Electrospinning of Chitosan for Antibacterial Applications—Current Trends
Abstract
:1. Introduction
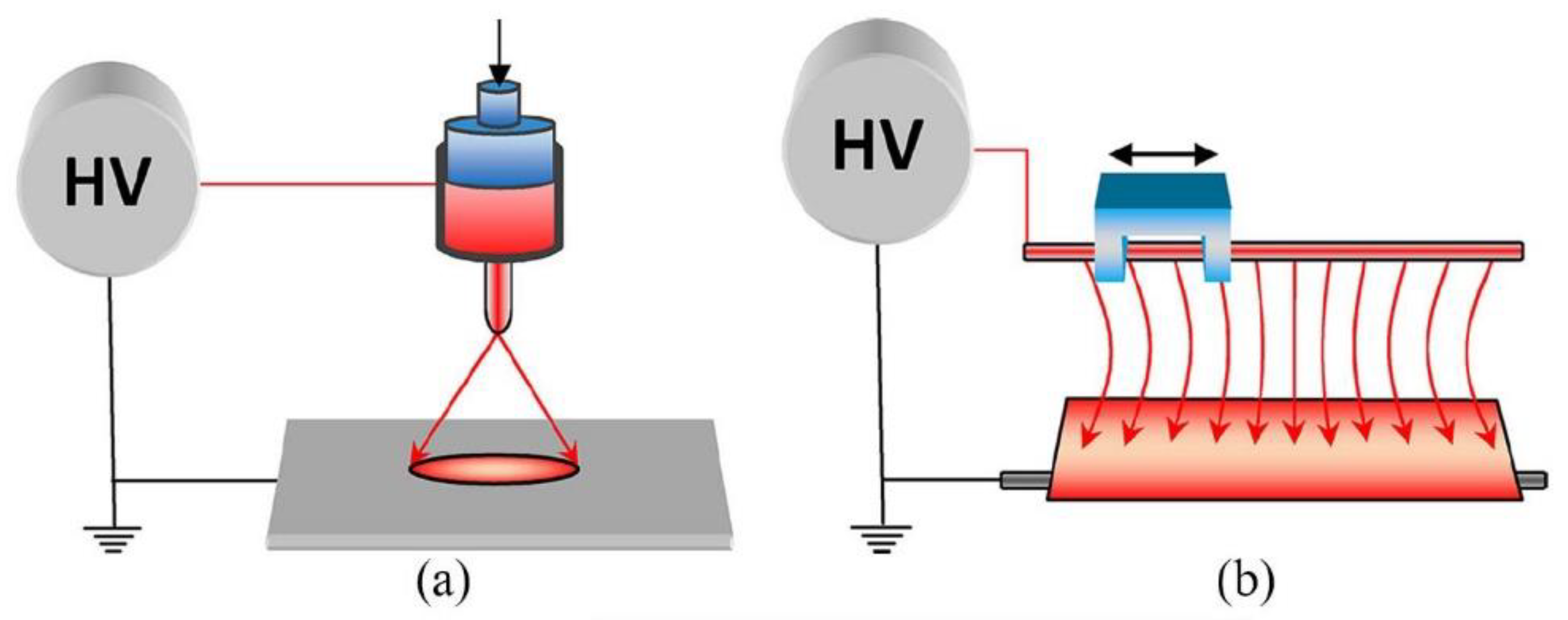
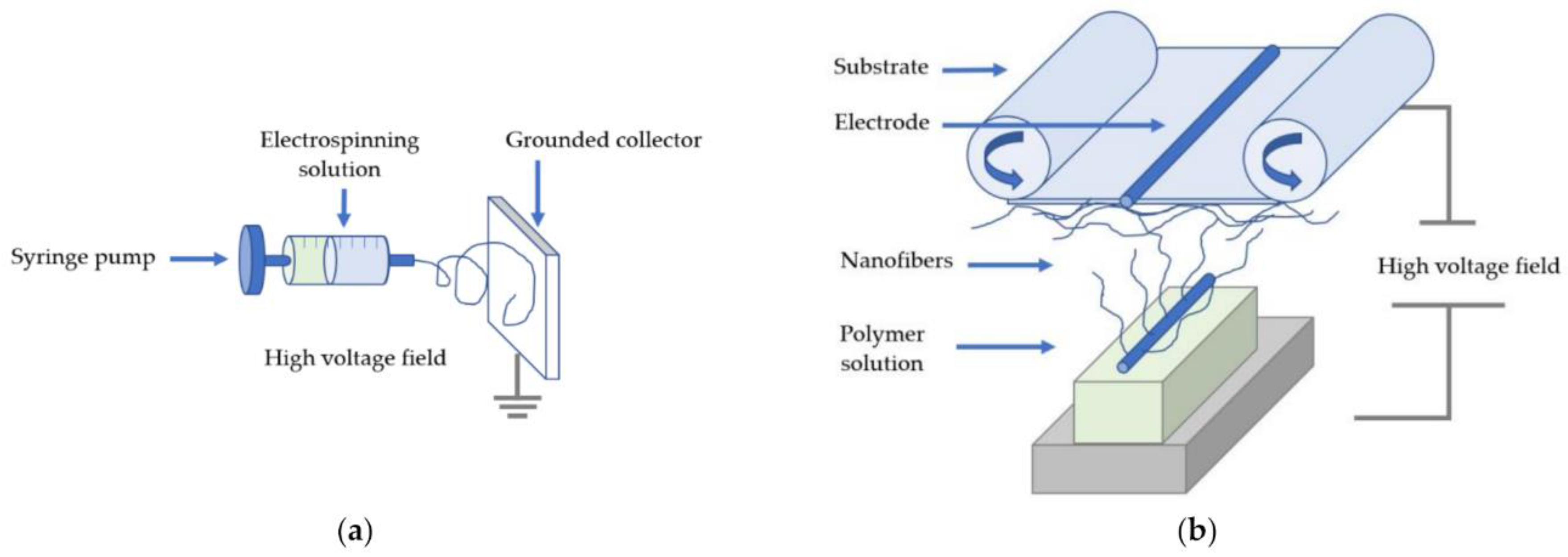
2. Electrospinning
2.1. Definition and Technical Setup
2.2. Industrial Electrospinning Techniques
3. Chitin
3.1. Extraction Procedure of Chitin from Crustaceans
3.2. Chitin Resources In Several Types of Mushrooms
4. Chitosan
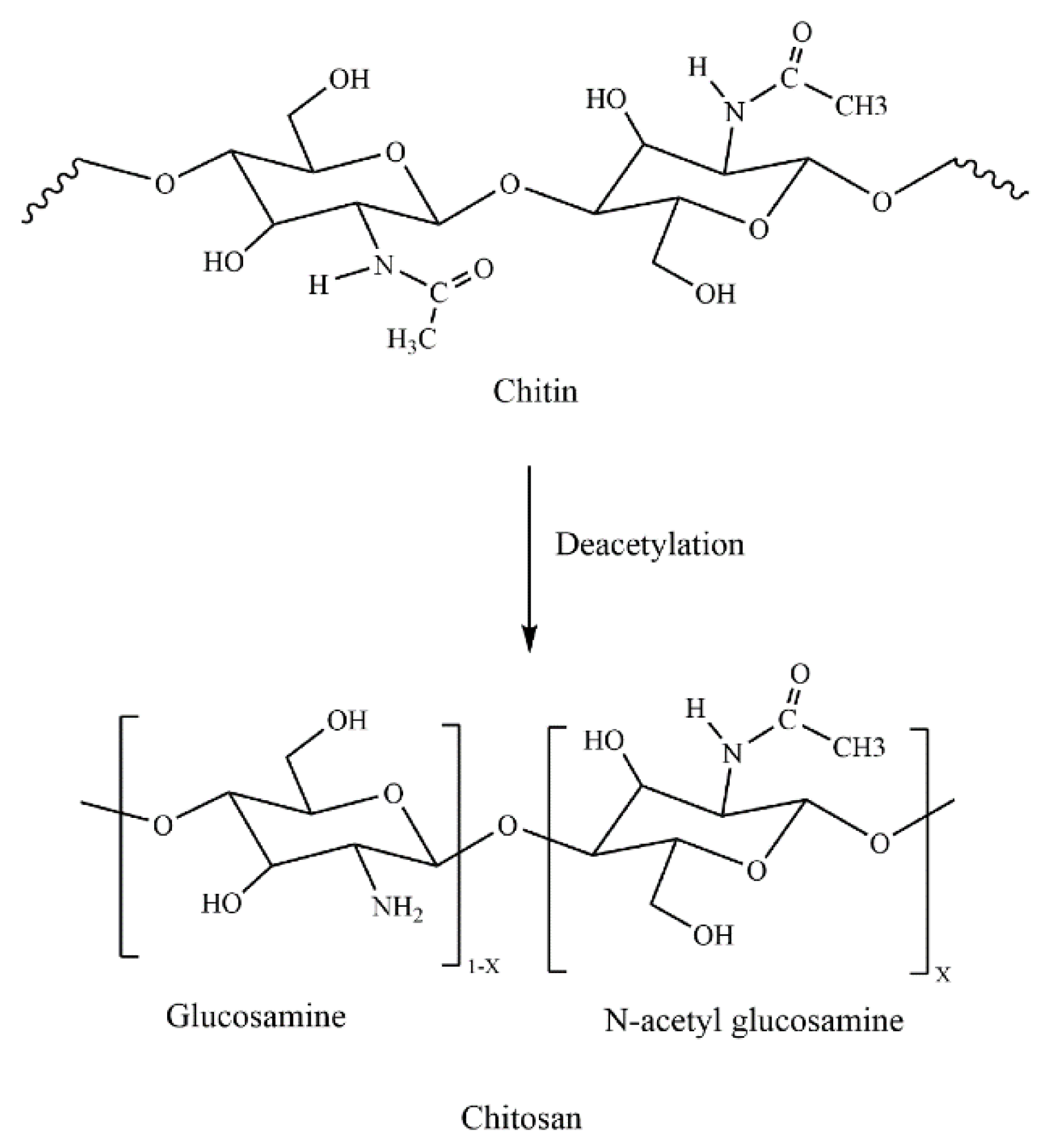
4.1. Parameters Influencing the Antibacterial Properties of Chitosan
4.1.1. pH of the Solution
4.1.2. Concentration
4.1.3. Molecular Weight
4.1.4. Degree of Deacetylation
5. Electrospinning of Chitosan
5.1. Voltage
5.2. Feed Rate
5.3. Distance
5.4. Viscosity
5.5. Molecular Weight
5.6. Concentration
5.7. Auxiliary Solvents and Composites
5.8. Discussions Regarding the Formation of Chitosan-Based Nanofibers for Defined Purposes
6. Antibacterial Effect of Chitosan Nanofibers
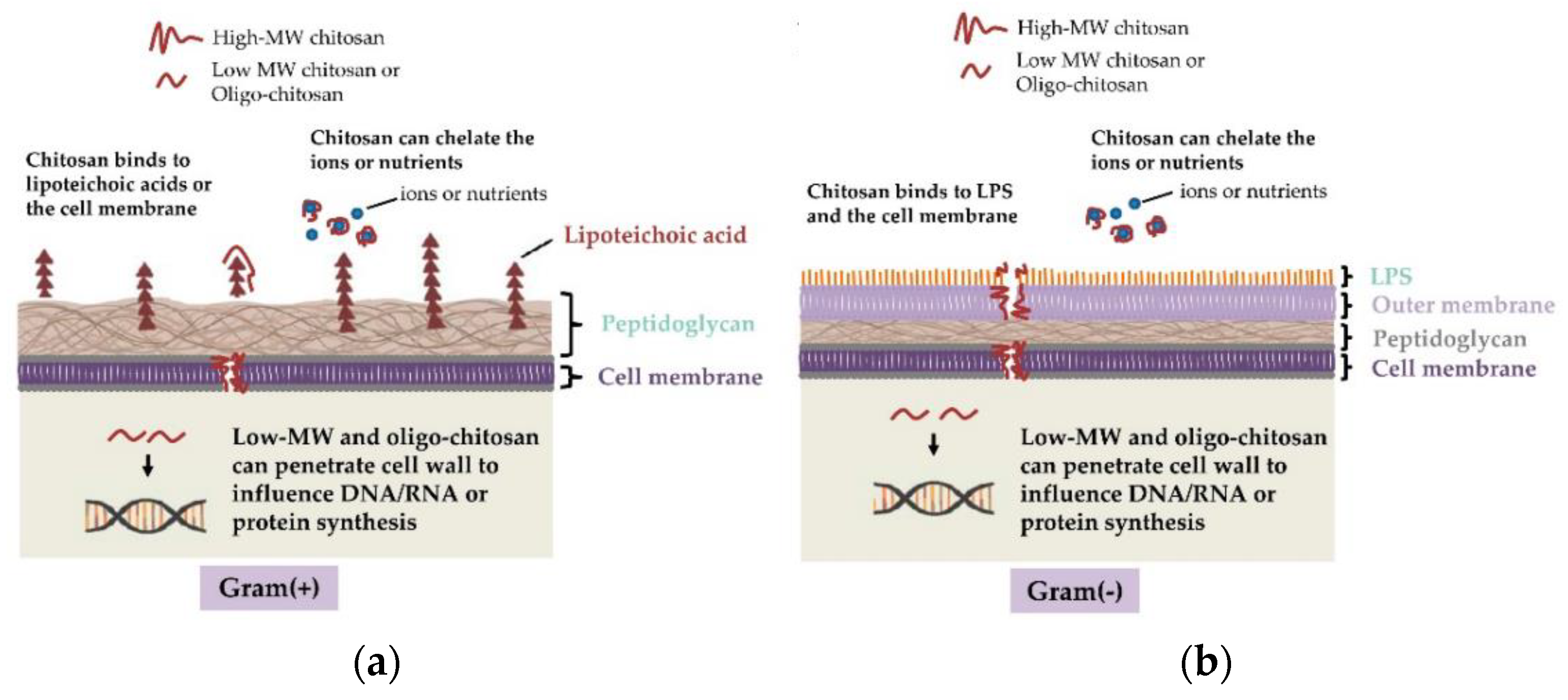
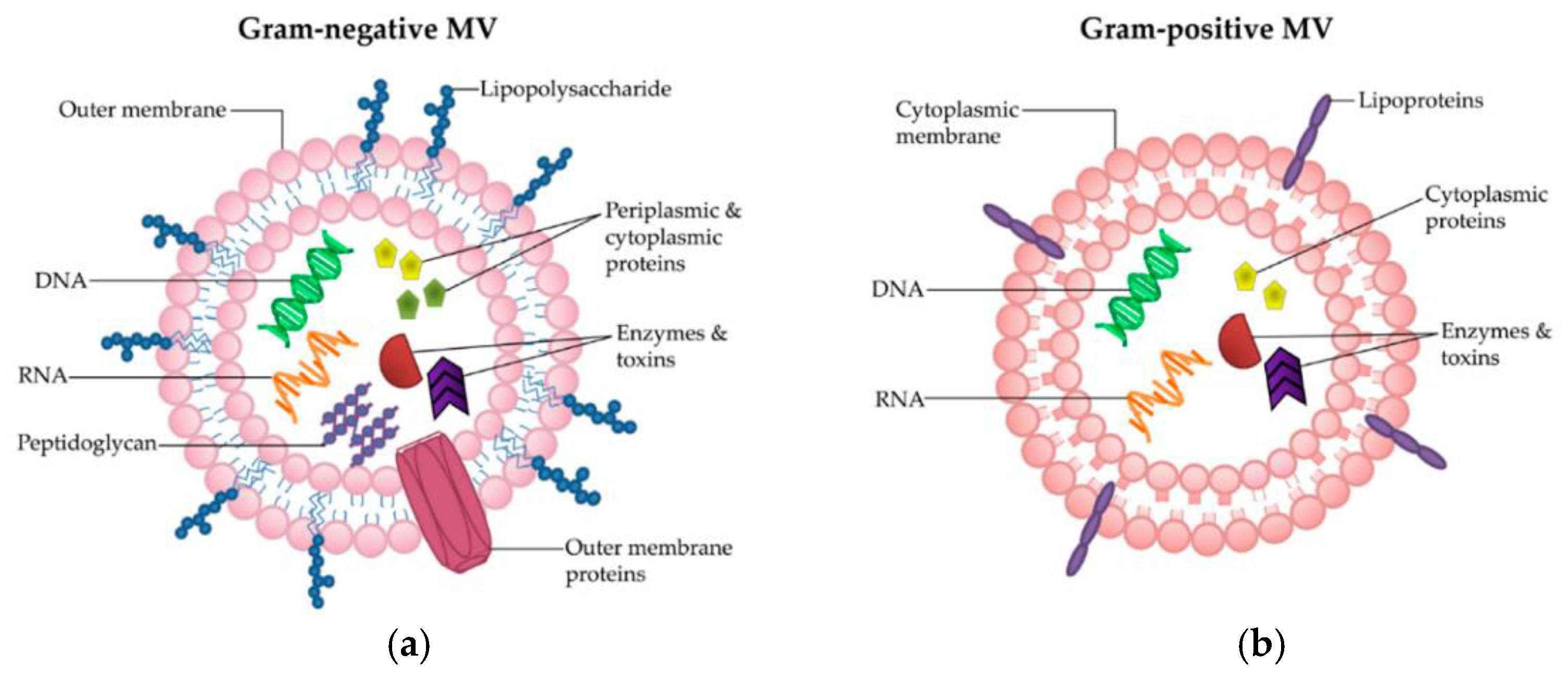
6.1. Mechanism of Antibacterial Activity of Chitosan
6.2. Susceptibility of Different Bacterial Strains to Chitosan
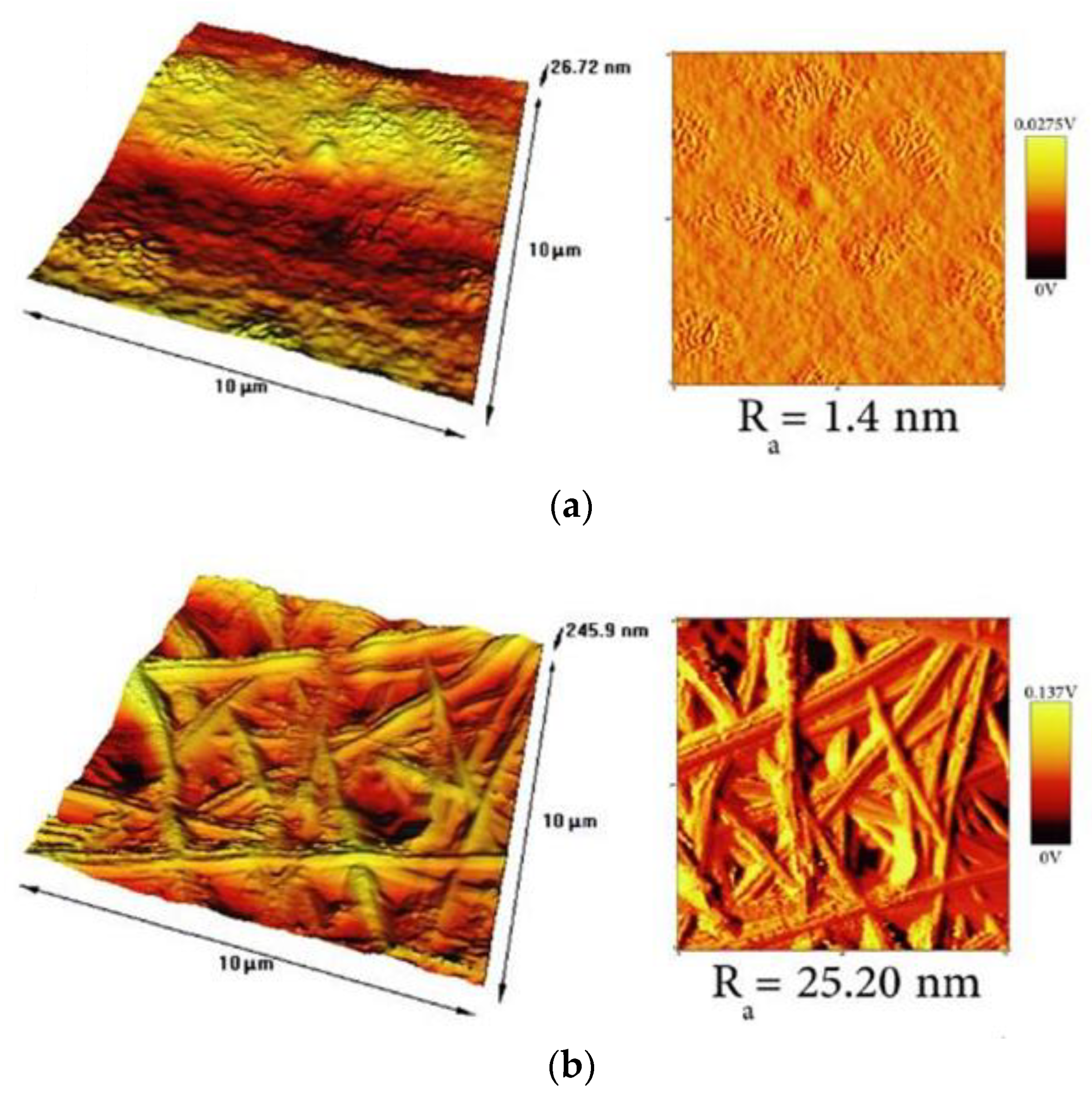
6.3. Effect of Surface Topography on the Antibacterial Activity
7. Application Fields of Electrospun Chitosan Nanofibers and Chitosan Derivatives
7.1. Water Filtration
7.1.1. Filtration Types and Membrane Criteria
- Microfiltration (MF) is extensively used in particle removal and degreasing processes;
- Ultrafiltration (UF) is a technique used for separating ions from oil, water, and emulsions without the use of chemicals. With UF, paints can be additionally recovered, and fats, oils, and greases from the food sector can be separated, which MF cannot remove. It can remove high-molecular-weight compounds, particles, colloids, and inorganic and organic polymers, but not low-molecular-weight ions such as magnesium chloride, calcium, or sodium [155];
- Reverse osmosis (RO) membranes are used as a popular filtering technique that removes large amounts of pollutants from water. A semipermeable membrane is used to separate dissolved solutes in this system. The distinction between RO membranes and other filtration membranes is that they do not need actual pores. These membranes are hydrophilic, which means that water may readily pass through them. They are easy to set up and provide for a high production capacity [156], but their limiting factors are biofouling and high transmembrane pressure requirements. The biological fouling increases as the number of eliminated microorganisms and other particles in water increases [157], which is one of the drawbacks of using RO membranes. Several studies have shown that at greater working pressures bacterial cells shrink in size, resulting in increased penetration through the filter. Therefore, to maintain antibacterial action during long-lasting application of the membrane, lower transmembrane pressure (TMP) is generally sought [8];
- Electrospun nanofibers provide the possibility of producing membranes of nanocomposites. The pore size and porosity as well as the antibacterial properties are adjustable by use of antibacterial components. Therefore, the easiest method to eliminate microorganisms, metal ions, and dyes while maintaining minimal pressure drops and high water flux is by nanofiber adsorption.
7.1.2. Electrospun Fibers from Chitosan–Polymer Mixtures for Water Filtration
7.1.3. Enhancing the Antibacterial Performance with Bactericidal Agents
7.2. Air Filtration
7.3. Food Storage
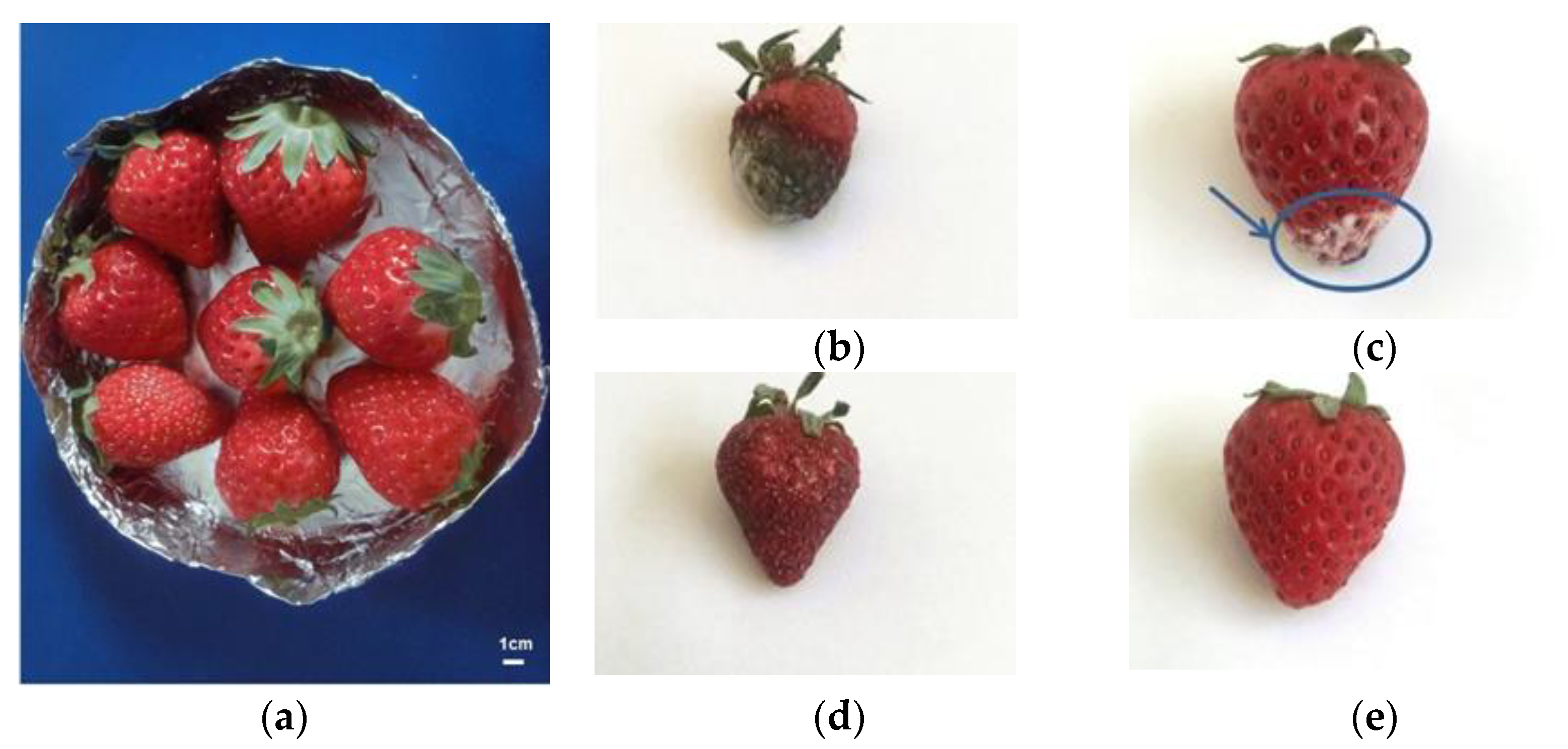
7.3.1. Fruit Freshness
7.3.2. Meat Shelf Life Prolongation
7.4. Biomedicine
7.4.1. Wound Dressings
7.4.2. Tissue Repair by Controlled Drug Release via Coaxial Electrospinning
7.5. Application Fields Summary
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozlowski, R.M.; Mackiewicz-Talarczyk, M. Handbook of Natural Fibres, 2nd ed.; Processing and Applications, The Textile Institute; Woodhead Publishing: Kidlington, UK, 2020; Volume 2. [Google Scholar]
- Storck, J.L.; Brockhagen, B.; Grothe, T.; Sabantina, L.; Kaltschmidt, B.; Tuvshinbayar, K.; Braun, L.; Tanzli, E.; Hütten, A.; Ehrmann, A. Stabilization and Carbonization of PAN Nanofiber Mats Electrospun on Metal Substrates. C 2021, 7, 12. [Google Scholar] [CrossRef]
- Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers 2021, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased Mechanical Properties of Carbon Nanofiber Mats for Possible Medical Applications. Fibers 2019, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Wehlage, D.; Blattner, H.; Mamun, A.; Kutzli, I.; Diestelhorst, E.; Rattenholl, A.; Gudermann, F.; Lütkemeyer, D.; Ehrmann, A. Cell growth on electrospun nanofiber mats from polyacrylonitrile (PAN) blends. AIMS Bioeng. 2020, 7, 43–54. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Tanzli, E.; Ehrmann, A. Electrospun Nanofibrous Membranes for Tissue Engineering and Cell Growth. Appl. Sci. 2021, 11, 6929. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Kou, Z.; Song, N.; Nie, G.; Wang, C.; Verpoort, F.; Mu, S. Rational design of electrospun nanofiber-typed electrocatalysts for water splitting: A review. Chem. Eng. J. 2022, 428, 131133. [Google Scholar] [CrossRef]
- Grimmelsmann, N.; Homburg, S.V.; Ehrmann, A. Electrospinning chitosan blends for nonwovens with morphologies between nanofiber mat and membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012007. [Google Scholar] [CrossRef] [Green Version]
- Nasreen, S.A.A.N.; Sundarrajan, S.; Nizar, S.A.S.; Balamurugan, R.; Ramakrishna, S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes 2013, 3, 266–284. [Google Scholar] [CrossRef]
