The Impact of Effective Microorganisms on Flesh Color and Chemical Composition of Raw Potato Tubers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation Practices
2.2. Growing Conditions
2.3. Characteristics of Microbiological Preparations
2.4. Color Assessment of Raw Tuber Pulp
2.5. Sampling for Chemical Analysis
2.6. Analysis of Selected Elements of the Chemical Composition of Tubers
2.7. Soil Sampling
2.8. Methodology for the Assessment of Soil Samples
2.9. Soil Analysis
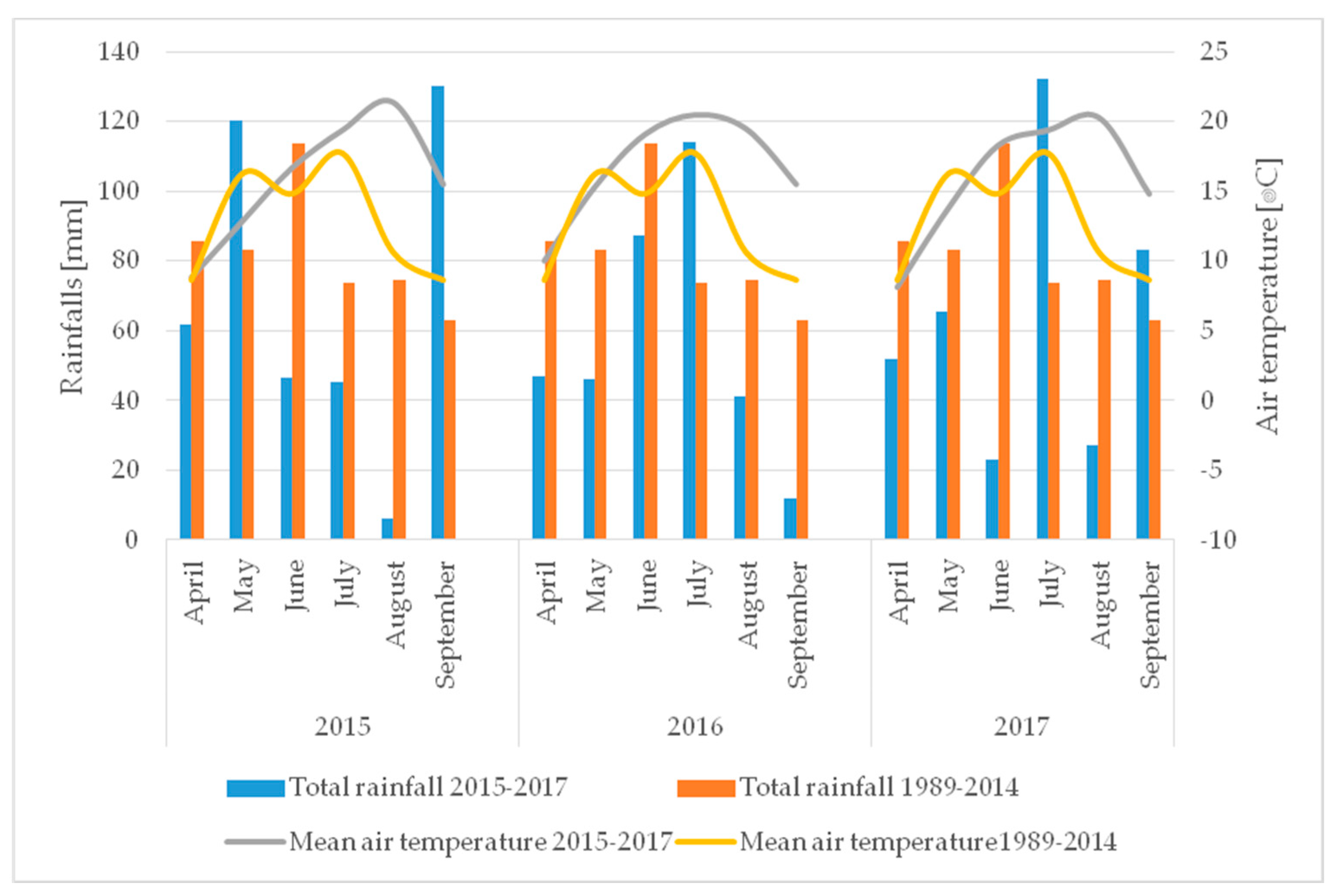
2.10. Meteorological Conditions
2.11. Statistical Calculations
3. Results
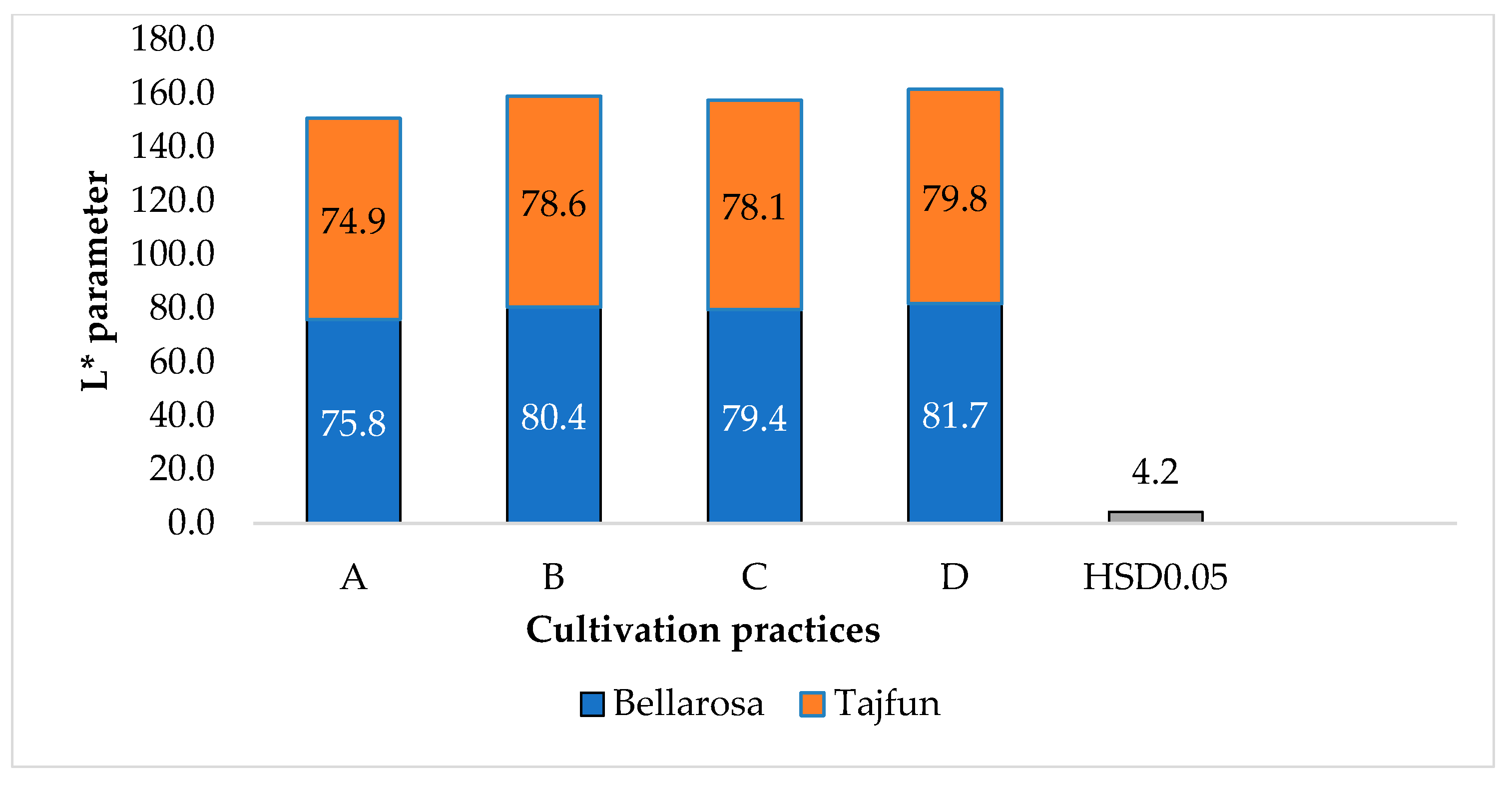
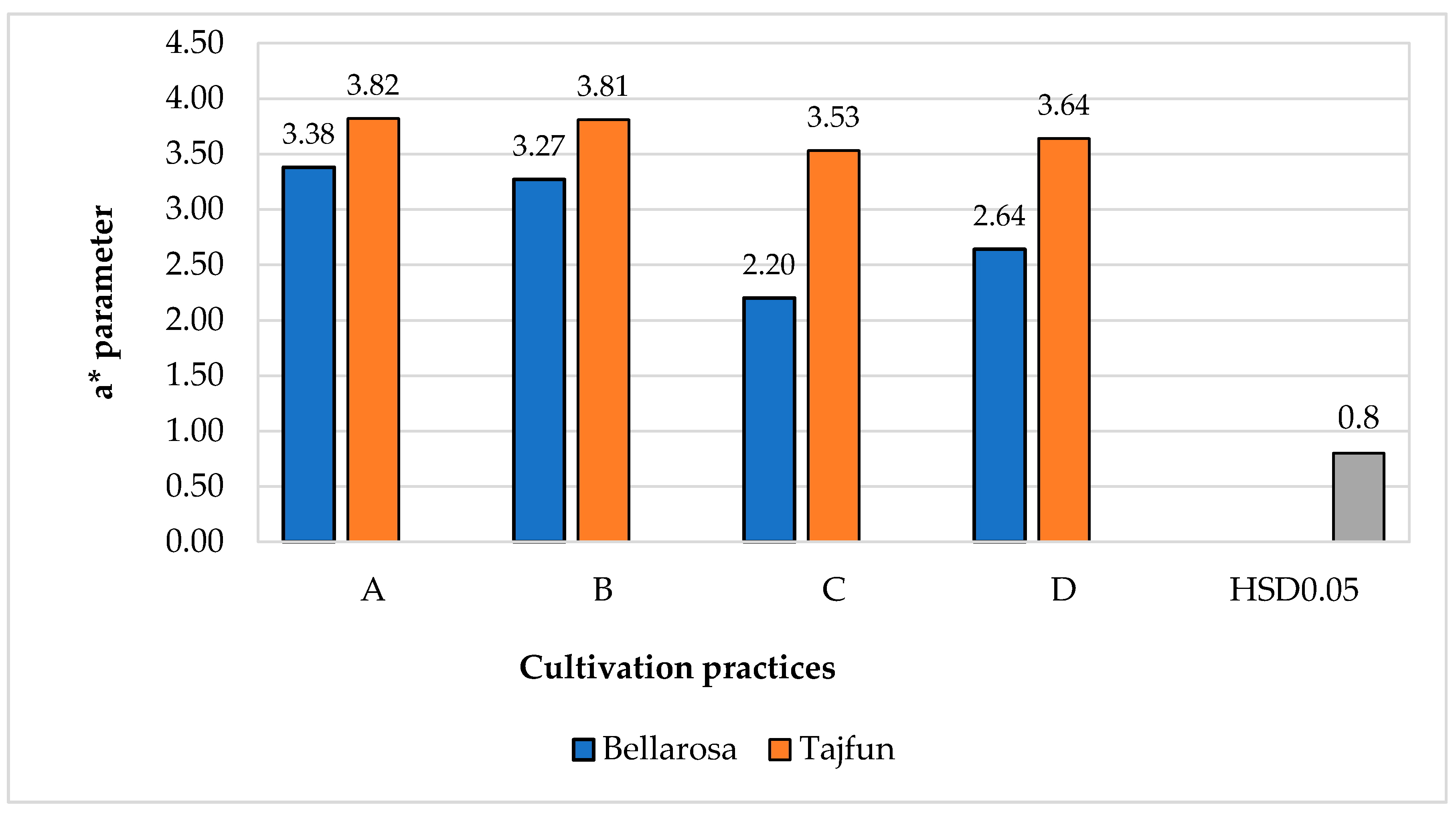
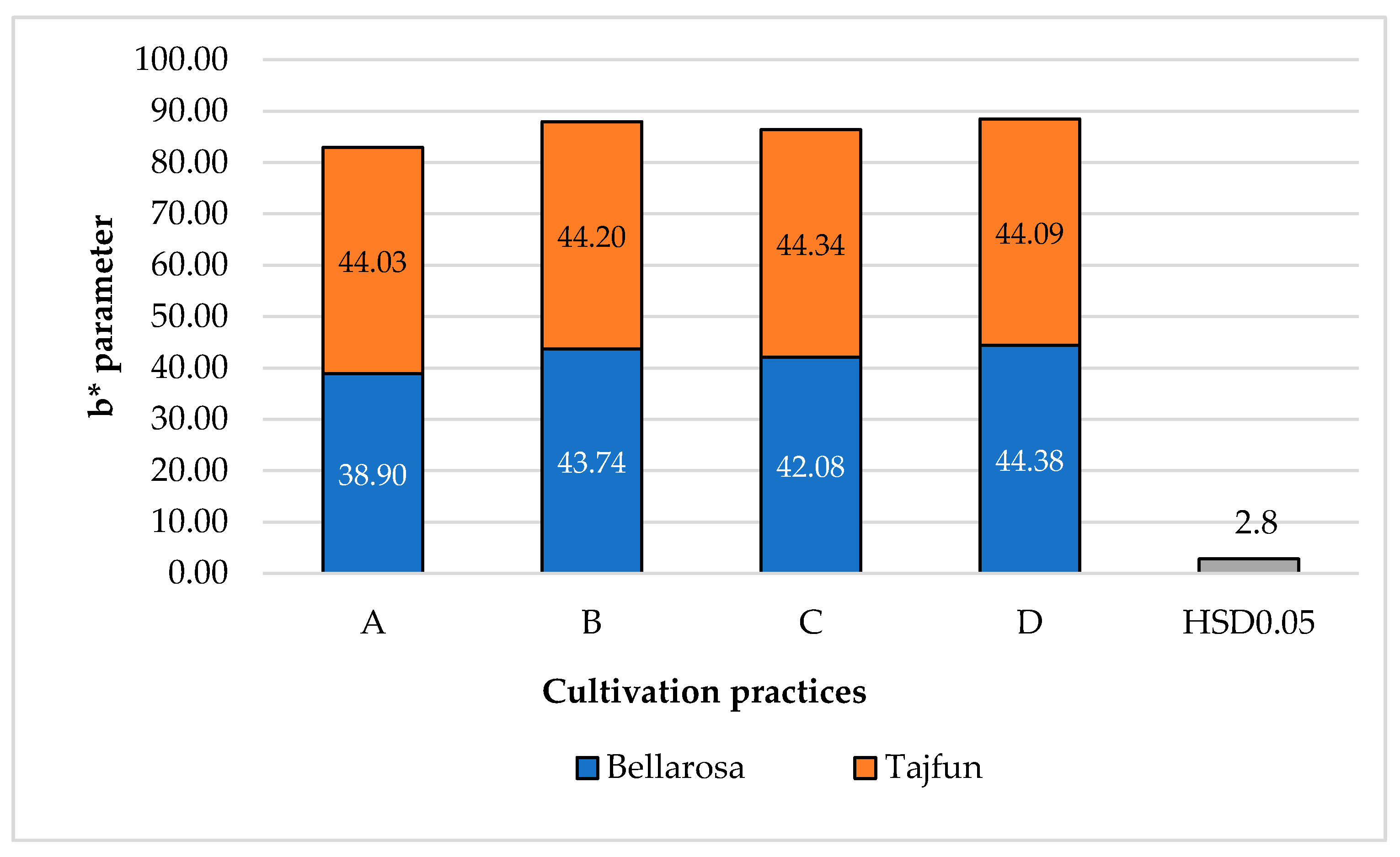
3.1. Influence of Cultivation Practices and Varieties on the Color Indices of Tuber Flesh
3.2. The Impact of Varieties and Weather Conditions, in the Years of Research Variations on Indicators of Tuber Flesh Color
