Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
2.3. Culture Maintenance
2.4. Preparation of Nanomaterials Stock Solution
2.5. Cytotoxicity Assay
2.6. Proliferation Assay
2.7. Gene Expression
2.8. In Vivo Toxicity
2.9. Statistical Analysis
3. Results and Discussion
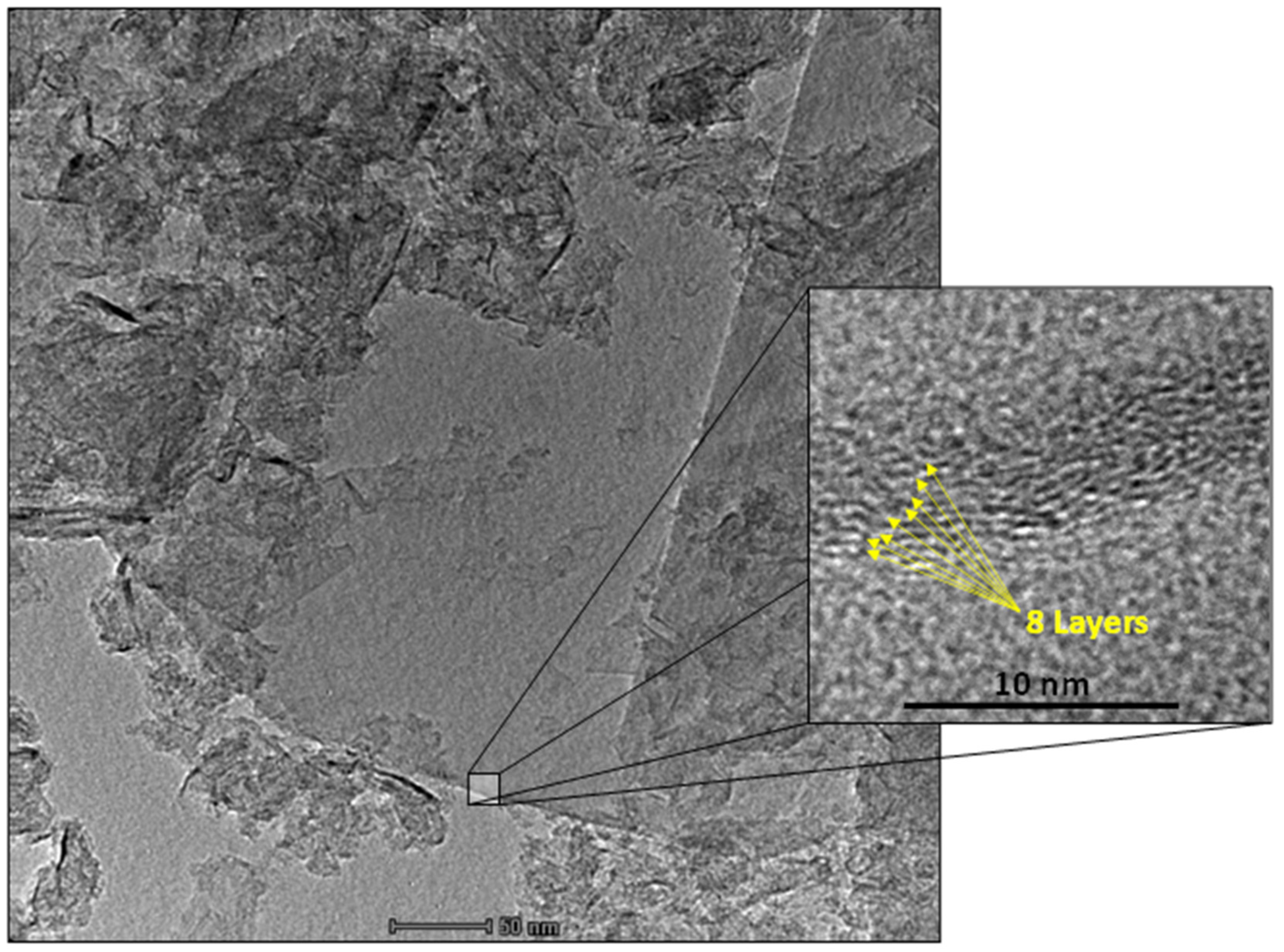
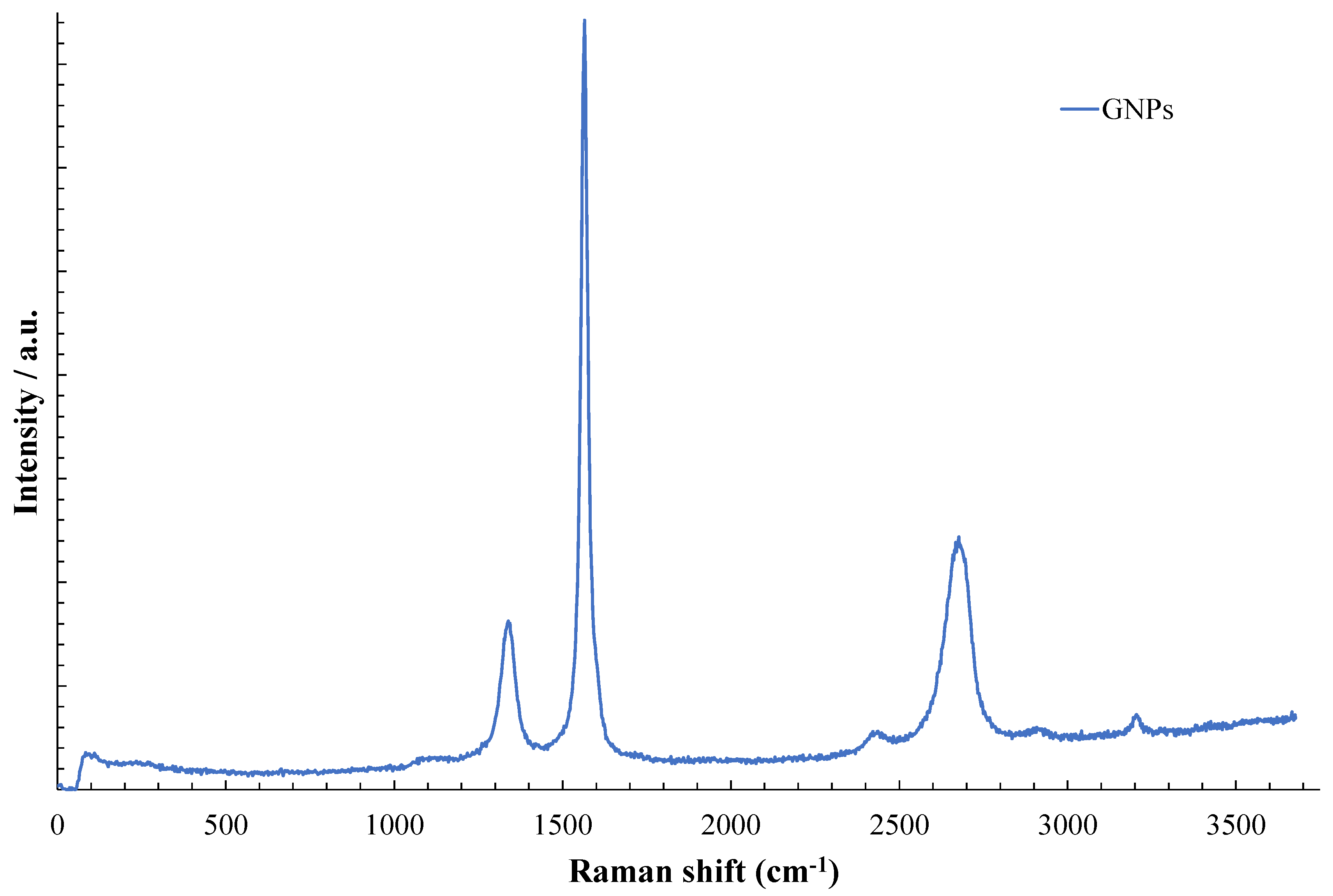
3.1. Material Characterization
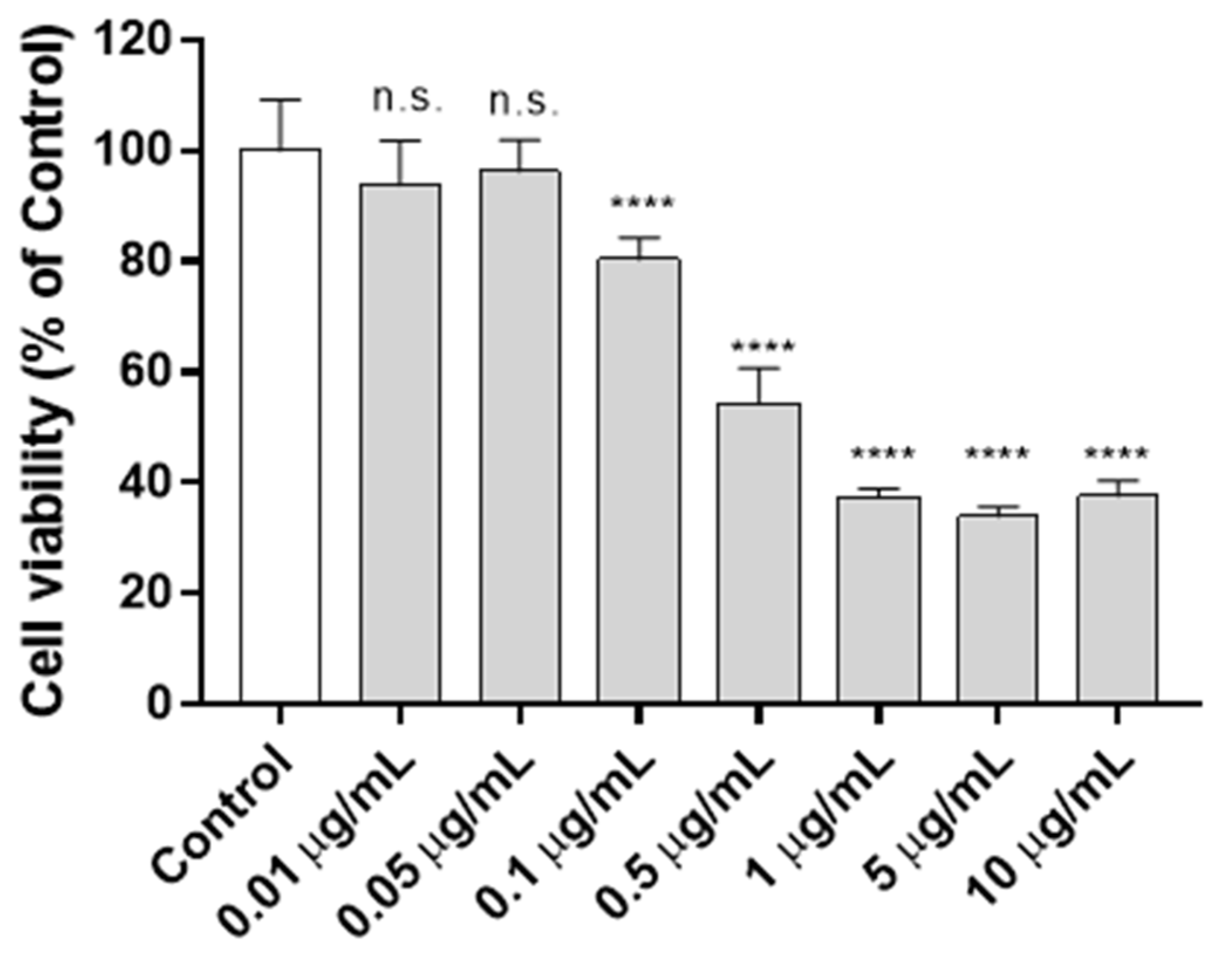
3.2. Cytotoxicity Assay
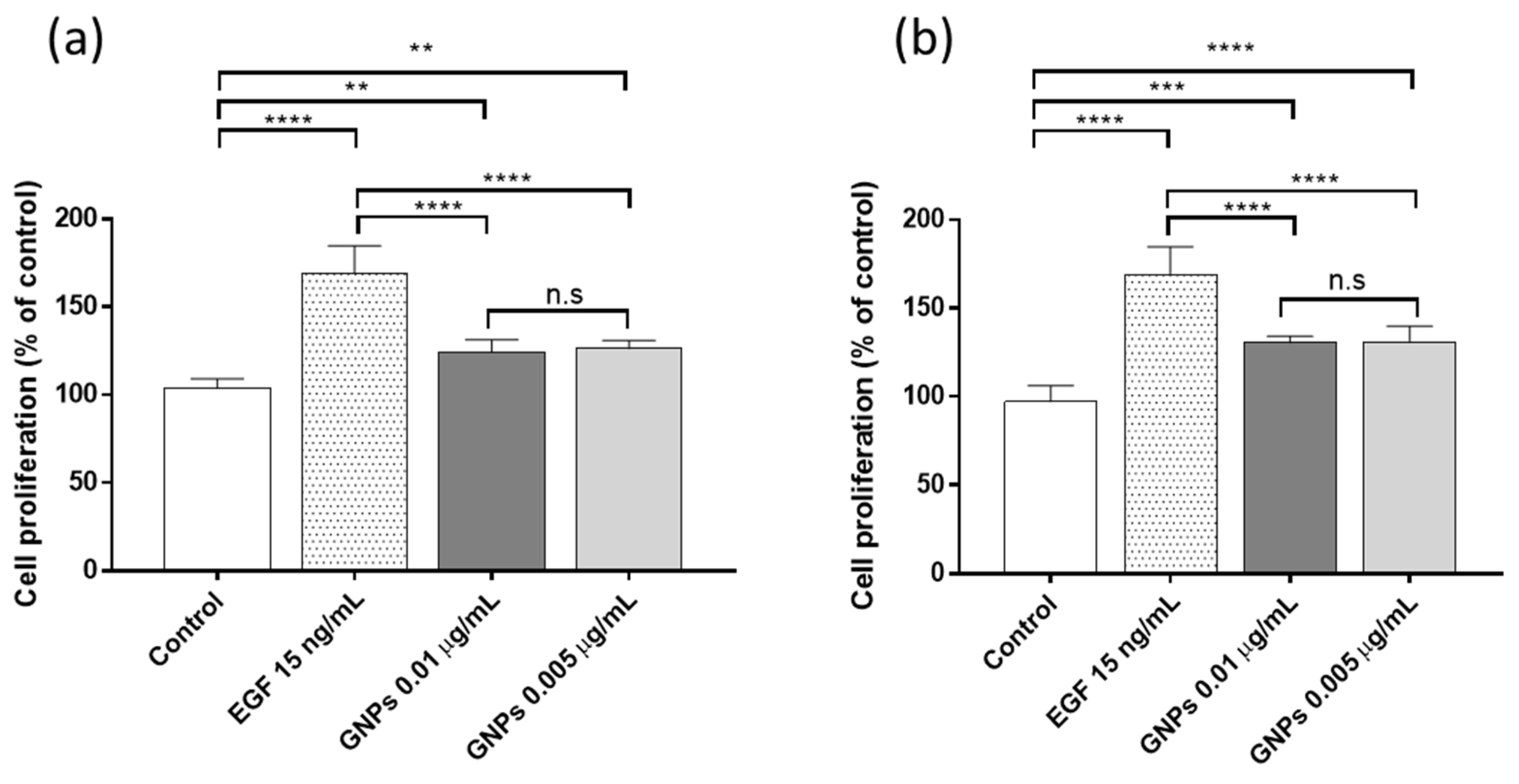
3.3. Proliferation Assay
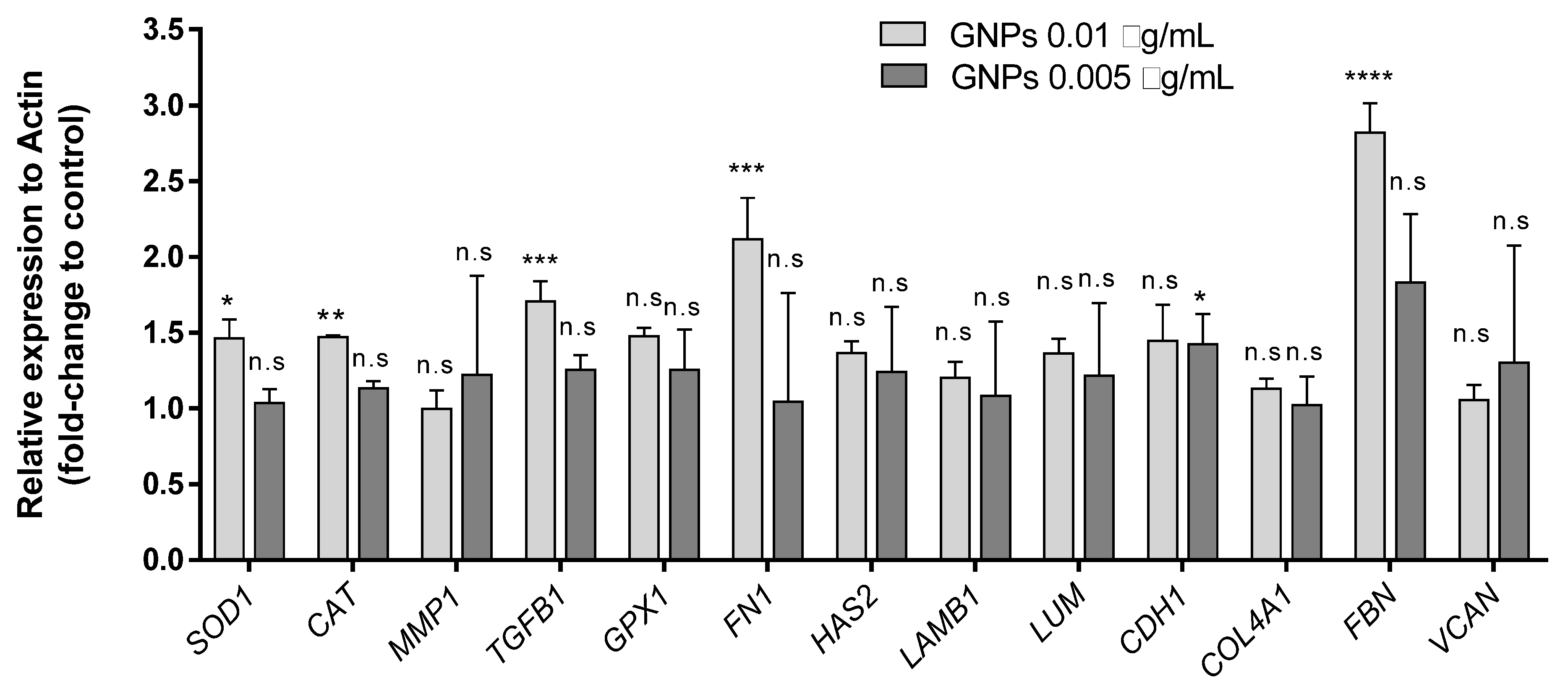
3.4. Gene Expression
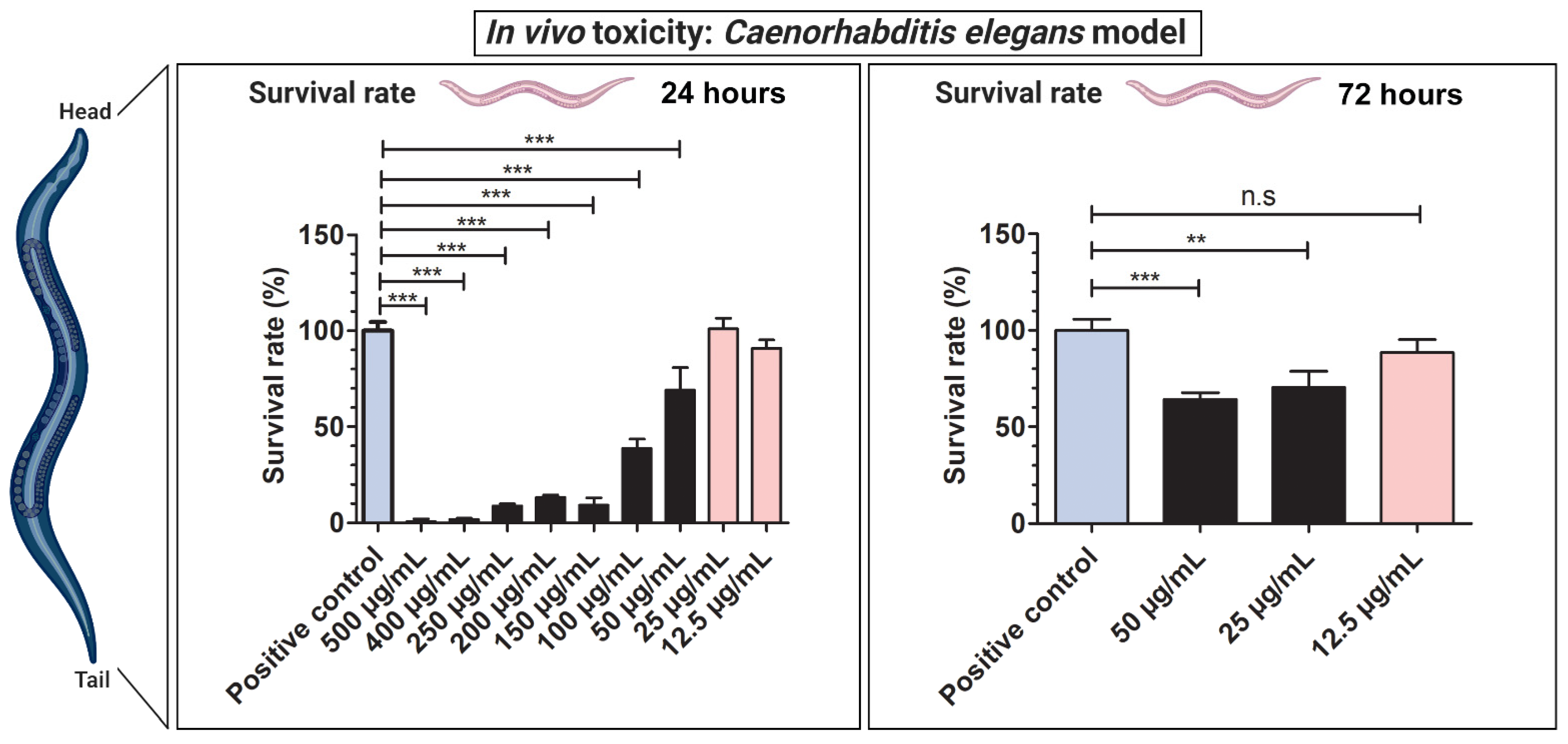
3.5. In Vivo Toxicity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene Symbol (Access Number) | Gene Name | Oligo Sequences | Function |
|---|---|---|---|
| ACTB (NM_001101) | Actin beta | 5′-CCATGCCCACCATCACGC-3′ | Highly conserved protein that is involved in cell motility, structure, and integrity |