- Yoshida, H.; Klinkhammer, K.; Matsusaki, M.; Möller, M.; Klee, D.; Akashi, M. Disulfide-Crosslinked Electrospun Poly(γ-glutamic acid) Non-wovens as Reduction-Responsive Scaffolds. Macromol. Biosci. 2009, 9, 568–574. [Google Scholar] [CrossRef]
- Klinkhammer, K.; Seiler, N.; Grafahrend, D.; Gerardo-Nava, J.; Mey, J.; Brook, G.A.; Möller, M.; Dalton, P.D.; Klee, D. Deposition of electrospun fibers on reactive substrates for in vitro investigations. Tissue Eng. Part C 2009, 15, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gerardo-Nava, J.; Führmann, T.; Klinkhammer, K.; Seiler, N.; Mey, J.; Klee, D.; Möller, M.; Dalton, P.D.; Brook, G.A. Human Neural Cell Interactions with Orientated Electrospun Nanofibers in vitro. Nanomedicine 2009, 4, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, A. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Ehrmann, A. Application of Electrospun Nanofibers for Fabrication of Versatile and Highly Efficient Electrochemical Devices: A Review. Polymers 2021, 13, 1741. [Google Scholar] [CrossRef]
- Kumar, A. (Ed.) Nanofibers; IntechOpen: London, UK, 2010; Volume 17. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Most recent developments in electrospun magnetic nanofibers: A review. J. Eng. Fibers Fabr. 2020, 15, 1558925019900843. [Google Scholar] [CrossRef]
- Dobrovolskaya, I.P.; Yudin, V.E.; Popryadukhin, P.P.; Ivan’kova, E.M.; Shabunin, A.C.; Kasatkin, I.A.; Morgantie, P. Effect of chitin nanofibrils on electrospinning of chitosan-based composite nanofibers. Carbohydr. Polym. 2018, 194, 260–266. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Naguib, H.F.; Morsi, R.E. Chitosan based nanofibers, review. Mater. Sci. Eng. C 2012, 32, 1711–1726. [Google Scholar] [CrossRef]
- Ditaranto, N.; Basoli, F.; Trombetta, M.; Cioffi, N.; Rainer, A. Electrospun Nanomaterials Implementing Antibacterial Inorganic Nanophases. Appl. Sci. 2018, 8, 1643. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Jia, X.; Liu, Q.; Kong, B.; Wang, H. Enhancing physical properties of chitosan/pullulan electrospinning nanofibers via green crosslinking strategies. Carbohydr. Polym. 2020, 247, 116734. [Google Scholar] [CrossRef]
- Patel, K.D.; Padalhin, A.R.; Franco, R.A.G.; Verisqa, F.; Kim, H.W.; Nguyen, L. Chapter One—Basic concepts and fundamental insights into electrospinning. In Biomedical Applications of Electrospinning and Electrospraying; Woodhead Publishing Series in Biomaterials; Kasoju, N., Ye, H., Eds.; Woodhead Publishing: Kidlington, UK, 2021; pp. 3–43. [Google Scholar] [CrossRef]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Effects of Solution Concentration, Emitting Electrode Polarity, Solvent Type, and Salt Addition on Electrospun Polyamide-6 Fibers: A Preliminary Report. Macromol. Symp. 2004, 216, 293–299. [Google Scholar] [CrossRef]
- Han, Y.; Shi, C.; Cui, F.; Chen, Q.; Tao, Y.; Li, Y. Solution properties and electrospinning of polyacrylamide and ε-polylysine complexes. Polymer 2020, 204, 122806. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Yi, X.; Duan, H. Morphological changes of nanofiber cross-sections due to surface tension. Extrem. Mech. Lett. 2021, 44, 101211. [Google Scholar] [CrossRef]
- Munir, M.W.; Ali, U. Classification of electrospinning methods, Morteza Sasani Ghamsari and Soumen Dhara. In Nanorods Nanocomposites; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/69037 (accessed on 13 December 2021). [CrossRef] [Green Version]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Lin, T. Needleless electrospinning: A practical way to mass production of nanofibers. J. Text. Sci. Eng. 2012, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mamun, A.; Blachowicz, T.; Sabantina, L. Electrospun Nanofiber Mats for Filtering Applications—Technology, Structure and Materials. Polymers 2021, 13, 1368. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achinivu, E.C.; Shamshina, J.L.; Rogers, R.D. Chitin extracted from various biomass sources: It’s not the same. Fluid Phase Equilibria 2021, 552, 113286. [Google Scholar] [CrossRef]
- Rinaudo, M. Materials based on chitin and chitosan. In Bio-Based Plastics: Materials and Applications; Kabasci, S., Ed.; John Wiley Sons: Chichester, UK, 2014; pp. 63–80. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Amery, F.; Ommeslag, S.; Vanhoutte, K.; Visser, R.; Robbens, J.; De Tender, C.; Debode, J. Chemically versus thermally processed brown shrimp shells or Chinese mitten crab as a source of chitin, nutrients or salts and as microbial stimulant in soilless strawberry cultivation. Sci. Total Environ. 2021, 771, 145263. [Google Scholar] [CrossRef]
- Álvarez, S.P.O.; Cadavid, D.A.R.; Sierra, D.M.E.; Orozco, C.P.O.; Vahos, D.F.R.; Ocampo, P.Z.; Atehortùa, L. Comparison of Extraction Methods of Chitin from Ganoderma lucidum Mushroom Obtained in Submerged Culture. BioMed Res. Int. 2014, 2014, 169071. [Google Scholar] [CrossRef] [Green Version]
- Vetter, J. Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem. 2007, 102, 6–9. [Google Scholar] [CrossRef]
- Mushi, N.E. A review on native well-preserved chitin nanofibrils for materials of high mechanical performance. Int. J. Biol. Macromol. 2021, 178, 91–606. [Google Scholar] [CrossRef]
- Nawawi, W.M.F.W.; Lee, K.Y.; Kontturi, E.; Murphy, R.J.; Bismarck, A. Chitin Nanopaper from Mushroom Extract: Natural Composite of Nanofibers and Glucan from a Single Biobased Source. ACS Sustain. Chem. Eng. 2019, 7, 6492–6496. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Sams, C.E. Chitin and chitosan value-added products from mushroom waste. J. Agric. Food Chem. 2004, 52, 7905–7910. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.T.; Mau, J.L. Selected physical properties of chitin prepared from shiitake stipes. LWT—Food Sci. Technol. 2007, 40, 558–563. [Google Scholar] [CrossRef]
