Effects of Recycled Fine Glass Aggregates on Alkali Silica Reaction and Thermo-Mechanical Behavior of Modified Concrete
Abstract
:1. Introduction
2. Test Samples and Experimental Methods Description
2.1. Materials
2.2. Specimens Preparation
2.3. Experimental Methods Description
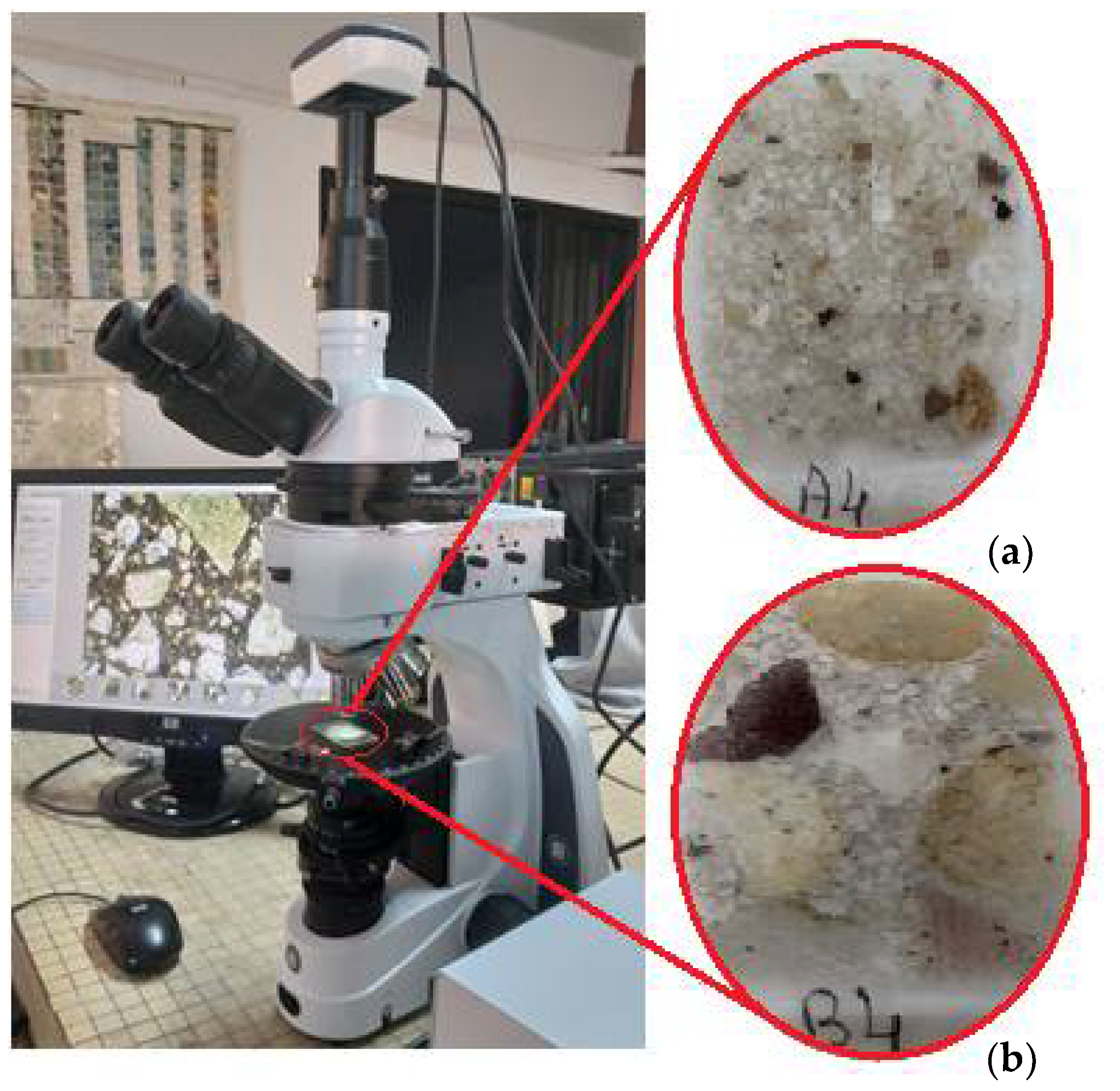