| 5′-CACAGAGCCTCGCCTTTG-3′ | |||
| CAT (NM_001752) | Catalase | 5′-TGAATGAGGAACAGAGGAAACG-3′ | Encodes catalase, a key antioxidant enzyme in the bodies defence against oxidative stress |
| 5′-AGATCCGGACTGCACAAAG-3′ | |||
| CDH1 (NM_001317184) | Cadherin 1 | 5′-AACAGCACGTACACAGCCCT-3′ | Loss of function of this gene is thought to contribute to cancer progression by increasing proliferation, invasion, and/or metastasis. |
| 5′-TCTGGTATGGGGGCGTTGTC-3′ | |||
| COL4A1 (NM_000088) | Collagen type I alpha 1 | 5′-CAAGGGCGACAGAGGTTTGC-3′ | Abundant in bone, cornea, dermis, and tendon. Mutations in this gene are associated with osteogenesis imperfect types I-IV |
| 5′-AAAACTCACCAGGCTCCCCC-3′ | |||
| FBN (NM_000138) | Fibrillin 1 | 5′-ATCCAACCACGTGCATCAGT-3′ | Extracellular matrix glycoprotein that is useful as a structural component of calcium-binding microfibrils, providing force-bearing structural support in elastic and nonelastic connective tissue throughout the body |
