Abstract
In the last two decades, a growing interest has been focused on gait and balance robot-assisted rehabilitation in children with neurological disabilities. Robotic devices allow the implementation of intensive, task-specific training fostering functional recovery and neuroplasticity phenomena. However, limited attention has been paid to the protocols used in this research framework. This systematic review aims to provide an overview of the existing literature on robotic systems for the rehabilitation of gait and balance in children with neurological disabilities and their rehabilitation applications. The literature search was carried out independently and synchronously by three authors on the following databases: MEDLINE, Cochrane Library, PeDro, Institute of Electrical and Electronics Engineers, ScienceDirect, and Google Scholar. The data collected included three subsections referring to clinical, technical, and regulatory aspects. Thirty-one articles out of 81 found on the primary literature search were included in the systematic review. Most studies involved children with cerebral palsy. Only one-third of the studies were randomized controlled trials. Overall, 17 devices (nine end-effector systems and eight exoskeletons) were investigated, among which only 4 (24%) were bore the CE mark. Studies differ on rehabilitation protocols duration, intensity, and outcome measures. Future research should improve both rehabilitation protocols’ and devices’ descriptions.
1. Introduction
Many neurologic disorders can affect children’s neuromotor development and their ability to participate actively in daily life (i.e., cerebral palsy, traumatic brain injury) impacting children’s social and cognitive development and increasing disability and caregiver burden [1].
In the last decades, extensive research on rehabilitation intervention in children with neurological disorders has been conducted. The focus of rehabilitation in this context is walking and mobility [2]. For this aim, the literature has increasingly emphasized the promotion of active therapies, including intensive, repetitive, and task-specific training to enhance gait recovery and neuroplasticity, which is the ultimate factor to consider when designing personalized neurorehabilitation interventions [3,4,5,6]. Neuroplasticity is the brain’s ability to reorganize itself by forming new neural connections due to learning in response to new situations or changes in their environment and a mechanism to compensate for brain injury [7].
Pediatric rehabilitation presents some peculiar aspects that differentiate it from rehabilitation intervention in adulthood. Remarkably, in the developmental age, since the children’s skills and competencies are not consolidated, the areas of development cannot be evaluated and treated individually. Their mutual interactions with each other should be taken into consideration. The field of neuromotor rehabilitation in children is rapidly evolving. This is confirmed by the relatively recent institution of the neuropsychomotor therapist, specialising in the motor, cognitive and emotional-relational rehabilitation of the child. In this dynamic rehabilitation context, new technologies such as robots for rehabilitation could be a valuable tool for improving and enriching rehabilitation approaches, especially when considering the specific needs of children with neurologic diseases.
A robot can be defined as an electromechanical device provided with actuators, a sensor system, and a control system [8]. The ability to mobilize limbs is provided by actuators to move the robot’s mechanical components in contact with the human body. Sensors acquire data on the mechanical systems and environment’s state, allowing interaction with the user [9]. Finally, the control system bridges actions and perception.
Robots can provide repetitive, intensive, task-oriented, and quantifiable training, essential features for a rehabilitation intervention to foster neuroplasticity and recovery in neurological patients [10,11]. In the last two decades, their use has increased significantly in various neurological conditions to improve either upper limb function or gait and balance. Results on the effectiveness of robot-assisted rehabilitation in different fields of application are promising. However, the literature is rather heterogeneous in terms of devices used, population investigated, and type of rehabilitation interventions. Moreover, in this solid but heterogeneous body of evidence, only a small fraction of previous research focused on rehabilitation in the developmental age [12].
A recent review suggested robots’ most clinically relevant features for rehabilitation [9]. Robotic devices can be classified according to their mechanical properties (i.e., end-effector systems, grounded exoskeletons, wearable exoskeletons, numbers of degrees of freedom, body segments involved), feedback modalities (i.e., haptic, visual, auditory), amount of assistance provided, and modalities of human–robot interaction (i.e., active, passive, assistive) [13]. Specifically, the exoskeleton-based devices are robots in which patient’s joints and robot’s mechanical structure are aligned. Consequently, the human-machine mechanical interface involves the entire limb. In contrast, the end-effector systems provide contact between the mechanical structure and patient only at the most distal segment of the involved limb (i.e., hand/foot) [12].
The European regulatory framework requires a robotic device to be registered as a medical device used for rehabilitation purposes. This requirement is ensured by the CE mark that allows its inclusion in the European Union market. Obtaining the CE mark ensures the fulfilment of European standards on quality and safety. As regards the US market, the Center for Devices and Radiological Health (CDRH) within the Food and Drug Administration is responsible for medical devices regulations and their sale in the United States.
Robots for rehabilitation have some relevant features that make them potentially relevant in the rehabilitation of children with neurological diseases. Firstly, the combination of robotic tasks with virtual reality environments provides an enriched experience that fosters higher focus and attention, novelty, fun, and challenge, which, together, maximize cognitive engagement, thus stimulating the child’s active voluntary participation and, most importantly, neuroplasticity [14]. Secondly, different feedback modalities of the patient’s performance, like auditive, visual and haptic, can enrich the treatment, fostering sensorimotor learning. In addition, an educational effort of healthcare professionals involved in the rehabilitation process should be put forward to improve knowledge on the specific technical features of these devices. The market offers many types of robots, and their technical characteristics are often not accompanied by specific clinical indications about the target functions, affecting, in turn, the development of the individualized neurorehabilitation program [15]. On one hand, the relentless technical innovation allows the creation of complex and sophisticated devices for neurological rehabilitation [16,17]. On the other hand, the lack of beneficial dialogue between developers and users limits the use of these devices in clinical practice [18].
Based on these considerations, it is conceivable that robot-assisted rehabilitation can be successfully applied to rehabilitation in children affected by neurological disorders. However, most of the literature on this topic is focused on adults, and further effort is needed to provide evidence on the developmental age [12,19].
Recently, a review was carried out on robotic devices for upper limb rehabilitation in children with neurological impairments [19]. The study presented an overview of the upper limb robotic device’s characteristics, their applications in the clinical setting, and results. However, to the best of our knowledge a similar study on gait and balance rehabilitation has not been published yet. Falzarano and colleagues [19] have pointed out that optimal control strategies and sensory-motor recovery in children with neurological diseases have not been defined yet. Moreover, rehabilitation protocols in this domain should be more detailed on specific contents and applications [12]. Most of the literature on this topic has focused on the type of device used to overlook treatment modalities. However, understanding the effects of different training modalities using robotic devices is crucial for improving rehabilitation care. It can foster the spread of these devices in the context of children’s rehabilitation.