3.3. Interaction of Experimental Factors with Flesh Color Indicators
3.4. Chemical Composition of Tubers
3.5. Share of Individual Variances in the Total (Phenotypic) Variance
3.6. Relationships between Flesh Color Indices and the Chemical Composition of Raw Potato Tuber Flesh
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| EM | effective microorganisms |
References
- Janas, R. Possibilities of using in organic production systems of cultivated crops. Prowl. Inż. Roln. 2009, 3, 111–119. [Google Scholar]
- Vaitkevičienė, N. The Effect of Biodynamic Preparations on the Accumulation of Biologically Active Compounds in the Tubers of Different Genotypes of Ware Potatoes. Ph.D. Thesis, Agricultural Sciences, Agronomy (01A), Aleksandras Stulginskij University, Akademija, Kaunas, Lithuania, 2016; p. 212. [Google Scholar]
- Vaitkevičienė, N.; Kulaitienė, J.; Jarienė, E.; Levickienė, D.; Danilčenko, H.; Średnicka-Tober, D.; Rembiałkowska, E.; Hallmann, E. Characterization of bioactive compounds in Colored potato (Solanum tuberosum L.) cultivars grown with conventional, organic, and biodynamic methods. Sustainability 2020, 12, 2701. [Google Scholar] [CrossRef] [Green Version]
- Vaitkevičienė, N.; Jarienė, E.; Danilčenko, H.; Sawicka, B. Effect of biodynamic preparations on the content of some mineral elements and starch in tubers of three colored potato cultivars. J. Elem. 2016, 21, 927–935. [Google Scholar] [CrossRef]
- Marszałek, K. The Study of Coordinates of Optical Filter Color with the Help of the MCalc Multilayer Calculation Program; Version 4.0.6; AGH University of Science and Technology, Thin Layer Technology Laboratory: Krakow, Poland, 2011; pp. 1–10. [Google Scholar]
- Ganczarski, J. CIE XYZ. 2004, p. 45. Available online: https://docplayer.pl/ (accessed on 23 September 2021).
