Tailored Nanoparticles as Vaccine Components
Abstract
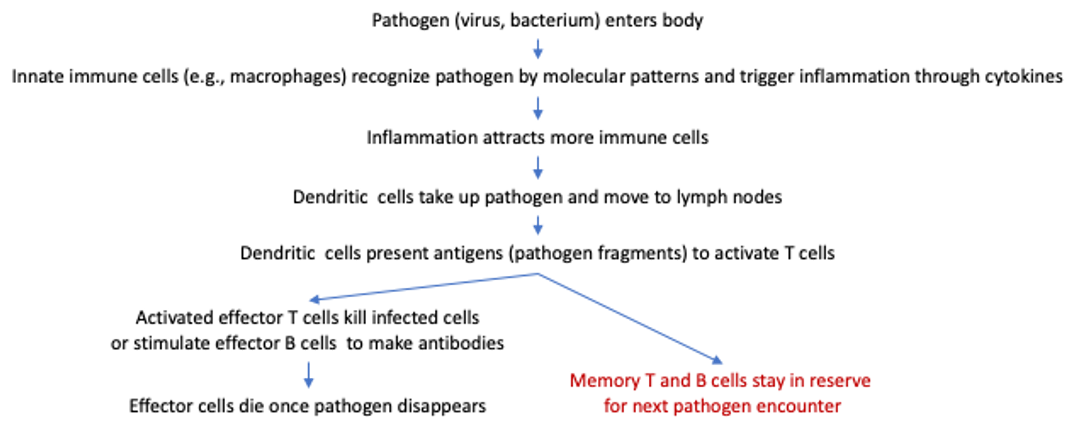
1. Vaccination
2. Forms of Antigen in a Vaccine
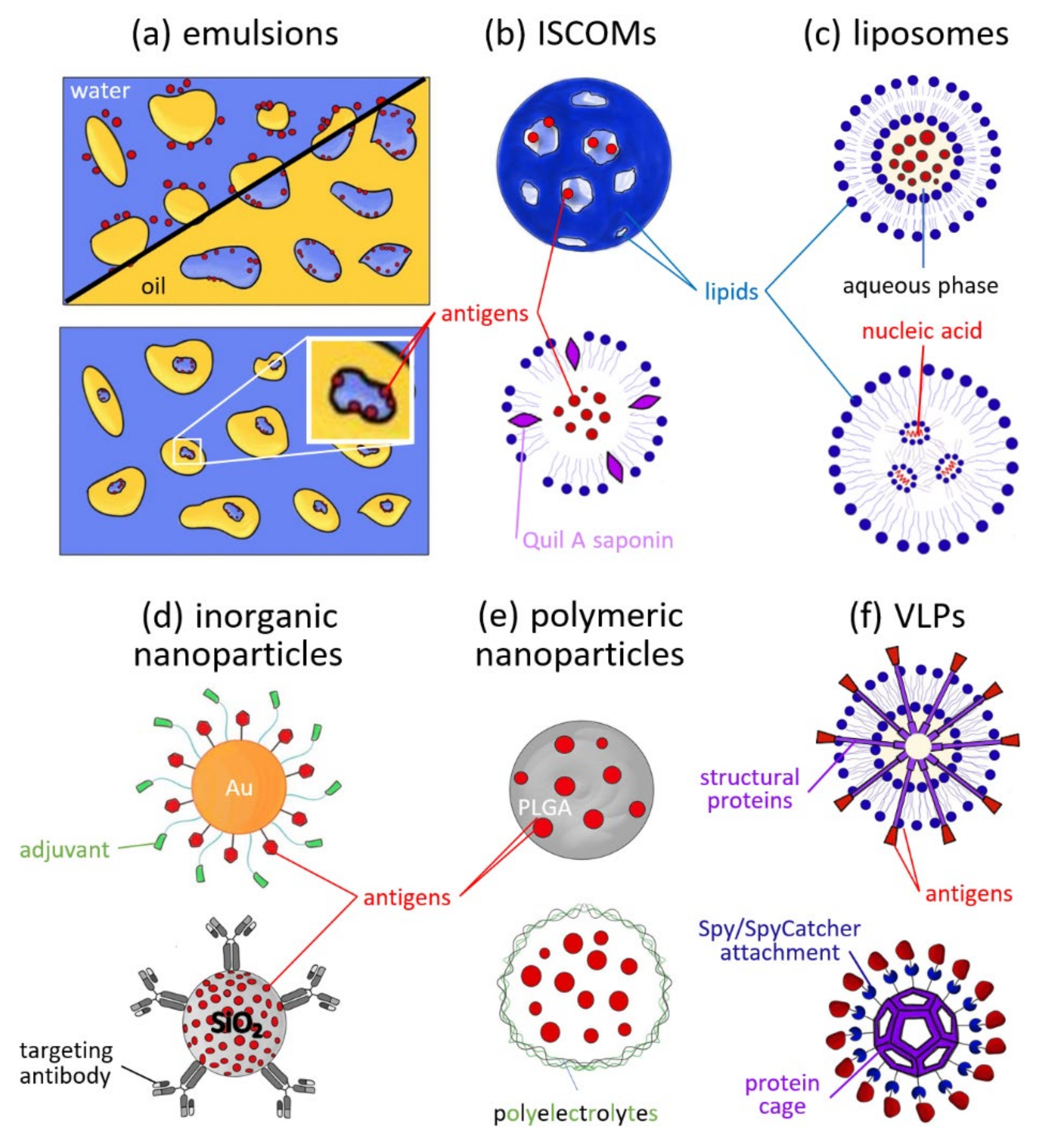
3. Types and Uses of Nanoparticles in Vaccines
3.1. Emulsions
3.2. ISCOMs
3.3. Liposomes
3.4. Inorganic Nanoparticles
3.5. Polymeric Nanoparticles
3.6. VLPs and Self-Assembled Protein Cages
4. Nanoparticles as Artificial Antigen-Presenting Cells—The Future of Immunotherapy?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beeraka, N.M.; Tulimilli, S.V.; Karnik, M.; Sadhu, S.P.; Pragada, R.R.; Aliev, G.; Madhunapantula, S.V. The Current Status and Challenges in the Development of Vaccines and Drugs against Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS-CoV-2). BioMed Res. Int. 2021, 2021, 8160860. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 2578. [Google Scholar] [CrossRef]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef] [PubMed]
- Sulczewski, F.B.; Liszbinski, R.B.; Romão, R.T.; Rodrigues, L.C. Junior Nanoparticle vaccines against viral infections. Arch. Virol. 2018, 163, 2313–2325. [Google Scholar] [CrossRef]
- Gomes, A.C.; Mohsen, M.; Bachmann, M.F. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front. Chem. 2020, 8, 1086. [Google Scholar] [CrossRef]
- Ho, J.K.-T.; Jeevan-Raj, B.; Netter, H.-J. Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Cutts, F.T.; Franceschi, S.; Goldie, S.; Castellsague, X.D.; De Sanjose, S.; Garnett, G.; Edmunds, W.J.; Claeys, P.; Goldenthal, K.L.; Harperi, D.M.; et al. Human papillomavirus and HPV vaccines: A review. Bull. World Health Organ. 2007, 85, 719–726. [Google Scholar] [CrossRef]
- Donaldson, B.; Lateef, Z.; Walker, G.F.; Young, S.L.; Ward, V.K. Virus-like particle vaccines: Immunology and formulation for clinical translation. Expert Rev. Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef]