This systematic review aims to provide an overview of the existing literature on robots and electromechanical devices used for the rehabilitation of gait and balance in children with neurological disability and their rehabilitation application, focusing on the type of robotic devices used and their application protocols.
A comprehensive perspective on the main results achieved by applying robot-assisted rehabilitation, which are the most successful methodologies implemented from which to lay the foundations for future studies on robot-based approaches, is provided.
2. Materials and Methods
The present study represents a new analysis in a subgroup of patients based on a recently published scoping review on robots for neurorehabilitation [12]. A new systematic literature search has been performed, and data on rehabilitation treatments ad protocols has been extracted. The review was carried out according to PRISMA guidelines, the present protocol was not registered.
2.1. Eligibility Criteria
A literature review was carried out. The inclusion criteria were as follow:
Population: children (age < 18 years old) affected by neurological diseases.
Intervention: robot-assisted rehabilitation.
Comparison: studies were included irrespective of the presence of a comparison.
Outcomes: studies included clinical and instrumental outcome measures, and feasibility and safety outcomes were included.
Study design: RCT, controlled and uncontrolled trials, case series studies were included.
Studies on robot-assisted rehabilitation on children (age < 18 years old) affected by neurological diseases were selected. Only English-written clinical trials, pilot studies, and observational studies were considered. Clinical studies including other non-invasive technologies (i.e., non-invasive brain stimulation and functional electrical stimulation) combined with robot-assisted approaches were excluded. Three authors carried out the assessment of eligibility independently. Where in disagreement, the option agreed by two out of three authors was chosen.
2.2. Information Sources
A first literature search was carried out from November 2019 to February 2021. No date restrictions were applied for the search. The search was carried out on the following database: MEDLINE, Cochrane Library and PeDro, Institute of electrical and electronics engineers, Science direct, Google Scholar.
2.3. Search Strategy
The following keywords were used: (pediatric OR child *) AND robot * AND (rehabilitation) AND (“Lower Extremity” [Mesh] OR balance).
2.4. Selection Process
Three authors independently and synchronously selected the included studies according to eligibility criteria. Selection, based on title, abstract, and eventually full-text reading, was performed sequentially.
2.5. Data Collection
For the included articles, the data-collection form included three subsections referring to clinical, technical, and rehabilitation intervention characteristics, respectively.
The first information subsection presents clinical features, including study authors, clinical characteristics of the population, and study design.
The second subsection presents technical features of the device such as the type of mechanical architecture (end-effector or exoskeleton), wearability, environment of the intervention (virtual or real), degrees of freedom (DoFs) for each limb, parameters recorded by the device, control system, and regulatory aspects such as the use of the CE mark according to the European Medical Device Regulation and relative classification, if available.
The third subsection presents the features of the rehabilitation intervention provided, such as modalities of human-robot interaction [20], type of feedback provided to the user, the possibility of adapting the level of training difficulty, intensity of the treatment (measured as minutes per session), number of sessions per day, duration of the treatment and rehabilitation aim of the device (assistive or interactive). An overview of the data extracted is reported in Table 1.

Table 1.
Overview of the extracted data on population, device, and rehabilitation features.
2.6. Study Quality Assessment
The quality of the included randomised controlled trials (RCTs) was assessed using the PEDro scale. This scale includes 11 criteria, of which only ten contribute to the total score (range 0–10). Studies with scores lower than five are considered poor quality and with a high risk of bias [21].
3. Results
The literature search identified 81 articles. After title and abstract reading, 35 articles met the requirements to proceed to the full-text reading stage. In this stage, four further articles were excluded due to the absence of a rehabilitation treatment [22,23,24,25]. This process led to 31 selected papers being included in the review (Table 2). Figure 1 shows the flow diagram that summarizes the process and results of the primary literature search. Almost a third (32%) of the studies were RCTs, suggesting an overall moderate-to-low quality of evidence. Among the ten included RCT studies, six were classified as good to excellent quality according to the PEDro score (score > 5) [26,27,28,29,30,31], three as fair quality (score = 5) [32,33,34] and one as poor quality (score < 5) (Table 3) [35]. Table 4 and Table 5 summarize the extracted data from the included studies regarding robotic features and treatment characteristics, respectively.

Table 2.
Overview of the included studies.
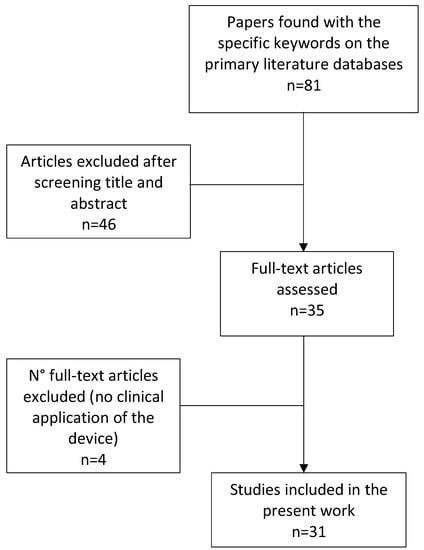
Figure 1.
Flow chart of the study selection process.
3.1. Clinical Features
Cerebral palsy (CP) was the most investigated disease, while five studies included subjects with acquired brain injury [32,36], SCI [37], Duchenne [38], peripheral nerves lesion [39]. The age of the included subjects ranged between 4 [40] and 16 years [41]. The included patients with CP showed a variety of dysfunctions, including diplegia, spastic diplegia, and hemiplegia. Notably, some studies included more than one population. Almost 30% of the studies (10/31) included less than six children [38,39,40,42,43,44,45,46,47,48].
3.2. Robotic Devices
Based on the analysis of the literature, 16 robots that have been developed beyond the proof-of-concept stage and were used for lower limb rehabilitation of children with neurological disorders were identified. The included devices’ technical characteristics are reported in Table 4. Only four devices were provided with the CE mark and, hence, are commercially available. Except for the Lokomat (Hocoma AG, Volketswil, Switzerland), which has been widely investigated in children with neurological disabilities [25,27,28,29,32,33,34,37,41,49,50], each of the other devices were investigated in few studies.
The 16 selected devices differ in architectural configuration (exoskeleton or end-effector) and the sets of targeted joints. There was an equal number of the exoskeleton and end-effector devices. As regards the mechanical structure, the selected robots have different degrees of freedom. Two main control schemes were used: impedance control and admittance control.
3.3. Rehabilitation Protocols Interventions
The rehabilitation protocols used in the included studies were analysed and are outlined in Table 5. In terms of the number of sessions provided, treatment duration varied significantly across studies between 1 [41] and 60 months [26]. The duration of each session has shown a wide range of variability ranging from 8 [41,51] to 75 min [52]. Noteworthy, in a relevant number of studies, some treatment information was missing. As for the assistance modality and the user’s feedback, the various devices can be considered relatively homogeneous (assistive) with both visual and haptic feedback provided.