2.3.1. Microscopic Characterization
2.3.2. Durability against Alkali-Silica Reactivity
2.3.3. Mechanical Properties
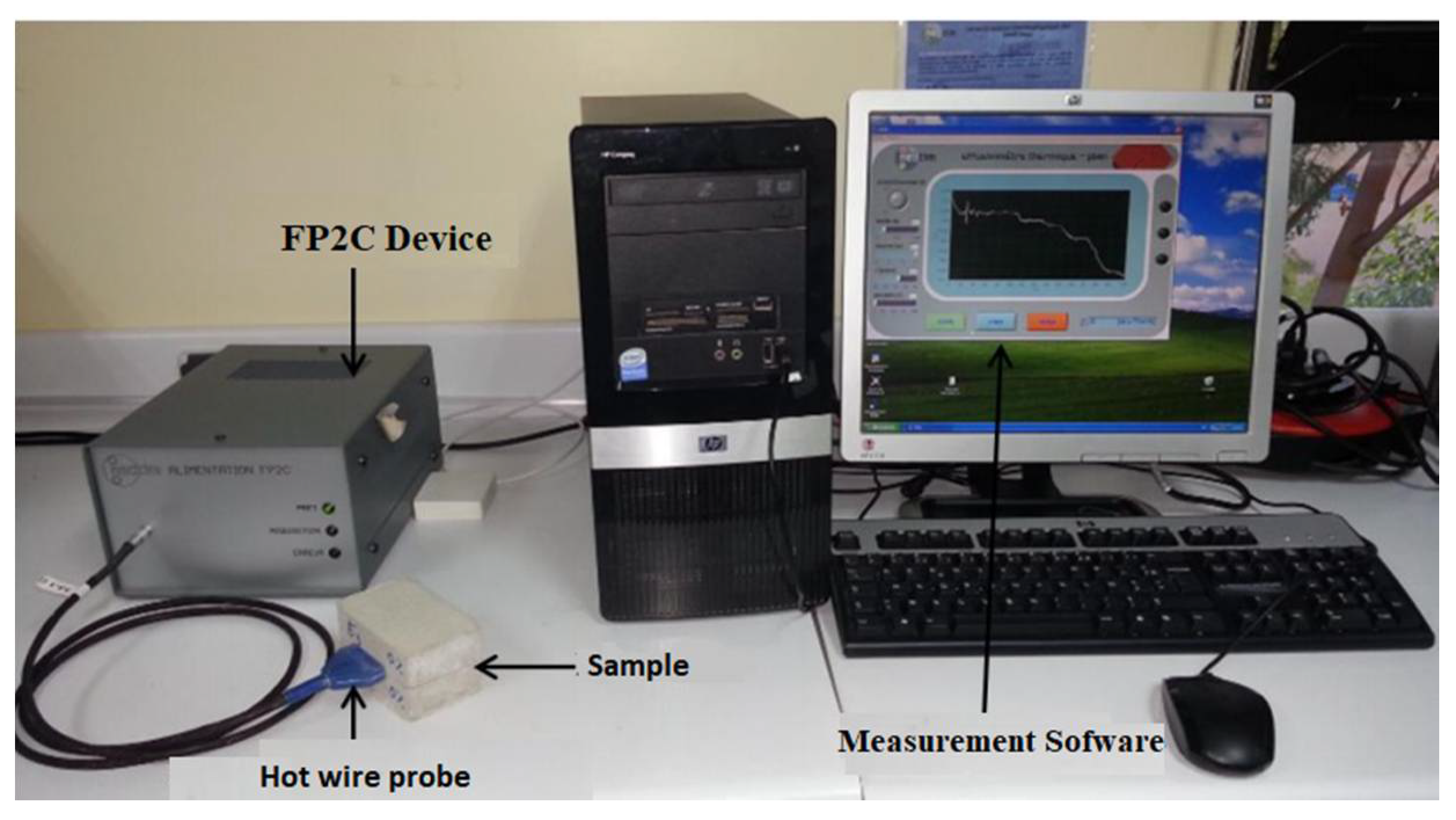
2.3.4. Thermo-Physical Properties
3. Results and Discussion
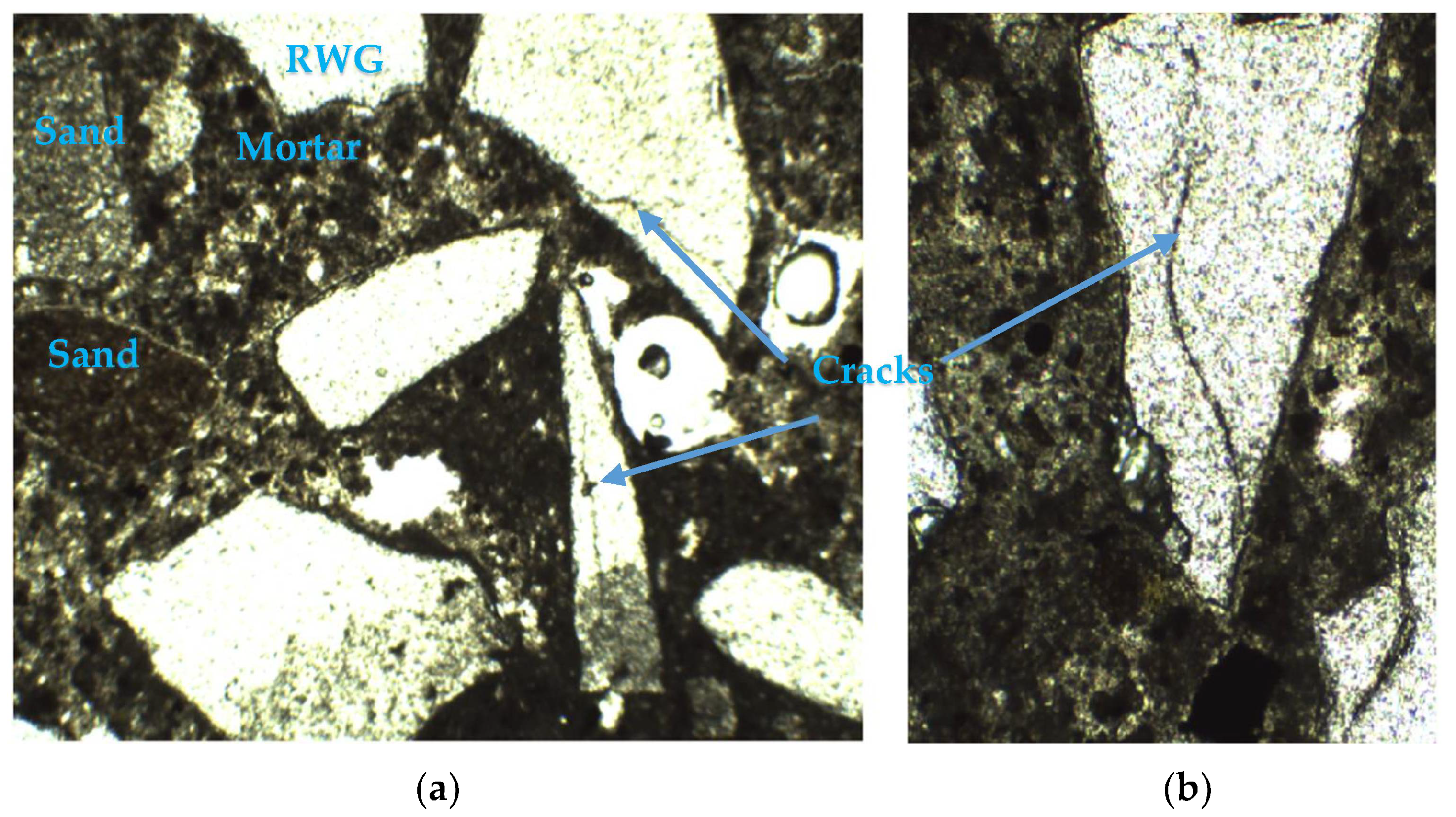
3.1. Microscopic Characterization Analysis
