Garlic Extract: Inhibition of Biochemical and Biophysical Changes in Glycated HSA
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Experiments
2.1.1. Preparation of Aqueous Garlic Extract
2.1.2. Total Phenol Content
2.1.3. Total Flavonoid Content
2.1.4. Hydrogen Peroxide Radical Scavenging
2.1.5. Modification of HSA by Glucose
2.1.6. Detection of Ketoamines by Nitroblue Tetrazolium (NBT) Reagent
2.1.7. Determination of Protein-Bound Carbonyl Content
2.1.8. Toxicity of Garlic Extract
2.2. Biophysical Experiments
2.2.1. Assay of AGE-Fluorophores
2.2.2. UV and Tryptophan Specific Spectral Studies
2.3. Statistical Analysis
3. Results
3.1. Biochemical Analysis
3.1.1. Phytochemical Screening
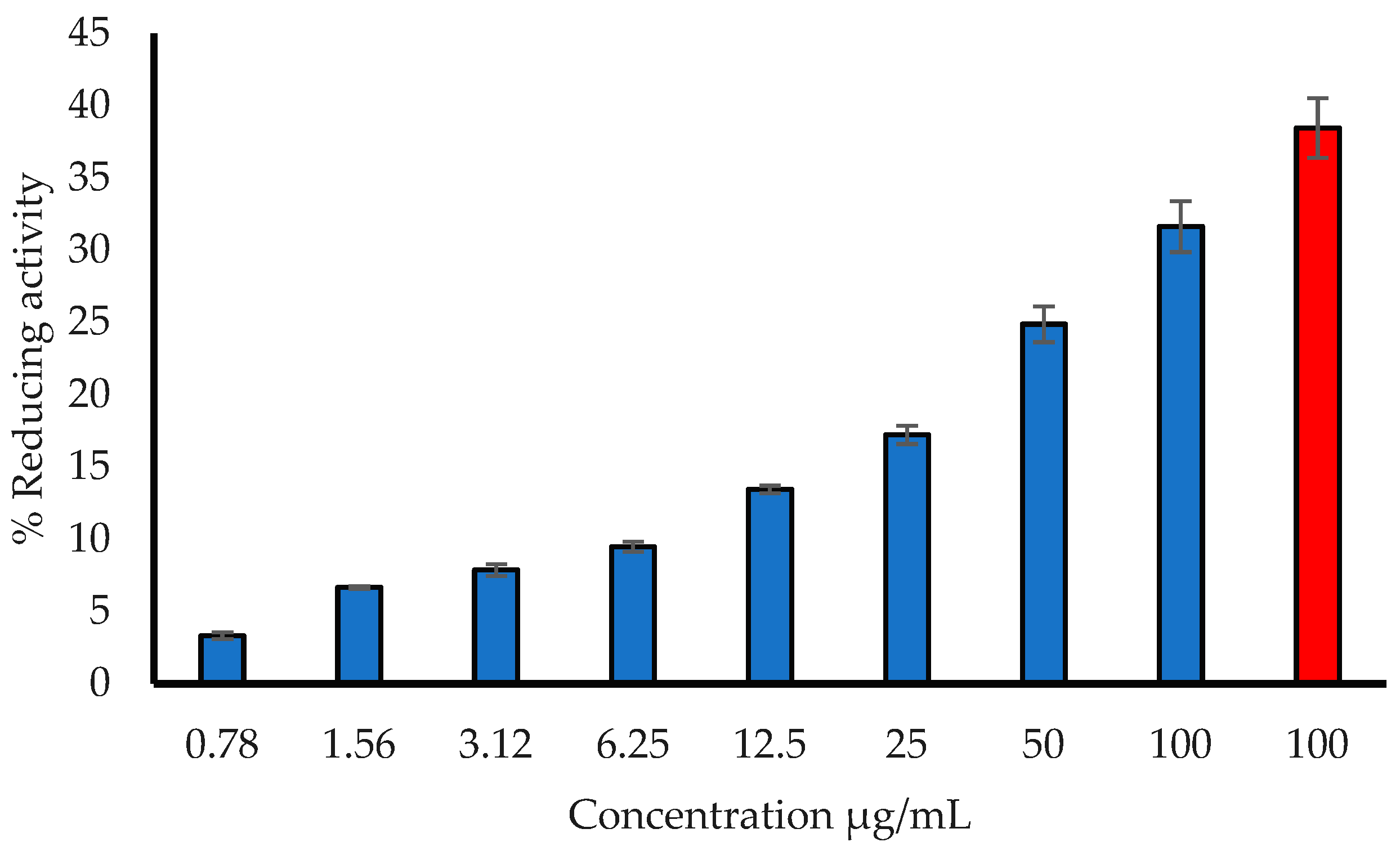
3.1.2. Hydrogen Peroxide (H2O2) Radical Reducing Ability
3.1.3. Formation of Ketoamines
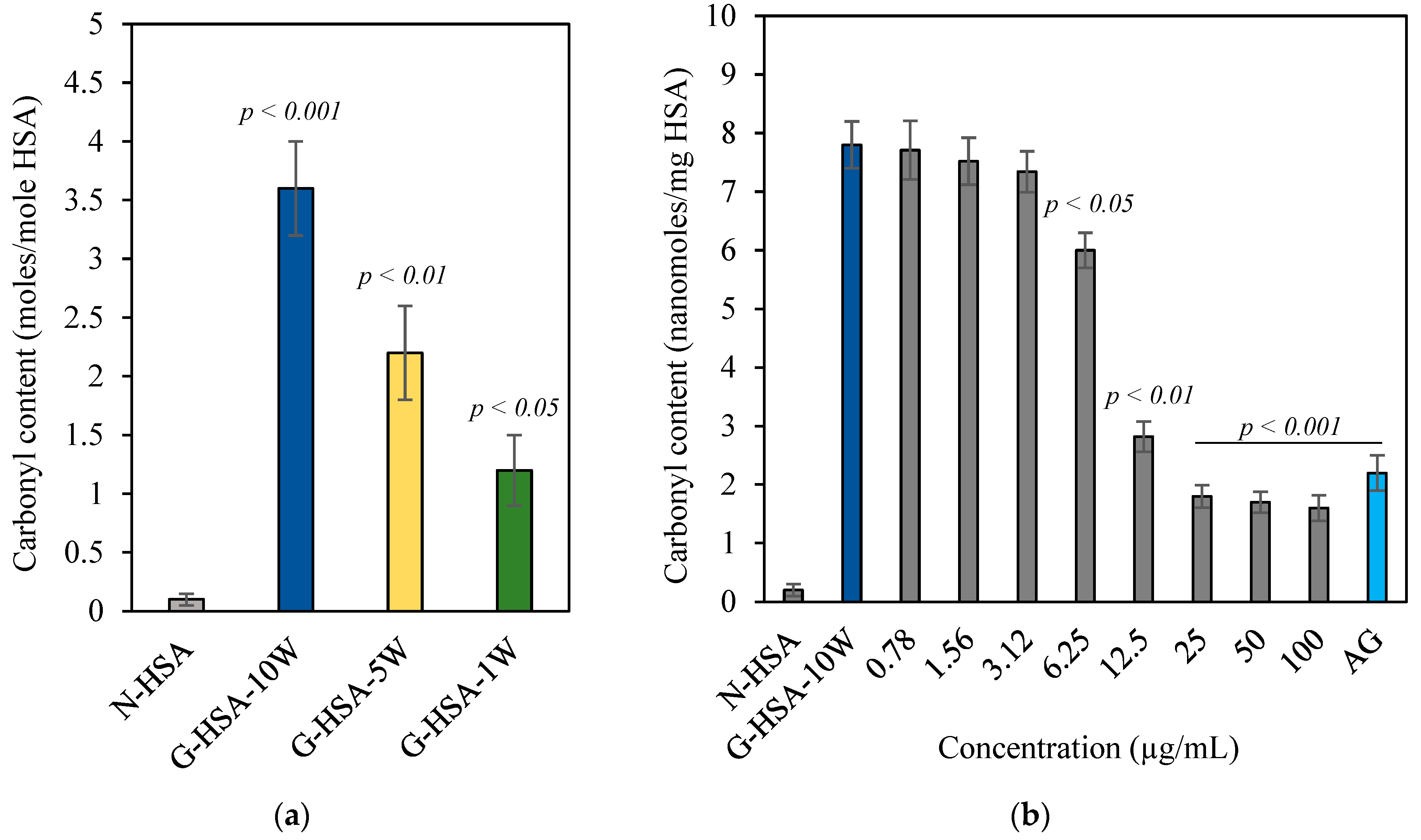
3.1.4. Estimation of Protein Bound Carbonyl Compounds
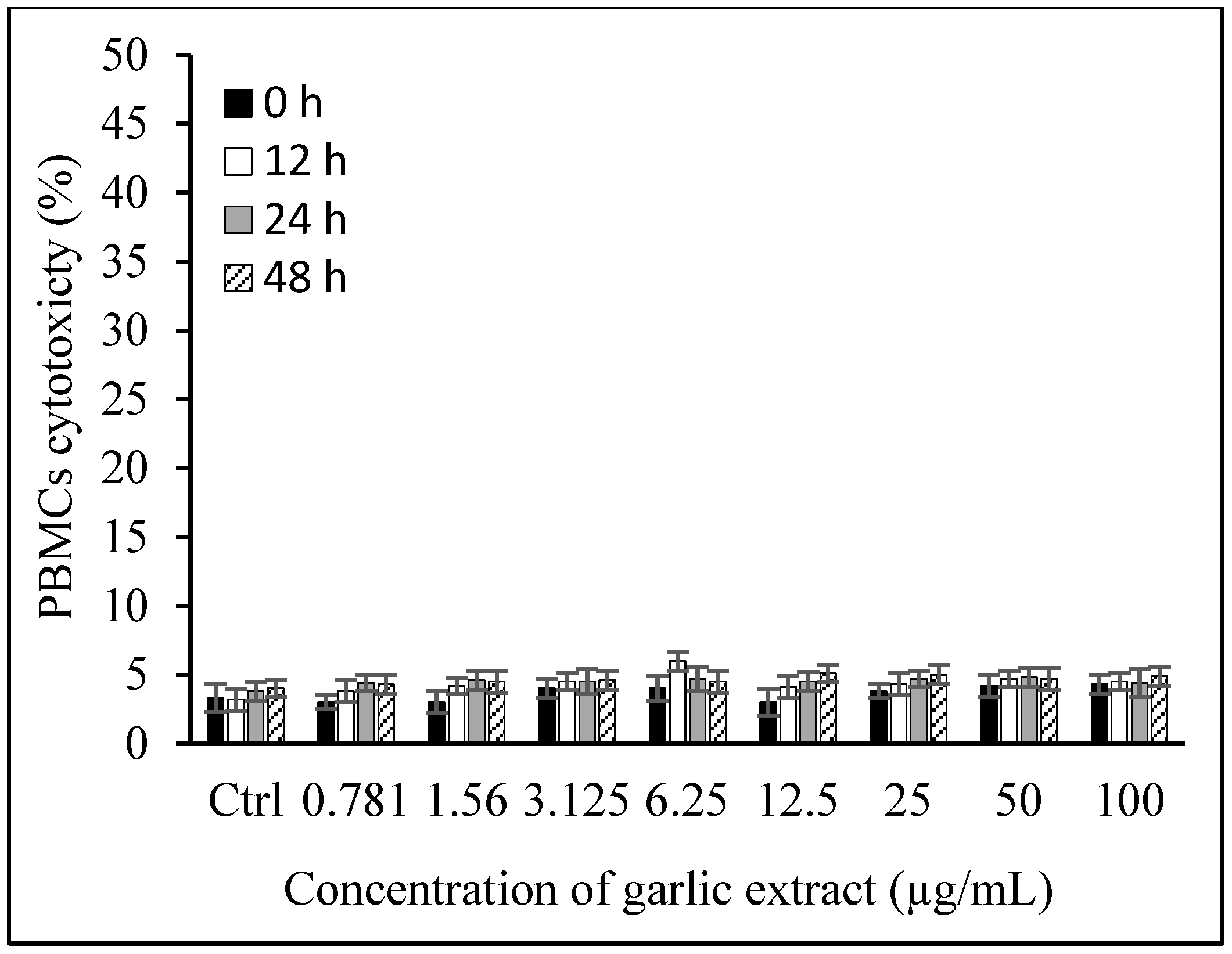
3.1.5. Toxicity of Garlic Extract
3.2. Biophysical Analyses
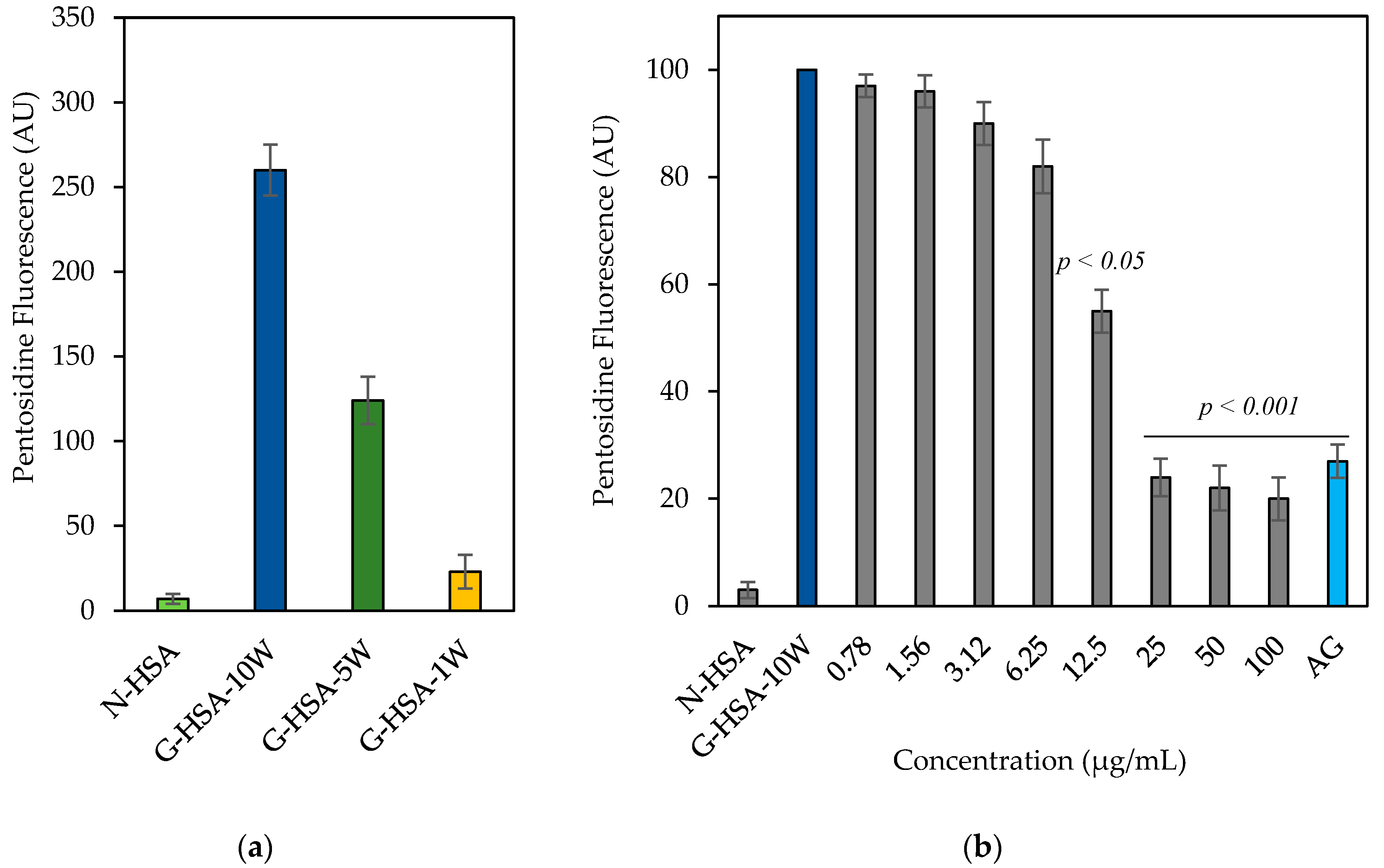
3.2.1. AGE Pentosidine Formation
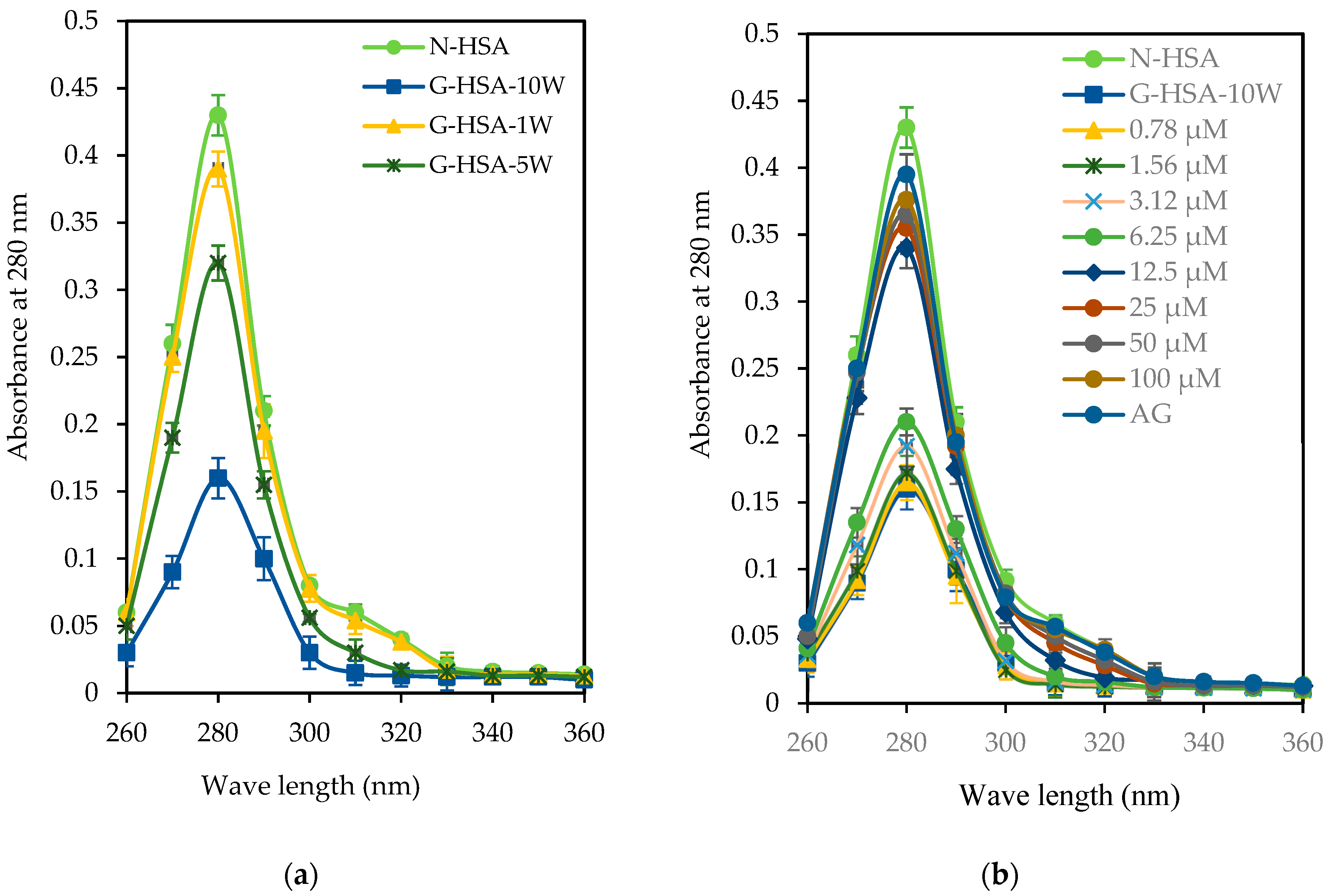
3.2.2. UV and Tryptophan Spectral Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.W.A.; Rasheed, Z.; Khan, W.A.; Ali, R. Biochemical, biophysical, and thermodynamic analysis of in vitro glycated human serum albumin. Biochemistry (Moscow) 2007, 72, 146–152. [Google Scholar] [CrossRef]