- Shyam, N.J. Chapter 2. Colour Measurements and Modeling. In Non-Destructive Evaluation of Food Quality; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–40. [Google Scholar] [CrossRef]
- Wszelaczyńska, E.; Wichrowska, D.; Pińska, M.; Rogozińska, I. Evaluation of enzymatic browning degree of edible potato tubers induced by herbicides, mechanical damages and storage by means of instrumental and sensory methods. Pol. J. Food Nutr. Sci. 2007, 57, 163–166. [Google Scholar]
- Kołodziejczyk, M. Effect of nitrogen fertilization and microbial populations on potato yielding. PSE 2014, 60, 379–386. [Google Scholar]
- Kołodziejczyk, M. Effect of nitrogen fertilization and microbial preparations on quality and storage losses in edible potato. Acta Agrophys. 2016, 23, 67–78. [Google Scholar]
- Pszczółkowski, P.; Sawicka, B. The effect of application of biopreparations and fungicides on the yield and selected parameters of seed value of seed potatoes. Acta Agrophys. 2018, 25, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Gałązka, A.; Kocoń, A. Evaluation of the effectiveness of preparations with beneficial microorganisms on the enzymatic activity of soil. Stud. Rep. IUNG PIB 2015, 45, 143–154. [Google Scholar] [CrossRef]
- Szembowski, B. Experiences of the farm in Trankwice with the EM—Farming TM biotechnology. In Natural Probiotic Microorganisms; Publishing House of the Ecosystem Association: Licheń, Poland, 2009; pp. 56–58. [Google Scholar]
- Roztropowicz, S.; Czerko, Z.; Głuska, A.; Goliszewski, W.; Gruczek, T.; Lis, B.; Lutomirska, B.; Nowacki, W.; Rykaczewska, K.; Sowa-Niedziałkowska, G.; et al. Methodic of Observation, Measurements and Sample Take in Agricultural Experiments with Potato; Plant Breeding Acclimatization Institute, Section Jadwisin: Jadwisin, Poland, 1999; p. 50. [Google Scholar]
- Higa, T. A Revolution in Protecting Our Planet; Development Foundation, SGGW: Warsaw, Poland, 2003; p. 132. [Google Scholar]
- Stępień, A.; Adamiak, E. Effective microorganisms (EM-1) and their influence on the occurrence of cereal disease. Progr. Plant Prot. 2009, 49, 2027–2030. [Google Scholar]
- Higa, T. Effective Microorganism: A New Dimension for Nature Farming. In Proceedings of the Second International Conference on Kyusei Nature Farming, Washington, DC, USA; 1994; pp. 20–22. [Google Scholar]
- Allahverdiyev, S.R.; Kırdar, E.; Gunduz, G.; Kadimaliyev, D.; Revin, V.; Filonenko, V.; Rasulova, D.A.; Abbasova, Z.I.; Gani-Zade, S.I.; Zeynalova, E.M. Effective Microorganisms (EM) Technology in Plants. Technology 2011, 14, 103–106. [Google Scholar]
- Boligłowa, E.; Gleń, K. Assessment of effective microorganism activity (EM) in winter wheat protection against fungal diseases. Ecol. Chem. Eng. A 2008, 15, 23–27. [Google Scholar]
- Badura, L. Do we know all the conditions for the functioning of microorganisms in terrestrial ecosystems? Cosmos. Probl. Nauk Biol. 2004, 53, 3–4, 264–265, 373–379. [Google Scholar]
- Paśmionka, I.; Kotarba, K. Possible application of effective microorganisms in environmental protection. Cosmos. Probl. Nauk Biol. 2015, 64, 173–184. [Google Scholar]
- Czopek, K.; Staniak, M.; Marcinek, B. Effect of preparations containing effective microorganisms for growth and development of floss flower (Ageratum Houstonianum Mill.). Fragm. Agron. 2018, 35, 7–14. [Google Scholar]
- Ji, B.; Hu, H.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of deep tillage and straw returning on soil microorganism and enzyme activities. Sci. World J. 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, J.; Peng, Y.; Xu, J.; Liao, L.; Yang, S. Storing and removing nitrogen in drainage from paddy field by using aquatic crops wetland. Paddy Water Environ. 2020, 18, 2661. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. Effect of the fungicides, microbiological preparations and extracts with herbs on the yield of potato. Fragm. Agron. 2018, 35, 81–93. [Google Scholar]
- CIE. Part 4: CIE 1976 L*a* b* Colour Space. In Colorimetry; CIE DS 014-4.3/E:2007; CIE Central Bureau: Vienna, Austria, 2007; pp. 1–12. [Google Scholar]
- Brainard, D.H.; Cottaris, N.P.; Radonji, A. The perception of color and material in naturalistic tasks. bioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensory Food Research. Basics—Methods—Applications, 2nd ed.; Scientific Publishers PTTŻ: Krakow, Poland, 2014; p. 375. ISBN 978-83-935421-3-0. [Google Scholar]
- AOAC. Official AOAC International Analysis Methods, 20th ed.; Issue 20; AOAC International: Rockville, MD, USA, 2016; p. 3172. ISBN 0935584870. [Google Scholar]
- Mocek, A. Soil Science; State Scientific Publisher: Warszawa, Poland, 2015; p. 571. [Google Scholar]
- Ryzak, M.; Bartmiński, P.; Bieganowski, A. Methods of determination of granulometric distribution of mineral soils. Acta Agrophysica Rozprawy i Monografie 2009, 175, 97. [Google Scholar]
- Polish Standard. PN-R-BS 0403. Chemical and Agricultural Analysis of Soil Sampling; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- KQ/PB-34; Version 4; Polish Accreditation Center: Warsaw, Poland, 2021.
- Polish Standard. PN-R-04023. Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- Polish Standard. PN-R-04022 1996 + AZ1 2002. Chemical and Agricultural Analysis of Soil. Determination of Available Potassium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Polish Standard. PN-R-04020 1994 + AZ1 2004. Chemical and Agricultural Analysis of Soil; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- Polish Standard. PN-R-04017. Content of Copper Soluble in HCl in Mineral Soil; Polish Committee for Standardization: Warsaw, Poland, 1992. [Google Scholar]
- Polish Standard. PN-R-04019. Content of Manganese Soluble in HCl in Mineral Soil; Polish Committee for Standardization: Warsaw, Poland, 1993. [Google Scholar]
- Polish Standard. PN-R-04021. The Content of Iron Soluble in HCl in Mineral Soil; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- Natural Resources Conservation Service Soils. Soil Texture Calculator. United States Department of Agriculture. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/?cid=nrcs142p2_054167 (accessed on 20 July 2021).
- Skowera, B.; Kopcińska, J.; Kopeć, B. Changes in thermal and precipitation conditions in Poland in 1971–2010. Ann. Warsaw Univ. Life Sci. 2014, 46, 153–162. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Koronacki, J. Statistics, for Students of Technical and Natural Sciences; Scientific and Technical Publishing House: Warsaw, Poland, 2009; p. 491. [Google Scholar]
- Pszczółkowski, P.; Sawicka, B.; Danilčenko, H.; Jarienė, E. The role of microbiological preparations in improving the quality of potato tubers. In Proceedings of the International Scientific Conference “New Trends in food Safety and Quality”, Aleksandras Stulginskis University, Akademija, Lithuania, 5–7 October 2017; pp. 22–23. [Google Scholar]
- Wojcieszyńska, D.; Wilczek, A. Phenolic compounds of natural origin. Sci. Technol. 2006, 1, 6–12. Available online: https://www.kiosk24.pl/download.html (accessed on 20 July 2021).