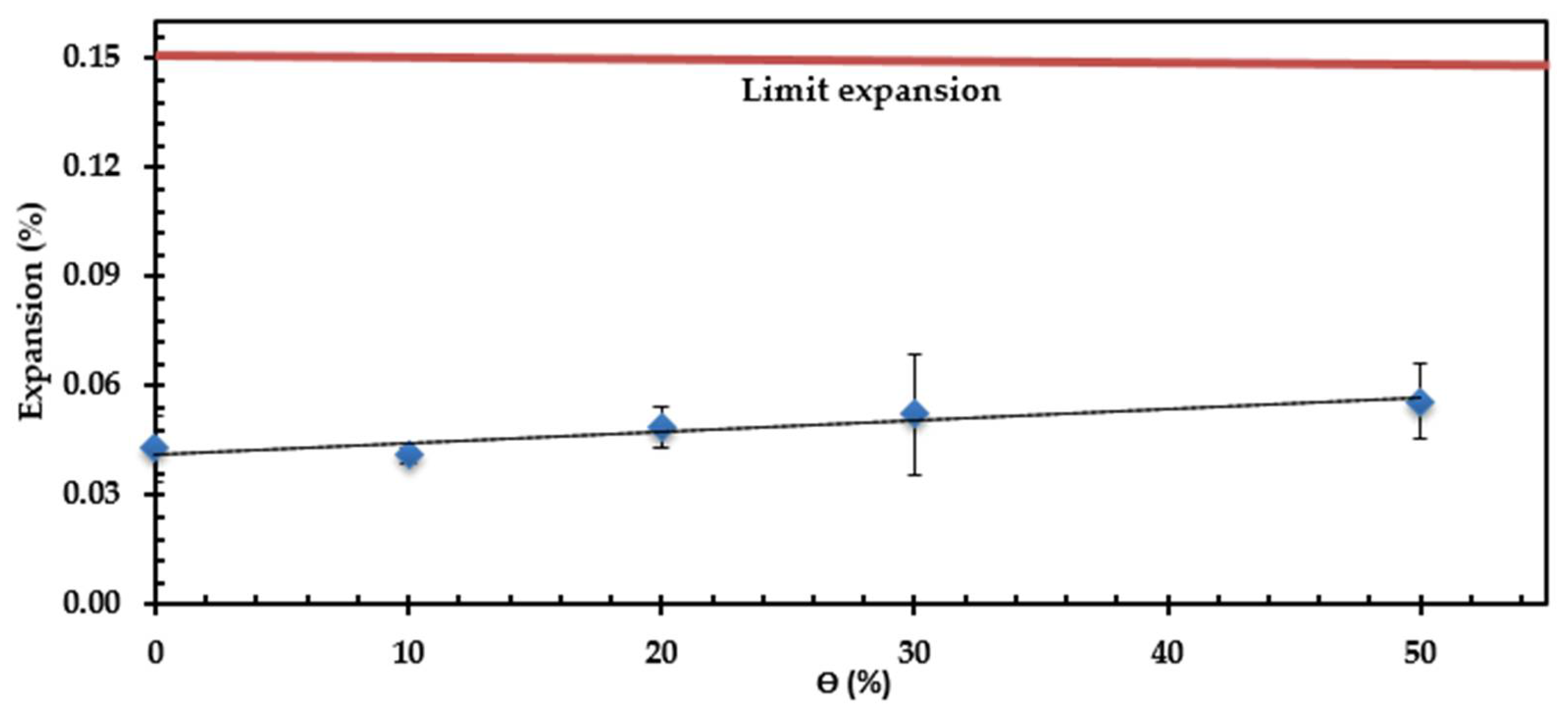
3.2. Durability against Alkali-Silica Reactivity
3.3. Experimental Results of Mechanical Properties
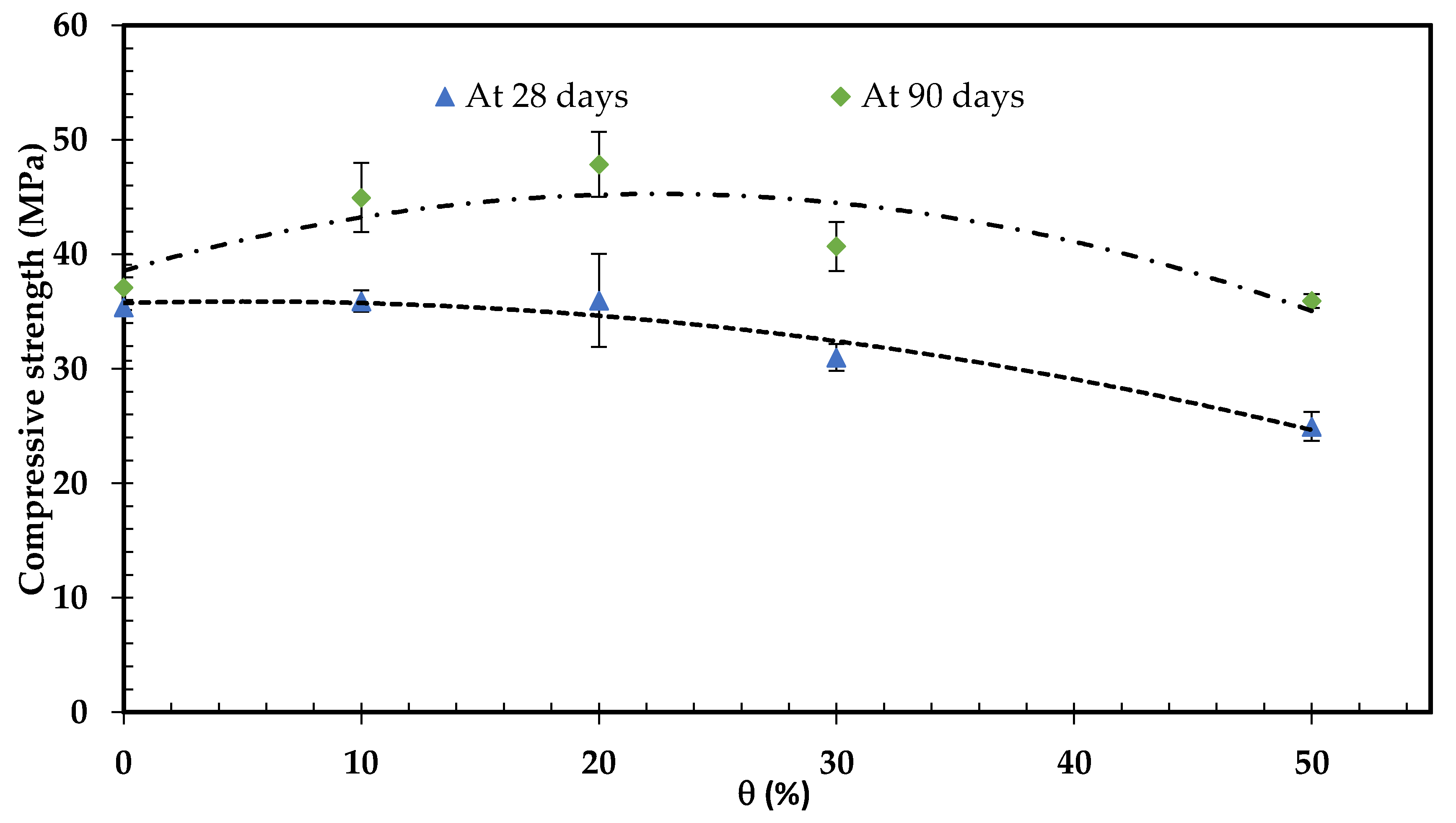
3.3.1. Compressive Strength
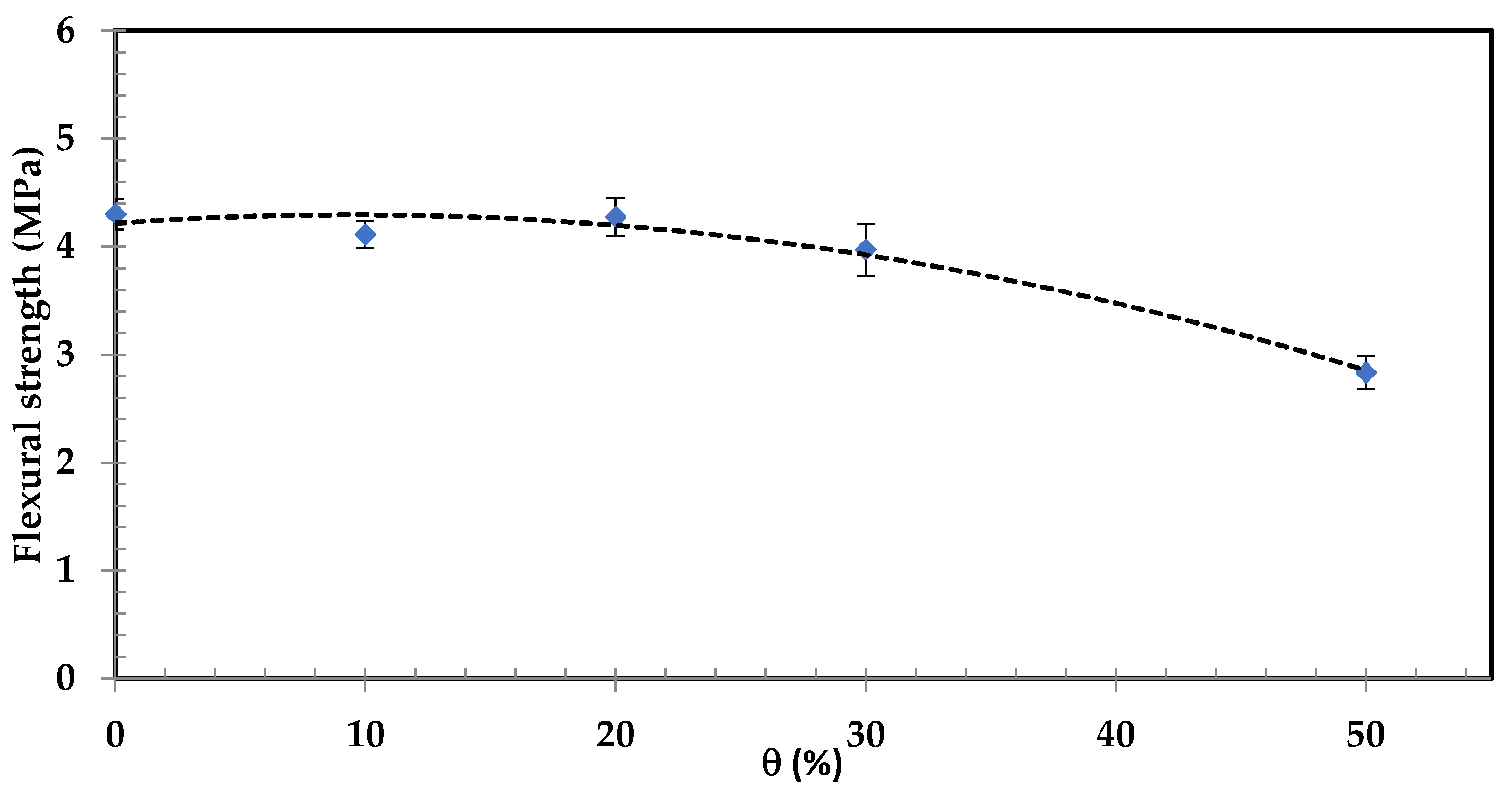
3.3.2. Flexural Strength
3.4. Experimental Results of Thermo-Physical Properties