- Khan, M.A.; Anwar, S.; Aljarbou, A.N.; Al-Orainy, M.; Aldebasi, Y.H.; Islam, S.; Younus, H. Protective effect of thymoquinone on glucose or methylglyoxal-induced glycation of superoxide dismutase. Int. J. Biol. Macromol. 2014, 65, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alam Khan, M.; Sadaf, A.; Younus, H. A structural study on the protection of glycation of superoxide dismutase by thymoquinone. Int. J. Biol. Macromol. 2014, 69, 476–481. [Google Scholar] [CrossRef]
- Khan, M.; Al Otaibi, A.; Sherwani, S.; Khan, W.; Alshammari, E.; Al-Zahrani, S.; Saleem, M.; Khan, S.; Alouffi, S. Glycation and Oxidative Stress Increase Autoantibodies in the Elderly. Molecules 2020, 25, 3675. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, V.V.; Mandlik, R.V.; Naik, S.R.; Maseeh, A. Nonenzymatic glycosylation: A biochemical link between chronic hyperglycemia and pathophysiologic processes associated with diabetic complications and aging related debilities. Biomed. Aging Pathol. 2012, 2, 133–142. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Qadrie, Z.L.; Khan, W.A. Antibodies against Gluco-Oxidatively Modified Human Serum Albumin Detected in Diabetes-Associated Complications. Int. Arch. Allergy Immunol. 2010, 153, 207–214. [Google Scholar] [CrossRef]
