Abstract
This work was conducted to study the chemical composition, antioxidant, antibacterial, and antifungal activities of essential oil and hydrolat from Withania frutescens. The essential oil was extracted by hydrodistillation. The chemical characterization was performed using gas chromatography-mass spectrometry (GC/MS). The antioxidant activity was studied using four different assays (DPPH, TAC, FRAP, and β-carotene bleaching). The antibacterial activity test was carried out on multidrug-resistant bacteria including Gram-negative and Gram-positive strains. Antifungal activity was tested on Candida albicans and Saccharomyces cerevisiae. The yield of essential oil (EO) obtained by hydrodistillation of W. frutescens was 0.31% majorly composed of camphor, α-thujone, carvacrol, and thymol. Regarding the antioxidant activities, the concentration of the sample required to inhibit 50% of radicals (IC50) of EO and hydrolat were 14.031 ± 0.012 and 232.081 ± 3.047 µg/mL (DPPH), 4.618 ± 0.045 and 8.997 ± 0.147 µg/mL (FRAP), 0.091 ± 0.007 and 0.131 ± 0.004 mg AAE/mg (TAC), 74.141 ± 1.040% and 40.850 ± 0.083% (β-carotene), respectively. Concerning the antibacterial activity of essential oil and hydrolat, the minimum inhibitory concentration (MIC) values found were 0.006 ± 0.001 and 6.125 ± 0.541 µg/mL (Escherichia coli 57), 0.003 ± 0.001 and 6.125 ± 0.068 µg/mL (Klebsiella pneumoniae), 0.001 ± 0.0 and 6.125 ± 0.046 µg/mL (Pseudomonas aeruginosa) and 0.012 ± 0.003 and 6.125 ± 0.571 µg/mL (Staphylococcus aureus), respectively. MIC values of essential oil and hydrolat vs. both C. albicans and S. cerevisiae were lower than 1/20,480 µg/mL. Based on the findings obtained, essential oils of Withania frutescens can be used as promising natural agents to fight free radical damage and nosocomial antibiotic-resistant microbes.
Keywords:
Withania frutescens; essential oil; hydrolat; nosocomial infection; bacteria; candidosis; yeasts 1. Introduction
Nosocomial infections caused by certain microbial strains remain a major cause of mortality and morbidity worldwide [1]. Many countries across the world have a great incidence of nosocomial diseases induced by multi-resistant microorganisms [2]. Several synthesized drugs have not been effective against nosocomial infections due to antibiotic-resistant bacteria [3]. It is thus fitting that medicines give high priority to alternative natural drugs to fight nosocomial antibiotic-resistant microbes [4]. Several studies have reported that plants could be promising sources of effective drugs against various microorganisms [5,6].
Antimicrobial resistance (AMR) is a complicated situation where bacteria and fungi develop strategies to defeat drugs designed to eliminate them, and therefore, the germs that are not killed continue to grow strongly, even more than before [7]. Over the past few decades, AMR has been considered as one of the biggest human health threats and was classified as belonging to the tenth challenging threat by the World Health Organization for 2019 [8,9]. Many factors are involved in the emergence of AMR including the unreasonable use of antibiotics in human health, animal husbandry, hygiene, and the food industry [10,11]. Effects of the drying pipeline of antibiotics have been also contributed to the aggravation of this issue. The phenomena of AMR are seriously alarming with complex threats, but regrettably with very few definite responses [12]. The death due to AMR infections is expected to reach 10 million by 2050 with a tremendous impact on the economy if no treatment options are conducted to contain AMR and its causative agents [13].
The studied bacteria in the present work are among the drug-resistant microbes known as the SAPEEKE group, an acronym of the following strains; Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp, Enterococcus faecium, Klebsiella pneumonia, and Escherichia coli pathogens. These species are multidrug-resistant, extensive drug-resistant, and even pan drug-resistant, as reported elsewhere [14,15,16]. It is well known that Candida spp. tested in this work is among the drug-resistant pathogens. A few years ago, mucosal candidiasis was responsible for affecting more than 90% of patients with AIDS. Due to the massive use of different drugs including oral azoles to fight mucosal candidiasis and invasive fungal infections, the resistance of Candida spp. is becoming largely recognized as one of the greatest growing health burdens [17].
Free radicals can be defined as molecules containing unpaired electrons of cellular oxygen metabolism in mammals. However, free radical-associated damage is largely involved in many pathological processes and cause damage to cells, membranes, and DNA. In this sense, free radical scavengers are required to fight free radicals. Free radical scavengers from natural sources including plants are regarded as potent antioxidant agents to control free radical damage [18].
In traditional medicine, Withania frutescens L. is frequently used by the indigenous population to fight bacterial infections, conjunctivitis, inflammation, tuberculosis, stress, bronchitis, anxiety, neurological disorders, and ulcers as well as liver and Parkinson’s disease [19]. Previously published studies reported some pharmacological activities of Withania frutescens (W. frutescens) including anti-inflammatory, analgesic, and healing activities [19]. W. frutescens.L extracts have been found to be rich in withanolides, which were isolated from the plant leaves. Moreover, the chemical analysis of the W. frutescens.L extract showed that this plant possessed pentacarbonyl (13.22%), 2-phenazine carbonitrile (10.64%), Terpinenol-4 (10.04%), 4H-1-benzopyran-4-one,2,3-dihydro-5,7-dihydroxy-2phenyl(S) (8.76%), and bicyclo [3.1.1]heptane, 6,6-dimethyl-2-methylene (28.48%) [20,21]. Earlier works showed no toxic effects in animals treated with the W. frutescens extract, therefore, the studied plant was considered safe in animals treated under both acute and subacute toxicity conditions [22,23,24]. Preliminary phytochemical screening of W. frutescens extracts showed the presence of some compound classes including tannins, coumarins, saponins, and mucilage [25].