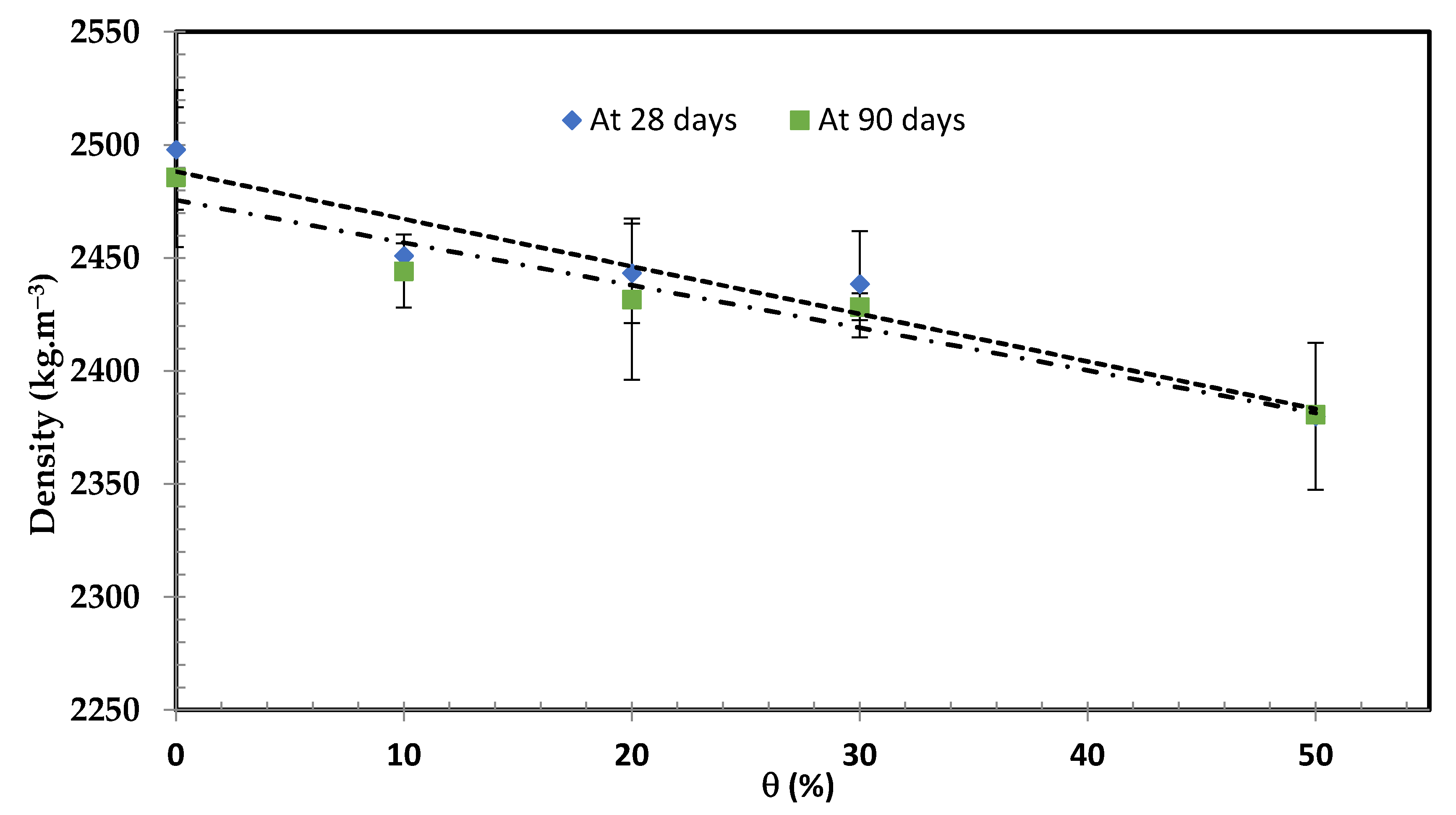
3.4.1. Density
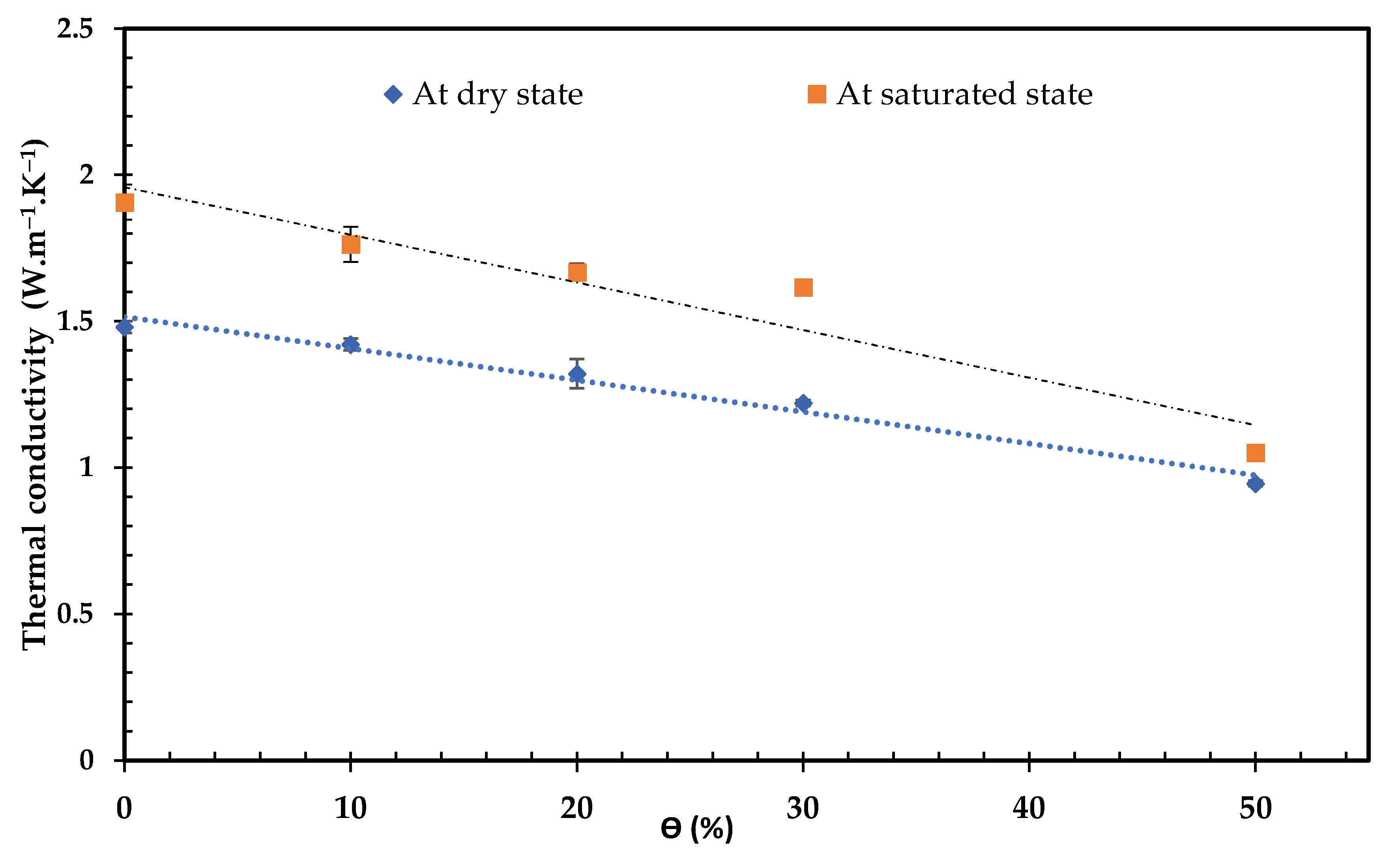
3.4.2. Thermal Conductivity
3.4.3. Thermal Effusivity
3.4.4. Thermal Diffusivity and Volumetric Thermal Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP. Sand and Sustainability: Finding New Solutions for Environmental Governance of Global Sand Resources; United Nations Environment Programme: Geneva, Switzerland, 2019. [Google Scholar]
- La Mafia du Sable au Maroc: Les Risques de L’extraction Illégale de Sable. Available online: https://www.globalriskintel.com/insights/sand-mafia-morocco-risks-illegal-sand-mining (accessed on 3 June 2021).