Table 3.
Study quality assessment.
Table 3.
Study quality assessment.
| Study | Eligibility Criteria | Randomization | Allocation Concealment | Baseline Comparison | Blinding of All Subjects | Blinding of All Therapists | Blinding of All Assessors | Follow up Completeness | Intention to Treat | Between-Group Comparison | Point Estimates and Variability | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beretta 2018 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Damiano 2017 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Druzbicki 2013 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Romei 2012 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Schroeder 2014 B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Wallard 2017 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Wallard 2018 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Chen 2016 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Smania 2011 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Yazici 2019 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |

Table 4.
Included devices’ characteristics.
Table 4.
Included devices’ characteristics.
| Device | Rehabilitation Aim | CE Class | CE MARK | CND | EE/Exo | Wearability | DoFs | Recorded Parameters | Control System | Device’s Main Features |
|---|---|---|---|---|---|---|---|---|---|---|
| Lokomat [27,28,30,32,33,34,37,49,50] | interactive | IIa | 1 | Z12069002 | Exo | no | (2 + 1) × 2 | kinematics, force | impedance, position | lower limbs gait orthosis with body weight support for treadmill walking |
| Lokomat + FreeD [41] | interactive | IIa | 1 | Z12069002 | Exo | no | (2 + 2) × 2 | ROM | impedance, position | lokomat + medio-lateral weight shifting support |
| MOTOMED [53] | interactive | IIa | 1 | Y034899 | EE | no | (2 + 1) × 2 | force | N/A | robotic cycling device |
| Hybrid Assistive Limb (HAL) [54] | interactive | IIb | 1 | N010299 | Exo | yes | (2 + 1) × 2 | - | force/kinematics, EMG | lower limbs wearable robot for overground walking |
| EksoGT [42] | interactive | IIa | 1 | Y99 | Exo | yes | (2 + 1) × 2 | kinematics | position | lower limbs wearable robot for overground walking |
| IntelliStretch [30,36,52,55,56,57] | interactive | - | - | - | EE | no | 1 + 0 | force, ROM | torque | device for ankle flexion-extension in sitting position with exergaming |
| Rutgers Ankle [43] | interactive | - | - | - | EE | no | 1 + 0 | ROM, force | position, force | device for ankle flexion-extension in sitting position with exergaming |
| PedBot [40] | interactive | - | - | - | EE | no | 2 + 0 | kinematics, force | force, torque | device for ankle assistive movement in sitting position with exergaming |
| ATLAS Exoskeleton [38] | interactive | - | - | - | Exo | yes | (3 + 0) ×2 | - | impedance | lower limbs wearable robot with weight support for overground walking |
| Exoskeleton for knee extension [47] | assistive | - | - | - | Exo | yes | 1 × 2 | kinematics, force | impedance | wearable robot for knee extension assistance during gait |
| Exoskeleton for ankle [45,46,51] | assistive | - | - | - | Exo | yes | 1 × 2 | torque, force | torque, force | wearable robot for ankle flexion-extension assistance during gait |
| pediAnklebot [39] | interactive | - | - | - | EE | no | 2 + 1 | kinematics, force | impedance | device for ankle assistive movement in sitting position with exergaming |
| FORTIS-102 [48] | interactive | - | - | - | EE | no | N/A | kinematics, force | N/A | device for simulation of horse riding |
| Gait Trainer GT1 [31] | assistive | - | - | - | EE | no | (1 + 2) × 2 | kinematics | position | gait assistive device with body weight support |
| Innowalk Pro [35] | interactive | - | - | - | Exo | yes | (1 + 2) × 2 | N/A | N/A | gait assistive device with body weight support |
| Trexo Home [44] | assistive | - | - | - | Exo | yes | (2 + 1) × 2 | N/A | N/A | lower limbs wearable robot for overground walking |
Legend: n: number, EE: End Effector, Exo: Exoskeleton, DoFs: Degrees of Freedom (active DoFs + passive DoFs), DNC: Device’s National Classification, N/A: Not Assessable.

Table 5.
Rehabilitation protocols of the included studies.
Table 5.
Rehabilitation protocols of the included studies.
| Robot, Study | Study Design | nP, Mean Age (±SD), Diagnosis | Number of Sessions Session/Week | Sessions/ Day | Sessions’ Duration [min/ses] | Environment | Assistance Modality | Feedback | Difficulty Level | Study’s Main Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Lokomat [27] | RCT | 26, 10.1y (2.2y) CP | 20 5/week | 1 | 45 | real | 0 | 2 | fixed | BG difference in the range of pelvic motion in the coronal plane on the right side No BG differences |
| Lokomat [28] | RCT | 14, 8.3y (1.2y) CP | 20 5/week | 1 | 40 | real | 0, 2 | 2 | adjustable | BG difference in balance in standing and walking (GMFM D-E) |
| Lokomat [29] | RCT | 14, 8.3y (1.2y) CP | 20 4 weeks | 1 | 40 | real | 0 | 2 | adjustable | BG difference in balance in standing and walking (GMFM D-E) |
| Lokomat [32] | RCT | 29, 11.2y (n.a) ABI | 20 5/week | 1 | 45 | real | 0 | 2 | adjustable | BG difference in balance in standing and walking (GMFM D-E) |
| Lokomat [34] | Uncontrolled trial | 18, 11.4y (4.9y) CP | 12 4/week | 1 | 30–60 | real | 0 | 2 | adjustable | WG improvement in balance in standing and walking (GMFM D-E) |
| Lokomat [37] | Uncontrolled trial | 14, 8.2y (5.4y) CP, SCI | 12 4/week | 1 | 50 | real | 0 | 2 | adjustable | WG improvement in balance in standing and walking (GMFM D-E) |
| Lokomat [49] | Uncontrolled trial | 22, 8.6y (2.1y) CP | 20 3–5/week | 1 | 45–60 | N/A | 0 | 2 | fixed | WG improvement in balance in standing (GMFM D) and walking speed |
| Lokomat [50] | Uncontrolled trial | 83, 10.8y (6.7y) CP | 12 4/week | 1 | 37 (±6) | real | 0 | 2 | adjustable | WG improvement in GMFM and COPM |
| Lokomat + FreeD [44] | Pilot comparative study | 15, 16y (2y) CP | 1 | 1 | 8 | real | 0, 6, 2 | 2 | adjustable | WG increase of proximal leg muscle activity when kinematic freedom of Lokomat was enlarged |
| MOTOMED [53] | RCT | 13, 9.2y (2.9y) CP | 60 5/week | 1 | 20 | real | 0, 2 | 2 | adjustable | BG difference in cadence while cycling—no differences in gait speed |