Many studies have reported that essential oils (EOs) possess pharmacological activities. However, few studies have reported on hydrolat. Unlike EO, studies on hydrolat (steam distillation in which an aqueous phase called hydrolat) are still limited despite the interest of the food, cosmetic, and phytotherapeutic industries. Several works have reported on the evaluation of hydrolat antioxidant power. However, this natural product can be advantageous not only as a promising source of therapeutic principles, but also as a potential preservative, especially in phyto-therapy, which includes aromatherapy [26].
To the best of our knowledge, no previous study has investigated the chemical composition and pharmacological activities of essential oils and hydrolat from W. frutescens. It is thus fitting that the present work aimed to study the chemical composition, antioxidant, antibacterial, and antifungal activities of both essential oils and hydrolat from this plant against antibiotic-resistant microbes.
2. Results and Discussion
2.1. Phytochemical Composition of Essential Oil
The yield of essential oil (EO) obtained by hydrodistillation of W. frutescens was 0.31% with 26 and 23 compounds identified by using HP-5MS and DB-HeawyWAX column, respectively (Figure 1 and Table 1). The gas chromatographic analysis showed that the monoterpene chemical classes constituted the major chemical groups in W. frutescens essential oils including 90.35 ± 1.72% and 88.93 ± 1.67% compounds identified by HP-5MS and DB-HeawyWAX column, respectively. For sesquiterpene classes, 3.52 ± 0.82% and 3.96 ± 0.51% were detected by HP-5MS and DB-HeawyWAX column, respectively. For the other compounds, 3.18 ± 74% and 4.22 ± 0.36% were detected by both columns, respectively.
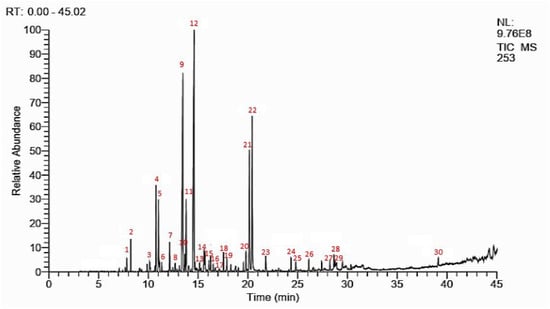
Figure 1.
GCMS chromatographic profile of W. frutescens EO.

Table 1.
Phytochemical compounds contained in the essential oil of W. frutescens.
The essential oils were mainly composed of camphor (24.26 ± 0.31% and 25.41 ± 0.22%), α-thujone (18.64 ± 0.07% and 17.45 ± 0.31%), carvacrol (12.57 ± 0.97% and 13.43 ± 0.21%), and thymol (9.53 ± 0.8% and 9.24 ± 0.83%) according to the detection by HP-5MS and DB-HeawyWAX, respectively.
Many potentially bioactive compounds with pharmacological activities were identified in the characterized EO including thymol, carvacrol, linalool, γ-terpinene, and p-cymene [6,27,28,29]. Some natural compounds reported in the studied oils like camphor possessed interesting pharmacological activities including analgesic, antiseptic, antispasmodic, anti-inflammatory, anti-infectious [30,31,32].
2.2. Antioxidant Activity
The antioxidant activity was assessed using the DPPH assay. The IC50 (the concentration of the sample required to inhibit 50% of free radicals) values of both EO and hydrolat were 14.031 ± 0.012 µg/mL and 232.081 ± 3.047 µg/mL, respectively. The IC50 value of BHT used as a positive control was 11.020 ± 0.903 µg/mL. ANOVA analysis showed no significant difference between the IC50 value of EO and that of BHT (p > 0.05). As shown in Figure 2, the essential oil showed interesting anti-radical activities when compared to BHT. In this sense, hydrolat showed limited antioxidant power when compared to the oil.
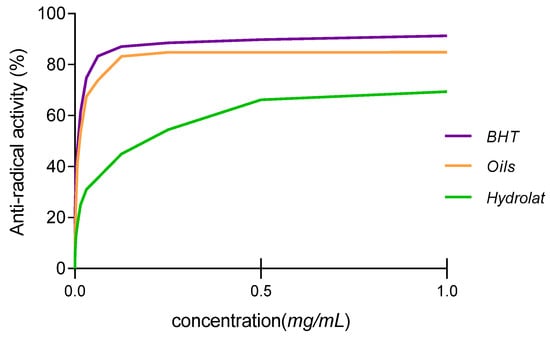
Figure 2.
Anti-free radical activity of essential oil and hydrolat from W. frutescens.
Essential oils are complex mixtures containing several compounds with different functional groups, different polarities, and different chemical behaviors. This chemical complexity could lead to different results depending on the test used. It is thus fitting that an approach with multiple assays aiming to evaluate the antioxidant potential of hydrolat and essential oils was conducted to validate the results obtained. The results obtained revealed that the total antioxidant capacity of EO and hydrolat were 0.091 ± 0.007 and 0.131 ± 0.004 mg AAE (ascorbic acid equivalent)/mg, respectively (Figure 3).
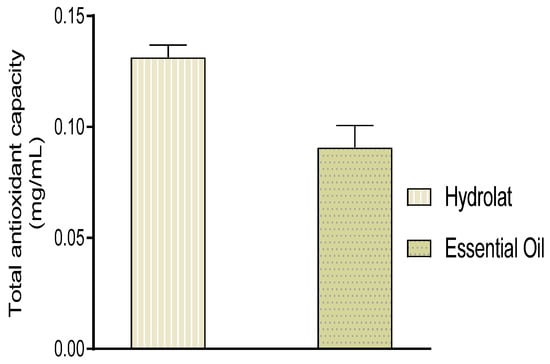
Figure 3.