- Alouffi, S.; Sherwani, S.; Al-Mogbel, M.S.; Sherwani, M.K.A.; Khan, M.W.A. Depression and Smoking Augment the Production of Circulating Autoantibodies against Glycated HSA in Rheumatoid Arthritis Patients. Int. Arch. Allergy Immunol. 2018, 177, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Schröter, D.; Höhn, A. Role of Advanced Glycation End Products in Carcinogenesis and their Therapeutic Implications. Curr. Pharm. Des. 2018, 24, 5245–5251. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does Accumulation of Advanced Glycation End Products Contribute to the Aging Phenotype? J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2010, 65A, 963–975. [Google Scholar] [CrossRef]
- Simm, A.; Wagner, J.; Gursinsky, T.; Nass, N.; Friedrich, I.; Schinzel, R.; Czeslik, E.; Silber, R.; Scheubel, R. Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery? Exp. Gerontol. 2007, 42, 668–675. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Grune, T.; Höhn, A. Accumulation of modified proteins and aggregate formation in aging. Exp. Gerontol. 2014, 57, 122–131. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Ueda, T.; Takeuchi, M. Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease. Sci. Rep. 2019, 9, 2121. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.; Sakasai-Sakai, A.; Takata, T.; Ueda, T.; Tsutsumi, M.; Hyogo, H.; Yamagishi, S. Involvement of the TAGE-RAGE system in non-alcoholic steatohepatitis: Novel treatment strategies. World J. Hepatol. 2014, 6, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Yamagishi, S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med. Hypotheses 2004, 63, 449–452. [Google Scholar] [CrossRef]