| HAL (Hybrid Assistive Limb) [54] | Uncontrolled trial | 6, 16.8y (3.5y) CP | 12 2–4/week | 1 | 20 | real | 3 | 2 | fixed | WG improvement of walking speed and spatiotemporal gait parameters |
| EksoGT [42] | Case study | 1, 17y CP | 12 3/week | 1 | 50 | real | 2 | 2, 3 | adjustable | WG improvement of speed and spatiotemporal gait parameters |
| IntelliStretch [30] | RCT | 18, 10.7y (6.0y) CP | 6 weeks 3/week | N/A | 40 | real | 0,2,7 | 1, 2 | adjustable | no BG differences (home vs lab robotic intervention) WG improvement in both groups for endurance, gait speed and balance (PBS) |
| IntelliStretch [52] | Uncontrolled trial | 28, 8.2y (3.6y) CP | 12 2/week | N/A | 75 | real | 0, 2, 7 | 1, 2 | adjustable | WG improvement in lower limb strength, spasticity (MAS), gait speed |
| IntelliStretch [55] | Uncontrolled trial | 23, 9yy (2.64) CP | 18 3/week | N/A | N/A | real | 0, 2, 7 | 1, 2 | adjustable | WG improvements in the ankle range of motion, muscle strength, spasticity (MAS) |
| IntelliStretch [36] | Uncontrolled trial | 10, 13.0y (3.9y) TBI | 15 3–5/week | N/A | 40 | real | 0, 2, 7 | 1, 2 | adjustable | WG improvements in the ankle range of motion, muscle strength, spasticity (MAS) |
| IntelliStretch [56] | Uncontrolled trial | 12, 8.6y (3.7y) CP | 18 3/week | N/A | 50 | real | 0, 2, 7 | 1, 2 | adjustable | WG improvements in the ankle range of motion, muscle strength, spasticity (MAS) |
| IntelliStretch [57] | Uncontrolled trial | 8, 13y (2.5y) CP | 18 3/week | N/A | 45–60 | real | 0, 3 | 1, 2, 3 | adjustable | WG improvement in spasticity (MAS) and balance (PBS) |
| Rutgers Ankle [43] | Uncontrolled trial | 1, 7y CP | 36 3/week | N/A | 40 | real | 1 | 1, 2 | adjustable | WG improvements in ankle strength and GMFM |
| PedBot [40] | Uncontrolled trial | 4, 13.7y (2.2y) CP | 20 N/A | N/A | 30 | virtual | 1, 3, 7 | 1, 2 | adjustable | WG improvement of ankle range of motion |
| ATLAS Exoskeleton [38] | Proof of concept | 2, 9y (12y) CP, Duchenne | N/A | N/A | N/A | real | 1, 3 | 2 | fixed | Not assessed |
| Exoskeleton for ankle [45] | Cohort study | 5, 5–30y CP | N/A | N/A | 25 | real | 2 | 2 | fixed | reducing of the metabolic cost of walking |
| Exoskeleton for ankle [46] | Uncontrolled trial | 5, 5–30y CP | N/A | N/A | N/A | real | 2 | 2 | fixed | WG increase of propulsive ankle joint power, reducing of plantar-flexor muscle iperactivity during walking |
| Exoskeleton for ankle [51] | Uncontrolled trial | 7, 14y (5y) CP | 1 | 1 | 8 | real | 2 | 2 | fixed | WG increasing in step length |
| pediAnklebot [39] | Uncontrolled trial | 3, 9y (n.a) CP, peroneal nerve lesion | N/A | N/A | N/A | real | 0, 3 | 1, 2 | adjustable | WG improvement device-assessed force parameters |
| Fortis-102 [48] | Case study | 1, 11y CP | 12 1/week | 1 | 45 | real | 0 | 2 | adjustable | increased abdominal muscle trophy and improve in static balance (stabilometry) |
| Gait trainer GT 1 [31] | RCT | 9, 13.9y (2.8y) CP | 10 5/week | N/A | 30 | real | 0 | 2 | adjustable | WG improvements in gait speed and endurance |
| Innowalk Pro [35] | CT | 12, 8.9y (n.a.) CP | 36 3/week | N/A | N/A | real | 1 | 2 | fixed | WG improvements in gait speed and endurance, no BG differences |
| Trexo Home [44] | Case study | 1, 7y CP | 36 3/week | N/A | 46 min/week | real | 0 | 2 | fixed | improved spasticity in knee flexion (MAS) |
Legend: n: number, CT: controlled trial, RCT: randomized controlled trial, Assistance modalities: 0: passive, 1: active, 2: assistive, 3: assist-as-needed, 4: passive-mirrored, 5: corrective, 6: perturbative, 7: resistive; Feedback modalities: 1: visual, 2: haptic, 3: auditory; CP: cerebral palsy, ABI: acquired brain injury, TBI: traumatic brain injury, SCI: spinal cord injury, PBS: Pediatric Balance Scale, COPM: Canadian Occupational Performance Measure, WG: within group, BG: between group.
4. Discussion
This work is a systematic review of the existing literature on robotic devices for gait and balance rehabilitation in children with neurological disabilities. Moreover, the present work reviews the rehabilitation protocols performed in these studies. Based on the literature search, a limited number of studies on this topic were found. Noteworthy, many of the included studies have been published in the last few years, thus suggesting that the interest in this field is rapidly increasing. However, compared with the extensive literature on adult robot-assisted neurorehabilitation, the number of studies on rehabilitation in childhood is still limited [12,58]. The rehabilitation interventions have varied substantially between studies, both in terms of intensity and modalities.
4.1. Population
CP was the most investigated pathology, reflecting the high prevalence of this disease in the developmental age [59]. Some studies have included other populations, but their sample sizes were generally limited except for the study carried out by [32], investigating a cohort of 29 children with ABI. Notably, some studies have included more than one population. Sample sizes were, overall, small, and 10/31 studies included five patients or fewer. The studies included in the present review have involved patients aged between 4 [40] and 16 years old [41]. This is in line with previous findings that have suggested that most of the research on the effectiveness of rehabilitation in childhood has been conducted with school-aged children. However, the majority of brain growth and development occurs in the first two years of life. This early period represents a critical window during which rehabilitation effectiveness might be maximized, but which is missed by modern rehabilitative approaches [4]. Therefore, further research on the early stage of development is needed. The heterogeneity of functional lower limbs’ impairments and age hampers the synthesis of the results. Moreover, inclusion criteria of some studies have included subjects’ anthropometry, pointing out a relevant issue with robot-assisted rehabilitation in developmental ages—that is, the variability in the height of the children involved.