Total antioxidant capacity of essential oils and hydrolat from W. frutescens.
Many studies have established a strong relationship between the chemical composition of essential oils and their antioxidant activity. The evaluation of interactions between natural antioxidant agents and other food components is an important step in the discussion of total antioxidant power in terms of health benefits. The large diversity of chemicals from a natural source, in addition to their potential interactions and action mode, make it difficult to assess the antioxidant effect by using a simple procedure [31]. It was reported that the antioxidant activity of essential oils was due to their chemical composition, particularly due to compounds with hydroxyl functions [32,33,34]. Consequently, essential oils that are higher in terpenes exhibit greater antioxidant power [35,36]. Metal ions are necessary for biochemical and physiological cellular functions, however, sometimes these ions go under wrong processes to cause lipid peroxidation, oxidative stress, or tissue injury in the absence of antioxidant agents [37].
In this study, the antioxidant power was also evaluated using the FRAP method. As shown in Table 2, The IC50 values of both essential oil and hydrolat were 4.618 ± 0.045 µg/mL and 8.997 ± 0.147 µg/mL respectively. Therefore, we could confirm that EO possessed strong antioxidant power when compared to hydrolat (p < 0.05). Thus, we can suggest that our EO can be highly effective against free radicals.

Table 2.
Antioxidant activities of essential oil and hydrolat from W. frutescens.
The degradation of fatty acids is one of the main causes of food spoilage as reported in many works. The inhibition of lipid oxidation is frequently ensured by the intervention of natural food preservatives. In the present research, lipid oxidation was assessed by measuring the inhibitory oxidation of linoleic acid in the presence of β-carotene, which was used as a marker. The results obtained showed that the absorbance of β-carotene gradually decreased in the presence of oils, hydrolat, and BHT. Moreover, the decrease in the negative control absorbance was the most important, followed by the hydrolat, essential oil, and BHT (Figure 4).
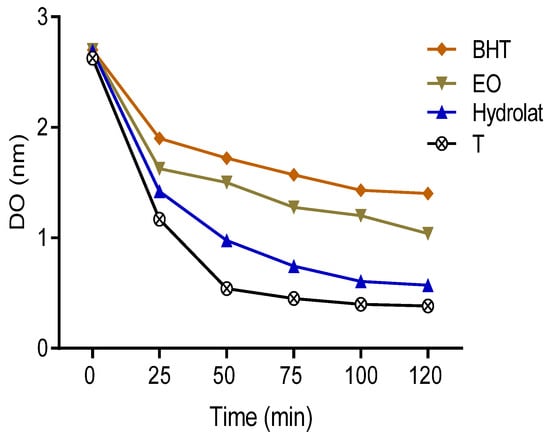
Figure 4.
The absorbance of β-carotene in the presence of the samples studied, BHT, and control.
The variation of β-carotene discoloration rate as a function of time may indicate that essential oils work against the oxidation of linoleic acid. The results obtained in this work showed that the percentage of free radical inhibition of both EO and hydrolat was 74.141 ± 1.040% and 40.850 ± 0.083%, respectively (Table 2). This activity remained significantly lower than that of BHT used as positive controls. These obtained findings were used to perform a comparison with those reported in the previous literature, which showed that species belonging to genera Withania possessed antioxidant power with 57% and 36% for the roots and leaves, respectively [22]. Antioxidants can exert a wide spectrum of biological functions (anti-allergic, anti-atherogenic, anti-inflammatory, antimicrobial, and antioxidant), and the identification and quantification of antioxidant content can be considered an important study to discover biological properties of natural compounds.
2.3. Antibacterial Activity of Essential Oil
The antibiotic resistance of strains used in the present work was well investigated before testing. All selected strains were found to be resistant to a large category of drugs, as shown in Table 3.

Table 3.
List of drug resistance applied to the studied bacteria.
In the present work, five concentrations of essential oil were used to evaluate the antibacterial activity using the agar diffusion method and minimum inhibitory concentration (MICs) assays. The results obtained are presented in Table 4 and Table 5.

Table 4.
Diameter of the inhibition zone of EO, hydrolat, and antibiotics (mm).

Table 5.
Minimum inhibitory concentration of hydrolat and essential oil of W. frutescens (MIC in µg/mL).
The results summarized in Table 4 and Table 5 show that the oil possesses a potent inhibitory effect on all selected bacteria, either Gram-negative or Gram-positive strains with different inhibition zone diameters. E. coli and S. aureus were the most sensitive bacteria to EO with inhibition zone diameters of 27 mm and 26 mm, respectively. The obtained results also revealed that the hydrolat was effective on E. coli 57, K. pneumonia, P. aeruginosa, and S. aureus except E. coli 97. A microdilution method was used to determine the minimal inhibitory concentration of the test sample (Table 5). The results obtained showed that the lowest inhibitory concentration of OE was recorded for P. aeruginosa with a concentration of 1/640 µg/mL, followed by K. pneumoniae with 1/320 µg/mL. Moreover, the studied OE was shown to be effective versus both types of Gram-positive and Gram-negative bacteria, unlike streptomycin and ampicillin, which were less effective [38]. Overall, the best antibacterial effect was shown by the EO vs. the broad spectrum of bacteria including both Gram-negative (E. coli 57, E. coli 97, K. pneumonia, S. aureus) and Gram-positive bacteria (P. aeruginosa). The antibacterial properties of essential oils observed in this work can be explained by the fact that the oil has a lipophilic character that makes it easy to penetrate the bacteria cell and ultimately lead to bacteria death. It was reported that the hydrocarbons make essential oil preferentially lodged in the biological membranes, which disturbs the membrane permeability, and ultimately leads to the immediate death of bacteria [38,39,40]. Closer data reported on the mechanism of action of oils with hydrocarbons can serve as a valuable reference for a better understating of oil mechanism actions toward bacteria [41]. Chemicals in the oil can work in synergy more than individually, as previous work showed that the antimicrobial activity of essential oils was found to be higher than its single compounds tested separately [42,43].