- Secrétariat Général du Gouvernement du MAROC. Loi n° 27-13 promulguée par le dahir n° 1-15-66 du 21 chaabane 1436 (9 juin 2015) relative aux carrières. BORM n°6422-1 2015, 151, 4424–4434. [Google Scholar]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; Urban Development Series; Knowledge Papers no. 15; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Statistical Report Glass-Alliance-Europe 2018–2019. Available online: https://www.glassallianceeurope.eu/en/statistical-data (accessed on 12 June 2021).
- Lelementarium. Verres. Available online: https://www.lelementarium.fr/product/verres/ (accessed on 15 May 2021).
- Byars, E.A.; Morales, B.; Zhu, H.Y. Conglasscrete. In Project Code: GLA2-006; The Waste and Resources Action Programme: Oxon, UK, 2004. [Google Scholar]
- Mohajerani, A.; Vajna, J.; Cheung, H.; Kurmus, T.H.H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Use of fine glass as ASR inhibitor in glass aggregate mortars. Constr. Build. Mater. 2010, 24, 1309–1312. [Google Scholar] [CrossRef]
- Lee, G.; Poon, C.S.; Wong, Y.L.; Ling, T.C. Effects of recycled fine glass aggregates on the properties of dry-mixed concrete blocks. Constr. Build. Mater. 2013, 38, 638–643. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, H.Y.; Chen, B.T.; Peng, Y.C. Study on the engineering properties and prediction models of an alkali-activated mortar material containing recycled waste glass. Constr. Build. Mater. 2017, 132, 130–141. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; AL-Hashmi, E.A. Recycling of waste glass as a partial replacement for fine aggregate in concrete. Waste Manag. 2009, 29, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Borhan, T.M. Properties of glass concrete reinforced with short basalt fibre. Mater. Des. 2012, 42, 265–271. [Google Scholar] [CrossRef]
- Olofinnade, O.M.; Ede, A.N.; Ndambuki, J.M.; Ngene, B.U.; Isaac, I. Strength and microstructure of eco-concrete produced using waste glass as partial and complete replacement for sand. Cogent. Eng. 2018, 5, 1–19. [Google Scholar] [CrossRef]
- Adaway, M.; Wang, Y. Recycled glass as a partial replacement for fine aggregate in structural concrete -Effects on compressive strength. Electron. J. Struct. Eng. 2015, 14, 116–122. [Google Scholar]
- Malik, M.I.; Manzoor, A.; Ali, R.; Ahmad, B.; Asima, S.; Bashir, M. Positive Potential of Partial Replacement of Fine Aggregates by Waste Glass (<600 Micron) in concrete. IJCIET 2014, 5, 146–153. [Google Scholar]
- Tamanna, N.; Tuladhar, R.; Sivakugan, N. Performance of recycled waste glass sand as partial replacement of sand in concrete. Constr. Build. Mater. 2020, 239, 117804. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, B.C.; Kim, J.H. Studies on mechanical properties of concrete containing waste glass aggregate. Cem. Concr. Res. 2004, 34, 2181–2189. [Google Scholar] [CrossRef]
- De Castro, S.; De Brito, J. Evaluation of the durability of concrete made with crushed glass aggregates. J. Clean. Prod. 2013, 41, 7–14. [Google Scholar] [CrossRef]
- Polley, B.C.; Cramer, S.M.; De la Cruz, R.V. Potential for using waste glass in portland cement concrete. J. Mater. Civ. Eng. 1998, 4, 210–219. [Google Scholar] [CrossRef]
- Tan, K.H.; Du, H. Use of waste glass as sand in mortar: Part I—Fresh, mechanical and durability properties. Cem. Concr. Compos. 2013, 35, 118–126. [Google Scholar] [CrossRef]
- Alani, A.; MacMullen, J.; Telik, O.; Zhang, Z.Y. Investigation into the thermal performance of recycled glass screed for construction purposes. Constr. Build. Mater. 2012, 29, 527–532. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.R.; Zujip, J.A. Thermal Conductivity and Microstructure of Concrete Using Recycle Glass as a Fine Aggregate Replacement. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 463–471. [Google Scholar]
- Guo, M.Z.; Chen, Z.; Ling, T.C.; Poon, C.S. Effects of recycled glass on properties of architectural mortar before and after exposure to elevated temperatures. J. Clean. Prod. 2015, 101, 158–164. [Google Scholar] [CrossRef]
- Al-Sibahy, A.; Edwards, R. Mechanical and thermal properties of novel lightweight concrete mixtures containing recycled glass and metakaolin. Constr. Build. Mater. 2012, 31, 157–167. [Google Scholar] [CrossRef]
- Chung, S.Y.; Elrahman, M.A.; Sikora, P.; Rucinska, T.; Horszczaruk, E.; Stephan, D. Evaluation of the effects of crushed and expanded waste glass aggregates on the material properties of lightweight concrete using image-based approaches. Materials 2017, 10, 1354. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ling, T.; Cui, H.; Sun, C. Influence of particle size of glass aggregates on the high temperature properties of dry-mix concrete blocks. Constr. Build. Mater. 2019, 209, 522–531. [Google Scholar] [CrossRef]
- Maraghechi, H.; Maraghechi, M.; Rajabipour, F.; Pantano, C.G. Pozzolanic reactivity of recycled glass powder at elevated temperatures: Reaction stoichiometry, reaction products and effect of alkali activation. Cem. Concr. Compos. 2014, 53, 105–114. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Tagnit-hamou, A. Pozzolanic properties of fine and coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Value-added utilisation of waste glass in concrete. Cem. Concr. Res. 2004, 34, 81–89. [Google Scholar] [CrossRef]