- Zapata, P.A.; Rojas, D.F.; Atehortua, L. Production of biomass, polysaccharides, and ganoderic acid using nonconventional carbon sources under submerged culture of the lingzhi or reishi medicinal mushroom, Ganoderma lucidum (W.Curt.:Fr.) P. Karst., (higher basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 197–203. [Google Scholar] [CrossRef]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2015, 138, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.; Boudrant, J.; Meyer, D.; Manno, N.; De Marchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Negoi, A.E.; Cristea, C.D.; Deșliu-Avram, M.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Extraction of Fungal Chitin Using Natural Deep Eutectic Solvents. Proceedings 2019, 29, 91. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, M.; Watanabe, A.; Asada, Y. Isolation, characterization, and expression analysis of a class IV chitin synthase gene from the edible basidiomycetous mushroom Pleurotus ostreatus. Mycoscience 2007, 48, 176–181. [Google Scholar] [CrossRef]
- Erdoğan, S.; Kaya, M.; Akata, I. Chitin extraction and chitosan production from cell wall of two mushroom species (Lactarius vellereus and Phyllophora ribis). AIP Conf. Proc. 2017, 1809, 020012. [Google Scholar] [CrossRef] [Green Version]
- Doğan, H.H.; Aydin, D. Some biological activities of Lactarius vellereus ın Turkey. Biol. Sci. 2013, 16, 1279–1286. [Google Scholar] [CrossRef] [Green Version]
- Khajavian, M.; Vatanpour, V.; Castro-Muñoz, R.; Boczkaj, G. Chitin and derivative chitosan-based structures—Preparation strategies aided by deep eutectic solvents: A review. Carbohydr. Polym. 2021, 275, 118702. [Google Scholar] [CrossRef]
- Lv, S.H. 7—High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Kidlington, UK, 2016; pp. 131–150. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, P.; Lv, X.; Liang, Y. Insight into the structure-function relationships of the solubility of chitin/chitosan in natural deep eutectic solvents. Mater. Today Commun. 2021, 27, 102374. [Google Scholar] [CrossRef]
- Yeul, V.S.; Rayalu, S.S. Unprecedented chitin and chitosan: A chemical overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Panariello, L.; Coltelli, M.B.; Buchignani, M.; Lazzeri, A. Chitosan and nano-structured chitin for biobased anti-microbial treatments onto cellulose based materials. Eur. Polym. J. 2019, 113, 328–339. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and characterization of chitin and chitosan—A review. J. Aquat. Food Prod. Technol. 1995, 2, 27–52. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; You, X.; Li, Q.; Qin, D.; Wang, M.; Yuan, S.; Chen, X.; Bi, S. Homogeneous deacetylation and degradation of chitin in NaOH/urea dissolution system. Int. J. Biol. Macromol. 2021, 189, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manag. 2022, 301, 113850. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Mi, Y.; Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of the acetylated chitosan derivatives containing sulfonium salts. Int. J. Biol. Macromol. 2020, 152, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.; Knoop, R.J.; Kappen, F.H.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Chenite, A.; Felt-Baeyens, O.; Mayer, J.M.; Gurny, R. Erratum to Pseudo-thermosetting chitosan hydrogels for biomedical application. Int. J. Pharm. 2005, 28, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jochelavicius, K.; Pereira, A.R.; Fiamingo, A.; Nobre, T.M.; Campana-Filho, S.P.; Oliveira, O.N. Chitosan effects on monolayers of zwitterionic, anionic and a natural lipid extract from E. coli at physiological pH. Colloids Surf. B Biointerfaces 2022, 209, 112146. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular interactions of chitosan: Degree of acetylation and molecular weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitosan-based dietary foods. Carbohydr. Polym. 1996, 29, 309–316. [Google Scholar] [CrossRef]
- Li, L.H.; Deng, J.C.; Deng, H.R.; Liu, Z.L.; Li, X.L. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem. Eng. J. 2010, 160, 378–382. [Google Scholar] [CrossRef]
- Reesha, K.V.; Panda, S.K.; Bindu, J.; Varghese, T.O. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhong, Z.; Zhao, Z. Preparation, characterization and antimicrobial activities of cyclic substituted chitosan derivatives. Int. J. Biol. Macromol. 2021, 193, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Cerón, A.A.; Nascife, L.; Norte, S.; Costa, S.A.; Oliveira do Nascimento, J.H.; Morisso, F.D.P.; Baruque-Ramos, J.; Oliveira, R.C.; Costa, S.M. Synthesis of chitosan-lysozyme microspheres, physicochemical characterization, enzymatic and antimicrobial activity. Int. J. Biol. Macromol. 2021, 185, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McClements, D.J. Characterization of β-lactoglobulin-chitosan interactions in aqueous solutions: A calorimetry, light scattering, electrophoretic mobility and solubility study. Food Hydrocoll. 2006, 20, 124–131. [Google Scholar] [CrossRef]
- Huang, P.; Huang, C.; Ma, X.; Gao, C.; Sun, F.; Yang, N.; Nishinari, K. Effect of pH on the mechanical, interfacial, and emulsification properties of chitosan microgels. Food Hydrocoll. 2021, 121, 106972. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Tikhonov, V.E.; Bezrodnykh, E.A.; Lopatin, S.A.; Varlamov, V.P. Comparative evaluation of antimicrobial activity of oligochitosans against Klebsiella pneumoniae. Russ. J. Bioorg. Chem. 2015, 41, 57–62. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Lim, C.S.; Azlan, A.Y.H.N.; Buang, F.; Busra, M.F.M. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cheng, X.; Liu, C.; Xing, K.; Zhang, J.; Sun, G.; Li, X.; Chen, X. Preparation, characterization, and antibacterial activity of oleic acid-grafted chitosan oligosaccharide nanoparticles. Front. Biol. China 2009, 4, 321–327. [Google Scholar] [CrossRef]
