Fermented Cranberry Fortified Buckwheat Product—Phenolic Composition, Antioxidant and Microbiological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Enumeration of Bacteria
2.3. Evaluation of Growth and Metabolic Parameters
2.4. Preparation of Buckwheat Substrate
2.5. Samples Fermentation
2.6. Extraction Procedure
2.7. Analysis of Total Phenolic Compounds
2.8. Analysis of Total Flavonoids
2.9. Analysis of Individual Phenolic Compounds
2.10. Analysis of Antioxidant Activity
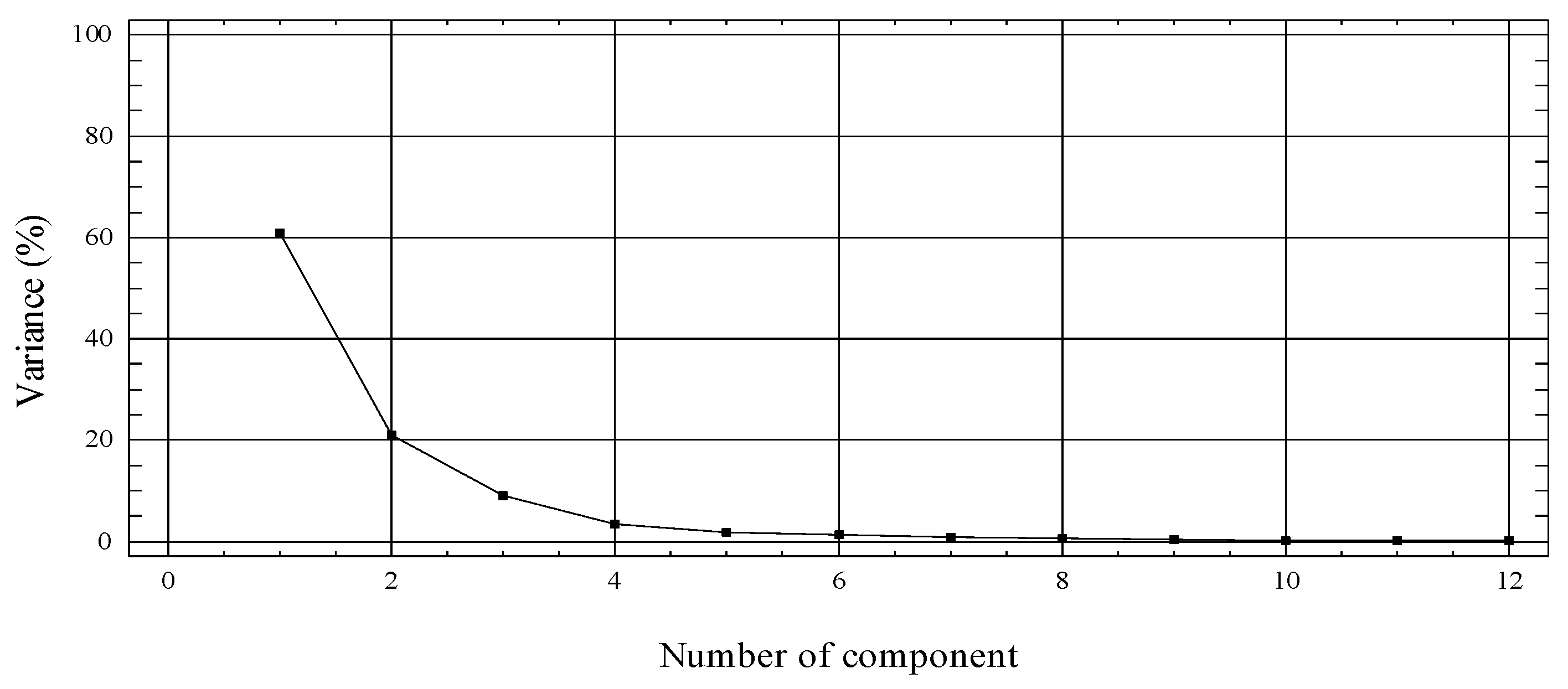
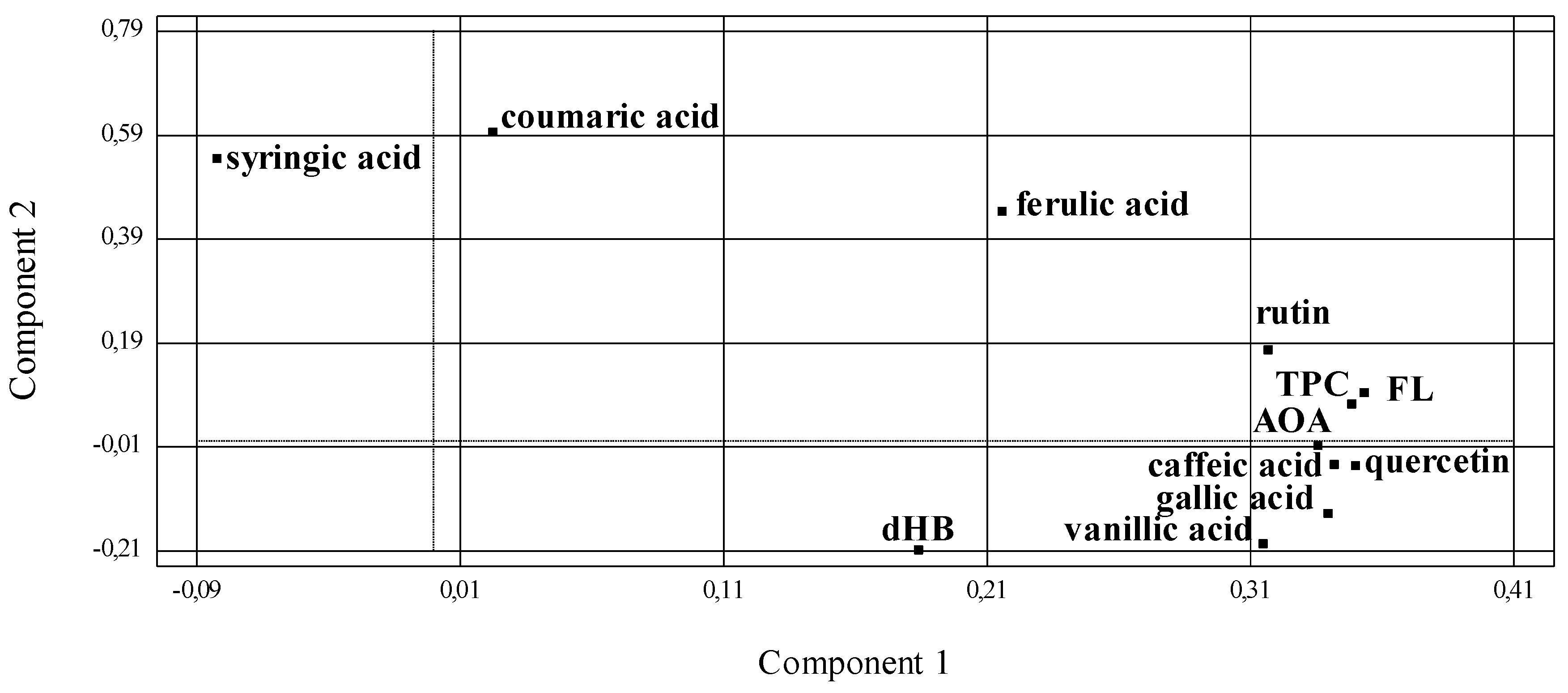
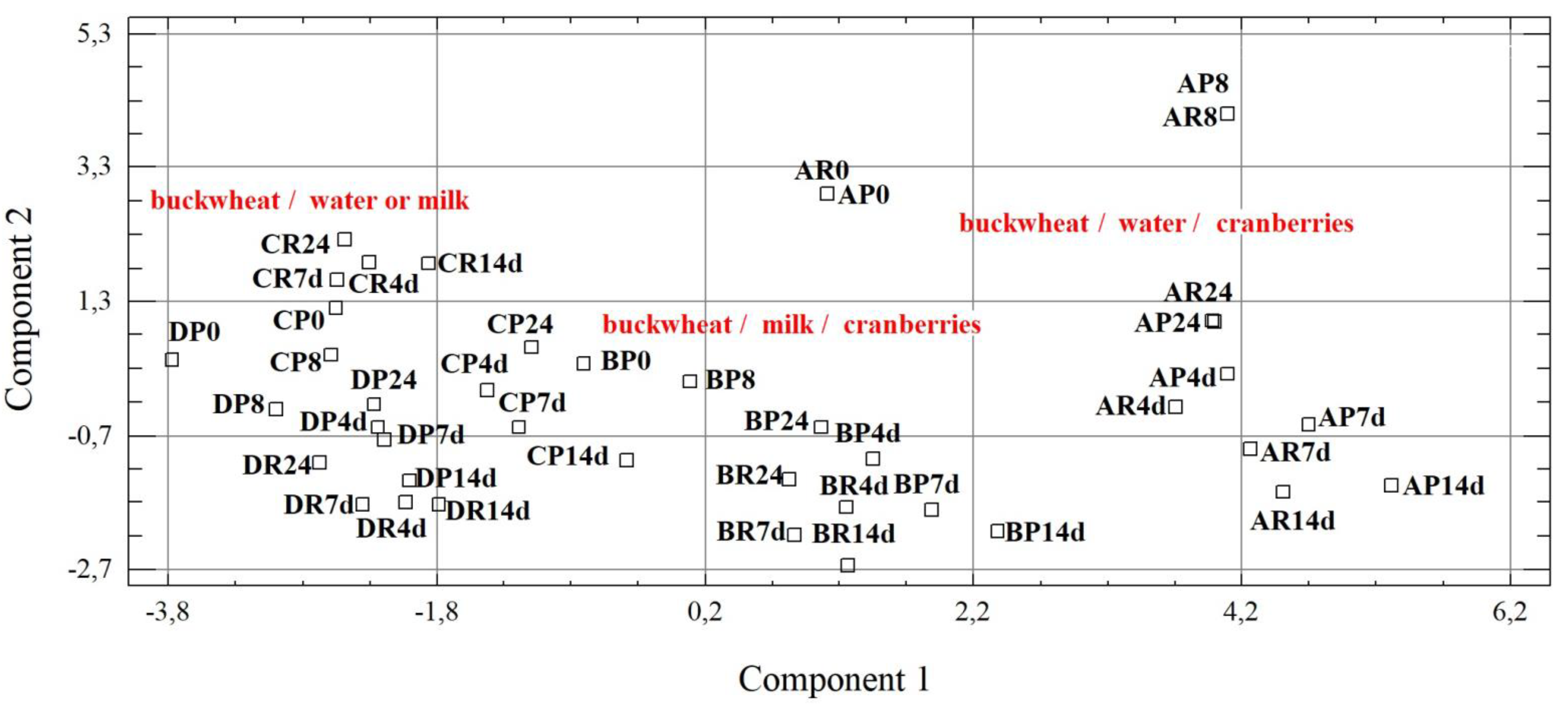
2.11. Statistical Analysis
3. Results
3.1. Fermentation Process
3.2. Total Phenolic Content
3.3. Total Flavonoid Content
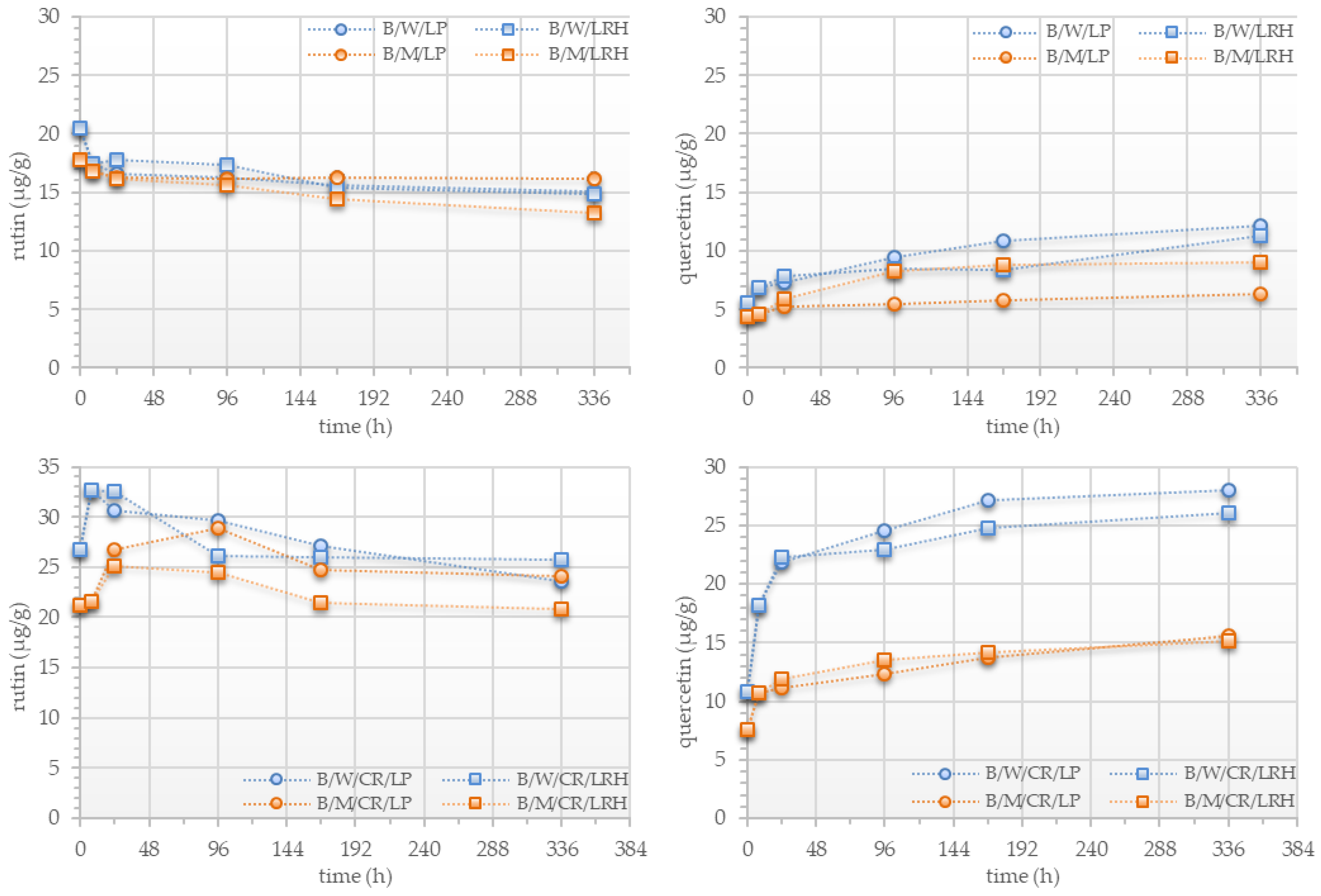
3.4. Phenolic Compounds Profile
3.5. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef]
- Liptáková, D.; Matejčeková, Z.; Valík, Ľ. Lactic acid bacteria and fermentation of cereals and pseudocereals, fermentation processes. In Fermentation Processes; Jozala, A.F., Ed.; InTech: Rijeka, Croatia, 2017; Volume 3, pp. 223–254. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Ghamry, M.; Li, L.; Zhao, W. A metabolomics comparison of Lactobacillus communities isolated from breast milk and camel milk and Lactobacillus apis isolated from bee gut during cereals-based fermentation vs. Lactobacillus plantarum as a reference. LWT-Food Sci. Technol. 2021, 146, 111400. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Fermentation of cereals for specific purpose. J. Food Nutr. Res. 2007, 46, 51–57. [Google Scholar]
- Mikulajová, A.; Šedivá, D.; Hybenová, E.; Mošovská, S. Buckwheat cultivars—Phenolic compounds profiles and antioxidant properties. Acta Chim. Slov. 2016, 9, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Kiprovski, B.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Zivcak, M.; Tran, L.S. The contribution of buckwheat genetic resources to health and dietary diversity. Curr. Genom. 2016, 17, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cer. Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Vollmannová, A.; Musilová, J.; Lidiková, J.; Árvay, J.; Šnirc, M.; Tóth, T.; Bojňanská, T.; Čičová, I.; Kreft, I.; Germ, M. Concentrations of phenolic acids are differently genetically determined in leaves, flowers, and grain of common buckwheat (Fagopyrum esculentum Moench). Plants 2021, 10, 1142. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D.; Berhow, M.; Lee, S. Antioxidant activity of commercial buckwheat flours and their free and bound phenolic compositions. Food Chem. 2011, 125, 923–929. [Google Scholar] [CrossRef]