- Sawicka, B.; Pszczółkowski, P.; Noaema, A.H.; Krochmal-Marczak, B.; Kiełtyka-Dadasiewicz, A. Effective Microorganisms in Agriculture and Food Processing Modern Research on the State of the Environment and the Therapeutic Use of Plants; Chwil, M., Skoczylas, M.M., Eds.; Publishing House University of Life Sciences in Lublin: Lublin, Poland, 2019; pp. 45–64. [Google Scholar] [CrossRef]
- Korzeniowska, K. Possibilities of using plant extracts containing phenolic compounds in organic farming. Chem. Ind. 2017, 1, 100–104. [Google Scholar] [CrossRef]
- Kowalska, J. Effect of fertilization and microbiological bio-stimulators on healthiness and yield of organic potato. Prog. Plant Prot. 2016, 56, 230–235. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Shahriari, R.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. Correlation and path analysis between yield and yield components in potato (Solanum tuberosum L.). Middle East J. Sci. Res. 2011, 7, 17–21. [Google Scholar]
- Sawicka, B.; Dolatowski, Z. Variability of the flesh darkening in tubers of new potato cultivars under the influence of ultrasounds. Probl. Prog. Agric. Sci. 2007, 517, 639–649. [Google Scholar]
- Frydecka-Mazurczyk, A.; Zgórska, K. Optical colour measurement of potato products. Acta Hortic. 2003, 600, 717–722. [Google Scholar] [CrossRef]
- Valarini, P.J.; Diaz Alvarez, M.C.; Gasco, J.M.; Guerrero, F.; Tokeshi, H. Assessment of soil properties by organic matter and EM- microorganism incorporation. Rev. Bras. Cienc. Solo. 2003, 27, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Zydlik, P.; Zydlik, Z. Impact of biological effective microorganism [EM] preparations on some physico-chemical properties of soil land vegetative growth of apple- tree rootstocks. Nauka Przyr. Technol. 2008, 2, 1–8. [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B.; Kiełtyka-Dadasiewicz, A.; Bienia, B. Influence of storage and climatic conditions on the quality tuber flesh potato cultivated in the organic system. Fragm. Agron. 2016, 33, 44–54. [Google Scholar]
- Wang-Pruski, G.; Nowak, J. Potato after-cooking darkening. Am. J. Potato Res. 2004, 81, 7–16. Available online: http://www.findarticles.com/p/articles/mi_qa4069/is_200401/ai_n9402757 (accessed on 24 September 2021). [CrossRef]
- Yoshi, O. Spectral color measurement. In Colorimetry: Understanding the CIE System; Schanda, J., Ed.; Wiley; John Wiley & Sons, Inc. Publication: Hoboken, NJ, USA, 2007. [Google Scholar]
- Schanda, J. CIE colorimetry. In Colorimetry: Understanding the CIE System; Schanda, J., Ed.; Wiley; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; p. 459. [Google Scholar] [CrossRef]
- Bienia, B.; Sawicka, B.; Krochmal-Marczak, B. Culinary quality of tubers of selected potato varieties depending on the foliar fertilization used. Acta Sci. Pol. Agric. 2020, 19, 123–136. [Google Scholar] [CrossRef]
- Sawicka, B. Dates of creation and development Phytophthora infestans [Mont.] De Bary in the changing conditions of the farmland. Acta Agroph 2005, 6, 537–547. [Google Scholar]
- Sawicka, B.; Skiba, D. Fluctuation of flesh darkening of raw and cooking tubers Helianthus tuberosus L. Ann. UMCS E Agric. 2009, 64, 15–22. [Google Scholar] [CrossRef]
- Spyrka, B.W. Influence of habitat conditions on the formation and size on mechanical damages of potato tubers for food processing industry. Ph.D. Thesis, Wrocław University of Environmental and Life Sciences, Wrocław Poland, 2013; p. 166. Available online: https://www.dbc.wroc.pl/Content/23989/Spyrka_B_doktor_110_DBC.pdf?handler=pdf (accessed on 24 September 2021). (In Polish).
- Wierzbicka, A.; Trawczyński, C. Effect of irrigation and soil’s microorganisms on the macro and micronutrient contents in organic potato tubers. Fragm. Agron. 2011, 28, 139–148. [Google Scholar]
- Grudzińska, M.; Zgórska, K. Effect of sugar content in potato tubers on colour of chips. Żywność Nauka Technol. Jakość 2008, 5, 107–115. [Google Scholar]