| 5′-AGAGCGGGTATCAACACAGC-3′ | |||
| FN1 (NM_001306129) | Fibronectin 1 | 5′-GGCCAGTCCTACAACCAGT-3′ | Involved in cell adhesion and migration processes including embryogenesis, wound healing, blood coagulation, host defence and metastasis. |
| 5′-CGGGAATCTTCTCTGTCAGC-3′ | |||
| GPX1 (NM_000581) | Glutathione peroxidase 1 | 5′-TTTGGGCATCAGGAGAACGC-3′ | Catalyse the reduction of organic hydroperoxides and hydrogen peroxide by glutathione, and thereby protect cells against oxidative damage |
| 5′-ACCGTTCACCTCGCACTTC-3′ | |||
| HAS2 (NM_005328) | Hyaluronan synthase 2 | 5′-CCGAGAATGGCTGTACAATGC-3′ | Serves a variety of functions, including space filling, lubrication of joints, and provision of a matrix through which cells can migrate |
| 5′-AGAGCTGGATTACTGTGGCAA-3′ | |||
| LAMB1 (NM_002291) | Laminin subunit beta 1 | 5′-CAGGGTGTGCAGTCAGGGAA-3′ | Implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signalling, neurite outgrowth and metastasis |
| 5′-TGTGTCTGCGTTGAGGGTGT-3′ | |||
| LUM (NM_002345) | Lumican | 5′-ACTTGGGTAGCTTTCAGGGCA-3′ | Is the major keratan sulfate proteoglycan of the cornea but is also distributed in interstitial collagenous matrices throughout the body |
| 5′-TTCCTGGCATTGATTGGTGGT-3′ | |||
| MMP1 (NM_001145938) | Matrix metallopeptidase 1 | 5′-GGACCATGCCATTGAGAAAG-3′ | Involved in the breakdown of extracellular matrix in normal physiological processes |
| 5′-TCCTCCAGGTCCATCAAAAG-3′ | |||
| SOD1 (NM_000454) | Superoxide dismutase 1 | 5′-GGTGTGGCCGATGTGTCT-3′ | The protein encoded by this gene binds to Cu2+ and Zn2+ cations and is one of two isozymes capable of destroying free superoxide radicals in the body |
| 5′-TCCACCTTTGCCCAAGTCA-3′ | |||
| TGFB1 (NM_000660) | Transforming growth factor beta 1 | 5′-AGCTGTACATTGACTTCCGCA-3′ | Regulates cell proliferation, differentiation, and growth |
