Aydin, M.; Candelise, C.; Coda, N.; Morone, G.; Antonucci, G.; Marinozzi, F.; Bini, F.; Paolucci, S.; Tieri, G. The Michelangelo Effect: Art Improves the Performance in a Virtual Reality Task Developed for Upper Limb Neurorehabilitation. Front. Psychol. 2021, 11, 611956. [Google Scholar] [CrossRef]
- Comani, S.; Velluto, L.; Schinaia, L.; Cerroni, G.; Serio, A.; Buzzelli, S.; Sorbi, S.; Guarnieri, B. Monitoring neuro-motor recovery from stroke with high-resolution EEG, robotics and virtual reality: A proof of concept. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 1106–1116. [Google Scholar] [CrossRef]
- Kim, W.S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.J.; Paik, N.J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef]
- Morone, G.; Spitoni, G.F.; De Bartolo, D.; Ghanbari Ghooshchy, S.; Di Iulio, F.; Paolucci, S.; Zoccolotti, P.; Iosa, M. Rehabilitative devices for a top-down approach. Expert Rev. Med. Devices 2019, 16, 187–195. [Google Scholar] [CrossRef]
- De Bartolo, D.; Spitoni, G.F.; Iosa, M.; Morone, G.; Ciancarelli, I.; Paolucci, S.; Antonucci, G. From movement to thought and back: A review on the role of cognitive factors influencing technological neurorehabilitation. Funct. Neurol. 2019, 34, 131–144. [Google Scholar]
- Torrisi, M.; Maggio, M.G.; De Cola, M.C.; Zichittella, C.; Carmela, C.; Porcari, B.; La Rosa, G.; De Luca, R.; Naro, A.; Calabrò, R.S. Beyond motor recovery after stroke: The role of hand robotic rehabilitation plus virtual reality in improving cognitive function. J. Clin. Neurosci. 2021, 92, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.E.; Zygman, M.L.; Rymer, W.Z.; Reinkensmeyer, D.J. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: A randomized controlled pilot study. J. Neuroeng. Rehabil. 2006, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Major, Z.Z.; Vaida, C.; Major, K.A.; Tucan, P.; Brusturean, E.; Gherman, B.; Birlescu, I.; Craciunaș, R.; Ulinici, I.; Simori, G.; et al. Comparative Assessment of Robotic versus Classical Physical Therapy Using Muscle Strength and Ranges of Motion Testing in Neurological Diseases. J. Pers. Med. 2021, 11, 953. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).