A recent device, the Trexo Home [44], represents an attempt to overcome this issue by providing different designs suitable for children from one year old up to a maximum of 170 cm. However, this remains a unique example, and, generally, the customization of devices size according to the children’s height is somewhat limited.
Along with its effectiveness, the safety and acceptability by patients and therapists represent other relevant aspects in robot-assisted rehabilitation. Borggraefe et al. [25] have investigated the safety and feasibility of robot-assisted treadmill training in 83 children and adolescents, and no severe side effects were found. In most patients, the adverse events were clinically insignificant and did not prevent children from undergoing the treatment. In contrast, none of the studies in the present review investigated patients’ and therapists’ acceptability of the robotic devices for rehabilitation. This aspect is crucial for fostering the spread of these devices in clinical settings and deserves a systematic assessment in future studies [41,60].
4.2. Robotic Devices
The increasing number of studies on neurological disabilities in children has paralleled the number of devices developed. However, most of the devices included in the present review were initially intended for adult users, with limited possibility to be adapted to child biomechanics, as they do not provide the possibility of adjusting the size, weight, and forces delivered. Despite this limited number of devices developed for the paediatric population, several studies have shown that their use can offer relevant opportunities to promote children’s sensorimotor recovery and prevent disease progression.
The wide range of subjects’ heights during childhood has possibly affected the diffusion of standard devices and has fostered the development of several customized robots. One of the main characteristics in pediatric rehabilitation is the patients’ continuous development and change in terms of anthropometric measures and rehabilitation needs. Consequently, developmental neuromotor rehabilitation is often a process that supports children from birth to adulthood.
The devices’ description in terms of technical features was limited in most of the included studies. Noteworthy, as previously suggested, it is conceivable that improving the reporting on robots’ characteristics in clinical trials may help other researchers and clinicians be aware of these technologies’ full potential and use them appropriately [12]. The knowledge of each robot training modalities and other technical aspects such as wearability or number of DoFs is crucial to understanding devices’ best implementations in clinical practice.
Moreover, in most of the literature examined, there was no clear distinction between assistive and purely rehabilitative devices (replacement of a function vs recovery of a function). While some devices were explicitly developed for rehabilitation, showing interactive features and adaptability to patient impairments, other devices seem to be designed with the original aim to reduce disability in impaired patients rather than being a rehabilitative tool. A significant example of the latter is the Ekso GT (Ekso Bionics, Richmond, CA, USA) [42]. Although provided with different treatment modalities to match the subject’s level of impairment, the device is poorly interactive. It does not properly provide task-oriented training per-se, which is an essential feature for inducing functional improvement and fostering neuroplasticity.
A remarkable attempt to describe the functioning of the rehabilitation robot used in their trial is represented by the study from [41]. In their study, the authors have evaluated new control modes by assessing leg muscle activation patterns and intensity and heart rate while walking with the Lokomat device. Specifically, two new control modes were introduced: the “Path Control” mode, which allows the patient to walk within a virtual tunnel surrounding the ideal movement trajectory, and the “FreeD”, which was developed to support weight shifting through the mediolaterally moveable pelvis and leg cuffs. Results indicate that the former seems promising for adolescent patients undergoing neurorehabilitation, as it increases proximal leg muscle activity while facilitating a physiological muscle activation.
Overall, the present review findings suggest an urgent need for new devices specifically designed and developed for children. Meeting this need is crucial for the development of this field and might improve rehabilitation. It would be essential to involve both engineers and therapists during the design stage, as regards technological innovation. This collaboration can provide both technical and clinical requirements with the aim of designing and programming devices increasingly suitable for rehabilitation aims and the needs of clinical relevance, always keeping the engagement and comfort of the child as the central reference point.
As summary and as perspective we can detail some considerations at a technical level to design robots for child neurorehabilitation that distinguish them from those for adults. The devices should be adaptable to the biomechanics of the child in terms of size and weight. This is crucial because, during development, these aspects change drastically. Regarding the forces delivered by the devices, they should be variable that implies the development of new types of motors. Finally, the rendering of the virtual reality projected on screen should be high to promote the engagement during the tasks.
4.3. Treatment Interventions
This review evaluates and reports the treatment modalities used in robot-assisted rehabilitation in children. Robotic devices can provide specific treatment features that foster recovery and neuroplasticity [13]. These devices allow performing highly repetitive, intensive, and task-oriented training, thus fostering the neuroplasticity process [15]. Notably, a recent meta-analysis on gait rehabilitation in children with CP has suggested that the body of literature for gait training has presented several limitations, including potentially insufficient duration, intensity, and total amount of gait training, which may have negatively biased the results [2]. Furthermore, the use of technology may combine purely motor rehabilitation with cognitive-behavioural exercises. Children can interact with the systems performing problem-solving activities, receiving rewards and maximizing their engagement. Indeed, robotic devices have been reported to provide a means of emotional expression for individuals with cognitive, physical, communication, or social impairments [61]. Above all, the interactive modalities provided by some devices and the combination of robotic tasks with VR environments may promote higher focus and attention, fun, and challenge. All these characteristics are of paramount importance in paediatric rehabilitation, and, all together, maximize cognitive engagement, thus stimulating the child’s active, voluntary participation and neuroplasticity [33,61].
Following the classification of architecture of robotic devices, the treatment interventions of the included studies can be roughly divided into two groups: rehabilitation treatments involving the whole lower limb and gait [27,28,30,31,32,33,34,35,37,38,41,42,44,50,53,54], and intervention targeted to specific joints like knee or ankle [30,36,39,40,43,45,46,47,51,52,55,56,57]. Although the importance of the features of robotic devices for rehabilitation, our analysis suggested that rehabilitation protocols are highly heterogeneous in terms of treatment duration, ranging from one [41] to 60 sessions [26] and duration of each session ranging from eight [41,51] to 75 min [52]. Moreover, in a limited number of studies, the difficulty level of the treatment was adjustable to the patient’s needs. Lastly, the assistance modality that characterizes the patient–machine interaction was often not reported or was limited to passive or active modalities. However, recent works on robot-assisted rehabilitation have suggested that the assistive modality could be the ideal strategy for fostering neuroplasticity [13]. Along with assistance modality, type of feedback provided, the number of repetitions executed, and control system used should also be specified when describing the rehabilitation intervention. Improving the reporting of the treatment and performing more complex and intensive rehabilitation protocols is crucial for taking full advantage of the available robotic devices.