Our findings showed that the bacterial strains tested whether Gram-negative or whether Gram-positive was found to be completely resistant to Ampicillin and partially to Streptomycin. These results agree with those reported in earlier works [44], which showed that the most threatening drug-resistant microbes including S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter spp., and E. coli pathogens. The microbes tested in this work are classified as being multidrug-resistant, extensive drug-resistant, and even pan-drug-resistant, as reported elsewhere [15,16]. The limited treatment strategies vs. drug-resistant infections require new and more effective antibiotics, however, the number of antibiotics approved for clinical use since 2000 has been limited and most of them respond only to Gram-positive bacteria, and their response against Gram-negative strains are reduced by the time, which can accentuate the emergence of a greater threat to human health [45,46].
To have a antimicrobial effect, the antimicrobial agent needs to reach and interact with target microbe sites. However, the drug–target interaction is frequently interrupted via various mechanisms in bacteria (multidrug-resistant, and extensive drug-resistant), which leads to the ineffectiveness of antimicrobial agents and ultimately help develop bacteria against the tested agents [44]. The low sensitivity of Gram-negative microorganisms to antibacterial agents may be explained by the fact that they have an outer membrane surrounding the cell wall, which limits the diffusion of hydrophobic compounds through its lipopolysaccharide coating. Due to their lipophilic nature, essential oils can easily cross the cell walls and the cytoplasmic membrane causing disorders of polysaccharide structure, fatty acids, and phospholipids as well as their permeability [47]. Our findings showed that essential oils have almost similar activity against Gram-positive and Gram-negative bacteria. Therefore, we could confirm that W. frutescens EO can be a promising weapon to fight nosocomial pathogenic and multidrug-resistant strains.
2.4. Antifungal Activity
Yeast infections have a high frequency in hospitalized patients worldwide with many risk factors associated with a poor prognosis. Several epidemiological studies conducted on yeast infections showed that Candida is responsible for many diseases. Candida species have been reported as the most common cause of invasive fungal infections among hospitalized patients, accounting for 8 to 10% of all nosocomial infections [48]. It was reported that invasive candidiasis is frequently associated with high crude death rates, and the control of these infections can be a great challenge since several antifungal options can no longer be effective toward the resistant strains [49]. S. cerevisiae has involved in human pathology causing vaginitis [50].
The antifungal activity of EO and hydrolat was conducted on C. albicans and S. cerevisiae yeasts. The inhibition of fungal growth was noted in the presence of essential oil and hydrolat. The results obtained showed that a strong essential oil inhibition was observed for C. albicans and S. cerevisiae with an inhibition zone diameter of 47 ± 3.120 mm and 40 ± 6.450 mm, respectively (Table 6). The drug used as the reference was less effective against the yeasts when compared to the essential oil with an inhibition zone diameter of 21.200 ± 4.200 mm against Candida and 27.650 ± 2.500 mm and Saccharomyces, respectively (Table 6). Our essential oil had a strong antifungal activity when compared to all standards used including fluconazole and copper sulfate. The minimum inhibitory concentration of essential oil was very low, as shown in Table 6. The results obtained revealed that our studied oil was more effective against both C. albicans and S. cerevisiae with a minimum inhibitory concentration of 10−4 mg/mL. The hydrolat also had a minimum inhibitory concentration of about 12.5 mg/mL. The hydrolat extract was moderately effective when compared to other results with an inhibition zone diameter of 9 ± 1.750 mm against both yeasts, C. albicans and S. cerevisiae, tested. The hydrolat extract also showed better activity by using the minimum inhibitory concentration assay on C. albicans of 0.400 ± 0.020 mg/mL and S. cerevisiae of 0.200 ± 0.010 mg/mL. The fungal strains investigated in the present work belong to the drug-resistant microbes since they showed high MICs, which existed in the range of 0.400 ± 0.020 and 0.400 ± 0.020 mg/mL for fluconazole and 10 ± 0.5–10 ± 0.25 for copper sulfate used as drug references (Table 6) [50].

Table 6.
Results of the antifungal activity of the essential oil and hydrolat.
Many studies have reported interesting data on the mechanism of action of essential oils in fungi for the understanding of the corresponding mechanisms of activity. It was concluded that oils with thymol and p-cymene penetrate cells, causing severe damage to the membrane [51]. The fungicidal activity results from direct damage to the membrane of cells rather than from metabolic impairment, leading to secondary damage of the cell membrane [52]. Such activity is in agreement with the chemical nature of monoterpenes, which most potentially act as a solvent of the cell membrane. In closer works, it was stated that fungicidal activity of oil with thymol and p-cymene vs. Candida spp. resulted from direct damage to the cytoplasmic membrane [53].
3. Materials and Methods
3.1. Chemicals
Ammonium molybdate, butylated hydroxytoluene (BHT), 2,2-diphenylpicrylhydrazyl (DPPH), sodium phosphate, quercetin, vitamin C, iron III chloride (FeCL3), 2,3,5-triphenyltetrazolium chloride (TTC), potassium ferricyanide (K3Fe (CN) 6), and β-carotene were purchased from Sigma Aldrich (Germany, Munich).