- Zheng, X.; Zeng, S.; Hu, J.; Wu, L.; Hou, X. Applications of silica-based nanoparticles for multimodal bioimaging. Appl. Spectrosc. Rev. 2018, 53, 377–394. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Rocha, R.; Gonçalves, R.; Ferreira, C.; Silva, B.; Castro, R.; Rodrigues, J.; Júnior, J.; Malaquias, L.; Abrahão, J.; et al. Nanoparticles as Vaccines to Prevent Arbovirus Infection: A Long Road Ahead. Pathogens 2021, 10, 36. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Liu, X.; Zhang, L.; Liu, C.; Liu, G. Cell membrane-encapsulated nanoparticles for vaccines and immunotherapy. Particuology 2021, in press. [Google Scholar] [CrossRef]
- Snapper, C.M. Distinct Immunologic Properties of Soluble Versus Particulate Antigens. Front. Immunol. 2018, 9, 598. [Google Scholar] [CrossRef]
- Li, M.; Kaminskas, L.M.; Marasini, N. Recent advances in nano/microparticle-based oral vaccines. J. Pharm. Investig. 2021, 51, 425–438. [Google Scholar] [CrossRef]
- Petkar, K.; Patil, S.; Chavhan, S.; Kaneko, K.; Sawant, K.; Kunda, N.; Saleem, I. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455. [Google Scholar] [CrossRef]
- Medina, K.L. Chapter 4—Overview of the immune system. In Handbook of Clinical Neurology; Pittock, S.J., Vincent, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 61–76. [Google Scholar] [CrossRef]
- Ghobadi, N.; Ghobadi, S.; Burkholder, M.B.; Habibipour, R. Nanoparticles development for pulmonary vaccination: Challenges and opportunities. Nanomed. J. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Ötjengerdes, R.M.; Roewe, J.; Mejias, R.; Marschall, A.L.J. Applying Antibodies Inside Cells: Principles and Recent Advances in Neurobiology, Virology and Oncology. Biodrugs 2020, 34, 435. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Immunological basis for enhanced immunity of nanoparticle vaccines. Expert Rev. Vaccines 2019, 18, 269–280. [Google Scholar] [CrossRef]
- Saroja, C.; Lakshmi, P.K.; Bhaskaran, S. Recent trends in vaccine delivery systems: A review. Int. J. Pharm. Investig. 2011, 1, 64–74. [Google Scholar] [CrossRef]
- Vaillant, A.A.J.; Grella, M.J. Vaccine (Vaccination); StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK532895/ (accessed on 13 August 2021).