- Anitha, A.; Rani, V.D.; Krishna, R.; Sreeja, V.; Selvamurugan, N.; Nair, S.; Tamura, H.; Jayakumar, R. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O-carboxymethyl and N,Ocarboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2009, 78, 672–677. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT—Food Sci. Technol. 2016, 71, 347–355. [Google Scholar] [CrossRef]
- El-tahlawy, K.F.; El-bendary, M.A.; Elhendawy, A.G.; Hudson, S.M. The antimicrobial activity of cotton fabrics treated with different crosslinking agents and chitosan. Carbohydr. Polym. 2005, 60, 421–430. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Junior, L.M.; de Souza, W.F.C.; Sato, H.H.; Alves, R.M.V.; Vieira, R.P. O-ATRP synthesized poly(β-pinene) blended with chitosan for antimicrobial and antioxidant bio-based films production. Int. J. Biol. Macromol. 2021, 193, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight-activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Bezrodnykh, E.A.; Antonov, Y.A.; Berezin, B.B.; Kulikov, S.N.; Tikhonov, W.E. Molecular features of the interaction and antimicrobial activity of chitosan in a solution containing sodium dodecyl sulfate. Carbohydr. Polym. 2021, 270, 118352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Feng, X.Q.; Yang, S.; Fu, G.Q.; Wanga, T.P.; Su, Z.X. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Eid, A.R.; Badr, M.M.; Marei, G.I.K. Structure and antimicrobial comparison between N-(benzyl) chitosan derivatives and N-(benzyl) chitosan tripolyphosphate nanoparticles against bacteria, fungi, and yeast. Int. J. Biol. Macromol. 2021, 186, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Niu, W.; Dong, Y.; Fu, Z.; Lv, J.; Wang, L.; Zhang, Z.; Huo, J.; Ju, J. Effects of molecular weight of chitosan on anti-inflammatory activity and modulation of intestinal microflora in an ulcerative colitis model. Int. J. Biol. Macromol. 2021, 193, 1927–1936. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf. B 2008, 65, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, M.; Khan, I.M.; Niazi, S.; Wang, B.; Ma, X.; Wang, Z.; Xia, W. Preparation and characterization of chitosan oligosaccharide derivatives containing cinnamyl moieties with enhanced antibacterial activities. LWT 2021, 147, 111663. [Google Scholar] [CrossRef]
- Affes, S.; Aranaz, I.; Acosta, N.; Heras, A.; Nasri, M.; Maalej, H. Chitosan derivatives-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 730–742. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Tien, N.D.; Lyngstadaas, S.P.; Mano, J.F.; Blaker, J.J.; Haugen, H.J. Recent Developments in Chitosan-Based Micro/Nanofibers for Sustainable Food Packaging, Smart Textiles, Cosmeceuticals, and Biomedical Applications. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.C.; Ajji, A. Antibacterial electrospun chitosan-based nanofibers: A bacterial membrane perforator. Food Sci. Nutr. 2017, 5, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Diestelhorst, E.; Mance, F.; Mamun, A.; Ehrmann, A. Chemical and morphological modification of PAN nanofibrous mats with addition of casein after electrospinning, stabilization and carbonization. Tekstilec 2020, 63, 38–49. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Conductive Electrospun Nanofiber Mats. Materials 2020, 13, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Grimmelsmann, N.; Grothe, T.; Homburg, S.V.; Ehrmann, A. Electrospinning and stabilization of chitosan nanofiber mats. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102006. [Google Scholar] [CrossRef]
- Sabantina, L.; Mirasol, J.R.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Investigation of Needleless Electrospun PAN Nanofiber Mats. AIP Conf. Ser. 2018, 1952, 020085. [Google Scholar] [CrossRef]
- Knidri, H.E.; Laajeb, A.; Lahsini, A. Chapter 2—Chitin and chitosan: Chemistry, solubility, fiber formation, and their potential applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Kidlington, UK, 2020; pp. 35–57. [Google Scholar] [CrossRef]
- Banner, J.; Dautzenberg, M.; Feldhans, T.; Hofmann, J.; Plümer, P.; Ehrmann, A. Water Resistance and Morphology of Electrospun Gelatine Blended with Citric Acid and Coconut Oil. Tekstilec 2018, 61, 129–135. [Google Scholar] [CrossRef]
- Coelho, S.C.; Benaut, P.; Laget, S.; Estevinho, B.N.; Rocha, F. Optimization of electrospinning parameters for the production of zein microstructures for food and biomedical applications. Micron 2022, 152, 103164. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Venkatraman, S.S. Importance of viscosity parameters in electrospinning: Of monolithic and core–shell fibers. Mater. Sci. Eng. C 2012, 32, 1037–1042. [Google Scholar] [CrossRef]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Liu, S.; Hua, T.; Luo, X.; Yi Lam, N.; Tao, X.-m.; Li, L. A novel approach to improving the quality of chitosan blended yarns using static theory. Text. Res. J. 2014, 85, 1022–1034. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Thien, D. Electrospun chitosan/pva nanofibers for drug delivery. Vietnam J. Sci. Technol. 2018, 54, 185. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Li, C.; Miyauchi, Y.; Kuwaki, O.; Shiratori, S. Formation of novel 2D polymer nanowebs via electrospinning. Nanotechnology 2006, 17, 3685–3691. [Google Scholar] [CrossRef]