- Koriyama, Y.; Furukawa, A.; Muramatsu, M.; Takino, J.-I.; Takeuchi, M. Glyceraldehyde caused Alzheimer’s disease-like alterations in diagnostic marker levels in SH-SY5Y human neuroblastoma cells. Sci. Rep. 2015, 5, 13313. [Google Scholar] [CrossRef]
- Takeuchi, M. Serum Levels of Toxic AGEs (TAGE) May Be a Promising Novel Biomarker for the Onset/Progression of Lifestyle-Related Diseases. Diagnostics 2016, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Takino, J.; Nagamine, K.; Hori, T.; Sakasai-Sakai, A.; Takeuchi, M. Contribution of the toxic advanced glycation end-products-receptor axis in nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2459–2469. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakasai-Sakai, A.; Takino, J.; Takata, T.; Ueda, T.; Tsutsumi, M. Toxic AGEs (TAGE) theory in the pathogenesis of NAFLD and ALD. Int. J. Diabetes Clin. Res. 2015, 2, 036. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takino, J.-I.; Sakasai-Sakai, A.; Takata, T.; Tsutsumi, M. Toxic AGE (TAGE) Theory for the Pathophysiology of the Onset/Progression of NAFLD and ALD. Nutrients 2017, 9, 634. [Google Scholar] [CrossRef]
- Rahbar, S.; Figarola, L.J. Novel Inhibitors of Advanced Glycation Endproducts. Arch. Biochem. Biophys. 2003, 419, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Indyk, D.; Bronowicka-Szydełko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Anwar, S.; Alzahrani, F.M.; Almatroudi, A.; Alfheeaid, H.