- Li, F.-H.; Yuan, Y.; Yang, X.-I.; Tao, S.-Y.; Ming, J. Phenolic profiles and antioxidant activity of buckwheat (Fagopyrum esculentum Möench and Fagopyrum tartaricum L. Gaerth) hulls, brans and flours. J. Integr. Agric. 2013, 12, 1684–1693. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and resilient cereals and pseudo-cereals for marginal agriculture: Molecular advances for improving nutrient bioavailability. Front. Genet. 2020, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.M.; Schober, T.J.; Dockery, P.; Arendt, E.K. Textural comparisons of gluten-free and wheat-based doughs, batters, and breads. Cereal Chem. 2004, 81, 567–575. [Google Scholar] [CrossRef]
- Feng, L.; Xie, Y.; Peng, C.; Liu, Y.; Wang, H. A novel antidiabetic food produced via solid-state fermentation of tartary buckwheat by L. plantarum TK9 and L. paracasei TK1501. Food Technol. Biotechnol. 2018, 56, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Ciesarová, Z.; Kukurová, K.; Zielinska, D.; Szawara-Nowak, D.; Starowicz, M.; Wronkowska, M. Effect of fermented and unfermented buckwheat flour on functional properties of gluten-free muffins. J. Food Sci. Technol. 2017, 54, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Vasile, A.; Bahrim, G.E.; Orpianesi, C.; Cresci, A. Effect of buckwheat flour and oat bran on growth and cell viability of the probiotic strains Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and their combination SYNBIO®, in synbiotic fermented milk. Int. J. Food Microbiol. 2013, 167, 261–268. [Google Scholar] [CrossRef]
- Kocková, M.; Valík, Ľ. Development of new cereal-, pseudocereal-, and cereal-leguminous-based probiotic foods. Czech J. Food Sci. 2014, 32, 391–397. [Google Scholar] [CrossRef]
- Liptáková, D.; Petruláková, M.; Pelikánová, J.; Valík, Ľ.; Krištúfková, K. Rast a prežívanie probiotickych laktobacilov v lisovanom pohánkovom produkte. Chem. Listy 2016, 110, 149–152. [Google Scholar]
- Moroni, A.V.; Arendt, E.K.; Bello, F.D. Biodiversity of lactic acid bacteria and yeast in spontaneously-fermented buckweath and teff sourdoughs. Food Microbiol. 2011, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Sut, S.; Pellizzaro, A.; Brun, P.; Voinovich, D.; Castagliuolo, I.; Dall’Acqua, S. The antiadhesive activity of cranberry phytocomplex studied by metabolomics: Intestinal PAC-A metabolites but not intact PAC-A are identified as markers in active urines against uropathogenic Escherichia coli. Fitoterapia 2017, 122, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Može, S.; Polak, T.; Gasperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Zhang, M.; Liu, L.; Dong, L.; Ma, Y.; Wei, Z.; Chi, J.; Zhang, R. Co-culture submerged fermentation by lactobacillus and yeast more effectively improved the profiles and bioaccessibility of phenolics in extruded brown rice than single-culture fermentation. Food Chem. 2020, 326, 126985. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Song, D. Co-microbiological regulation of phenolic release through solid-state fermentation of corn kernels (Zea mays L.) to improve their antioxidant activity. LWT-Food Sci. Technol. 2021, 142, 111003. [Google Scholar] [CrossRef]