- Alfagih, I.M.; Aldosari, B.; AlQuadeib, B.; Almurshedi, A.; Alfagih, M.M. Nanoparticles as Adjuvants and Nanodelivery Systems for mRNA-Based Vaccines. Pharmaceutics 2020, 13, 45. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of Vaccine Immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Jarząb, A.; Skowicki, M.; Witkowska, D. Subunit vaccines—Antigens, carriers, conjugation methods and the role of adjuvants. Postepy Hig. Med. Dosw. 2013, 67, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Khan, S. Conjugate vaccines and polysaccharide response. CMAJ Can. Med. Assoc. J. 2006, 174, 976–977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection—Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef]
- Gregory, A.E.; Williamson, D.; Titball, R. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Lou, P.; Hu, Z.; Qu, P.; Li, D.; Li, Q.; Xu, Y.; Niu, J.; He, Y. A Nanoparticle-Based Hepatitis C Virus Vaccine With Enhanced Potency. J. Infect. Dis. 2020, 221, 1304–1314. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Briquez, P.S.; Hauert, S.; De Titta, A.; Gray, L.T.; Alpar, A.T.; Swartz, M.A.; Hubbell, J.A. Engineering Targeting Materials for Therapeutic Cancer Vaccines. Front. Bioeng. Biotechnol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Wang, X.-Y.; Zhu, G. Nanovaccines for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1559. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59® adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Aucouturier, J.; Dupuis, L.; Deville, S.; Ascarateil, S.; Ganne, V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines 2002, 1, 111–118. [Google Scholar] [CrossRef]
- Zeng, B.J.; Chuan, Y.P.; O’Sullivan, B.; Caminschi, I.; Lahoud, M.H.; Thomas, R.; Middelberg, A.P.J. Receptor-specific delivery of protein antigen to dendritic cells by a nanoemulsion formed using top-down non-covalent click self-assembly. Small Weinh. Bergstr. Ger. 2013, 9, 3736–3742. [Google Scholar] [CrossRef]
- Mischler, R.; Metcalfe, I.C. Inflexal® V a trivalent virosome subunit influenza vaccine: Production. Vaccine 2002, 20, B17–B23. [Google Scholar] [CrossRef]
- Epaxal: A Virosomal Vaccine to Prevent Hepatitis A Infection—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18844588/ (accessed on 20 September 2021).
- Zhang, J.; Sun, X.; Shao, R.; Liang, W.; Gao, J.; Chen, J. Polycation liposomes combined with calcium phosphate nanoparticles as a non-viral carrier for siRNA delivery. J. Drug Deliv. Sci. Technol. 2015, 30, 1–6. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Stone, J.W.; Thornburg, N.J.; Blum, D.L.; Kuhn, S.J.; Wright, D.W.; Crowe, J.E. Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology 2013, 24, 295102. [Google Scholar] [CrossRef]
- An, M.; Li, M.; Xi, J.; Liu, H. Silica Nanoparticle as a Lymph Node Targeting Platform for Vaccine Delivery. ACS Appl. Mater. Interfaces 2017, 9, 23466–23475. [Google Scholar] [CrossRef]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesner, U. Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett. 2005, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Niut, Y.; Popatt, A.; Yu, M.; Karmakar, S.; Gu, W.; Yu, C. Recent advances in the rational design of silica-based nanoparticles for gene therapy. Ther. Deliv. 2012, 3, 1217–1237. [Google Scholar] [PubMed]
- Morato, Y.L.; Paredes, K.O.; Chamizo, L.L.; Marciello, M.; Filice, M. Recent Advances in Multimodal Molecular Imaging of Cancer Mediated by Hybrid Magnetic Nanoparticles. Polymers 2021, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- PLGA-Particle Vaccine Carrying TLR3/RIG-I Ligand Riboxxim Synergizes with Immune Checkpoint Blockade for Effective Anti-Cancer Immunotherapy|Nature Communications. Available online: https://www.nature.com/articles/s41467-021-23244-3 (accessed on 13 August 2021).