3.2. Selection and Identification of Plant Material
W. frutescens was collected at the end of March 2019 from the region of Fez-Morocco. The botanical identification was carried out by the botanist Amina BARI and given the voucher number BPRN69 before being deposited at the herbarium of the Faculty of Sciences, Sidi Mohamed Ben Abdellah Dhar El-Mahraz Fez University, Morocco. The aerial parts of the studied plant were dried in the shade at room temperature for 10 days before being subject to extraction.
3.3. Extraction of Essential Oils
A total of 200 g of the aerial part were finely cut and placed in a round-bottomed flask with 750 mL of distilled water. The mixture was boiled for 2 h and then the EO obtained was separated from the water before being stored at 4 °C in the darkness until further use.
3.4. Preparation of Hydrolat
Extraction of hydrolat was carried out by liquid–liquid extraction. Briefly, 200 mL recovered from the solution obtained by hydrodistillation was successively extracted again three times with 100 mL of diethyl ether at room temperature to obtain the hydrolat extract. Afterward, the organic layer was evaporated and then the remains were dried out using Na2SO4 to obtain the oil (0.03%).
3.5. Chemical Characterization of Essential Oil by GC/MS
The identification of different chemical compounds contained in essential oils was carried out by gas chromatography coupled to a mass spectrometer. W. frutescens oil was analyzed using a Thermo Fischer capillary gas chromatograph directly coupled to the mass spectrometer system (model GC ULTRA S/N 20062969; Polaris QS/N 210729) using two columns, a non-polar HP-5MS capillary fused silica column (60 m 0.32 mm, 0.25 mm film thickness) and a DB-HeawyWAX column (30 m × 0.25 mm, film thickness 0.25 µm), in addition to GC-FID (flame ionization detector). GC-MS operating conditions were maintained as follows: initial temperature of 40 °C/2 min; speed of 2 C/min; a final temperature of 260 °C/10 min; injector temperature of 250 °C; and carrier gas of helium 1 mL/min. The essential oil was diluted in hexane with a dilution ratio of 10:100. The volume of the sample injected was 1 mL with the fractional injection technique; ionization energy was 70 eV, ionization mode; ion source temperature of 200 C, sweep mass range m/z 40–650, and interface line temperature of 300 C. Retention indices (RI) were determined with reference to a homologous series of n-alkanes and by matching their recorded mass spectra with those stored in the spectrometer database (NIST MS Library v. 2.0) [38,54,55].
3.6. In Vitro Antioxidant Activity of Essential Oils
The antioxidant power of essential oil was evaluated in vitro using four assays: DPPH-, reducing power, total antioxidant capacity, and the β-carotene discoloration.
3.7. Diphenyl-1-Picrylhydrazyl Assay
The DPPH test was carried out according to the method described by BEKTAS [56]. Briefly, 100 µL of EO diluted in methanol was used with different concentrations. Each test portion was mixed with 750 µL of DPPH solution (0.004%). After 30 min of incubation at room temperature, the absorbance was read at 517 nm. Results were expressed as percentage inhibition according to the following formula.
where PI is the inhibition percentage; A0 is the DPPH absorbance without the sample (negative control); and A is the DPPH absorbance with the sample.
PI(%) = (A0 − A/A0) × 100
3.8. Ferric Reducing Antioxidant Power Test
This test was carried out according to the previously reported method [56]. Briefly, 500 µL was recovered from phosphate buffer solution (0.2 MPH = 6.6) and mixed with 500 µL of potassium ferricyanide [K3Fe(CN)6] (1%). Afterward, the whole was added to 100 µL of oil diluted in methanol before being incubated at 50 °C for 20 min within the water bath. The sample was mixed again with 500 µL of aqueous solution (10% TCA) including 500 µL of distilled water and 100 µL of 0.1% (FeCl3) to be ready for analysis. The absorbance was determined at 700 nm against a blank. The results obtained were expressed as an effective concentration. The median effective concentration (EC-50) was performed from the graph.
3.9. Total Antioxidant Capacity Test
Twenty-five µL of EO was mixed with 1 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). Afterward, the solution obtained was incubated at 95 °C for 90 min. The absorbance was measured at 695 nm [57]. The total antioxidant capacity was expressed in milligrams of ascorbic acid equivalent per gram of essential oil (mg EAA/g EO). The experiment was performed in triplicate.
3.10. Beta-Carotene Discoloration Test
One milliliter of the solution of β-carotene solubilized in chloroform (2 mg/10 mL) was introduced into a vial containing 10 µL linoleic acid and 100 mg Tween 80. The chloroform was evaporated at 45 °C for 5 min using a rotary evaporator under empty. Next, 25 mL of hydrogen peroxide was added to the residue before adding water to obtain an emulsion. A total of 2.5 mL of the mixture obtained was mixed with 100 µL of essential oil diluted in methanol and was then incubated in the water bath at 50 °C for 2 h with the BHT (positive control). The percentage of antioxidant activity was calculated according to the following equation [58].
where AA% is the percentage of antioxidant activity; AE is the absorbance of the sample; and ABHT is the absorbance of the sample with the positive control (BHT).
AA% = (AE/ABHT) × 100
3.11. Antibacterial Activity
The antibacterial activity of essential oil was tested on five strains: Gram-negative bacteria including E. coli (ATB:57) B6N (CHU, Fez), E. coli (ATB:97) BGM (CHU, Fez), K. pneumoniae (LM, FMP, Fez), and P. aeruginosa (LM, FMP, Fez); Gram-positive bacteria including S. aureus (LM, FMP, Fez). All strains tested were clinically isolated from lung, urinary tract, and surgical site infections (University Hospital Complex, Fez, Morocco). These bacterial strains have been reported as multidrug-resistant, extensive drug-resistant, and even pan-drug-resistant [14,15,16]. The disk diffusion method was used to determine the inhibition zone. Briefly, after incubation of the inoculated plates for 30 min, the experiments were conducted as follows: sterile discs (6 mm) impregnated with 10 µL of the test material, ampicillin 1.67 mg/disc, and streptomycin 0.02 mg/disc were placed on the agar surface. Afterward, the plates were incubated again at 37 °C. At the end of the experiment, the antimicrobial power was evaluated by measuring the growth inhibition zones in mm [38].