- Ravisankar, S.; Dizlek, H.; Awika, J.M. Changes in extractable phenolic profile during natural fermentation of wheat, sorghum and teff. Food Res. Int. 2021, 145, 110426. [Google Scholar] [CrossRef]
- Santetti, G.S.; Dacoreggio, M.V.; Inácio, H.P.; Biduski, B.; Hoff, R.B.; Fritzen Freire, C.B.; Gutkoski, L.C.; Amboni, R.D.M.C. The addition of yerba mate leaves on bread dough has influences on fermentation time and the availability of phenolic compounds? LWT-Food Sci. Technol. 2021, 146, 111442. [Google Scholar] [CrossRef]
- Liptáková, D.; Koreňová, J.; Hornická, M.; Valík, Ľ. Mikrobiologická analýza materského mlieka. Lek. Obz. 2016, 65, 227–231. (In Slovak) [Google Scholar]
- Matejčeková, Z.; Mikulajová, A.; Vlková, E.; Liptáková, D.; Moškovská, S.; Hybenová, E.; Valík, Ľ. Design of bacterial cultures in fermented functional maize product formulation. Pol. J. Food Nutr. Sci. 2019, 69, 417–426. [Google Scholar] [CrossRef]
- EN ISO 15214. Microbiology of Food and Animal Feeding Stuffs. In Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. Colony-Count Technique at 30 °C; International Organization of Standardization (ISO): Geneva, Switzerland, 2002; pp. 1–12. [Google Scholar]
- Baranyi, J.; Roberts, A.T. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995, 26, 199–218. [Google Scholar] [CrossRef] [Green Version]
- Mikulajová, A.; Takácsová, M.; Alexy, P.; Brindzová, L. Optimalization of extraction of phenolic compounds from buckwheat on the basis of results of experimental design method. Chem. Listy 2007, 101, 563–568. [Google Scholar]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef]
- Mikulajová, A.; Takácsová, M.; Rapta, P.; Brindzová, L.; Zalibera, M.; Németh, K. Total phenolic contents and antioxidant capacities of cereal and pseudocereal genotypes. J. Food Nutr. Res. 2007, 46, 150–157. [Google Scholar]
- Kreft, S.; Štrukelj, B.; Gaberščik, A.; Kreft, I. Rutin in buckwheat herbs grown at different UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002, 53, 1801–1804. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their mutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Michalak, M.; Kubik-Komar, A.; Waśko, A.; Polak-Berecka, M. Starter culture for curly kale juice fermentation selected using principal component analysis. Food Biosci. 2020, 35, 100602. [Google Scholar] [CrossRef]
- Srisukchayakul, P.; Charalampopoulos, D.; Karatzas, K.A. Study on the effect of citric acid adaptation toward the subsequent survival of Lactobacillus plantarum NCIMB 8826 in low pH fruit juices during refrigerated storage. Food Res. Int. 2018, 111, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Gueimonde, M.; Delgado, S.; Mayo, B.; Ruas-Madiedo, P.; Margolles, A.; Reyes-Gavilán, C.G. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 2004, 37, 839–850. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Chen, T.; Piao, M.; Rahman, E.S.M.; Zhang, L.; Deng, Y. Influence of fermentation on antioxidant and hypolipidemic properties of maifanite mineral water-cultured common buckwheat sprouts. Food Chem. 2020, 321, 126741. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhai, F.-H.; Li, X.-X.; Cao, J.-L.; Han, J.-R. Total phenolic contents and antioxidant properties of buckwheat fermented by three strains of Agaricus. J. Cereal Sci. 2017, 73, 138–142. [Google Scholar] [CrossRef]
- Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Schiattarella, P.; Budelli, A.; Nigro, R. Lactic fermentation of cereal flour: Feasibility tests on rice, oat and wheat. Appl. Food Biotechnol. 2019, 3, 165–172. [Google Scholar] [CrossRef]
- Rathore, S.; Salmeron, I.; Pandiella, S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Hur, S.; Lee, S.Y.; Kim, Y.; Choi, I.; Kim, G. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Hansen, H.B.; Andreasen, M.F.; Nielsen, M.M.; Larsen, L.M.; Bach Knudsen, K.E.; Meyer, A.S.; Christensen, L.P.; Hansen, Å. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Abraha, H.S.; Gabaza, M.; Vandevelde, J.; Raes, K. Soluble and bound phenolic contents and antioxidant capacity of tef injera as affected by traditional fermentation. J. Food Compost. Anal. 2017, 58, 52–59. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of substrate composition and inoculum on the fermentation kinetics and flavour compound profiles of potentially non-dairy probiotic formulations. LWT-Food Sci. Technol. 2014, 55, 240–247. [Google Scholar] [CrossRef]
- Pelikánová, J.; Liptáková, D.; Valík, Ľ. Suitability of lactic acid bacteria for fermentation of maize and amaranth. J. Food Nutr. Res. 2015, 54, 354–364. [Google Scholar]
- Matejčeková, Z.; Liptáková, D.; Valík, Ľ. Functional probiotic products based on fermented buckwheat with Lactobacillus rhamnosus. LWT-Food Sci. Technol. 2017, 81, 35–41. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Saharan, P.; Sadh, P.K.; Duhan, J.S. Comparative assessment of effect of fermentation on phenolics, flavanoids and free radical scavenging activity of commonly used cereals. Biocatal. Agric. Biotechnol. 2017, 12, 236–240. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry Products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2019, 49, 741–781. [Google Scholar] [CrossRef] [PubMed]
| Buckwheat Substrate | Nend [log CFU/mL] | kd [log CFU/mL h] |
|---|---|---|
| water + cranberries + L. plantarum HM1 | 5.78 a | −0.011 |
| water + cranberries + L. rhamnouss GG | 6.00 b | −0.010 |
| milk + cranberries + L. plantarum HM1 | 6.86 d | −0.011 |
| milk + cranberries + L. rhamnouss GG | 6.78 c | −0.007 |
| water + L. plantarum HM1 | 7.62 g | −0.005 |
| water + L. rhamnouss GG | 7.58 f | −0.006 |
| milk + L. plantarum HM1 | 7.43 e | −0.018 |
| milk + L. rhamnosus GG | 7.69 h | −0.001 |
| Time | ||||||
|---|---|---|---|---|---|---|
| Buckwheat Substrate | 0 h | 8 h | 24 h | 4 d | 7 d | 14 d |
| water + cranberries | ||||||