- Hraber, P.; Bradfute, S.; Clarke, E.; Ye, C.; Pitard, B. Amphiphilic block copolymer delivery of a DNA vaccine against Zika virus. Vaccine 2018, 36, 6911–6917. [Google Scholar] [CrossRef]
- Dölen, Y.; Gileadi, U.; Chen, J.-L.; Valente, M.; Creemers, J.H.A.; Van Dinther, E.A.W.; van Riessen, N.K.; Jäger, E.; Hruby, M.; Cerundolo, V.; et al. PLGA Nanoparticles Co-encapsulating NY-ESO-1 Peptides and IMM60 Induce Robust CD8 and CD4 T Cell and B Cell Responses. Front. Immunol. 2021, 12, 641703. [Google Scholar] [CrossRef]
- Trimaille, T.; Verrier, B. Micelle-Based Adjuvants for Subunit Vaccine Delivery. Vaccines 2015, 3, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, A.; Phelip, C.; Coolen, A.-L.; Monge, C.; Boisgard, A.-S.; Paul, S.; Verrier, B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, W.; Cruz, J.G.; Marasini, N.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Development of Polyelectrolyte Complexes for the Delivery of Peptide-Based Subunit Vaccines against Group A Streptococcus. Nanomaterials 2020, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Taber, L.; Umerska, A. Polyelectrolyte complexes as nanoparticulate drug delivery systems. Eur. Pharm. Rev. 2015, 20, 36–40. [Google Scholar]
- Sukhorukov, G.B.; Rogach, A.L.; Garstka, M.A.; Springer, S.; Parak, W.J.; Muñoz-Javier, A.; Kreft, O.; Skirtach, A.; Susha, A.S.; Ramaye, Y.; et al. Multifunctionalized polymer microcapsules: Novel tools for biological and pharmacological applications. Small Weinh. Bergstr. Ger. 2007, 3, 944–955. [Google Scholar] [CrossRef]
- Timin, A.S.; Gould, D.J.; Sukhorukov, G.B. Multi-layer microcapsules: Fresh insights and new applications. Expert Opin. Drug Deliv. 2017, 14, 583–587. [Google Scholar] [CrossRef]
- Protein A Functionalized Polyelectrolyte Microcapsules as a Universal Platform for Enhanced Targeting of Cell Surface Receptors|ACS Applied Materials & Interfaces. Available online: https://pubs.acs.org/doi/10.1021/acsami.7b01313 (accessed on 20 September 2021).
- Serradell, M.C.; Rupil, L.L.; Martino, R.A.; Prucca, C.G.; Carranza, P.G.; Saura, A.; Fernández, E.A.; Gargantini, P.R.; Tenaglia, A.H.; Petiti, J.P.; et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat. Commun. 2019, 10, 361. [Google Scholar] [CrossRef]
- Chu, K.-B.; Quan, F.-S. Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites. Curr. Top. Microbiol. Immunol. 2021, 433, 77–106. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-derived virus-like particles in vaccine development. NPJ Vaccines 2017, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Santi, L.; Huang, Z.; Mason, H. Virus like particles production in green plants. Methods 2006, 40, 66–76. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.F.; Zhou, J.; Chen, S.; Cai, L.L.; Bao, Q.Y.; Zheng, F.Y.; Lu, J.Q.; Padmanabha, J.; Hengst, K.; Malcolm, K.; et al. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine 2000, 18, 1051–1058. [Google Scholar] [CrossRef]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- André, F.E. Overview of a 5-year clinical experience with a yeast-derived hepatitis B vaccine. Vaccine 1990, 8, S74–S78. [Google Scholar] [CrossRef]
- Landry, N.; Ward, B.J.; Trepanier, S.; Montomoli, E.; Dargis, M.; Lapini, G.; Vézina, L.-P. Preclinical and Clinical Development of Plant-Made Virus-Like Particle Vaccine against Avian H5N1 Influenza. PLoS ONE 2010, 5, e15559. [Google Scholar] [CrossRef]
- Wetzel, D.; Rolf, T.; Suckow, M.; Kranz, A.; Barbian, A.; Chan, J.-A.; Leitsch, J.; Weniger, M.; Jenzelewski, V.; Kouskousis, B.; et al. Establishment of a yeast-based VLP platform for antigen presentation. Microb. Cell Factories 2018, 17, 17. [Google Scholar] [CrossRef]