- Condor, A.F.; Perez, P.G.; Lokare, C. Effective Microorganisms: Myth or reality. Rev. Peru. Biol. 2006, 14, 315–319. [Google Scholar]
- Martyniuk, S. Effective and ineffective microbiological preparations used in the protection and cultivation of plants and reliable and unreliable methods of their assessment. Post. Mikrobiol. 2011, 50, 321–328. [Google Scholar]
- Martyniuk, S.; Księżak, J. Evaluation of pseudomicrobial biopreparations used in plant cultivation. Pol. J. Agron. 2011, 6, 27–33. [Google Scholar]
- Saggin, R.; Coupland, J.N. Non-contact ultrasonic measurements in food materials. Food Res. Int. 2001, 34, 865–870. [Google Scholar] [CrossRef]
- Liu, J.X.; Yao, J.; Yan, B.J.; Shi, Z.Q.; Wang, X.Q.; Yu, J.Q. Mulberry Leaf Supplement for Sheep Fed Ammoniated Rice Straw; Animal Production and Health, FAO: Rome, Italy, 2002; Volume 147, pp. 189–201. Available online: http://www.fao.org/3/x9895e/x9895e0i.htm (accessed on 24 September 2021).
- Sawicka, B.; Pszczółkowski, P.; Danilčenko, H.; Jariene, E. Impact of ultrasound on the physicochemical properties of potato tubers. Agron. Sci. 2020, 75, 85–104. [Google Scholar] [CrossRef]
- Allahverdiev, S.R.; Minkova, N.O.; Yarigin, D.V.; Gündüz, G. The Silent Heroes: Effective microorganisms. Orman. Derg. 2015, 10, 24–28. [Google Scholar]

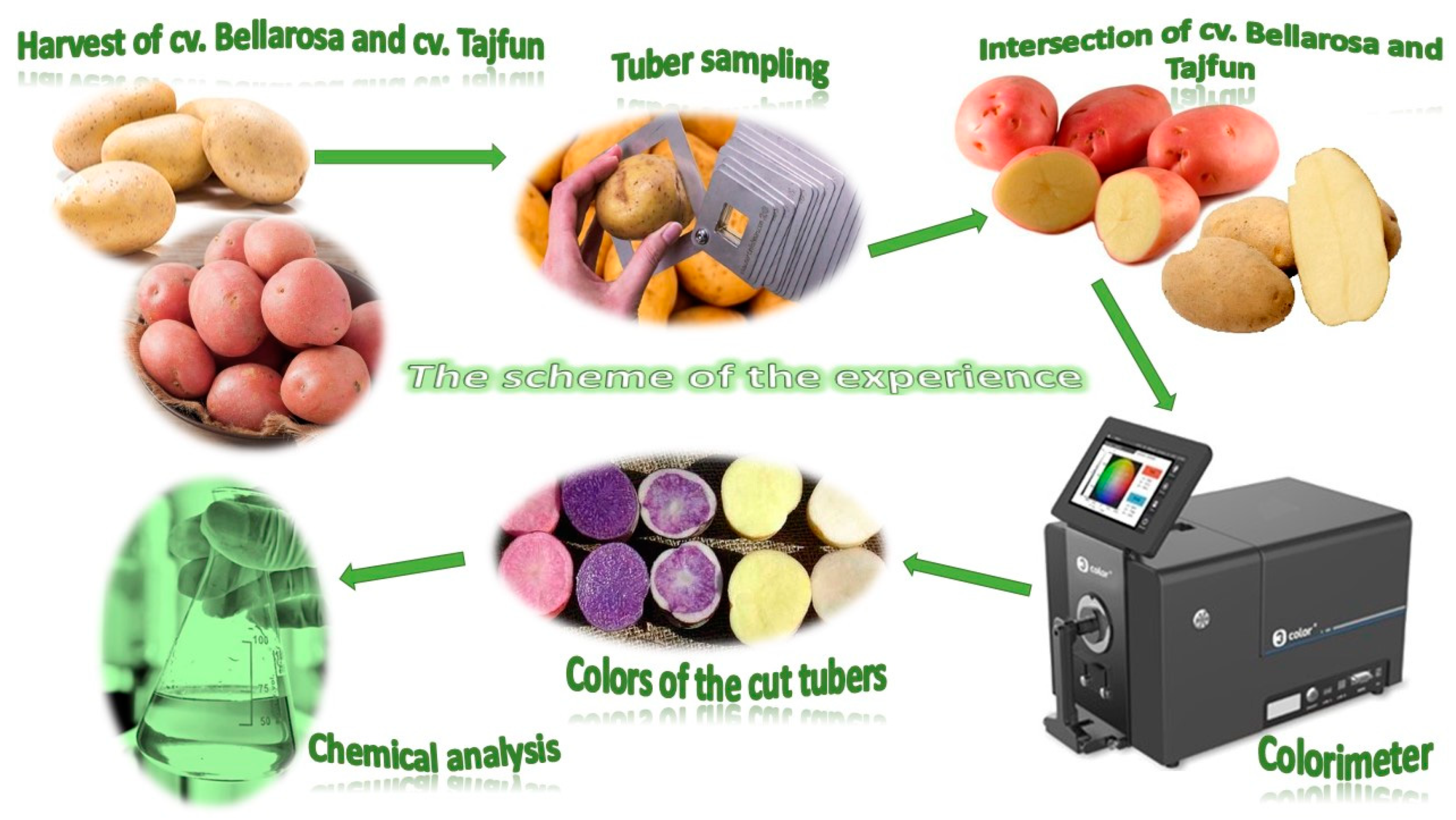


| 2015 | 2016 | 2017 |
|---|---|---|
| mandipropamid 0.150 kg·ha−1 benalaksyl 0.160 kg·ha−1 + mankozeb 1.3 kg·ha−1 famoksat 0.175 kg·ha−1 + cymoksanil 0.175 kg·ha−1 | mandipropamid 0.150 kg·ha−1 benalaksyl 0.160 kg·ha−1 + mankozeb 1.3 kg·ha−1 famoksat 0.175 kg·ha−1+ cymoksanil 0.175 kg·ha−1 | mandipropamid 0.150 kg ha−1 benalaksyl 0.160 kg·ha−1 + mankozeb 1.3 kg·ha−1 famoksat 0.175 kg·ha−1+ cymoksanil 0.175 kg·ha−1 |
| Characteristics/Cultivar | Bellarosa | Tajfun |
|---|---|---|
| Maturity time | early | medium early |
| Flesh color | yellow | yellow |
| Skin color | rose | yellow |
| Tuber shape | round oval | oval |
| Cooking type * | AB | B-BC |
| Resistance to late blight on a 9° scale | susceptible (2°) | susceptible (5°) |
| Resistance to PVY on a 9° scale | resistant (8°) | field resistant (7°) |
| Resistance to PLRV on a 9° scale | medium resistant (5.5°) | resistant (8°) |
| Resistance to nematode | resistant to the potato cyst nematode Ro1. | quite resistant to the potato cyst nematode |
| Year of Research | Macronutrient Content [mg·100 g−1 Soil] | Humus Content [g·kg−1] | pH [KCL] | Micronutrient Content [mg·kg−1 Soil] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | Cu | Mn | Zn | Fe | B | |||
| 2015 | 8.9 | 10.9 | 7.8 | 0.94 | 5.9 | 7.51 | 318 | 40.1 | 3760 | 7.24 |
| 2016 | 8.3 | 9.1 | 7.0 | 1.06 | 5.8 | 4.92 | 337 | 56.7 | 3925 | 5.28 |
| 2017 | 10.6 | 9.8 | 6.3 | 1.03 | 6.6 | 8.99 | 166 | 41.1 | 3600 | 6.04 |
| Mean | 9.3 | 9.9 | 7.0 | 1.02 | - | 7.02 | 274 | 46.0 | 3762 | 6.17 |
| Month | Year | ||
|---|---|---|---|
| 2015 | 2016 | 2017 | |
| April | 2.3 | 1.6 | 2.1 |
| May | 3.0 | 1.0 | 1.5 |
| June | 0.9 | 1.5 | 0.4 |
| July | 0.8 | 1.8 | 2.2 |
| August | 0.1 | 0.7 | 0.4 |
| September | 2.8 | 0.3 | 1.9 |
| Specification | L* | a* | b* | |
|---|---|---|---|---|
| Cultivation practices ** | A | 75.23b ± 1.20 | 3.26a ± 0.05 | 40.69b ± 0.67 |
| B | 79.39a ± 2.57 | 3.20a ± 0.08 | 43.25a ± 1.04 | |
| C | 78.63a ± 0.68 | 2.54c ± 0.06 | 42.88a ± 0.49 | |
| D | 80.63a ± 1.62 | 2.81b ± 0.13 | 43.46a ± 1.71 | |
| Cultivars | ‘Bellarosa’ | 79.3a ± 1.03 | 2.58b ± 0.14 | 41.04b ± 0.57 |
| ‘Tajfun’ | 77.6b ± 1.86 | 3.32a ± 0.10 | 43.90a ± 0.51 | |
| Years | 2015 | 78.80a ± 0.16 | 2.70b ± 0.20 | 41.40b ± 0.23 |
| 2016 | 76.40b ± 2.10 | 3.02a ± 0.13 | 42.45b ± 0.85 | |
| 2017 | 80.20b ± 1.22 | 3.14a ± 0.07 | 43.55a ± 1.18 | |
| Experimental Factors | 2015 | 2016 | 2017 | |
|---|---|---|---|---|
| Cultivation practices ** | A | 76.52a ± 1.27 | 72.25a ± 1.14 | 76.90a ± 2.72 |
| B | 78.78a ± 0.85 | 78.26a ± 4.21 | 81.14a ± 3.36 | |
| C | 78.95a ± 1.61 | 76.59a ± 1.75 | 80.36a ± 2.25 | |
| D | 80.97a ± 1.07 | 78.50a ± 3.57 | 82.41a ± 3.34 | |
| HSD p0.05 | ns *** | |||