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Health Promoting Effect of Phyllanthus emblica and Azadiractha indica against Advanced Glycation End Products Formation. Appl. Sci. 2021, 11, 8819. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; AlRumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotective Effects of Garlic Extract against Carbon Tetrachloride (CCl4)-Induced Liver Injury via Modulation of Antioxidant, Anti-Inflammatory Activities and Hepatocyte Architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.K.; Js, D.; Ali, M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2015, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.E.; Wang, W.; Qin, J. Anti-hyperlipidemia of garlic by reducing the level of total cholesterol and low-density lipoprotein: A meta-analysis. Medicine (Baltimore) 2018, 97, e0255. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Myasoedova, V.A.; Iltchuk, M.I.; Zhang, D.-W.; Orekhov, A.N. Therapeutic effects of garlic in cardiovascular atherosclerotic disease. Chin. J. Nat. Med. 2019, 17, 721–728. [Google Scholar] [CrossRef]
- Moutia, M.; Habti, N.; Badou, A. In Vitro and In Vivo Immunomodulator Activities of Allium sativum L. Evid.-Based Complement. Altern. Med. 2018, 2018, 4984659. [Google Scholar] [CrossRef]
- Abu Almaaty, A.; Rashed, H.; Soliman, M.; Fayad, E.; Althobaiti, F.; El-Shenawy, N. Parasitological and Biochemical Efficacy of the Active Ingredients of Allium sativum and Curcuma longa in Schistosoma mansoni Infected Mice. Molecules 2021, 26, 4542. [Google Scholar] [CrossRef]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Joseph, R.J.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Taie, H.A.A.; Salama, Z.A.-E.R.; Radwan, S. Potential activity of basil plants as a source of antioxidants and anticancer agents as affected by organic and bio-organic fertilization. Not. Bot. Horti Agrobot. 2010, 38, 119–127. [Google Scholar]
- Ruch, R.J.; Cheng, S.-J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Mashiba, S.; Uchida, K.; Okuda, S.; Tomita, S. Measurement of glycated albumin by the nitroblue tetrazolium colorimetric method. Clin. Chim. Acta 1992, 212, 3–15. [Google Scholar] [CrossRef]
- Ahmed, N.; Furth, J.A. A microassay for protein glycation based on the periodate method. Analyt. Biochem. 1991, 192, 109–111. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef]
- Otaibi, A.A.; Sherwani, S.; Al-Zahrani, S.A.; Alshammari, E.M.; Khan, W.A.; Alsukaibi, A.K.D.; Khan, S.N.; Khan, M.W.A. Biologically Active α-Amino Amide Analogs and γδ T Cells-A Unique Anticancer Approach for Leukemia. Front. Oncol. 2021, 11, 706586. [Google Scholar] [CrossRef]
- Liggins, J.; Furth, J.A. Role of protein-bound carbonyl groups in the formation of advanced glycation end products. Biochim. Biophys. Acta 1997, 1361, 123–130. [Google Scholar] [CrossRef]