| + L. plantarum HM1 | 0.596 ± 0.002 a | 0.887 ± 0.019 b | 0.715 ± 0.004 c | 0.706 ± 0.002 d | 0.734 ± 0.001 e | 0.737 ± 0.001 f,x |
| + L. rhamnosus GG | 0.596 ± 0.002 a | 0.887 ± 0.019 b | 0.793 ± 0.006 c | 0.708 ± 0.011 d | 0.707 ± 0.001 d | 0.718 ± 0.004 e,y |
| milk + cranberries | ||||||
| + L. plantarum HM1 | 0.542 ± 0.001 a | 0.593 ± 0.005 b | 0.628 ± 0.001 c | 0.630 ± 0.001 c | 0.638 ± 0.001 d | 0.642 ± 0.001 e,x |
| + L. rhamnosus GG | 0.542 ± 0.001 a | 0.593 ± 0.005 b | 0.611 ± 0.007 c | 0.613 ± 0.005 c | 0.628 ± 0.002 d | 0.634 ± 0.001 e,y |
| water | ||||||
| + L. plantarum HM1 | 0.267 ± 0.001 a | 0.322 ± 0.003 b | 0.372 ± 0.006 c | 0.380 ± 0.002 c | 0.430 ± 0.005 d | 0.578 ± 0.009 e,x |
| + L. rhamnosus GG | 0.267 ± 0.001 a | 0.322 ± 0.003 b | 0.384 ± 0.002 c | 0.389 ± 0.001 d | 0.356 ± 0.004 e | 0.412 ± 0.006 f,y |
| milk | ||||||
| + L. plantarum HM1 | 0.154 ± 0.002 a | 0.235 ± 0.001 b | 0.344 ± 0.006 c | 0.347 ± 0.003 c | 0.351 ± 0.002 d | 0.356 ± 0.001 e,x |
| + L. rhamnosus GG | 0.154 ± 0.002 a | 0.235 ± 0.001 b | 0.230 ± 0.00 c | 0.235 ± 0.002 b | 0.244 ± 0.001 d | 0.254 ± 0.001 e,y |
| Time | ||||||
|---|---|---|---|---|---|---|
| Buckwheat Substrate | 0 h | 8 h | 24 h | 4 d | 7 d | 14 d |
| water + cranberries | ||||||
| + L. plantarum HM1 | 0.113 ± 0.001 a | 0.158 ± 0.002 b | 0.128 ± 0.000 c | 0.121 ± 0.000 d | 0.124 ± 0.001 e | 0.134 ± 0.001 f,x |
| + L. rhamnosus GG | 0.113 ± 0.001 a | 0.158 ± 0.002 b | 0.137 ± 0.000 c | 0.130 ± 0.000 d | 0.130 ± 0.000 d | 0.128 ± 0.001 e,y |
| milk + cranberries | ||||||
| + L. plantarum HM1 | 0.092 ± 0.000 a | 0.099 ± 0.000 b | 0.107 ± 0.000 c | 0.102 ± 0.001 d | 0.103 ± 0.000 d | 0.108 ± 0.000 d,x |
| + L. rhamnosus GG | 0.092 ± 0.000 a | 0.099 ± 0.000 b | 0.101 ± 0.000 c | 0.104 ± 0.000 d | 0.099 ± 0.001 b | 0.107 ± 0.000 e,y |
| water | ||||||
| + L. plantarum HM1 | 0.043 ± 0.000 a | 0.050 ± 0.001 b | 0.058 ± 0.000 c | 0.055 ± 0.000 d | 0.057 ± 0.000 e | 0.059 ± 0.000 f,x |
| + L. rhamnosus GG | 0.043 ± 0.000 a | 0.050 ± 0.001 b | 0.050 ± 0.000 b | 0.049 ± 0.001 b | 0.052 ± 0.000 c | 0.055 ± 0.001 d,y |
| milk | ||||||
| + L. plantarum HM1 | 0.035 ± 0.001 a | 0.039 ± 0.000 b | 0.041 ± 0.000 c | 0.040 ± 0.000 b | 0.043 ± 0.000 d | 0.045 ± 0.001 d,x |
| + L. rhamnosus GG | 0.035 ± 0.001 a | 0.039 ± 0.000 b | 0.040 ± 0.000 c | 0.039 ± 0.000 b | 0.042 ± 0.001 c | 0.043 ± 0.001 c,x |
| Buckwheat Substrate | Gallic | dHB | Vanillic | Syringic | Caffeic | p-Coumaric | Ferulic | |
|---|---|---|---|---|---|---|---|---|
| water + cranberries | 0 h | 8.995 ± 0.113 | 11.778 ± 0.002 | 6.624 ± 0.019 | 16.936 ± 0.040 | 16.376 ± 0.107 | 27.736 ± 0.050 | 6.537 ± 0.051 |
| + L. plantarum HM1 | 8 h | 12.216 ± 0.036 * | 11.629 ± 0.012 | 6.647 ± 0.014 | 14.497 ± 0.094 * | 21.676 ± 0.042 * | 38.251 ± 0.190 * | 8.729 ± 0.024 * |
| 24 h | 14.136 ± 0.100 * | 12.467 ± 0.129 * | 6.839 ± 0.020 | 9.877 ± 0.030 * | 24.439 ± 0.097 * | 22.987 ± 0.165 * | 7.225 ± 0.039 * | |