The methodological quality of the studies included is low to moderate, with only 10 RCTs. The limited quality of evidence affects the attempt to synthesize the effectiveness of robot-assisted neurorehabilitation in these subjects, and future RCTs should provide solid rehabilitation protocols and technical descriptions. However, some common pattern in studies that used similar devices and treatment modalities can be found. Specifically, intervention using robot for gait rehabilitation, such as Lokomat and Gait-Trainer, showed patients’ improvement in functional gait parameters like speed and endurance [28,29,31,32,34,37,42,49,50,54]. In contrast, single-joint devices generally showed improvements in active and passive joint-specific movement features, like muscle strength or spasticity [36,40,43,45,52,55,56,57]. From a clinical perspective, therefore, clinicians should be aware of technical difference between different devices and choose the most appropriate one (when possible) accordingly. It is relevant to underline that most of the studies included in the present review were uncontrolled trials, hence, it is hard to disentangle the treatment effects specifically induced by the robotic device.
As for the design of the technical features of robots for child neurehabilitation, we can summarize the design methodology, at the conceptual level the rehabilitation protocols, that differentiates them from the adult ones. The protocols should take into account the influence of early intervention on neurodevelopmental that implies emphasizing treatments focused on social and environmental engagement. As, during development, the consequences are more critical at the sensory and cognitive levels, the protocols should focus particularly on the quantitative functional assessment of those features. This corresponds to also robustly measuring the baseline of healthy, age-matched controls.
5. Conclusions
The present review suggests that robot-assisted rehabilitation may be helpful and feasible for neurorehabilitation in children. Moreover, our analysis suggests a need for new devices specifically designed and developed for children. When using robotic devices, clinicians should design intensive, task-oriented, personalized treatment to foster neuroplasticity and recovery. Meeting this need is crucial for developing and improving rehabilitation outcomes, and, ultimately, contributing to increasing the quality of life of children with neurological disabilities.
The lack of information regarding devices’ descriptions and details of rehabilitation treatment has hampered data extraction in the present work. It may affect the replication of the experimental rehabilitation treatments in other clinical settings.
Author Contributions
All authors contributed to the conceptualization and methodology; writing—original draft preparation: N.V., M.G., L.V., S.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors report no involvement in the research by the sponsor, which could have influenced the outcome of this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The present study was conducted within the framework of the Italian Consensus Conference on ‘Rehabilitation assisted by robotic and electromechanical devices for persons with disability of neurological origin’ (CICERONE), as promoted by the Italian Society of Physical and Rehabilitation Medicine (SIMFER, Società Italiana di Medicina Fisica e Riabilitativa,) and Italian Society of Neurological Rehabilitation (SIRN, Società Italiana di Riabilitazione Neurologica) (2019–2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leonard, H.C. The Impact of Poor Motor Skills on Perceptual, Social and Cognitive Development: The Case of Developmental Coordination Disorder. Front. Psychol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, N.G.; Bodkin, A.W.; Bjornson, K.; Hobbs, A.; Soileau, M.; Lahasky, K. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children with Cerebral Palsy: Systematic Review and Meta-analysis. Phys. Ther. 2016, 96, 1938–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, B.R.; Elliott, E.J.; Coggan, S.; Pinto, R.Z.; Jirikowic, T.; McCoy, S.W.; Latimer, J. Interventions to improve gross motor performance in children with neurodevelopmental disorders: A meta-analysis. BMC Pediatr. 2016, 16, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, L.; Rose, S.E.; Boyd, R.N. Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nat. Rev. Neurol. 2015, 11, 390–400. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Blank, R.; Smits-Engelsman, B.; Polatajko, H.; Wilson, P. European Academy for Childhood Dis-ability (EACD): Recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version). Dev. Med. Child Neurol. 2012, 54, 54. [Google Scholar] [CrossRef]
- YouRong, S.S.; Veeravagu, A.; Grant, G. Neuroplasticity after Traumatic Brain Injury in Translational Research in Traumatic Brain Injury; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Siciliano, B.; Sciavicco, L.; Villani, L.; Oriolo, G. Robotics: Modelling, Planning and Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Papaleo, E.; Zollo, L.; Garcia-Aracil, N.; Badesa, F.J.; Morales, R.; Mazzoleni, S.; Sterzi, S.; Guglielmelli, E. Upper-limb kinematic reconstruction during stroke robot-aided therapy. Med. Biol. Eng. Comput. 2015, 53, 815–828. [Google Scholar] [CrossRef]
- Gandolfi, M.; Formaggio, E.; Geroin, C.; Storti, S.F.; Boscolo Galazzo, I.; Bortolami, M.; Saltuari, L.; Picelli, A.; Waldner, A.; Manganotti, P.; et al. Quantification of Upper Limb Motor Recovery and EEG Power Changes after Robot-Assisted Bilateral Arm Training in Chronic Stroke Patients: A Prospective Pilot Study. Neural Plast. 2018, 2018, 8105480. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.; Formaggio, E.; Geroin, C.; Storti, S.F.; Galazzo, I.B.; Waldner, A.; Manganotti, P.; Smania, N. Electroencephalographic Changes of Brain Oscillatory Activity After Upper Limb Somatic Sensation Training in a Patient with Somatosensory Deficit After Stroke. Clin. EEG Neurosci. 2014, 46, 347–352. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Posteraro, F.; Morone, G.; Dell’Orco, A.; Botticelli, A.; Dimitrova, E.; Gervasoni, E.; Goffredo, M.; Zenzeri, J.; et al. State of the art and challenges for the classification of studies on electromechanical and robotic devices in neurorehabilitation: A scoping review. Eur. J. Phys. Rehabil. Med. 2021, 57. [Google Scholar] [CrossRef]