- Khan, M.W.; Al Otaibi, A.; Al-Zahrani, S.A.; Alshammari, E.M.; Haque, A.; Alouffi, S.; Khan, W.A.; Khan, S.N. Experimental and theoretical insight into resistance to glycation of bovine serum albumin. J. Mol. Struct. 2021, 1230, 129645. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural Products: Implication in Cancer Prevention and Treatment through Modulating Various Biological Activities. Anti-Cancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Khan, S.; Adil, M.; Saeed, M.; Srivastava, A.K. Structure based molecular inhibition of Caspase-8 for treatment of multi-neurodegenerative diseases using known natural compounds. Bioinformation 2014, 10, 191–195. [Google Scholar] [CrossRef][Green Version]
- Garima, G.; Moin, S.; Aggarwal, N.K.; Gupta, P.K.; Dwivedi, S.; Gupta, U. a study on the role of dietary agent aged garlic extract on protein glycation. World J. Pharm. Res. 2015, 4, 468–476. [Google Scholar]
- Anwar, S.; Younus, H. Inhibitory effect of alliin from Allium sativum on the glycation of superoxide dismutase. Int. J. Biol. Macromol. 2017, 103, 182–193. [Google Scholar] [CrossRef]
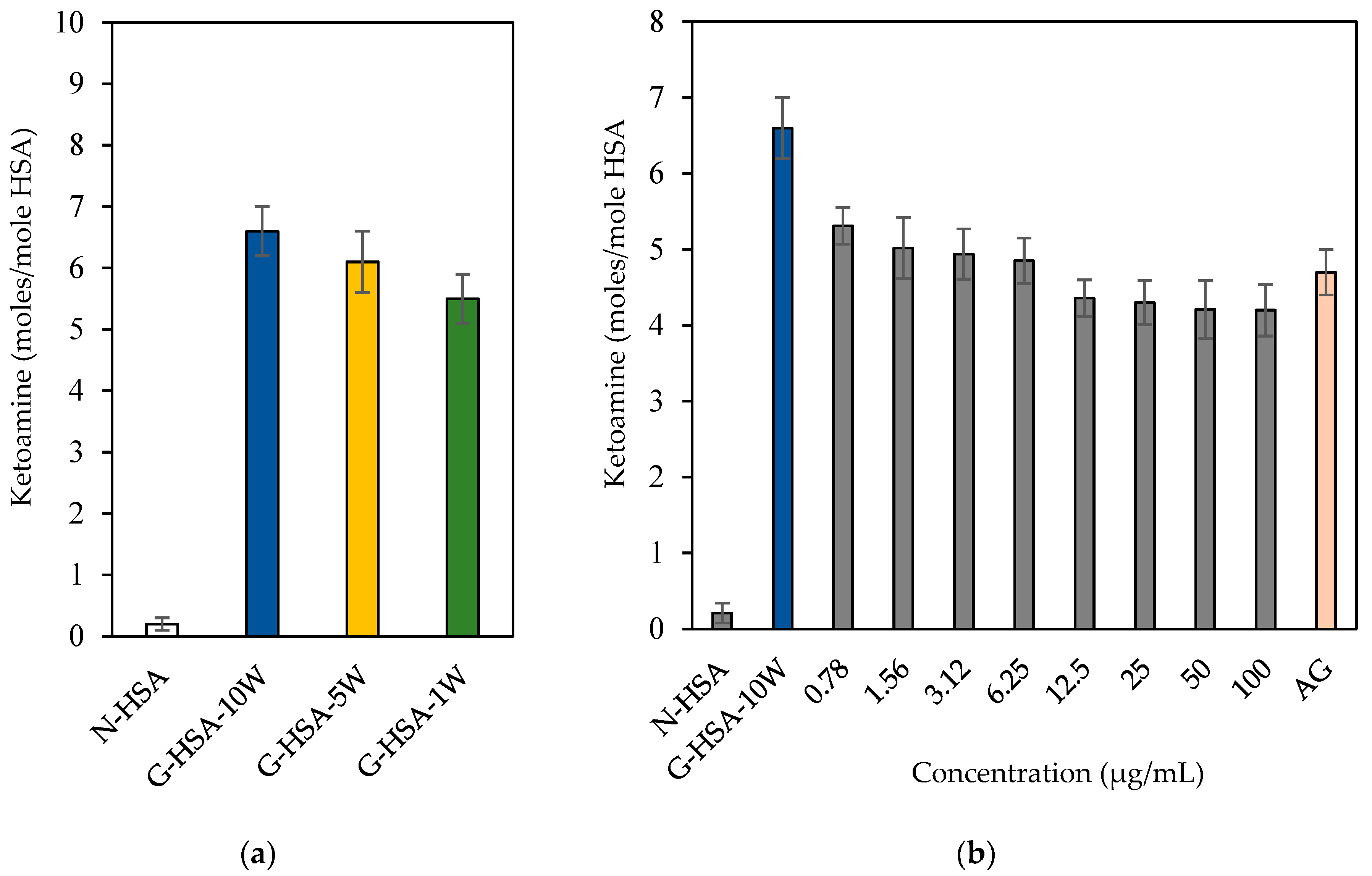

| Preliminary Screening | Garlic Extract |
|---|---|
| Weight of dry powder | 50 g |
| Yield | 5.19% |
| Extract | Aqueous |
| Flavonoid | + |
| Polyphenolic compounds | + |
| Total phenolic compounds | 21.45 ± 0.02 mg gallic acid equivalent/g dry weight of extract |