- Arkan, E.; Azandaryani, A.H.; Moradipour, P.; Behbood, L. Bio macromolecular based fibers in nanomedicine: A combination of drug delivery and tissue engineering. Curr. Pharm. Biotechnol. 2018, 18, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Perry, S.L.; Schiffman, J.D. Electrospinning nanofibers from chitosan/hyaluronic acid complex coacervates. Biomacromolecules 2019, 20, 4191–4198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.I.; Kim, C.H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, J.D.; Stulga, L.A.; Schauer, C.L. Chitin and Chitosan: Transformations due to the electrospinning process. Polym. Eng. Sci. 2009, 49, 1918–1928. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.H.; Ko, W.K.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.C.; et al. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef]
- Ma, G.; Liu, Y.; Fang, D.; Chen, J.; Peng, C.; Fei, X.; Nie, J. Hyaluronic acid/chitosan polyelectrolyte complexes nanofibers prepared by electrospinning. Mater. Lett. 2012, 74, 78–80. [Google Scholar] [CrossRef]
- Lam, N.Y.K.; Zhang, M.; Guo, H.F.; Ho, C.P.; Li, L. Effect of fiber length and blending method on the tensile properties of ring spun chitosan–cotton blend yarns. Text. Res. J. 2016, 87, 244–257. [Google Scholar] [CrossRef]
- Liu, Q.; Ouyang, W.C.; Zhou, X.H.; Jin, T.; Wu, Z.W. Antibacterial Activity and Drug Loading of Moxifloxacin-Loaded Poly(Vinyl Alcohol)/Chitosan Electrospun Nanofibers. Front. Mater. 2021, 8, 36. [Google Scholar] [CrossRef]
- Wawro, D.; Skrzetuska, E.; Włodarczyk, B.; Kowalski, K.; Kruci’nska, I. Processing of Chitosan Yarn into Knitted Fabrics. Fibres Text. East. Eur. 2016, 6, 52–57. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Molnár, K.; Szebényi, G.; Szolnoki, B.; Marosi, G.; Vas, L.M.; Toldy, A. Enhanced conductivity composites for aircraft applications: Carbon nanotube inclusion both in epoxy matrix and in carbonized electrospun nanofibers. Polym. Adv. Technol. 2014, 25, 981–988. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Peksel, A. Fabrication of PVA-chitosan-based nanofibers for phytase immobilization to enhance enzymatic activity. Int. J. Biol. Macromol. 2020, 164, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Ahmed, M.K. Wound healing activity of Chitosan/ Polyvinyl Alcohol embedded by gold nanoparticles prepared by nanosecond laser ablation. J. Mol. Struct. 2020, 1217, 128401. [Google Scholar] [CrossRef]
- Bao, D.; Liu, L.; Sun, T.; Han, Y.; Meng, F.; Zhao, M.; Yu, Y.; Guo, J.; Zhang, S. Solid solid phase change (SSPC) chitosan-g-mPEG fiber with improved mechanical performance via in-situ wet spinning process. Carbohydr. Polym. 2020, 240, 116313. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K.S.; Cha, D.; Kim, H.Y.; Nishida, A.K.; Yamamoto, H.Y. Electrospinning of chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship on electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Au, H.T.; Pham, L.N.; Vu, T.H.T.; Park, J.S. Fabrication of an antibacterial non-woven mat of a poly(lactic acid)/chitosan blend by electrospinning. Macromol. Res. 2012, 20, 51–58. [Google Scholar] [CrossRef]
- Alipour, S.M.; Nouri, M.; Mokhtari, J.; Bahrami, S.H. Electrospinning of poly(vinyl alcohol)–water-soluble quaternized chitosan derivative blend. Carbohydr. Res. 2009, 344, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Camacho, A.; Cortez-Rocha, M.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, W. Preparation, characterization and antibacterial activity of water-soluble O-fumaryl-chitosan. Carbohydr. Polym. 2011, 83, 1169–1173. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Hild, M.; Toskas, G.; Aibibu, D.; Wittenburg, G.; Meissner, H.; Cherif, C.; Hund, R.-D. Chitosan/gelatin micro/nanofiber 3D composite scaffolds for regenerative medicine. Compos. Interfaces 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Li, B.; Logan, B.E. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf. B Biointerfaces 2004, 36, 81–90. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Bitto, N.J.; Kaparakis-Liaskos, M. The Therapeutic Benefit of Bacterial Membrane Vesicles. Int. J. Mol. Sci. 2017, 18, 1287. [Google Scholar] [CrossRef] [Green Version]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Tao, Y.; Qian, L.H.; Xie, J. Effect of chitosan on membrane permeability and cell morphology of Pseudomonas aeruginosa and Staphylococcus aureus. Carbohydr. Polym. 2011, 86, 969–974. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Tomás, J.M.; Ciurana, B.; Benedí, V.J.; Juarez, A. Role of lipopolysaccharide and complement in susceptibility of Escherichia coli and Salmonella typhimurium to non-immune serum. Microbiology 1988, 134, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Shahriari, S.; Monajjemi, M.; Zare, K. Increasing the efficiency of some antibiotics on penetrating bacteria cell membrane. Ukr. J. Ecol. 2018, 8, 671–679. [Google Scholar] [CrossRef]
- Nokhasteh, A.S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Sadeghi-Avalshahr, A. Preparation of PVA/Chitosan samples by electrospinning and film casting methods and evaluating the effect of surface morphology on their antibacterial behavior. Mater. Res. Express 2019, 7, 015401. [Google Scholar] [CrossRef]
- Bazaka, K.; Crawford, R.J.; Ivanova, E.P. Do bacteria differentiate between degrees of nanoscale surface roughness. Biotechnol. J. 2011, 6, 1103–1114. [Google Scholar] [CrossRef]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun polystyrene fiber diameter influencing bacterial attachment, proliferation, and growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; De la Rua, A.; Garralon, G.; Plaza, F.; Hontoria, E.; Gomez, M.A. Urban wastewater disinfection by filtration technologies. Desalination 2006, 190, 16–28. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, J.; Menkhaus, T.J.; Varadaraju, H.; Sun, Y.; Fong, H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J. Membr. Sci. 2011, 369, 499–505. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Liu, L.Q.; Zhang, Z. Needleless electro-spun nanofibers used for filtration of small particles. Express Polym. Lett. 2013, 7, 683–689. [Google Scholar] [CrossRef]