- Thrane, S.; Janitzek, C.M.; Matondo, S.; Resende, M.; Gustavsson, T.; De Jongh, W.A.; Clemmensen, S.; Roeffen, W.; Van De Vegte-Bolmer, M.; Van Gemert, G.J.; et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J. Nanobiotechnol. 2016, 14, 30. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Tissot, A.; Lechner, F.; Sebbel, P.; Erdmann, I.; Kündig, T.; Bächi, T.; Storni, T.; Jennings, G.; Pumpens, P.; et al. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine 2002, 20, 3104–3112. [Google Scholar] [CrossRef]
- Tan, T.K.; Rijal, P.; Rahikainen, R.; Keeble, A.H.; Schimanski, L.; Hussain, S.; Harvey, R.; Hayes, J.W.P.; Edwards, J.C.; McLean, R.K.; et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat. Commun. 2021, 12, 542. [Google Scholar] [CrossRef]
- Hsia, Y.; Bale, J.; Gonen, S.; Shi, D.; Sheffler, W.; Fong, K.K.; Nattermann, U.; Xu, C.; Huang, P.-S.; Ravichandran, R.; et al. Design of a hyperstable 60-subunit protein icosahedron. Nature 2016, 535, 136–139. [Google Scholar] [CrossRef]
- Neal, L.R.; Bailey, S.; Wyatt, M.; Bowers, J.; Majchrzak, K.; Nelson, M.H.; Haupt, C.; Paulos, C.M.; Varela, J.C. The Basics of Artificial Antigen Presenting Cells in T Cell-Based Cancer Immunotherapies. J. Immunol. Res. Ther. 2017, 2, 68–79. [Google Scholar]
- Eggermont, L.J.; Paulis, L.E.; Tel, J.; Figdor, C.G. Towards efficient cancer immunotherapy: Advances in developing artificial antigen-presenting cells. Trends Biotechnol. 2014, 32, 456–465. [Google Scholar] [CrossRef]
- Hickey, J.W.; Kosmides, A.K.; Schneck, J. Chapter Six—Engineering Platforms for T Cell Modulation. In International Review of Cell and Molecular Biology; Galluzzi, L., Rudqvist, N.-P., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 341, pp. 277–362. [Google Scholar] [CrossRef]
- Horwitz, D.A.; Bickerton, S.; La Cava, A. Strategies to Use Nanoparticles to Generate CD4 and CD8 Regulatory T Cells for the Treatment of SLE and Other Autoimmune Diseases. Front. Immunol. 2021, 12, 681062. [Google Scholar] [CrossRef]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef] [PubMed]
| Particle Type | Characteristics | Size |
|---|---|---|
| Emulsions | Oil-in-water or water-in-oil. Used as adjuvants in vaccine delivery. | 50–600nm |
| ISCOMS | Made of saponin, phospholipids, and cholesterol. Cage-like particles. | 40–60 nm |
| Liposomes | Biodegradable lipidic NPs. Encapsulation and controlled release of antigens. | 200–1000 nm |
| Inorganic NPs | Controllable synthesis and rigid structure. Non- biodegradable | 2–150 nm |
| Polymeric NPs | Synthetic or natural polymers. Biodegradable. Allows controlled release of antigens | Variable |
| VLPs and Self-assembled protein cages | Self-assembling viral capsids lacking genetic material. Fold into complex quaternary structures. | Variable 10–40 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Springer, S. Tailored Nanoparticles as Vaccine Components. Appl. Sci. 2021, 11, 11898. https://doi.org/10.3390/app112411898
Popa A, Springer S. Tailored Nanoparticles as Vaccine Components. Applied Sciences. 2021; 11(24):11898. https://doi.org/10.3390/app112411898
Chicago/Turabian StylePopa, Alina, and Sebastian Springer. 2021. "Tailored Nanoparticles as Vaccine Components" Applied Sciences 11, no. 24: 11898. https://doi.org/10.3390/app112411898
APA StylePopa, A., & Springer, S. (2021). Tailored Nanoparticles as Vaccine Components. Applied Sciences, 11(24), 11898. https://doi.org/10.3390/app112411898