The bacterial strains selected were cultured in tubes containing 9 mL of MHB (Mueller–Hinton broth) before being incubated at 37 °C for 18–24 h. A drop of the culture was seeded onto Petri dishes containing nutrient agar before being incubated again at 37 °C for 18–24 h. The bacterial suspension (inoculum) was prepared from the pure cultures as follows: identical colonies from wells were isolated and then discharged into 10 mL sterile physiological water with 0.9% NaCl. The optical density of bacterial suspension was adjusted to be between 0.08 and 0.1 nm, which corresponded to 107 to 108 CFU/mL according to McFarland. The minimum inhibitory concentrations (MICs) were determined using a microdilution method [59].
3.12. Antifungal Activity
The antifungal activity of both EO and hydrolat on Candida albicans and Saccharomyces cerevisiae was performed using the direct contact method. Different concentrations of the test sample were incorporated into the agar culture medium.
The blastospores of strain cultures (5-day cultures) were recovered from Petri dishes before counting the blast strains obtained using the Malassez cell. The optical density (OD) of the fungal spore suspension was measured using a spectrophotometer with 630 nm to standardize the spore suspension at 107 blastospores/mL. Fluconazole and copper sulfate were used as positive controls with 5 mg/mL.
Different concentrations (v/v) of 1/100; 1/200; 1/400; 1/800; 1/1600; 1/3200 of oils were incorporated into the PDA (potato dextrose agar) culture medium. Afterward, 10 µL of the inoculum spot was deposited in each medium. Petri dishes were sealed with parafilm before being incubated at 27 °C for six days. The minimum inhibitory concentration was observed in the solid medium [60]. Petri dishes with concentrations that showed a total absence of mycelial growth were selected to determine the minimum inhibitory concentrations (MIC).
In the present work, the obtained results showed that the oil from W. frutescens was found to exhibit a potent antioxidant activity in all bioassays used such as DPPH, FRAP, TAC, and β-carotene. The antioxidant activity of the essential oil tested was strongly correlated with the antimicrobial and antibacterial activity. However, hydrolat showed limited effects toward fungal and bacteria tested as well as the antioxidant activity.
3.13. Statistical Analysis
The mean values ± standard deviations were calculated using GraphPad Prism 7 (Microsoft Software, GraphPad Software Inc.; San Diego, CA, USA). The results were compared using one-way ANOVA and the Tukey-test as the post-hoc test. The difference at p < 0.05 was considered to be significant.
4. Conclusions
In the current research study, we investigated the chemical composition, antioxidant, antibacterial, and antifungal activities of essential oil and hydrolat from W. frutescens on nosocomial antibiotic-resistant microbes. The results obtained showed that the oil was found to be rich in bioactive compounds with promising activities on both yeasts and bacteria. However, hydrolat was not more active toward the fungal and bacteria tested nor in antioxidant activity. Finally, we confirmed that oil in W. frutescens can serve as medicines as it provides potentially active agents to fight free radical damage, bacterial, and fungal infections.
Author Contributions
A.E.M., F.Z.J., M.B.: Writing—original draft; R.U., A.B. (Ahmed Bari), H.M.M., M.S., M.A.M.A.-S., E.E. and G.A.E.M.: Writing, reviewing, and funding acquisition. A.M.S., B.S. and A.R.: Formal analysis; D.B. and A.B. (Amina Bari): Methodology and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia via grant number RG-1435-072 and article processing charge (APC) was also supported by the Deanship of Scientific Research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data reported here is available from the authors upon request.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding this work through the research group project number RG-1435-072.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jenkins, D.R. Nosocomial Infections and Infection Control. Medicine 2017, 45, 629–633. [Google Scholar] [CrossRef]
- Maoulainine, F.M.R.; Elidrissi, N.S.; Chkil, G.; Abba, F.; Soraa, N.; Chabaa, L.; Amine, M.; Aboussad, A. Épidémiologie De L’Infection Nosocomiale Bactérienne Dans Un Service De Réanimation Néonatale Marocain. Arch. Pediatrie 2014, 21, 938–943. [Google Scholar] [CrossRef]
- Chan, M. A Global Health Guardian: Climate Change, Air Pollution and Antimicrobial Resistance. In Ten Years Public Health 2007–2017; World Health Organization: Geneva, Switzerland, 2017; pp. 135–145. ISBN 978-92-4-151244-2. Available online: https://www.who.int/publications/10-year-review/chapter-guardian.pdf (accessed on 4 January 2021).
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Gherraf, N.; Zellagui, A.; Kabouche, A.; Lahouel, M.; Salhi, R.; Rhouati, S. Chemical Constituents and Antimicrobial Activity of Essential Oils of Ammodaucus Leucotricus. Arab. J. Chem. 2017, 10, S2476–S2478. [Google Scholar] [CrossRef]
- Siddique, S.; Parveen, Z.; Firdaus-e-Bareen, X.; Mazhar, S. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oils from Leaves of Three Melaleuca Species of Pakistani Flora. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K. Synchronous Application of Antibiotics and Essential Oils: Dual Mechanisms of Action as a Potential Solution to Antibiotic Resistance. Crit. Rev. Microbiol. 2018, 44, 414–435. [Google Scholar] [CrossRef] [PubMed]
- Shriram, V.; Khare, T.; Bhagwat, R.; Shukla, R.; Kumar, V. Inhibiting Bacterial Drug Efflux Pumps via Phyto-Therapeutics to Combat Threatening Antimicrobial Resistance. Front. Microbiol. 2018, 9, 2990. [Google Scholar] [CrossRef] [PubMed]
- Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 9 January 2021).