| 4 d | 14.302 ± 0.058 * | 12.658 ± 0.040 * | 7.253 ± 0.037 * | 8.938 ± 0.045 * | 25.582 ± 0.140 * | 20.132 ± 0.261 * | 6.537 ± 0.017 | |
| 7 d | 15.702 ± 0.147 * | 13.836 ± 0.129 * | 7.652 ± 0.018 * | 7.002 ± 0.010 * | 26.326 ± 0.055 * | 18.382 ± 1.006 * | 6.899 ± 0.013 * | |
| 14 d | 18.283 ± 0.147 * | 16.420 ± 0.111 * | 8.200 ± 0.067 * | 7.946 ± 0.010 * | 29.574 ± 0.016 * | 16.106 ± 0.083 * | 5.625 ± 0.029 * | |
| water + cranberries | 0 h | 8.995 ± 0.113 | 11.778 ± 0.002 | 6.624 ± 0.019 | 16.936 ± 0.040 | 16.376 ± 0.107 | 27.736 ± 0.050 | 6.537 ± 0.051 |
| + L. rhamnosus GG | 8 h | 12.216 ± 0.036 * | 11.629 ± 0.012 | 6.647 ± 0.014 | 14.497 ± 0.094 * | 21.676 ± 0.042 * | 38.251 ± 0.190 * | 8.729 ± 0.024 * |
| 24 h | 14.633 ± 0.210 * | 13.084 ± 0.104 * | 6.687 ± 0.054 | 10.021 ± 0.024 * | 21.729 ± 0.008 * | 23.665 ± 0.165 * | 6.711 ± 0.020 * | |
| 4 d | 15.786 ± 0.250 * | 13.254 ± 0.003 * | 7.738 ± 0.013 * | 8.536 ± 0.006 * | 21.301 ± 0.145 * | 17.694 ± 0.049 * | 6.610 ± 0.037 | |
| 7 d | 17.303 ± 0.103 * | 13.374 ± 0.046 * | 8.485 ± 0.041 * | 7.408 ± 0.019 * | 21.529 ± 0.134 * | 14.405 ± 0.102 * | 6.830 ± 0.028 * | |
| 14 d | 17.636 ± 0.063 * | 13.959 ± 0.123 * | 8.545 ± 0.009 * | 7.320 ± 0.021 * | 24.438 ± 0.069 * | 12.080 ± 0.073 * | 5.715 ± 0.019 * | |
| milk + cranberries | 0 h | 7.365 ± 0.109 | 8.764 ± 0.046 | 5.691 ± 0.029 | 11.224 ± 0.032 | 13.680 ± 0.118 | 16.467 ± 0.134 | 4.060 ± 0.009 |
| + L. plantarum HM1 | 8 h | 7.961 ± 0.037 | 9.819 ± 0.023 | 5.734 ± 0.058 | 11.125 ± 0.010 | 15.816 ± 0.197 * | 15.419 ± 0.043 * | 4.001 ± 0.008 |
| 24 h | 11.343 ± 0.025 * | 10.048 ± 0.002 * | 6.666 ± 0.023 * | 10.950 ± 0.073 * | 17.161 ± 0.214 * | 10.839 ± 0.043 * | 3.334 ± 0.011 * | |
| 4 d | 11.498 ± 0.037 * | 9.335 ± 0.033 * | 8.079 ± 0.049 * | 9.541 ± 0.024 * | 17.747 ± 0.183 * | 9.649 ± 0.006 * | 3.238 ± 0.004 * | |
| 7 d | 12.049 ± 0.078 * | 11.758 ± 0.064 * | 9.408 ± 0.049 * | 9.220 ± 0.028 * | 18.143 ± 0.083 * | 8.385 ± 0.011 * | 3.186 ± 0.048 * | |
| 14 d | 13.371 ± 0.068 * | 13.366 ± 0.108 * | 9.752 ± 0.018 * | 9.405 ± 0.033 * | 19.001 ± 0.058 * | 8.309 ± 0.027 * | 2.881 ± 0.050 * | |
| milk + cranberries | 0 h | 7.365 ± 0.109 | 8.764 ± 0.046 | 5.691 ± 0.029 | 11.224 ± 0.032 | 13.680 ± 0.118 | 16.467 ± 0.134 | 4.060 ± 0.009 |
| + L. rhamnosus GG | 8 h | 7.961 ± 0.037 | 9.819 ± 0.023 | 5.734 ± 0.058 | 11.125 ± 0.010 | 15.816 ± 0.197 * | 15.419 ± 0.043 * | 4.001 ± 0.008 |
| 24 h | 8.860 ± 0.078 * | 9.862 ± 0.015 * | 7.105 ± 0.024 * | 6.373 ± 0.016 * | 15.767 ± 0.144 * | 12.344 ± 0.018 * | 3.450 ± 0.021 * | |
| 4 d | 9.224 ± 0.033 * | 10.837 ± 0.038 * | 7.988 ± 0.015 * | 5.743 ± 0.001 * | 16.098 ± 0.053 * | 11.099 ± 0.044 * | 3.651 ± 0.027 * | |
| 7 d | 10.839 ± 0.064 * | 9.658 ± 0.046 * | 6.444 ± 0.051 * | 5.280 ± 0.040 * | 17.777 ± 0.131 * | 10.157 ± 0.037 * | 2.393 ± 0.009 * | |
| 14 d | 12.004 ± 0.004 * | 11.161 ± 0.033 * | 6.388 ± 0.018 * | 5.069 ± 0.025 * | 18.865 ± 0.058 * | 8.867 ± 0.045 * | 2.069 ± 0.008 * | |