| 5′-TGTCCAGGCTCCAAATGTAGG-3′ | |||
| VCAN (NM_001126336) | Versican | 5′-CTGGTCTCCGCTGTATCCTG-3′ | Involved in cell adhesion, proliferation, migration, and angiogenesis and plays a central role in the morphogenesis and maintenance of tissue |
| 5′-ATCGCTGCAAAATGAACCCG-3′ |
References
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioff, N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [Green Version]
- Weiss, C.; Carriere, M.; Fusco, L.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Pasquali, M.; Pasquali, M.; Scott, J.A.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar]
- Serrano-Aroca, Á.; Takayama, K.; Tuñón-Molina, A.; Seyran, M.; Hassan, S.S.; Pal Choudhury, P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palù, G.; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Salesa, B.; Llorens-Gámez, M.; Serrano-Aroca, Á. Study of 1D and 2D carbon nanomaterial in alginate films. Nanomaterials 2020, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Gardea, F.; Naraghi, M.; Lagoudas, D. Effect of thermal interface on heat flow in carbon nanofiber composites. ACS Appl. Mater. Interfaces 2014, 6, 1061–1072. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [PubMed]
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Aachmann, F.L.; Serrano-Aroca, Á. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of Carbon Nanofibers (0.1% w/w). Appl. Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 40572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salesa, B.; Serrano-Aroca, Á. Multi-Layer Graphene Oxide in Human Keratinocytes: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Coatings 2021, 11, 414. [Google Scholar] [CrossRef]
- Salesa, B.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Biomedicines 2021, 9, 1155. [Google Scholar] [CrossRef]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.F.; Pestana, M.; Magalhães, F.D.; Pinto, A.M.; Gonçalves, I.C. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z.; Then, Y.Y.; Loo, Y.Y. Reinforcement of graphene nanoplatelets on plasticized poly(lactic acid) nanocomposites: Mechanical, thermal, morphology, and antibacterial properties. J. Appl. Polym. Sci. 2015, 132, 41652. [Google Scholar] [CrossRef]
- Kuwabara, P.E.; O’Neil, N. The use of functional genomics in C. elegans for studying human development and disease. J. Inherit. Metab. Dis. 2001, 24, 127–138. [Google Scholar] [CrossRef]
- Yin, J.; Hong, X.; Ma, L.; Liu, R.; Bu, Y. Non-targeted metabolomic profiling of atrazine in Caenorhabditis elegans using UHPLC-QE Orbitrap/MS. Ecotoxicol. Environ. Saf. 2020, 206, 111170. [Google Scholar] [CrossRef]
- NCBI Primer Designing Tool. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 5 August 2021).