| Cultivars | ‘Bellarosa’ | 79.00a ± 0.44 | 76.20a ± 0.48 | 82.80a ± 3.64 |
| ‘Tajfun’ | 78.60a ± 0.42 | 76.60a ± 3.13 | 77.60b ± 1.85 | |
| HSD p0.05 | 2.1 | |||
| Experimental Factors | 2015 | 2016 | 2017 | |
|---|---|---|---|---|
| Cultivation practices ** | A | 3.02a ± 0.14 | 3.30a ± 0.11 | 3.45a ± 0.07 |
| B | 2.96ab ± 0.12 | 3.28a ± 0.13 | 3.38a ± 0.10 | |
| C | 2.26b ± 0.08 | 2.64b ± 0.10 | 2.71b ± 0.09 | |
| D | 2.55a ± 0.16 | 2.88a ± 0.08 | 3.00a ± 0.13 | |
| HSD p0.05 | ns *** | |||
| Cultivars | ‘Bellarosa’ | 2.45b ± 0.09 | 2.48b ± 0.05 | 2.81b ± 0.04 |
| ‘Tajfun’ | 2.94a ± 0.03 | 3.57a ± 0.07 | 3.47a ± 0.18 | |
| HSD p0.05 | 0.6 | |||
| Experimental Factors | 2015 | 2016 | 2017 | |
|---|---|---|---|---|
| Cultivation practices ** | A | 39.85a ± 0.23 | 40.49a ± 1.04 | 41.72a ± 1.57 |
| B | 42.56a ± 0.16 | 42.84.a ± 1.74 | 44.34a ± 1.25 | |
| C | 41.50a ± 0.28 | 42.36a ± 1.16 | 43.58a ± 1.81 | |
| D | 41.70a ± 0.65 | 44.11a ± 0.83 | 44.56a ± 1.37 | |
| HSD p0.05 | ns *** | |||
| Cultivars | ‘Bellarosa’ | 40.80a ± 0.03 | 40.90b ± 0.69 | 41.40b ± 2.24 |
| ‘Tajfun’ | 42.00a ± 0.49 | 44.00a ± 0.92 | 45.70a ± 1.19 | |
| HSD p0.05 | 2.1 | |||
| Specification | DM (%) | Starch (%) | Vitamin C (mg·kg−1) | Total Sugars (%) | Reducing Sugars (%) | Saccharose (%) | |
|---|---|---|---|---|---|---|---|
| Cultivation practices * | A | 22.3b ± 0.1 | 15.2b ± 0.2 | 14.8 a ± 0.1 | 0.66 a ± 0.02 | 0.37 a ± 0.02 | 0.29 a ± 0.02 |
| B | 22.7a ± 0.1 | 15.6a ± 0.3 | 15.1 a ± 0.1 | 0.60 b ± 0.01 | 0.34 b ± 0.01 | 0.26 b ± 0.01 | |
| C | 22.7a ± 0.2 | 15.5ab ± 0.3 | 14.9 a ± 0.1 | 0.60 b ± 0.02 | 0.34 b ± 0.01 | 0.26 b ± 0.01 | |
| D | 22.6ab ± 0.1 | 15.4ab ± 0.1 | 14.9 a ± 0.1 | 0.60 b ± 0.01 | 0.34 b ± 0.01 | 0.26 b ± 0.01 | |
| HSD p0.05 | 0.4 | 0.4 | ns ** | 0.05 | 0.03 | 0.03 | |
| Cultivars | ’Bellarosa’ | 21.1b ± 0.1 | 14.2b ± 0.2 | 11.9 b ± 0.1 | 0.65 a ± 0.02 | 0.37 a ± 0.02 | 0.28 a ± 0.02 |
| ‘Tajfun’ | 24.1a ± 0.3 | 16.6a ± 0.1 | 18.0 a ± 0.1 | 0.58 b ± 0.01 | 0.33 b ± 0.01 | 0.25 b ± 0.01 | |
| HSD p0.05 | 0.2 | 0.2 | 0.2 | 0.03 | 0.02 | 0.01 | |
| Years | 2015 | 23.0a ± 0.2 | 15.1b ± 0.2 | 13.9 b ± 0.1 | 0.57 b ± 0.01 | 0.32 b ± 0.01 | 0.25 b ± 0.01 |
| 2016 | 21.7b ± 0.3 | 15.6a ± 0.3 | 14.1 b ± 0.1 | 0.87 a ± 0.02 | 0.49 a ± 0.02 | 0.38 a ± 0.02 | |
| 2017 | 23.1a ± 0.2 | 15.6a ± 0.2 | 16.7 a ± 0.1 | 0.41 c ± 0.01 | 0.23 c ± 0.01 | 0.18 c ± 0.01 | |
| HSD p0.05 | 0.3 | 0.3 | 0.3 | 0.04 | 0.03 | 0.02 | |
| Experimental Factors | DM (%) | Starch (%) | Vitamin C (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years | ||||||||||
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | ||
| Cultivation practices * | A | 22.7a | 21.4b | 22.7a | 14.9a | 15.4a | 15.3a | 13.7b | 13.9b | 16.7a |
| B | 23.3a | 21.8b | 23.1a | 15.2a | 15.8a | 15.9a | 14.2b | 14.5b | 16.7a | |
| C | 23.2a | 21.8b | 23.2a | 15.2a | 15.7a | 15.7a | 13.9b | 14.1b | 16.6a | |
| D | 23.2a | 21.6b | 23.1a | 15.1a | 15.6a | 15.6a | 13.9b | 14.1b | 16.6a | |
| HSD p0.05 | 1.2 | ns ** | 1.3 | |||||||
| Cultivars | ‘Bellarosa’ | 21.4b | 20.3b | 21.6b | 14.5b | 14.2b | 13.9b | 10.3b | 12.3b | 13.0b |
| ‘Tajfun’ | 24.7a | 23.0a | 24.4a | 15.7a | 17.0a | 17.3a | 17.5a | 16.0a | 20.4a | |
| HSD p0.05 | 0.6 | 0.6 | 0.7 | |||||||
| Experimental Factors | Total Sugars (%) | Reducing Sugars (%) | Saccharose (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years | ||||||||||
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | ||
| Cultivation practices * | A | 0.62b | 0.93a | 0.45c | 0.35b | 0.52a | 0.26b | 0.27b | 0.40a | 0.20b |
| B | 0.55b | 0.86a | 0.39c | 0.31b | 0.49a | 0.22b | 0.24b | 0.37a | 0.17b | |
| C | 0.55b | 0.86a | 0.40c | 0.31b | 0.48a | 0.22b | 0.24b | 0.37a | 0.17b | |
| D | 0.56b | 0.86a | 0.40c | 0.32b | 0.48a | 0.22b | 0.24b | 0.37a | 0.17b | |
| HSD p0.05 | 0.16 | 0.10 | 0.09 | |||||||
| Cultivars | ‘Bellarosa’ | 0.62a | 0.92a | 0.42a | 0.35a | 0.52a | 0.24a | 0.27a | 0.40a | 0.18a |
| ‘Tajfun’ | 0.52b | 0.83b | 0.40a | 0.30b | 0.47b | 0.22a | 0.23b | 0.36b | 0.17a | |