- Buckner, M.M.C.; Ciusa, M.L.; Piddock, L.J.V. Strategies to Combat Antimicrobial Resistance: Anti-Plasmid and Plasmid Curing. FEMS Microbiol. Rev. 2018, 42, 781–804. [Google Scholar] [CrossRef] [PubMed]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial Resistance in Healthcare, Agriculture and the Environment: The Biochemistry behind the Headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Islam, S.; Aldstadt, J.; Aga, D. Global Antimicrobial Resistance: A Complex and Dire Threat with Few Definite Answers. Trop. Med. Int. Health 2019, 24, 658–662. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://www.biomerieuxconnection.com/wp-content/uploads/2018/04/Tackling-Drug-Resistant-Infections-Globally_-Final-Report-and-Recommendations.pdf (accessed on 4 January 2021).
- Mapara, N.; Sharma, M.; Shriram, V.; Bharadwaj, R.; Mohite, K.C.; Kumar, V. Antimicrobial Potentials of Helicteres Isora Silver Nanoparticles against Extensively Drug-Resistant (XDR) Clinical Isolates of Pseudomonas Aeruginosa. Appl. Microbiol. Biotechnol. 2015, 99, 10655–10667. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Thomas, V.M.; Brown, R.M.; Ashcraft, D.S.; Pankey, G.A. Synergistic Effect between Nisin and Polymyxin B against Pandrug-Resistant and Extensively Drug-Resistant Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2019, 53, 663–668. [Google Scholar] [CrossRef]
- Maenza, J.R.; Merz, W.G.; Romagnoli, M.J.; Keruly, J.C.; Moore, R.D.; Gallant, J.E. Infection Due to Fluconazole-Resistant Candida in Patients with AIDS: Prevalence and Microbiology. Clin. Infect. Dis. 1997, 24, 28–34. [Google Scholar] [CrossRef]
- Bourhia, M.; Laasri, F.E.; Aourik, H.; Boukhris, A.; Ullah, R.; Bari, A.; Ali, S.S.; El Mzibri, M.; Benbacer, L.; Gmouh, S. Antioxidant and Antiproliferative Activities of Bioactive Compounds Contained in Rosmarinus Officinalis Used in the Mediterranean Diet. Evid. Based Complement. Altern. Med. 2019, 2019, 7623830. [Google Scholar] [CrossRef] [PubMed]
- Jamal, B. The Traditional Moroccan Pharmacopee, Ancient Arab Medicine and Popular Knowledge; IBIS Press: Paris, France, 1998. [Google Scholar]
- El Moussaoui, A.; Jawhari, F.Z.; Bourhia, M.; Maliki, I.; Sounni, F.; Mothana, R.A.; Bousta, D.; Bari, A. Withania Frutescens: Chemical Characterization, Analgesic, Anti-Inflammatory, and Healing Activities. Open Chem. 2020. [Google Scholar] [CrossRef]
- EL Moussaoui, A.; Jawhari, F.; EL Ouahdani, K.; Es-Safi, I.; Bousta, D.; Bari, A. Valorization of the Pharmacological Potential of Phytochemical Compounds Contained in the Crude Extract of the Root of a Plant of Withania Frutescens L. Phytothérapie 2019. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, Antifungal and Antioxidant Activity of Total Polyphenols of Withania Frutescens L. Bioorg. Chem. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, A.E.L.; Bourhia, M.; Jawhari, F.Z.; Es-safi, I.; Ali, S.S.; Bari, A.; Mahmood, H.M.; Bousta, D.; Bari, A. Withania Frutescens. L Extract: Phytochemical Characterization and Acute and Repeated Dose 28-Day Oral Toxicity Studies in Mice. BioMed Res. Int. 2020, 2020, 1976298. [Google Scholar] [CrossRef] [PubMed]
- EL Moussaoui, A.; Bourhia, M.; Jawhari, F.Z.; Mechchate, H.; Slighoua, M.; Bari, A.; Ullah, R.; Mahmood, H.M.; Ali, S.S.; Ibenmoussa, S.; et al. Phytochemical Identification, Acute, and Sub-Acute Oral Toxicity Studies of the Foliar Extract of Withania Frutescens. Molecules 2020, 25, 4528. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, A.; Jawhari, F.Z.; Bousta, D.; Bari, M. Phytochemical characterization and antioxidant activity of the northern moroccan species: Withania frutescens L. Asian J. Pharm. Clin. Res. 2019, 12, 276–279. [Google Scholar] [CrossRef]
- Hay, Y.-O.; Abril-Sierra, M.A.; Sequeda-Castañeda, L.G.; Bonnafous, C.; Raynaud, C. Evaluation of Combinations Essential Oils and with Evaluation of Combinations Essential Oils and with Hydrosols on Antimicrobial and Antioxidant Activities. J. Pharm. Pharmacogn. Res. 2018, 6, 216–230. [Google Scholar]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme Essential Oils from Spain: Aromatic Profile Ascertained by GC–MS, and Their Antioxidant, Anti-Lipoxygenase and Antimicrobial Activities. J. Food Drug Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef]
- Zuccarini, P. Camphor: Risks and Benefits of a Widely Used Natural Product. J. Appl. Sci. Environ. Manag. 2009, 13, 69–74. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Camphor (Cinnamomum Camphora), a Traditional Remedy with the History of Treating Several Diseases. Int. J. Case Rep. Images 2013, 4, 86–89. [Google Scholar] [CrossRef]
- Sherkheli, M.A.; Benecke, H.; Doerner, J.F.; Kletke, O.; Vogt-Eisele, A.K.; Gisselmann, G.; Hatt, H. Monoterpenoids Induce Agonist-Specific Desensitization of Transient Receptor Potential Vanilloid-3 (TRPV3) Ion Channels. J. Pharm. Pharm. Sci. 2009, 12, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A. Study Approach of Antioxidant Properties in Foods: Update and Considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef]