- Wang, J.; Pan, K.; Giannelis, E.P.; Cao, B. Polyacrylonitrile/polyaniline core–shell nanofiber mat for removal of hexavalent chromium from aqueous solution: Mechanism and applications. RSC Adv. 2013, 3, 8978–8987. [Google Scholar] [CrossRef]
- Qin, X.H.; Wang, S.Y. Electrospun Nanofibers from Crosslinked Poly (vinyl alcohol) and Its Filtration Efficiency. J. Appl. Polym. Sci. 2008, 109, 951–956. [Google Scholar] [CrossRef]
- Gafri, H.S.F.; Zuki, F.M.; Aroua, M.K.; Hashim, N.A. Mechanism of bacterial adhesion on ultrafiltration membrane modified by natural antimicrobial polymers (chitosan) and combination with activated carbon (PAC). Rev. Chem. Eng. 2018, 35, 421–443. [Google Scholar] [CrossRef]
- Wenten, G.I. Recent development in membrane science and its industrial applications. Membr. Sci. Technol. 2002, 24, 1009–1025. [Google Scholar]
- Pandey, S.J.; Jegatheesan, V.; Baskaran, K.; Shu, L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: A review. Rev. Environ. Sci. Biotechnol. 2012, 11, 125–145. [Google Scholar] [CrossRef]
- Ghani, M.; Gharehaghaji, A.A.; Arami, M.; Takhtkuse, N.; Rezaei, B. Fabrication of electrospun polyamide-6/chitosan nanofibrous membrane toward anionic dyes removal. J. Nanotechnol. 2014, 2014, 278418. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, M.; Moya, W.; Tkacik, G. Removal of Microorganisms from Samples Using Nanofibers Filtration Media. U.S. Patent No. US 9,889,214 B2, 13 February 2018. [Google Scholar]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Jabur, A.R.; Abbas, L.K.; Moosa, S.A. Fabrication of Electrospun Chitosan/Nylon 6 Nanofibrous Membrane toward Metal Ions Removal and Antibacterial Effect. Adv. Mater. Sci. Eng. 2016, 2016, 5810216. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.; Oldinski, R.; Ma, H.; Bryers, J.D.; Miqin, M. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohyd Polym 2013, 92, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.J.; Araújo, P.A.; Correia, P.B.; Ramsey, M.M.; Kruithof, J.C.; van Loosdrecht, M.C.M.; Freeman, B.D.; Paul, D.R.; Whiteley, M.; Vrouwenvelder, J.S. Short-term adhesion and long-term biofouling testing of polydopamine and poly(ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water Res. 2012, 46, 3737–3753. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Davidson, P.M.; Zivanovic, S.; Meyer, H. Nanofibrous chitosan non-wovens for filtration applications. Polymer 2009, 50, 3661–3669. [Google Scholar] [CrossRef]
- Felse, P.A.; Panda, T. Studies on applications of chitin and its derivatives. Bioprocess Eng. 1999, 20, 505–512. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food application of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Xiao, B.; Wan, Y.Y.; Zhao, M.; Liu, Y.; Zhang, S. Preparation and characterization of antimicrobial chitosan-N-arginine with different degrees of substitution. Carbohydr. Polym. 2011, 83, 144–150. [Google Scholar] [CrossRef]
- Higazy, A.; Hashem, M.; Elshafei, A.; Shaker, N.; Hady, M.A. Development of antimicrobial jute packaging using chitosan and chitosan-metal complex. Carbohydr. Polym. 2010, 79, 867–874. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, H.; Ye, H.; Li, J.; Huang, J. Preparation, antibacterial, and antioxidant activities of silver/chitosan composites. J. Carbohydr. Chem. 2014, 33, 298–312. [Google Scholar] [CrossRef]
- Sanpui, P.; Murugadoss, A.; Prasad, P.V.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- Cao, X.L.; Cheng, C.; Ma, Y.L.; Zhao, C.S. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med. 2010, 21, 2861–2868. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Bezir, N.C.; Bozkurt, B.; Evcin, A.; Özcan, B.; Kır, E.; Akarca, G.; Ceylan, O. Enhanced antibacterial activity of silver-doped chitosan nanofibers. AIP Conf. Proc. 2019, 2178, 030003. [Google Scholar] [CrossRef]
- Potara, M.; Jakab, E.; Damert, A.; Popescu, O.; Canpean, V.; Astilean, S. Synergistic antibacterial activity of chitosan–silver nanocomposites on Staphylococcus aureus. Nanotechnology 2011, 22, 135101. [Google Scholar] [CrossRef] [PubMed]
- Adibzadeh, S.; Bazgir, S.; Katbab, A.-A. Fabrication and characterization of chitosan/poly(vinyl alcohol) electrospun nanofibrous membranes containing silver nanoparticles for antibacterial water filtration. Iran. Polym. J. 2014, 23, 645–654. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.; Kumar, D.; Mukherjee, A.; Malakar, D.; Kalarikka, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Zhang, C.; Li, P. Characterisation and cooperative antimicrobial properties of chitosan/nano-ZnO composite nanofibrous membranes. Food Chem. 2012, 132, 419–427. [Google Scholar] [CrossRef]
- Makaremi, M.; Lim, C.X.; Pasbakhsh, P.; Lee, S.M.; Goh, K.-L.; Chang, H.; Chan, E.S. Electrospun Functionalized Polyacrylonitrile-Chitosan Bi-layer Membranes for Water Filtration Applications. RSC Adv. 2016, 59, 53882–53893. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Yue, Y.; He, W.; Jiang, F.; Lin, C.-H.; Pui, D.Y.H.; Liang, Y.; Wang, J. The antibacterial performance of positively charged and chitosan dipped air filter media. Build. Environ. 2020, 180, 107020. [Google Scholar] [CrossRef]