- Poole, A.B. Alkali-Silica Reactivity Mechanisms of Gel Formation and Expansion. In Proceedings of the 9th International Conference on Alkali-Aggregate Reaction, London, UK, 27–31 July 1992; pp. 782–787. [Google Scholar]
- Ke, G.; Li, W.; Li, R.; Li, Y.; Wang, G. Mitigation effect of waste glass powders on alkali–silica reaction (ASR) expansion in cementitious composite. Int. J. Concr. Struct. Mater. 2018, 12, 67. [Google Scholar] [CrossRef]
- Maraghechi, H.; Shafaatian, S.; Fischer, G.; Rajabipour, F. The role of residual cracks on alkali silica reactivity of recycled glass aggregates. Cem. Concr. Compos. 2012, 34, 41–47. [Google Scholar] [CrossRef]
- Rajabipour, F.; Asce, M.; Maraghechi, H.; Fischer, G. Investigating the alkali silica reaction of recycled glass aggregates in concrete materials. J. Mater. Civ. Eng. 2010, 22, 1201–1208. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Tao, Z. A comprehensive review on performance of cementitious and geopolymeric concretes with recycled waste glass as powder, sand or cullet. Resour. Conserv. Recycl. 2021, 172, 105664. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Use of waste glass as sand in mortar: Part II—Alkali-silica reaction and mitigation methods. Cem. Concr. Compos. 2013, 35, 109–117. [Google Scholar] [CrossRef]
- Douaissia, Z.; Merzoud, M.; Benazzouk, A. Effect of glass powder and silica fume on mechanical properties and on alkali-silica reaction of recycled glass mortars. MATEC. Web. Conf. 2020, 330, 01050. [Google Scholar] [CrossRef]
- Nasiru, S.; Jiang, L.; Yu, L.; Chu, H.; Huang, Y.; Pei, C.; Gu, Y.; Jin, W.; Klu, E.E.; Guo, M. Properties of cement mortar containing recycled glass and rice husk ash. Constr. Build. Mater. 2021, 299, 123900. [Google Scholar] [CrossRef]
- AFNOR. Granulats: Analyse Granulométrique par Tamisage NF P 18-560; AFNOR: Paris, France, 1992. [Google Scholar]
- AFNOR. Bétons: Essai D’affaissement NF P18-45; AFNOR: Paris, France, 1981. [Google Scholar]
- AFNOR. Bétons: Essai D’étude, de Convenance et de Contrôle, Confection et Conservation des Eprouvettes; AFNOR: Paris, France, 1981. [Google Scholar]
- Grattan-Bellew, P.E. A Critical Review of Ultra-accelerated Alkali-silica Reactivity. Cem. Concr. Compos. 1997, 19, 403–414. [Google Scholar] [CrossRef]
- AFNOR. Granulats: Méthodes d’Essai de Réactivité aux Alcalis NF P 18-594; AFNOR: Paris, France, 2015. [Google Scholar]
- AFNOR. Béton: Spécification, Performances, Production et Conformité NF P 18-325-1; AFNOR: Paris, France, 2004. [Google Scholar]
- AFNOR. Essai sur Béton Durci: Partie 5: Résistance à la Flexion sur Eprouvettes; AFNOR: Paris, France, 2001. [Google Scholar]
- Boumhaout, M.; Boukhattem, L.; Hamdi, H.; Benhamou, B.; Ait Nouh, F. Thermomechanical characterization of a bio-composite building material: Mortar reinforced with date palm fibers mesh. Constr. Build. Mater. 2017, 135, 241–250. [Google Scholar] [CrossRef]
- AFNOR. Essai Pour Béton Durci: Partie 7: Masse Volumique du Béton NF P 18-435; AFNOR: Paris, France, 2001. [Google Scholar]
- Kashani, A.; Ngo, T.D.; Hajimohammadi, A. Effect of recycled glass fines on mechanical and durability properties of concrete foam in comparison with traditional cementitious fines. Cem. Concr. Compos. 2019, 99, 120–129. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Effect of particle size on alkali-silica reaction in recycled glass mortars. Constr. Build. Mater. 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Guo, S.; Dai, Q.; Sun, X.; Xiao, X.; Si, R.; Wang, J. Reduced alkali-silica reaction damage in recycled glass mortar samples with supplementary cementitious materials. J. Clean. Prod. 2018, 172, 3621–3633. [Google Scholar] [CrossRef]
- Rivard, P.; Ballivy, G. Application de méthodes pétrographiques à l’évaluation de l’état de dégradation du béton affecté par l’alcali-réaction. Bull. Lab. Ponts Chaussees 2003, 244–245, 73–90. [Google Scholar]
- Topçu, I.B.; Boga, A.R.; Bilir, T. Alkali-silica reactions of mortars produced by using waste glass as fine aggregate and admixtures such as fly ash and Li2CO3. Waste Manag. 2008, 28, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ishiyama, K. Maximum Dosage of Glass Cullet as Fine Aggregate in Mortar. Achiev. Sustain. Constr. 2005, 185–192. [Google Scholar]
- Chen, C.H.; Huang, R.; Wu, J.K.; Yang, C.C. Waste E-glass particles used in cementitious mixtures. Cem. Concr. Res. 2006, 36, 449–456. [Google Scholar] [CrossRef]
- Dames, M. Glass Feedstock Evaluation Project; The Clean Washington Center: Washington, DC, USA, 1993. [Google Scholar]
- Sakami, S.; Boukhattem, L.; Boumhaout, M.; Benhamou, B. Development of alfa fiber-based mortar with improved thermo-mechanical properties. Appl. Sci. 2020, 10, 8021. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Beaucour, A.L.; Ortola, S.; Noumowé, A. Experimental study on the thermal properties of lightweight aggregate concretes at different moisture contents and ambient temperatures. Constr. Build. Mater. 2017, 151, 720–731. [Google Scholar] [CrossRef]
- Materials Thermal Properties Database. Available online: https://thermtest.com/materials-database (accessed on 1 June 2021).