- Turner, D.L.; Ramos-Murguialday, A.; Birbaumer, N.; Hoffmann, U.; Luft, A. Neurophysiology of robot-mediated training and therapy: A perspective for future use in clinical populations. Front. Neurol. 2013, 4, 184. [Google Scholar] [CrossRef] [Green Version]
- Rose, F.D.; Johnson, D.A.; Attree, E.A. Rehabilitation of the head-injured child: Basic research and new technology. Pediatric Rehabil. 1997, 1, 3–7. [Google Scholar] [CrossRef]
- Morone, G.; Cocchi, I.; Paolucci, S.; Iosa, M. Robot-assisted therapy for arm recovery for stroke patients: State of the art and clinical implication. Expert Rev. Med. Devices 2020, 17, 223–233. [Google Scholar] [CrossRef]
- Pisla, D.; Nadas, I.; Tucan, P.; Albert, S.; Carbone, G.; Antal, T.; Banica, A.; Gherman, B. Development of a Control System and Functional Validation of a Parallel Robot for Lower Limb Rehabilitation. Actuators 2021, 10, 277. [Google Scholar] [CrossRef]
- Vaida, C.; Birlescu, I.; Pisla, A.; Ulinici, I.-M.; Tarnita, D.; Carbone, G.; Pisla, D. Systematic Design of a Parallel Robotic System for Lower Limb Rehabilitation. IEEE Access 2020, 8, 34522–34537. [Google Scholar] [CrossRef]
- Shirota, C.; Jansa, J.; Diaz, J.; Balasubramanian, S.; Mazzoleni, S.; Borghese, N.A.; Melendez-Calderon, A. On the assessment of coordination between upper extremities: Towards a common language between rehabilitation engineers, clinicians and neuroscientists. J. Neuroeng. Rehabil. 2016, 13, 80. [Google Scholar] [CrossRef] [Green Version]
- Falzarano, V.; Marini, F.; Morasso, P.; Zenzeri, J. Devices and Protocols for Upper Limb Robot-Assisted Rehabilitation of Children with Neuromotor Disorders. Appl. Sci. 2019, 9, 2689. [Google Scholar] [CrossRef] [Green Version]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.A.; Buurke, J.H.; Prange, G.B.; Amirabdollahian, F. Training modalities in robot-mediated upper limb rehabilitation in stroke: A framework for classification based on a systematic review. J. Neuroeng. Rehabil. 2014, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Hariohm, K.; Prakash, V.; Saravankumar, J. Quantity and quality of randomized controlled trials published by Indian physiotherapists. Perspect. Clin. Res. 2015, 6, 91–97. [Google Scholar] [CrossRef]
- Krebs, H.I.; Rossi, S.; Kim, S.-J.; Artemiadis, P.; Williams, D.; Castelli, E.; Cappa, P. Pediatric anklebot. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 27 June–1 July 2011; pp. 1–5. [Google Scholar]
- Herrero, P.; Asensio, A.; García, E.; Marco, A.; Oliván, B.; Ibarz, A.; Gómez-Trullén, E.M.; Casas, R. Study of the therapeutic effects of an advanced hippotherapy simulator in children with cerebral palsy: A randomized controlled trial. BMC Musculoskelet. Disord. 2010, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Gasparri, G.M.; Luque, J.; Lerner, Z.F. Proportional Joint-Moment Control for Instantaneously Adaptive Ankle Exoskeleton Assistance. IEEE Trans. Neural. Syst. Rehabil. Eng. 2019, 27, 751–759. [Google Scholar] [CrossRef]
- Borggraefe, I.; Klaiber, M.; Schuler, T.; Warken, B.; Schroeder, S.A.; Heinen, F.; Meyer-Heim, A. Safety of robotic-assisted treadmill therapy in children and adolescents with gait impairment: A bi-centre survey. Dev. Neurorehabilit. 2010, 13, 114–119. [Google Scholar] [CrossRef]
- Damiano, D.L. Activity, activity, activity: Rethinking our physical therapy approach to cerebral palsy. Phys. Ther. 2006, 86, 1534–1540. [Google Scholar] [CrossRef]
- Drużbicki, M.; Rusek, W.; Snela, S.; Dudek, J.; Szczepanik, M.; Zak, E.; Durmala, J.; Czernuszenko, A.; Bonikowski, M.; Sobota, G. Functional effects of robotic-assisted locomotor treadmill therapy in children with cerebral palsy. J. Rehabil. Med. 2013, 45, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Robotic-assisted gait training improves walking abilities in diplegic children with cerebral palsy. Eur. J. Paediatr. Neurol. 2017, 21, 557–564. [Google Scholar] [CrossRef]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Effect of robotic-assisted gait rehabilitation on dynamic equilibrium control in the gait of children with cerebral palsy. Gait Posture 2018, 60, 55–60. [Google Scholar] [CrossRef]
- Chen, K.; Wu, Y.-N.; Ren, Y.; Liu, L.; Gaebler-Spira, D.; Tankard, K.; Lee, J.; Song, W.; Wang, M.; Zhang, L.-Q. Home-Based Versus Laboratory-Based Robotic Ankle Training for Children with Cerebral Palsy: A Pilot Randomized Comparative Trial. Arch. Phys. Med. Rehabil. 2016, 97, 1237–1243. [Google Scholar] [CrossRef] [Green Version]
- Smania, N.; Bonetti, P.; Gandolfi, M.; Cosentino, A.; Waldner, A.; Hesse, S.; Werner, C.; Bisoffi, G.; Geroin, C.; Munari, D. Improved Gait After Repetitive Locomotor Training in Children with Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2011, 90, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Beretta, E.; Molteni, E.; Biffi, E.; Morganti, R.; Avantaggiato, P.; Strazzer, S. Robotically-driven orthoses exert proximal-to-distal differential recovery on the lower limbs in children with hemiplegia, early after acquired brain injury. Eur. J. Paediatr. Neurol. 2018, 22, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Romei, M.; Montinaro, A.; Piccinini, L.; Maghini, C.; Germiniasi, C.; Bo, I.; Turconi, A.C. Efficacy of robotic-assisted gait training compared with intensive task-oriented physiotherapy for children with Cerebral Palsy. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1890–1894. [Google Scholar]
- Schroeder, A.S.; Von Kries, R.; Riedel, C.; Homburg, M.; Auffermann, H.; Blaschek, A.; Jahn, K.; Heinen, F.; Borggraefe, I.; Berweck, S. Patient-specific determinants of responsiveness to robot-enhanced treadmill therapy in children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 2014, 56, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, M.; Livanelioğlu, A.; Gücüyener, K.; Tekin, L.; Sümer, E.; Yakut, Y. Effects of robotic rehabilitation on walking and balance in pediatric patients with hemiparetic cerebral palsy. Gait Posture 2019, 70, 397–402. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, B.; Ren, Y.; Dvorkin, A.; Gaebler-Spira, D.; Sisung, C.; Zhang, L. Ankle passive and active movement training in children with acute brain injury using a wearable robot. J. Rehabil. Med. 2018, 50, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Borggraefe, I.; Kiwull, L.; Schaefer, J.S.; Koerte, I.; Blaschek, A.; Meyer-Heim, A.; Heinen, F. Sustainability of motor performance after robotic-assisted treadmill therapy in children: An open, non-randomized baseline-treatment study. Eur. J. Phys. Rehabil. Med. 2010, 46, 125–131. [Google Scholar] [CrossRef]