- Dotti, F.; Varesano, A.; Montarsolo, A.; Aluigi, A.; Tonin, C.; Mazzuchetti, G. Electrospun Porous Mats for High Efficiency Filtration. J. Ind. Text. 2007, 37, 151–162. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Feng, G.; Gang, P. Needleless electrospinning for scaled-up production of ultrafine chitosan hybrid nanofibers used for air filtration. RSC Adv. 2016, 6, 105988–105995. [Google Scholar] [CrossRef] [Green Version]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Caner, C. The effect of edible eggshell coatings on egg quality and consumer perception. J. Sci. Food Agric. 2005, 85, 1897–1902. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef] [PubMed]
- De Farias, B.S.; Cadaval Jr, T.R.S.A.; de Almeida Pinto, L.A. Chitosan-functionalized nanofibers: A comprehensive review on challenges and prospects for food applications. Int. J. Biol. Macromol. 2019, 123, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Farhoodi, M. Nanocomposite Materials for Food Packaging Applications: Characterization and Safety Evaluation. Food Eng. Rev. 2016, 8, 35–51. [Google Scholar] [CrossRef]
- Yue, T.-T.; Li, X.; Wang, X.-X.; Xu, Y.; Yu, M.; Ma, J.-W.; Zhou, Y.; Ramakrishna, S.; Long, Y.-Z. Electrospinning of Carboxymethyl Chitosan/Polyoxyethylene Oxide Nanofibers for Fruit Fresh-Keeping. Nanoscale Res. Lett. 2018, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of Action of Electrospun Chitosan-Based Nanofibers against Meat Spoilage and Pathogenic Bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun Non-Woven Nanofibrous Hybrid Mats Based on Chitosan and PLA for Wound-Dressing Applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Nie, J.; Yang, D. Electrospun poly(lactic acid)/chitosan core–shell structure nanofibers from homogeneous solution. Carbohydr. Polym. 2012, 90, 1445–1451. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Coopman, K.; Georgiadou, S. Biocompatibility assessment of conducting PANI/chitosan nanofibers for wound healing applications. Polymers 2017, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Moutsatsou, P.; Coopman, K.; Georgiadou, S. Chitosan & Conductive PANI/Chitosan Composite Nanofibers—Evaluation of Antibacterial Properties. Curr. Nanomater. 2019, 4, 6–20. [Google Scholar] [CrossRef]
- Cai, Z.X.; Mo, X.M.; Zhang, K.H.; Fan, L.-P.; Yin, A.-L.; He, C.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol. 2020, 160, 352–363. [Google Scholar] [CrossRef]
- Trinca, R.B.; Westin, C.B.; Fracassi da Silva, J.A.; Moraes, A.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Lin, T.-C.; Lin, F.-H.; Lin, J.-C. In vitro feasibility study of the use of a magnetic electrospun chitosan nanofiber composite for hyperthermia treatment of tumor cells. Acta Biomater. 2012, 8, 2704–2711. [Google Scholar] [CrossRef]
- An, J.; Zhang, H.; Zhang, J.; Zhao, Y.; Yuan, X. Preparation and antibacterial activity of electrospun chitosan/poly(ethylene oxide) membranes containing silver nanoparticles. Colloid Polym. Sci. 2009, 287, 1425–1434. [Google Scholar] [CrossRef]
- Zhuang, X.P.; Cheng, B.W.; Kang, W.M.; Xu, X. Electrospun chitosan/gelatin nanofibers containing silver nanoparticles. Carbohydr. Polym. 2010, 82, 524–527. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [Green Version]
- Abbaspour, M.; Makhmalzadeh, B.S.; Rezaee, B.; Shoja, S.; Ahangari, Z. Evaluation of the Antimicrobial Effect of Chitosan/Polyvinyl Alcohol Electrospun Nanofibers Containing Mafenide Acetate. Jundishapur J. Microbiol. 2015, 8, e24239. [Google Scholar] [CrossRef] [Green Version]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun matrices for localized drug delivery. Curr. Technol. Sel. Biomed. Appl. 2012, 81, 1–13. [Google Scholar] [CrossRef]
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R.M.; Fortunato, G. Nylon-6/chitosan core–shell antimicrobial nanofibers for the prevention of mesh-associated surgical site infection. J. Nanobiotechnol. 2020, 18, 51. [Google Scholar] [CrossRef]
- Asiedu-Gyekye, I.J.; Mahmood, A.S.; Awortwe, C.; Nyarko, A.K. Toxicological assessment of polyhexamethylene biguanide for water treatment. Interdiscip Toxicol. 2016, 8, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. ChemPlusChem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmad, A.; Khan, S.M.; Gul, S.-E.; Jamil, T.; Islam, A.; Hussain, T. Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan. Desalination 2014, 351, 59–69. [Google Scholar] [CrossRef]

| Water Filtration System | Technology | Species size | Filtered spezies (examples) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Microfiltration | Membrane | 0.1–5 µm [160] | Particles, large bacteria and pathogens, grease | Low-pressure filtration, low energy consumption, no chemicals required, low production costs [160] | (Bio)fouling reduces efficiency, pre-treatment and chemical cleaning are necessary [156,160] |
| Ultrafiltration | Membrane | 0.01–0.1 µm [160] | High-molecular weight material, colloids, particles, endotoxins, some viruses, oil | No chemicals required, low-pressure filtration, low energy consumption, low production costs [155,156] | (Bio)fouling limits performance, pre-desinfection or membrane modification is necessary [155] |
| Reverse Osmosis | Membrane | 0.0001–0.001 µm [160] | Salts, metal ions, solids, colloids, organics | Most important potable system, ultra-pure water [157] | (Bio)fouling limits performance, pre-treatment is necessary, eg. by UV-irradiation [157] |
| Nanofiltration | Nanofibers, nanofibrous membranes | 0.001–0.01 µm [160] | Particles, endotoxins, microorganism, dyes, disinfection, desalination | High surface-to-area ratio, high porosity, small driving forces needed, high water flux, low energy consumption, usage of antimicrobial chitosan reduces biofouling, no further treatment necessary, improved hydrophilicity [158,161,162] | Bio(fouling) is possible |
| Material | Method | Applications, Antibacterial Tests | Reference |