- Stiernagle, T. Maintenance of C. elegans, WormBook, ed. The C. elegans Research Community, WormBook. 2006. Available online: http://www.wormbook.org (accessed on 12 December 2021). [CrossRef] [Green Version]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar]
- Aguilar, T.; Sani, E.; Mercatelli, L.; Carrillo-Berdugo, I.; Torres, E.; Navas, J. Exfoliated graphene oxide-based nanofluids with enhanced thermal and optical properties for solar collectors in concentrating solar power. J. Mol. Liq. 2020, 306, 112862. [Google Scholar] [CrossRef]
- Franqui, L.S.; De Farias, M.A.; Portugal, R.V.; Costa, C.A.R.; Domingues, R.R.; Souza Filho, A.G.; Coluci, V.R.; Leme, A.F.P.; Martinez, D.S.T. Interaction of graphene oxide with cell culture medium: Evaluating the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessment and nanobiointeractions. Mater. Sci. Eng. C 2019, 100, 363–377. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Li, H.; Xing, X.; Jin, L.; Cao, Q.; Li, P. Direct exfoliation of graphite into graphene in aqueous solution using a novel surfactant obtained from used engine oil. J. Mater. Sci. 2018, 53, 2484–2496. [Google Scholar] [CrossRef]
- Skaltsas, T.; Ke, X.; Bittencourt, C.; Tagmatarchis, N. Ultrasonication induces oxygenated species and defects onto exfoliated graphene. J. Phys. Chem. C 2013, 117, 23272–23278. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; He, C.; Dai, L.; Liu, J.; Wang, L. Rationally designed surfactants for few-layered graphene exfoliation: Ionic groups attached to electron-deficient π-conjugated unit through alkyl spacers. ACS Nano 2014, 8, 6663–6670. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, G.H.; Han, B.S.; Lee, B.S.; Lee, S.; Cho, M.H.; Kim, J.H.; Kim, D.W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2015, 89, 1557–1568. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; De Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [PubMed]
- Mehrali, M.; Moghaddam, E.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Latibari, S.T.; Metselaar, H.S.C.; Kadri, N.A.; Zandi, K.; Osman, N.A.A. Mechanical and in vitro biological performance of graphene nanoplatelets reinforced calcium silicate composite. PLoS ONE 2014, 9, e106802. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Lu, Y.; Yu, W.; Luo, J.; Xiao, Z.; Xiao, F.; Wang, X. Clinical value of decreased superoxide dismutase 1 in patients with epilepsy. Seizure 2012, 21, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.J.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.H.; Lee, J.H.; Lee, D.H.; Jung, K.; Yang, J.Y.; Shin, H.S.; Lee, J.; Jeong, J.; Oh, J.H. Skin sensitization evaluation of carbon-based graphene nanoplatelets. Toxics 2021, 9, 62. [Google Scholar] [CrossRef]
- Domenech, J.; Hernández, A.; Demir, E.; Marcos, R.; Cortés, C. Interactions of graphene oxide and graphene nanoplatelets with the in vitro Caco-2/HT29 model of intestinal barrier. Sci. Rep. 2020, 10, 2793. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [PubMed]
- Sonnhammer, E.L.L.; Durbin, R. Analysis of Protein Domain Families inCaenorhabditis elegans. Genomics 1997, 46, 200–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, V.M.; Orsi, G.; Brown, A.; Pritchard, D.I.; Aylott, J.W. Mapping the pharyngeal and intestinal pH of Caenorhabditis elegans and real-time luminal pH oscillations using extended dynamic range pH-sensitive nanosensors. ACS Nano 2013, 7, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Stutz, K.; Kaech, A.; Aebi, M.; Künzler, M.; Hengartner, M.O. Disruption of the C. elegans Intestinal Brush Border by the Fungal Lectin CCL2 Phenocopies Dietary Lectin Toxicity in Mammals. PLoS ONE 2015, 10, e0129381. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zou, X.; Ding, Y.; Wang, H.; Wu, X.; Liang, B. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 2013, 14, 164. [Google Scholar] [CrossRef] [Green Version]

| pH | GNPs |
|---|---|
| 12 | −38, 6 |
| 10 | −33, 2 |
| 7 | −20, 9 |
| 5 | −2, 13 |
| 3 | 2, 69 |
| Material | DLS (nm) | PdI | ||
|---|---|---|---|---|
| Water | DMEM | Water | DMEM | |
| GNPs | 625, 8 | 2042 | 0, 557 | 0, 137 |
| Exposure (h) | EC50 (µg/mL) | 95% CI | R Square |
|---|---|---|---|
| 12 | 1.142 | 0.837–1.564 | 0.8961 |
| 24 | 0.760 | 0.585–1.004 | 0.9176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salesa, B.; Tuñón-Molina, A.; Cano-Vicent, A.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression. Appl. Sci. 2022, 12, 720. https://doi.org/10.3390/app12020720
Salesa B, Tuñón-Molina A, Cano-Vicent A, Assis M, Andrés J, Serrano-Aroca Á. Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression. Applied Sciences. 2022; 12(2):720. https://doi.org/10.3390/app12020720
Chicago/Turabian StyleSalesa, Beatriz, Alberto Tuñón-Molina, Alba Cano-Vicent, Marcelo Assis, Juan Andrés, and Ángel Serrano-Aroca. 2022. "Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression" Applied Sciences 12, no. 2: 720. https://doi.org/10.3390/app12020720
APA StyleSalesa, B., Tuñón-Molina, A., Cano-Vicent, A., Assis, M., Andrés, J., & Serrano-Aroca, Á. (2022). Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression. Applied Sciences, 12(2), 720. https://doi.org/10.3390/app12020720