- Garcia, E.; Cestari, M.; Sanz-Merodio, D. Wearable exoskeletons for the physical treatment of children with quad-riparesis. In Proceedings of the 2014 IEEE-RAS International Conference on Humanoid Robots, Madrid, Spain, 18–20 November 2014; pp. 425–430. [Google Scholar]
- Michmizos, K.P.; Rossi, S.; Castelli, E.; Cappa, P.; Krebs, H.I. Robot-Aided Neurorehabilitation: A Pediatric Robot for Ankle Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Cleary, K.; Monfaredi, R.; Salvador, T.; Talari, H.F.; Coley, C.; Kovelman, S.; Belschner, J.; Alyamani, S.; Schladen, M.; Evans, S.H. Pedbothome: Robotically-Assisted Ankle Rehabilitation System for Children with Cerebral Palsy. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; Volume 2019, pp. 13–20. [Google Scholar]
- Aurich-Schuler, T.; Grob, F.; Van Hedel, H.J.; Labruyère, R. Can Lokomat therapy with children and adolescents be improved? An adaptive clinical pilot trial comparing Guidance force, Path control, and FreeD. J. Neuroeng. Rehabil. 2017, 14, 76. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, K.K.; Ehrenberg, N.; Cheng, J.; Nolan, K.J. Effects of Robotic Exoskeleton Gait Training on an Adolescent with Brain Injury. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 4445–4448. [Google Scholar]
- Cioi, D.; Kale, A.; Burdea, G.; Engsberg, J.; Janes, W.; Ross, S. Ankle control and strength training for children with cerebral palsy using the Rutgers Ankle CP: A case study. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 27 June–1 July 2011. [Google Scholar]
- Diot, C.M.; Thomas, R.L.; Raess, L.; Wrightson, J.G.; Condliffe, E.G. Robotic lower extremity exoskeleton use in a non-ambulatory child with cerebral palsy: A case study. Disabil. Rehabil. Assist. Technol. 2021, 1–5. [Google Scholar] [CrossRef]
- Lerner, Z.F.; Gasparri, G.M.; Bair, M.O.; Lawson, J.L.; Luque, J.; Harvey, T.A.; Lerner, A.T. An Untethered Ankle Exoskeleton Improves Walking Economy in a Pilot Study of Individuals with Cerebral Palsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1985–1993. [Google Scholar] [CrossRef]
- Lerner, Z.F.; Harvey, T.A.; Lawson, J.L. A Battery-Powered Ankle Exoskeleton Improves Gait Mechanics in a Feasibility Study of Individuals with Cerebral Palsy. Ann. Biomed. Eng. 2019, 47, 1345–1356. [Google Scholar] [CrossRef]
- Lerner, Z.F.; Damiano, D.L.; Park, H.-S.; Gravunder, A.J.; Bulea, T.C. A Robotic Exoskeleton for Treatment of Crouch Gait in Children with Cerebral Palsy: Design and Initial Application. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 650–659. [Google Scholar] [CrossRef]
- Park, J.-H.; You, J.H. Innovative robotic hippotherapy improves postural muscle size and postural stability during the quiet stance and gait initiation in a child with cerebral palsy: A single case study. NeuroRehabilitation 2018, 42, 247–253. [Google Scholar] [CrossRef]
- Meyer-Heim, A.; Ammann-Reiffer, C.; Schmartz, A.; Schäfer, J.; Sennhauser, F.H.; Heinen, F.; Knecht, B.; Dabrowski, E.; Borggraefe, I. Improvement of walking abilities after robotic-assisted locomotion training in children with cerebral palsy. Arch. Dis. Child. 2009, 94, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.S.; Homburg, M.; Warken, B.; Auffermann, H.; Koerte, I.; Berweck, S.; Jahn, K.; Heinen, F.; Borggraefe, I. Prospective cohort study to evaluate changes of function, activity and participation in patients with bilateral spastic cerebral palsy after Robot-enhanced repetitive treadmill therapy. Eur. J. Paediatr. Neurol. Off. J. Eur. Paediatr. Neurol. Soc. 2014, 18, 502–510. [Google Scholar] [CrossRef]
- Fang, Y.; Lerner, Z.F. Feasibility of Augmenting Ankle Exoskeleton Walking Performance with Step Length Bio-feedback in Individuals with Cerebral Palsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 442–449. [Google Scholar] [CrossRef]
- Sukal-Moulton, T.; Clancy, T.; Zhang, L.Q.; Gaebler-Spira, D. Clinical application of a robotic ankle training pro-gram for cerebral palsy compared to the research laboratory application: Does it translate to practice? Arch. Phys. Med. Rehabil. 2014, 95, 1433–1440. [Google Scholar] [CrossRef] [Green Version]
- Damiano, D.L.; Stanley, C.J.; Ohlrich, L.; Alter, K.E. Task-Specific and Functional Effects of Speed-Focused El-liptical or Motor-Assisted Cycle Training in Children with Bilateral Cerebral Palsy: Randomized Clinical Trial. Neurorehabilit. Neural Repair 2017, 31, 736–745. [Google Scholar] [CrossRef]
- Matsuda, M.; Iwasaki, N.; Mataki, Y.; Mutsuzaki, H.; Yoshikawa, K.; Takahashi, K.; Enomoto, K.; Sano, K.; Kubota, A.; Nakayama, T.; et al. Robot-assisted training using Hybrid Assistive Limb® for cerebral palsy. Brain Dev. 2018, 40, 642–648. [Google Scholar] [CrossRef]
- Chen, K.; Ren, Y.; Gaebler-Spira, D.; Zhang, L.Q. Home-based tele-assisted robotic rehabilitation of joint impair-ments in children with cerebral palsy. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 5288–5291. [Google Scholar]
- Wu, Y.-N.; Hwang, M.; Ren, Y.; Gaebler-Spira, D.; Zhang, L.-Q. Combined Passive Stretching and Active Movement Rehabilitation of Lower-Limb Impairments in Children with Cerebral Palsy Using a Portable Robot. Neurorehabilit. Neural Repair 2011, 25, 378–385. [Google Scholar] [CrossRef]
- Lee, S.J.; Jin, D.; Kang, S.H.; Gaebler-Spira, D.; Zhang, L.Q. Combined ankle/knee stretching and pivoting stepping training for children with cerebral palsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1743–1752. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar]
- Morrissey, K.; Fairbrother, H. Severe Traumatic Brain Injury in Children: An Evidence-Based Review of Emergency Department Management. Pediatr. Emerg. Med. Pr. 2016, 13, 1–28. [Google Scholar]
- Iosa, M.; Morone, G.; Cherubini, A.; Paolucci, S. The three laws of neurorobotics: A review on what neurorehabilitation robots should do for patients and clinicians. J. Med. Biol. Eng. 2016, 36, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, F.; Niedzwecki, C.; Gaebler-Spira, D. Technological Advancements in Cerebral Palsy Rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 117–129. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).