| Total flavonoid content | 16.58 ± 0.03 mg quercetin equivalent/g dry weight of the extract |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.W.A.; Otaibi, A.A.; Alhumaid, A.F.M.; Alsukaibi, A.K.D.; Alshamari, A.K.; Alshammari, E.M.; Al-Zahrani, S.A.; Almudyani, A.Y.M.; Sherwani, S. Garlic Extract: Inhibition of Biochemical and Biophysical Changes in Glycated HSA. Appl. Sci. 2021, 11, 11028. https://doi.org/10.3390/app112211028
Khan MWA, Otaibi AA, Alhumaid AFM, Alsukaibi AKD, Alshamari AK, Alshammari EM, Al-Zahrani SA, Almudyani AYM, Sherwani S. Garlic Extract: Inhibition of Biochemical and Biophysical Changes in Glycated HSA. Applied Sciences. 2021; 11(22):11028. https://doi.org/10.3390/app112211028
Chicago/Turabian StyleKhan, Mohd W. A., Ahmed A. Otaibi, Arwa F. M. Alhumaid, Abdulmohsen K. D. Alsukaibi, Asma K. Alshamari, Eida M. Alshammari, Salma A. Al-Zahrani, Ahmed Y. M. Almudyani, and Subuhi Sherwani. 2021. "Garlic Extract: Inhibition of Biochemical and Biophysical Changes in Glycated HSA" Applied Sciences 11, no. 22: 11028. https://doi.org/10.3390/app112211028
APA StyleKhan, M. W. A., Otaibi, A. A., Alhumaid, A. F. M., Alsukaibi, A. K. D., Alshamari, A. K., Alshammari, E. M., Al-Zahrani, S. A., Almudyani, A. Y. M., & Sherwani, S. (2021). Garlic Extract: Inhibition of Biochemical and Biophysical Changes in Glycated HSA. Applied Sciences, 11(22), 11028. https://doi.org/10.3390/app112211028