- Bouhdid, S.; Skali, S.N.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial and Antioxidant Activities of Origanum Compactum Essential Oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar] [CrossRef]
- Gülçin, Ì.; Şat, I.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.I. Comparison of Antioxidant Activity of Clove (Eugenia Caryophylata Thunb) Buds and Lavender (Lavandula Stoechas, L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic Antioxidants from Clonal Oregano (Origanum Vulgare) with Antimicrobial Activity against Helicobacter Pylori. Process. Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Zhuang, S.R.; Chen, S.L.; Tsai, J.H.; Huang, C.C.; Wu, T.C.; Liu, W.S.; Tseng, H.C.; Lee, H.S.; Huang, M.C.; Shane, G.T.; et al. Effect of Citronellol and the Chinese Medical Herb Complex on Cellular Immunity of Cancer Patients Receiving Chemotherapy/Radiotherapy. Phytother. Res. 2009, 23, 785–790. [Google Scholar] [CrossRef]
- Fayed, S.A. Antioxidant and Anticancer Activities of Citrus Reticulate (Petitgrain Mandarin) and Pelargonium Graveolens (Geranium) Essential Oils. Res. J. Agric. Biol. Sci. 2009, 5, 740–747. [Google Scholar]
- Tiwari, A.K. Imbalance in Antioxidant Defence and Human Diseases: Multiple Approach of Natural Antioxidants Therapy. Curr. Sci. 2001, 81, 1179–1187. [Google Scholar]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, Antioxidant and Antibacterial Activities of Two Moroccan Teucrium Polium, L. Subspecies: Preventive Approach against Nosocomial Infections. Arab. J. Chem. 2019. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial Activity of Individual and Mixed Fractions of Dill, Cilantro, Coriander and Eucalyptus Essential Oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 453–462. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-Vitro Antimicrobial Activity and Chemical Composition of Sardinian Thymus Essential Oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The Alarming Antimicrobial Resistance in ESKAPEE Pathogens: Can Essential Oils Come to the Rescue? Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the Clinical Pipeline at the End of 2015. J. Antibiot. 2017, 70, 3–24. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Securing New Drugs for Future Generations: The Pipeline of Antibiotics. Antibiotic Resistance Threats in the United States. 2013. Available online: https://amr-review.org/sites/default/files/SECURING%20NEW%20DRUGS%20FOR%20FUTURE%20GENERATIONS%20FINAL%20WEB_0.pdf (accessed on 4 January 2021).
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Vidal, L.; Goldberg, E.; Leibovici, L.; Paul, M. Treatment of Invasive Candidal Infections: Systematic Review and Meta-Analysis. Mayo Clin. Proc. 2008, 83, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Kett, D.H.; Cubillos, G.F. Anidulafungin in the Treatment of Patients with Invasive Candidiasis. Int. J. Antimicrob. Agents 2008, 32, S99–S102. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S. Caralluma Europaea (Guss.) NE Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef]
- Green, L.J.; Marder, P.; Mann, L.L.; Chio, L.-C.; Current, W.L. LY303366 Exhibits Rapid and Potent Fungicidal Activity in Flow Cytometric Assays of Yeast Viability. Antimicrob. Agents Chemother. 1999, 43, 830–835. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Sansonetty, F.; Martinez-De-Oliveira, J.; Fonseca, A.F.; Mårdh, P.-A. Antifungal Activity of Local Anesthetics against Candida Species. Infect. Dis. Obstet. Gynecol. 2000, 8, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Martinez-De-Oliveira, J.; Fonseca, A.F.; Mårdh, P.-A. Antifungal Activity of Ibuprofen Alone and in Combination with Fluconazole against Candida Species. J. Med. Microbiol. 2000, 49, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-11-4. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and Antioxidant Activities of the Essential Oil and Various Extracts of Salvia Tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Moattar, F.S.; Sariri, R.; Yaghmaee, P.; Giahi, M. Enzymatic and Non-Enzymatic Antioxidants of Calamintha Officinalis Moench Extracts. J. Appl. Biotechnol. Rep. 2016, 3, 489–494. [Google Scholar]
- Mašković, P.Z.; Manojlović, N.T.; Mandić, A.I.; Mišan, A.Č.; Milovanović, I.L.; Radojković, M.M.; Cvijović, M.S.; Solujić, S.R. Phytochemical Screening and Biological Activity of Extracts of Plant Species Halacsya Sendtneri (Boiss.). Dörfl. Hem. Ind 2012, 66, 43–51. [Google Scholar] [CrossRef]
- Dayal, B.; Purohit, R.M. Screening of Some Indian Essential Oils for Their Antifungal Properties. Flavour Ind. 1971, 2, 484–485. [Google Scholar]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and Antioxidant Properties of the Essential Oils and Methanol Extract from Mentha Longifolia, L. Ssp. Longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Remmal, A.; Bouchikhi, T.; Rhayour, K.; Ettayebi, M.; Tantaoui-Elaraki, A. Improved Method for the Determination of Antimicrobial Activity of Essential Oil in Agar Medium. J. Essent. Oil Res. 1993, 5, 179–184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).