|---|---|---|---|
| Filtration Systems | |||
| Chitosan (MW: 190–310 kDa) + PEO (MW: 600 kDa) | Needle-free, Nanospider Lab | Filtration | Grimmelsmann et al. 2017 [10] |
| Water Filtration | |||
| Chitosan (DDA: 85%) + PCL | Needle-based electrospinning | Antibacterial efficiency of nanofibrous membranes tested with S. aureus for water filtration | Cooper et al. 2013 [162] |
| Chitosan (MW: 338 kDa, DDA ≥ 90%) + Nylon-6 (MW: 25 kDa) (100:0, 80:20, 70:30) | Needle-based electrospinning | Removal of metal ions (Pb(NO3)2, NaCl) and antibacterial efficiency against E. coli for water filtration | Jabur et al. 2016 [161] |
| Chitosan solution + PVA solution (70:30)] ± AgNO3 solution (1, 2, 3, 4, 5%) (Ag-doped chitosan nanofibers) | Home-made needle-based electrospinning setup | Antibacterial efficiency against S. aureus | Bezir et al. 2019 [173] |
| Chitosan (DDA: 75–85%) + PVA (MW: 72 kDa)] ± AgNO3 solution (0, 2, 4%) (Ag-doped chitosan nanofibers) | Needle-based electrospinning, rotating steel collector, covered with polyester substrate | Antibacterial efficiency tested with E. coli for static and dynamic water filtration | Adibzadeh et al. 2014 [175] |
| Polyacrylonitril (PAN, MW: 150 kDa) ± ZnO-nanoparticles (Zano®20)] ± [chitosan (MW: 50 kDa, DDA: 75–85%) + PEO (MW: 900 kDa) | Needle-based electrospinning | Antibacterial efficiency of PAN/ZnO–Cs membranes tested with E. coli and Enterococcus faecalis for water filtration | Makaremi et al. 2016 [178] |
| Air Filtration | |||
| Nylon-6 + chitosan (80:20) solution or pure nylon-6 solution for electrospinning; compared with nylon-6 nanofibrous filter media, dipped in chitosan solution | Multi-jet electrospinning with three spinnerets, rotating drum collector | Survival rate of E. coli and B. subtilis on hydrophilic and hydrophobic blankets and on air filtration media | Sun et al. 2020 [179] |
| Chitosan (DDA: 80–95%, MW: 590 kDa) + PVA (86–90% hydrolysed, MW: 118–124 kDa) (1:2–3:1) ± AgNO3 (0.04–1.0%) ± TiO2 (0.04%) | Home-made needle-less electrospinning | Filtration efficiency tested for removal of nanoparticles aerosols for air filtration, antibacterial efficiency tested with E. coli and S. aureus | Wang et al. 2016 [154] |
| Food Storage | |||
| Carboxymethyl chitosan (MW: 80–250 kDa, DDA: 95%) + PEO (MW: 5000 kDa) | Needle-based electrospinning | Nanofiber membranes for fruit freshness | Yue et al. 2018 [187] |
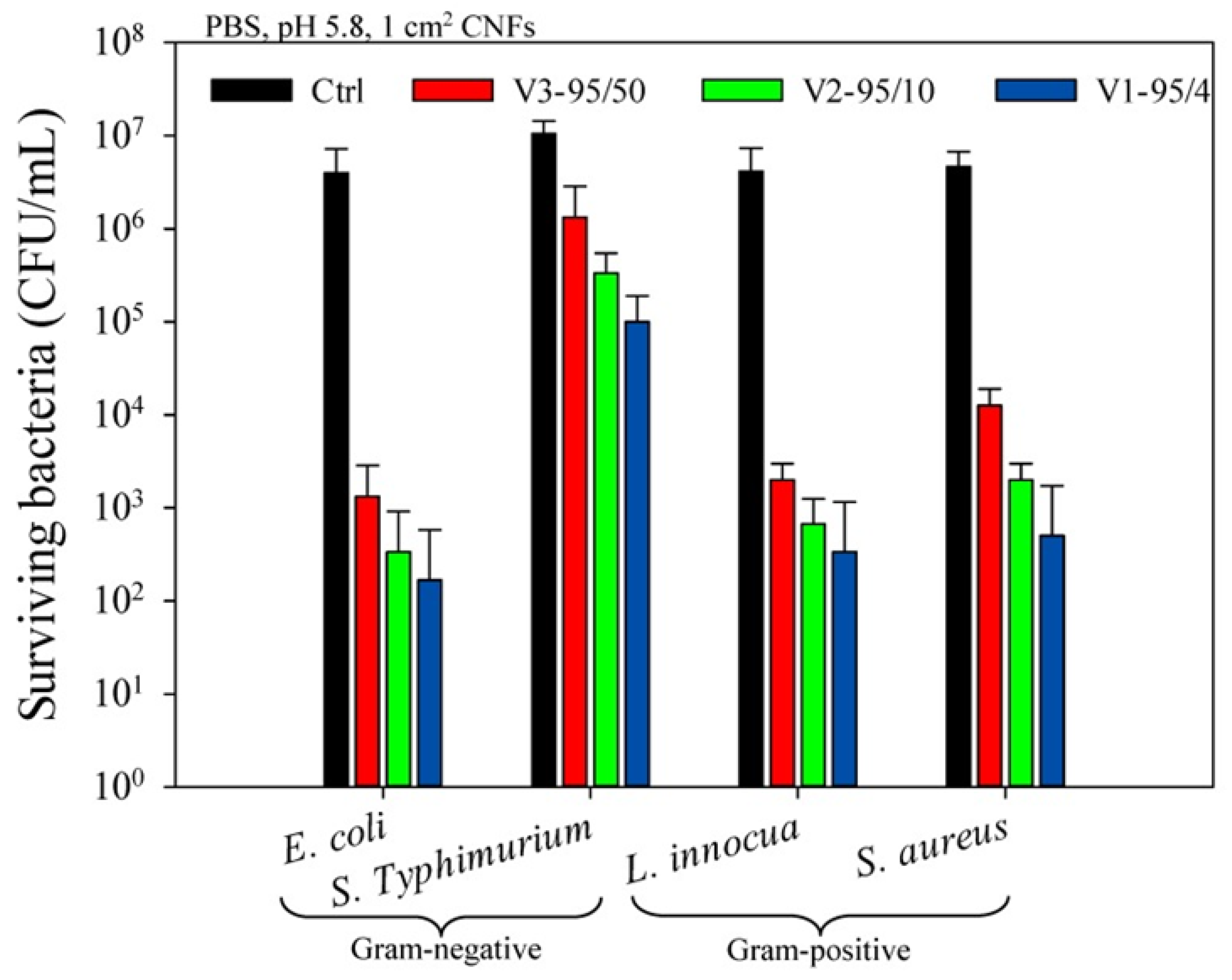
| Chitosan (DDA: 95%, MW: 4, 10, 50 kDa) + PEO (MW: 600 kDa) (50:50, 60:40, 70:30, 80:20, and 90:10) | Home-made needle-based electrospinning | Antibacterial efficiency against E. coli, Salmonella enterica serovar Typhimurium, S. aureus, Listeria innocua for food protection | Arkoun et al. 2017 [188] |
| Wound Dressing/Tissue Engineering | |||
| Chitosan (deacetylation degree 80–90%) + PVA (polymerization degree 1700, alcoholysis degree 88%) + Moxifloxacin hydrchloride | Needle-based electrospinning | Drug encapsulation, antibacterial material | Liu et al. 2021 [118] |
| Chitosan (50 kDa, DDA: 95%) + PEO (MW: 600 kDa) (80:20) | Horizontal needle-based electrospinning | Antibacterial efficiency tested with E. coli, S. aureus, L. innocua, and S. Typhimurium (food contamination and skin infections) | Arkoun et al. 2017 [94] |
| Chitosan (MW: 600–800 kDa, DDA: 90%) ± Polyaniline emeraldine base (PANI, MW: 50 kDa) | Needle-based electrospinning | Antibacterial efficiency tested with E. coli, B. subtilis for wound dressing | Moutsatsou et al. 2019 [194] |
| Chitosan (MW: 190–310 kDa, DDA: 77%) ± MXene (Ti3C2Tz flakes) | Needle-based electrospinning; fibers crosslinked with glutaraldehyde | Antibacterial efficiency tested with E. coli, S. aureus for wound dressing | Mayerberger et al. 2018 [201] |
| Chitosan (viscosity < 500 cp) + PVA (MW: 72 kDa) ± mafenide acetate | Needle-based electrospinning | Antibacterial efficiency tested with S. aureus and Pseudomonas aeruginosa for wound healing | Abbaspour et al. 2015 [202] |
| Single fibers: PA6, chitosan/PEO (80:20), PA6-PHBM, chitosan-5CLO8Q core–shell fibers with core: PA6-PHMB shell: chitosan-5CLO8Q + PEO | Single needle-based electropinning, co-axial needle-based electrospinning | Antimicrobial efficiency tested in vitro against S. aureus and P. aeruginosa in surgical site infections | Keirouz et al. 2020 [204] |
| Chitosan (medium MW, DDA: 75–85%) + silk generated from cocoons of Bombyx mori silkworm | Needle-based electrospinning | Antibacterial efficiency tested with Escherichia coli and Staphylococcus aureus for wound dressing | Cai et al. 2010 [195] |
| Double layer scaffold 1. layer: PCL ± cellulose acetate (10%) 2. chitosan + PEO | Needle-based electrospinning | Dressing for skin lesions, low cytotoxicity to L929 fibroblasts, promotion of cell proliferation | Trinca et al. 2017 [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antaby, E.; Klinkhammer, K.; Sabantina, L. Electrospinning of Chitosan for Antibacterial Applications—Current Trends. Appl. Sci. 2021, 11, 11937. https://doi.org/10.3390/app112411937
Antaby E, Klinkhammer K, Sabantina L. Electrospinning of Chitosan for Antibacterial Applications—Current Trends. Applied Sciences. 2021; 11(24):11937. https://doi.org/10.3390/app112411937
Chicago/Turabian StyleAntaby, Eliconda, Kristina Klinkhammer, and Lilia Sabantina. 2021. "Electrospinning of Chitosan for Antibacterial Applications—Current Trends" Applied Sciences 11, no. 24: 11937. https://doi.org/10.3390/app112411937
APA StyleAntaby, E., Klinkhammer, K., & Sabantina, L. (2021). Electrospinning of Chitosan for Antibacterial Applications—Current Trends. Applied Sciences, 11(24), 11937. https://doi.org/10.3390/app112411937