| HSD p0.05 | 0.08 | 0.05 | 0.04 | |||||||
| Trait | Significance of the Influence | Percentage of Variance Share in the Total Variance (Phenotypical One) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Varieties | Years | Cultivation Practices | Varieties × Cultivation Practices | Cultivations Practices × Years | Varieties × Cult. Practice × Years | Varieties | Years | Cultivation Practices | Varieties × Cultivation Practices | Cultivations Practices × Years | Varieties × Cultivation Practices × Years | |
| L* | ** | ** | ** | ** | ** | ** | 22.8 | 15.7 | 8.7 | 23.0 | 11.2 | 17.8 |
| a* | ** | * | ** | ** | ** | ** | 23.3 | 6.2 | 9.6 | 15,3 | 15.7 | 29.2 |
| b* | ** | * | * | ** | ** | ** | 17.5 | 8.5 | 6.4 | 15.9 | 16.8 | 34.9 |
| DM | ** | ** | ** | ** | ** | ** | 5.7 | 8.6 | 11.4 | 22.8 | 34.3 | 17.1 |
| Starch | ** | * | ** | ** | ns | ** | 5.4 | 8.2 | 10.9 | 23.6 | 3.4 | 36.9 |
| Vitamin C | ** | ** | ns | ** | ** | ** | 6.3 | 9.4 | 2.8 | 25.1 | 37.6 | 18.8 |
| Total sugars | ** | ** | ** | ** | ** | ** | 10.2 | 15.3 | 20.3 | 40.7 | 61.0 | 30.5 |
| Reducing sugars | ** | * | ** | ** | ** | ** | 5.7 | 8.8 | 11.4 | 22.9 | 34.1 | 17.0 |
| Saccharose | ** | * | * | ** | ** | ** | 4.7 | 7.0 | 9.4 | 18.7 | 28.1 | 14.0 |
| Specification | x1 | x2 | x3 | x4 | x5 | x6 | y1 | y2 | y3 |
|---|---|---|---|---|---|---|---|---|---|
| Average | 22.58 | 15.43 | 0.62 | 0.35 | 0.27 | 14.92 | 77.38 | 3.01 | 42.02 |
| Median | 22.35 | 14.99 | 0.59 | 0.33 | 0.26 | 14.51 | 77.38 | 3.13 | 41.90 |
| Standard deviation | 1.65 | 1.35 | 0.20 | 0.11 | 0.09 | 3.43 | 3.43 | 0.61 | 2.55 |
| Kurtosis | 1.42 | −1.55 | −1.38 | −1.38 | −1.38 | −1.21 | 0.48 | −0.54 | −0.35 |
| Skewness | 0.05 | 0.30 | 0.37 | 0.37 | 0.37 | 0.26 | 0.12 | −0.52 | −0.38 |
| Range | 5.00 | 4.05 | 0.60 | 0.34 | 0.26 | 10.76 | 17.37 | 2.38 | 11.20 |
| Minimum | 19.90 | 13.57 | 0.36 | 0.20 | 0.16 | 10.05 | 69.55 | 1.63 | 35.51 |
| Maximum | 24.90 | 17.63 | 0.96 | 0.54 | 0.42 | 20.81 | 86.92 | 4.01 | 46.71 |
| RSD (%) | 7.29 | 8.74 | 32.37 | 32.37 | 32.37 | 22.99 | 4.43 | 20.31 | 6.06 |
| Specification | x1 | x2 | x3 | x4 | x5 | x6 | y1 | y2 | y3 |
|---|---|---|---|---|---|---|---|---|---|
| x1 | 1.00 | ||||||||
| x2 | 0.77 ** | 1.00 | |||||||
| x3 | −0.53 ** | −0.12 | 1.00 | ||||||
| x4 | −0.53 ** | −0.12 | 0.99 ** | 1.00 | |||||
| x5 | −0.53 ** | −0.12 | 0.98 ** | 0.99 ** | 1.00 | ||||
| x6 | 0.88 ** | 0.83 ** | −0.40 * | −0.40 ** | −0.40 ** | 1.00 | |||
| y1 | 0.01 | −0.21 | −0.46 ** | −0.46 ** | −0.46 ** | −0.12 | 1.00 | ||
| y2 | 0.03 | 0.20 | 0.11 | 0.11 | 0.11 | 0.23 | −0.25 | 1.00 | |
| y3 | 0.13 | 0.24 | −0.27 * | −0.27 * | −0.27 * | 0.31 * | 0.37 * | 0.34 * | 1.00 |
| Dependent Variables | Regression Equation | Significance Level | Coefficient of Determination (%) |
|---|---|---|---|
| y1 | Y1 = 89.134 − 10.36x1 − 0.359x4 | 0.001 | 66.42 |
| y2 | Y2= 2.798 − 0.339x1 | 0.035 | 31.07 |
| y3 | Y3= 44.125 − 3.471x1 | 0.022 | 37.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczółkowski, P.; Krochmal-Marczak, B.; Sawicka, B.; Pszczółkowski, M. The Impact of Effective Microorganisms on Flesh Color and Chemical Composition of Raw Potato Tubers. Appl. Sci. 2021, 11, 8959. https://doi.org/10.3390/app11198959
Pszczółkowski P, Krochmal-Marczak B, Sawicka B, Pszczółkowski M. The Impact of Effective Microorganisms on Flesh Color and Chemical Composition of Raw Potato Tubers. Applied Sciences. 2021; 11(19):8959. https://doi.org/10.3390/app11198959
Chicago/Turabian StylePszczółkowski, Piotr, Barbara Krochmal-Marczak, Barbara Sawicka, and Mateusz Pszczółkowski. 2021. "The Impact of Effective Microorganisms on Flesh Color and Chemical Composition of Raw Potato Tubers" Applied Sciences 11, no. 19: 8959. https://doi.org/10.3390/app11198959
APA StylePszczółkowski, P., Krochmal-Marczak, B., Sawicka, B., & Pszczółkowski, M. (2021). The Impact of Effective Microorganisms on Flesh Color and Chemical Composition of Raw Potato Tubers. Applied Sciences, 11(19), 8959. https://doi.org/10.3390/app11198959