- Ling, T.; Poon, C.; Kou, S. Feasibility of using recycled glass in architectural cement mortars. Cem. Concr. Compos. 2011, 33, 848–854. [Google Scholar] [CrossRef]
- Laloui, L.; Rotta Loria, A.F.B. Heat and mass transfers in the context of energy geostructures. In Analysis and Design of Energy Geostructures, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–135. [Google Scholar]








| % | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | MnO | TiO2 | P2O5 | Na2O | Cr2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RWG | 50–70 | 5–25 | <1 | 5–25 | 1–5 | 1–5 | <0.5 | <0.5 | <0.5 | 5–25 | <0.5 |
| Sand | 68.51 | 13.55 | 2.83 | 1.62 | 2.64 | 3.95 | - | 0.7 | 0.16 | 2.72 | - |
| Nature | Gravel | Sand | RWG |
|---|---|---|---|
| Flakiness coefficient FC | 13 | - | - |
| Superficial cleanness SC (%) | 0.7 | - | - |
| Los Angeles abrasion test LA (%) | 21 | - | - |
| Fineness modulus | - | 2.62 | - |
| Equivalent of sand ES | - | 75 | - |
| Specific weight (T.m−3) | 2.63 | 2.68 | 2.5 |
| Water absorption (%) | 0.5 | 2.5 | 0 |
| Bulk density (kg.m−3) | 1455 | 1500 | 1300 |
| Composite | Materials (kg) | Water (kg) | Superplasticizer (kg) | |||
|---|---|---|---|---|---|---|
| RWG | Gravel | Sand | Cement | |||
| RC | - | 995.56 | 797.10 | 400 | 180 | 6.80 |
| CRWG10 | 74.35 | 995.56 | 717.39 | 400 | 172 | 6.08 |
| CRWG20 | 148.71 | 995.56 | 637.68 | 400 | 162.79 | 5.04 |
| CRWG30 | 223.06 | 995.56 | 575.97 | 400 | 161.66 | 5.96 |
| CRWG50 | 371.78 | 995.56 | 398.55 | 400 | 172.72 | 6.4 |
| RM | MRWG10 | MRWG20 | MRWG30 | MRWG50 | |||
|---|---|---|---|---|---|---|---|
| Composite Constituents | Sand classified with grain size (g) | 0.16–0.31 | 120 | 108 | 96 | 84 | 60 |
| 0.31–0.63 | 120 | 108 | 96 | 84 | 60 | ||
| 0.63–1.25 | 300 | 270 | 240 | 210 | 150 | ||
| 1.25–2.50 | 300 | 270 | 240 | 210 | 150 | ||
| 2.50–5.00 | 360 | 324 | 288 | 252 | 180 | ||
| Waste glass (g) | 0 | 111.94 | 223.88 | 335.82 | 559.70 | ||
| Cement (g) | 600 | 600 | 600 | 600 | 600 | ||
| Water with NaOH Addition (L) | 300 | 300 | 300 | 300 | 300 | ||
| Spots/ASR gel | Chemical Components (Weight %) | Ca/Si (W %) Present Work | Ca/Si (W %) [51] | CaO/SiO2 (W %) [50] | CaO/SiO2 (W %) [35] | ||
|---|---|---|---|---|---|---|---|
| Ca | Si | Na | |||||
| spot 1-RWG | 6.85 | 29.91 | 12.15 | 0.21 | |||
| spot 2-Cement paste | 81 | 15 | 0 | 5.4 | |||
| spot 3-Glass-paste interface | 18.99 | 12.55 | 4.03 | 1.51 | |||
| spot 4-Glass-paste interface | 44.74 | 23.84 | 2.39 | 1.87 | |||
| ASR gel | 0.58 | 0.42 | 0.27 | ||||
| Composite | ||||
|---|---|---|---|---|
| At Dry State | At Saturated State | At Dry State | At Saturated State | |
| RC | ||||
| CRWG10 | ||||
| CRWG20 | ||||
| CRWG30 | ||||
| CRWG50 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abalouch, I.; Sakami, S.; Elabbassi, F.-E.; Boukhattem, L. Effects of Recycled Fine Glass Aggregates on Alkali Silica Reaction and Thermo-Mechanical Behavior of Modified Concrete. Appl. Sci. 2021, 11, 9045. https://doi.org/10.3390/app11199045
Abalouch I, Sakami S, Elabbassi F-E, Boukhattem L. Effects of Recycled Fine Glass Aggregates on Alkali Silica Reaction and Thermo-Mechanical Behavior of Modified Concrete. Applied Sciences. 2021; 11(19):9045. https://doi.org/10.3390/app11199045
Chicago/Turabian StyleAbalouch, Ibtissam, Siham Sakami, Fatima-Ezzahra Elabbassi, and Lahcen Boukhattem. 2021. "Effects of Recycled Fine Glass Aggregates on Alkali Silica Reaction and Thermo-Mechanical Behavior of Modified Concrete" Applied Sciences 11, no. 19: 9045. https://doi.org/10.3390/app11199045
APA StyleAbalouch, I., Sakami, S., Elabbassi, F.-E., & Boukhattem, L. (2021). Effects of Recycled Fine Glass Aggregates on Alkali Silica Reaction and Thermo-Mechanical Behavior of Modified Concrete. Applied Sciences, 11(19), 9045. https://doi.org/10.3390/app11199045