| water | 0 h | 5.649 ± 0.0042 | 9.685 ± 0.038 | 4.272 ± 0.030 | 13.672 ± 0.037 | 14.280 ± 0.065 | 20.237 ± 0.165 | 4.424 ± 0.022 |
| + L. plantarum HM1 | 8 h | 6.972 ± 0.004 * | 10.021 ± 0.079 * | 3.620 ± 0.020 * | 11.958 ± 0.046 * | 12.748 ± 0.212 * | 19.592 ± 0.027 * | 3.622 ± 0.029 * |
| 24 h | 7.011 ± 0.064 * | 12.309 ± 0.115 * | 5.421 ± 0.019 * | 11.388 ± 0.074 * | 17.531 ± 0.059 * | 19.527 ± 0.132 * | 6.245 ± 0.014 * | |
| 4 d | 8.733 ± 0.071 * | 10.567 ± 0.086 * | 5.106 ± 0.019 * | 10.588 ± 0.099 * | 16.220 ± 0.100 * | 18.528 ± 0.051 * | 4.301 ± 0.020 | |
| 7 d | 11.185 ± 0.066 * | 11.354 ± 0.129 * | 4.328 ± 0.035 | 10.157 ± 0.062 * | 16.021 ± 0.042 * | 16.724 ± 0.057 * | 3.510 ± 0.029 * | |
| 14 d | 13.268 ± 0.014 * | 12.728 ± 0.036 * | 4.941 ± 0.012 * | 9.982 ± 0.025 * | 16.195 ± 0.076 * | 14.592 ± 0.121 * | 3.513 ± 0.026 * | |
| water | 0 h | 5.649 ± 0.042 | 9.685 ± 0.038 | 4.272 ± 0.030 | 13.672 ± 0.037 | 14.280 ± 0.065 | 20.237 ± 0.165 | 4.424 ± 0.022 |
| + L. rhamnosus GG | 8 h | 6.972 ± 0.004 * | 10.021 ± 0.079* | 3.620 ± 0.020 * | 11.958 ± 0.046 * | 12.748 ± 0.212 * | 19.592 ± 0.027 * | 3.622 ± 0.029 * |
| 24 h | 6.412 ± 0.020 * | 7.990 ± 0.009 * | 3.143 ± 0.025 * | 12.474 ± 0.047 * | 11.952 ± 0.047 * | 29.509 ± 0.110 * | 5.244 ± 0.027 * | |
| 4 d | 7.235 ± 0.024 * | 9.874 ± 0.039 * | 3.266 ± 0.016 * | 12.592 ± 0.014 * | 12.345 ± 0.085 * | 29.117 ± 0.100 * | 4.962 ± 0.047 * | |
| 7 d | 6.888 ± 0.032 * | 8.958 ± 0.049 * | 3.341 ± 0.008 * | 10.753 ± 0.073 * | 10.465 ± 0.055 * | 26.628 ± 0.243 * | 6.006 ± 0.043 * | |
| 14 d | 7.588 ± 0.012 * | 10.457 ± 0.024 * | 3.645 ± 0.065 * | 13.391 ± 0.165 * | 13.081 ± 0.043 * | 27.001 ± 0.143 * | 5.693 ± 0.050 * | |
| milk | 0 h | 4.642 ± 0.039 | 7.524 ± 0.045 | 3.928 ± 0.024 | 11.369 ± 0.094 | 13.411 ± 0.013 | 16.983 ± 0.073 | 3.669 ± 0.003 |
| + L. plantarum HM1 | 8 h | 6.426 ± 0.038 * | 11.114 ± 0.084 * | 4.418 ± 0.014 * | 10.395 ± 0.036 * | 14.001 ± 0.024 * | 18.181 ± 0.099 * | 3.169 ± 0.040 * |
| 24 h | 7.237 ± 0.007 * | 11.232 ± 0.066 * | 4.701 ± 0.027 * | 10.843 ± 0.034 * | 14.310 ± 0.158 * | 16.772 ± 0.000 | 3.776 ± 0.021 | |
| 4 d | 7.186 ± 0.002 * | 13.306 ± 0.097 * | 4.601 ± 0.027 * | 11.509 ± 0.034 * | 13.850 ± 0.021 * | 15.175 ± 0.132 * | 3.273 ± 0.023 * | |
| 7 d | 7.405 ± 0.010 * | 11.650 ± 0.115 * | 3.883 ± 0.035 | 9.107 ± 0.056 * | 13.966 ± 0.014 * | 13.159 ± 0.106 * | 4.123 ± 0.003 * | |
| 14 d | 8.564 ± 0.040 * | 11.910 ± 0.012 * | 4.049 ± 0.001 * | 8.036 ± 0.036 * | 14.215 ± 0.037 * | 12.507 ± 0.030 * | 3.236 ± 0.012 * | |
| milk | 0 h | 4.642 ± 0.039 | 7.524 ± 0.045 | 3.928 ± 0.024 | 11.369 ± 0.094 | 13.411 ± 0.013 | 16.983 ± 0.073 | 3.669 ± 0.003 |
| + L. rhamnosus GG | 8 h | 6.426 ± 0.038 * | 11.114 ± 0.084 * | 4.418 ± 0.014 * | 10.395 ± 0.036 * | 14.001 ± 0.024 * | 18.181 ± 0.099 * | 3.169 ± 0.040 * |
| 24 h | 6.424 ± 0.015 * | 11.988 ± 0.007 * | 4.690 ± 0.031 * | 7.844 ± 0.017 * | 13.289 ± 0.085 | 16.355 ± 0.132 * | 3.299 ± 0.017 * | |
| 4 d | 8.647 ± 0.003 * | 14.955 ± 0.050 * | 5.448 ± 0.051 * | 8.771 ± 0.087 * | 14.107 ± 0.049 * | 13.561 ± 0.075 * | 3.200 ± 0.020 * | |
| 7 d | 7.404 ± 0.041 * | 13.063 ± 0.029 * | 5.270 ± 0.039 * | 7.392 ± 0.012 * | 12.421 ± 0.022 * | 12.931 ± 0.050 * | 3.373 ± 0.014 * | |
| 14 d | 8.943 ± 0.058 * | 15.424 ± 0.007 * | 5.573 ± 0.019 * | 8.666 ± 0.002 * | 13.581 ± 0.044 | 14.669 ± 0.104 * | 3.362 ± 0.017 * |
| Time | ||||||
|---|---|---|---|---|---|---|
| Buckwheat Substrate | 0 h | 8 h | 24 h | 4 d | 7 d | 14 d |
| water + cranberries | ||||||
| + L. plantarum HM1 | 1.157 ± 0.006 a | 1.391 ± 0.010 b | 1.393 ± 0.005 b | 1.353 ± 0.006 c | 1.425 ± 0.005 d | 1.462 ± 0.012 e,x |
| + L. rhamnosus GG | 1.157 ± 0.006 a | 1.391 ± 0.010 b | 1.220 ± 0.037 c | 1.194 ± 0.005 d | 1.314 ± 0.002 e | 1.334 ± 0.005 f,y |
| milk + cranberries | ||||||
| + L. plantarum HM1 | 1.112 ± 0.002 a | 1.259 ± 0.003 b | 1.258 ± 0.005 b | 1.220 ± 0.002 c | 1.255 ± 0.001 b | 1.274 ± 0.005 d,x |
| + L. rhamnosus GG | 1.112 ± 0.002 a | 1.259 ± 0.003 b | 1.282 ± 0.010 c | 1.278 ± 0.005 c | 1.312 ± 0.006 d | 1.329 ± 0.006 e,y |
| water | ||||||
| + L. plantarum HM1 | 0.609 ± 0.006 a | 0.658 ± 0.005 b | 0.724 ± 0.006 c | 0.716 ± 0.004 c | 0.768 ± 0.007 d | 0.819 ± 0.011 e,x |
| + L. rhamnosus GG | 0.609 ± 0.006 a | 0.658 ± 0.005 b | 0.751 ± 0.009 c | 0.651 ± 0.004 b | 0.623 ± 0.010 d | 0.727 ± 0.003 e,y |
| milk | ||||||
| + L. plantarum HM1 | 0.315 ± 0.012 a | 0.353 ± 0.001 b | 0.667 ± 0.011 c | 0.660 ± 0.002 c | 0.721 ± 0.003 d | 0.776 ± 0.006 e,x |
| + L. rhamnosus GG | 0.315 ± 0.012 a | 0.353 ± 0.001 b | 0.484 ± 0.005 c | 0.400 ± 0.001 d | 0.473 ± 0.017 c | 0.609 ± 0.024 e,y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulajová, A.; Matejčeková, Z.; Mošovská, S.; Kohajdová, Z.; Valík, Ľ.; Hybenová, E. Fermented Cranberry Fortified Buckwheat Product—Phenolic Composition, Antioxidant and Microbiological Properties. Appl. Sci. 2021, 11, 9241. https://doi.org/10.3390/app11199241
Mikulajová A, Matejčeková Z, Mošovská S, Kohajdová Z, Valík Ľ, Hybenová E. Fermented Cranberry Fortified Buckwheat Product—Phenolic Composition, Antioxidant and Microbiological Properties. Applied Sciences. 2021; 11(19):9241. https://doi.org/10.3390/app11199241
Chicago/Turabian StyleMikulajová, Anna, Zuzana Matejčeková, Silvia Mošovská, Zlatica Kohajdová, Ľubomír Valík, and Eva Hybenová. 2021. "Fermented Cranberry Fortified Buckwheat Product—Phenolic Composition, Antioxidant and Microbiological Properties" Applied Sciences 11, no. 19: 9241. https://doi.org/10.3390/app11199241
APA StyleMikulajová, A., Matejčeková, Z., Mošovská, S., Kohajdová, Z., Valík, Ľ., & Hybenová, E. (2021). Fermented Cranberry Fortified Buckwheat Product—Phenolic Composition, Antioxidant and Microbiological Properties. Applied Sciences, 11(19), 9241. https://doi.org/10.3390/app11199241







