Metal Binding and Sources of Humic Substances in Recent Sediments from the Cananéia-Iguape Estuarine-Lagoon Complex (South-Eastern Brazil)
Abstract
1. Introduction
2. Materials and Methods
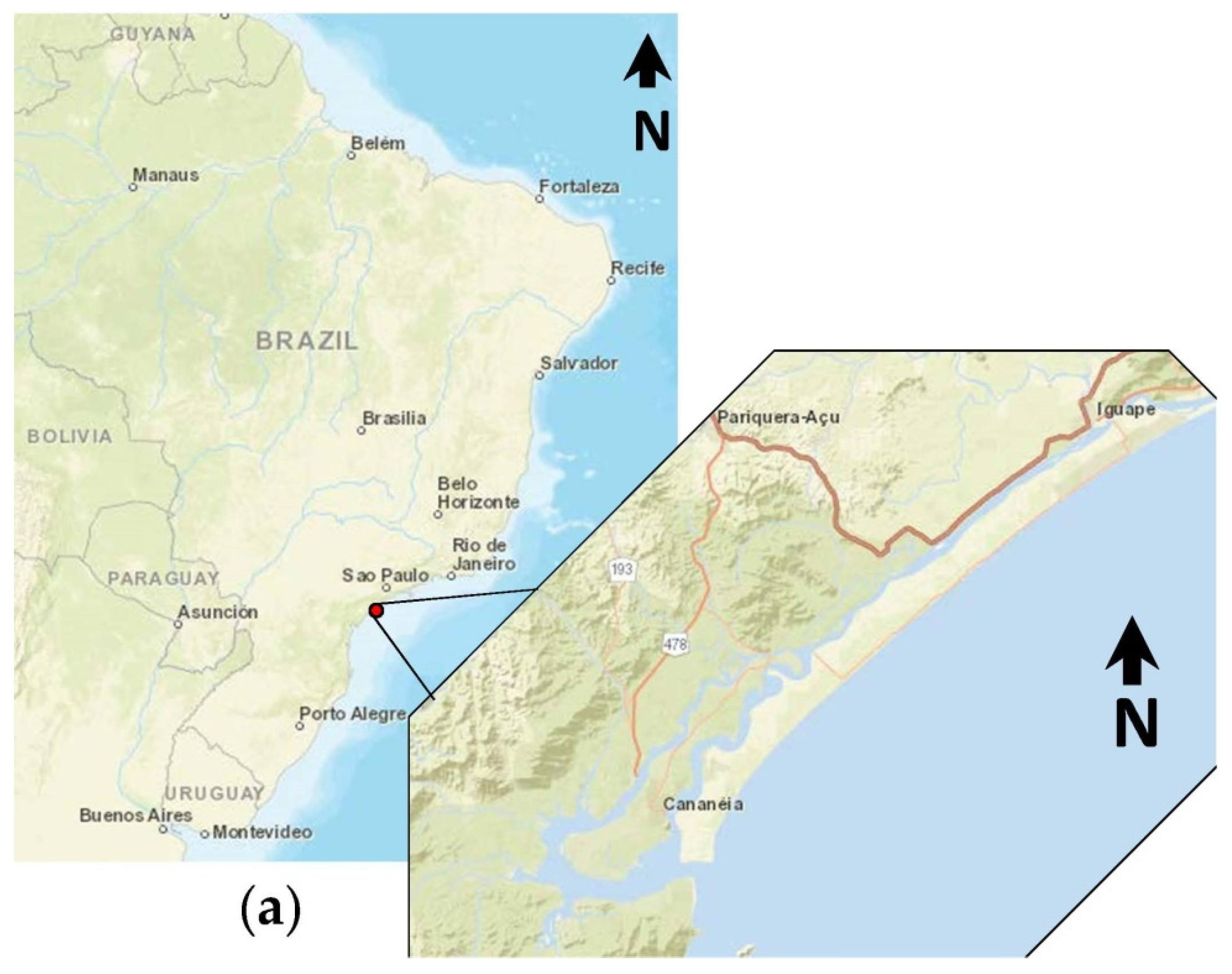
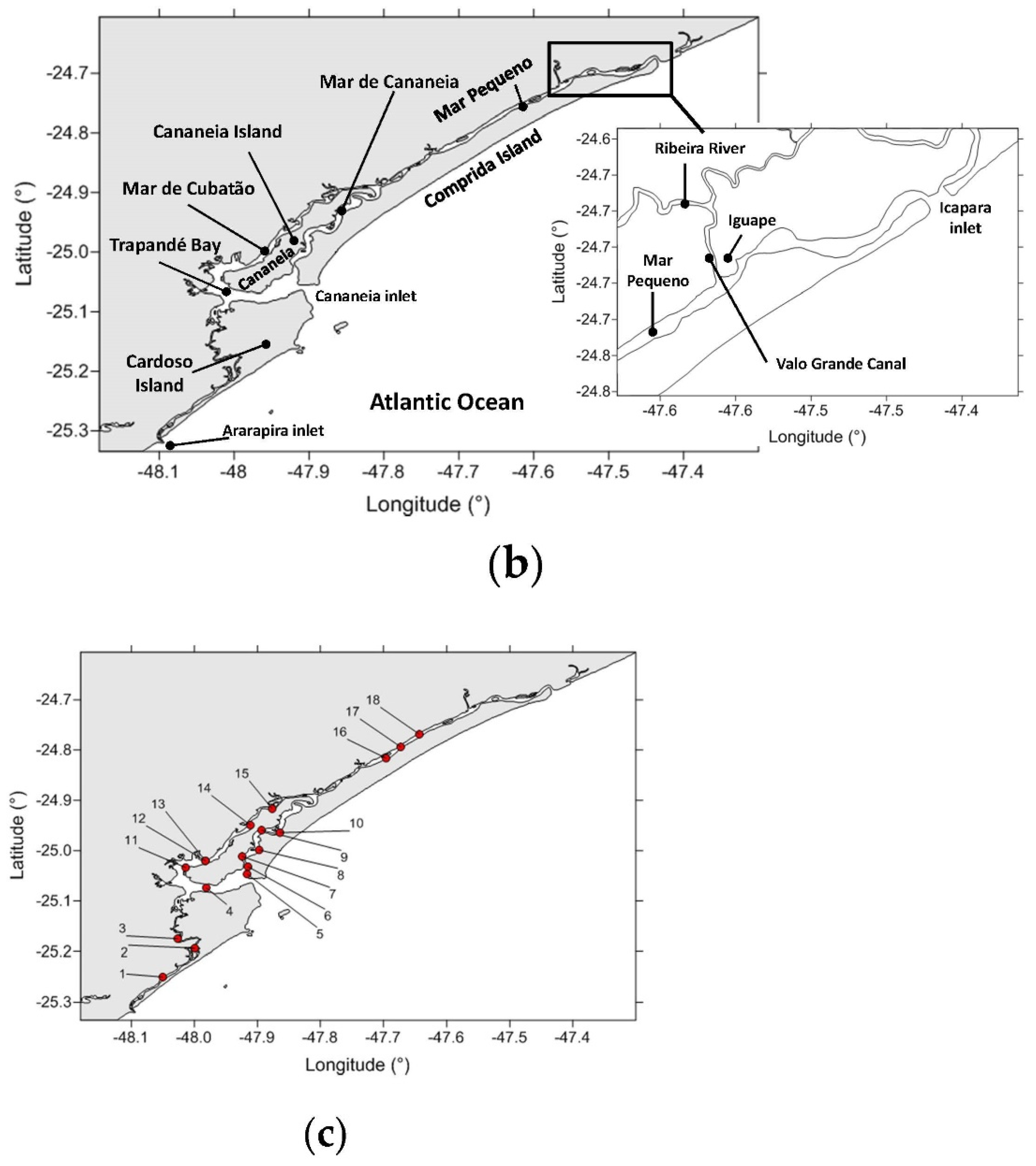
2.1. Study Area
2.2. Sediment Sampling
2.3. Grain Size Analysis of Sediments
2.4. Calcium Carbonate Content (CaCO3%)
2.5. TOC, TN and δ13C Values of SOM, FHA, BHA, and FA
2.6. Extraction of FHA, BHA, and FA from Sediments
2.7. Analysis of Metals Content in FHA, BHA, and FA
2.8. Statistical Data Analysis
3. Results
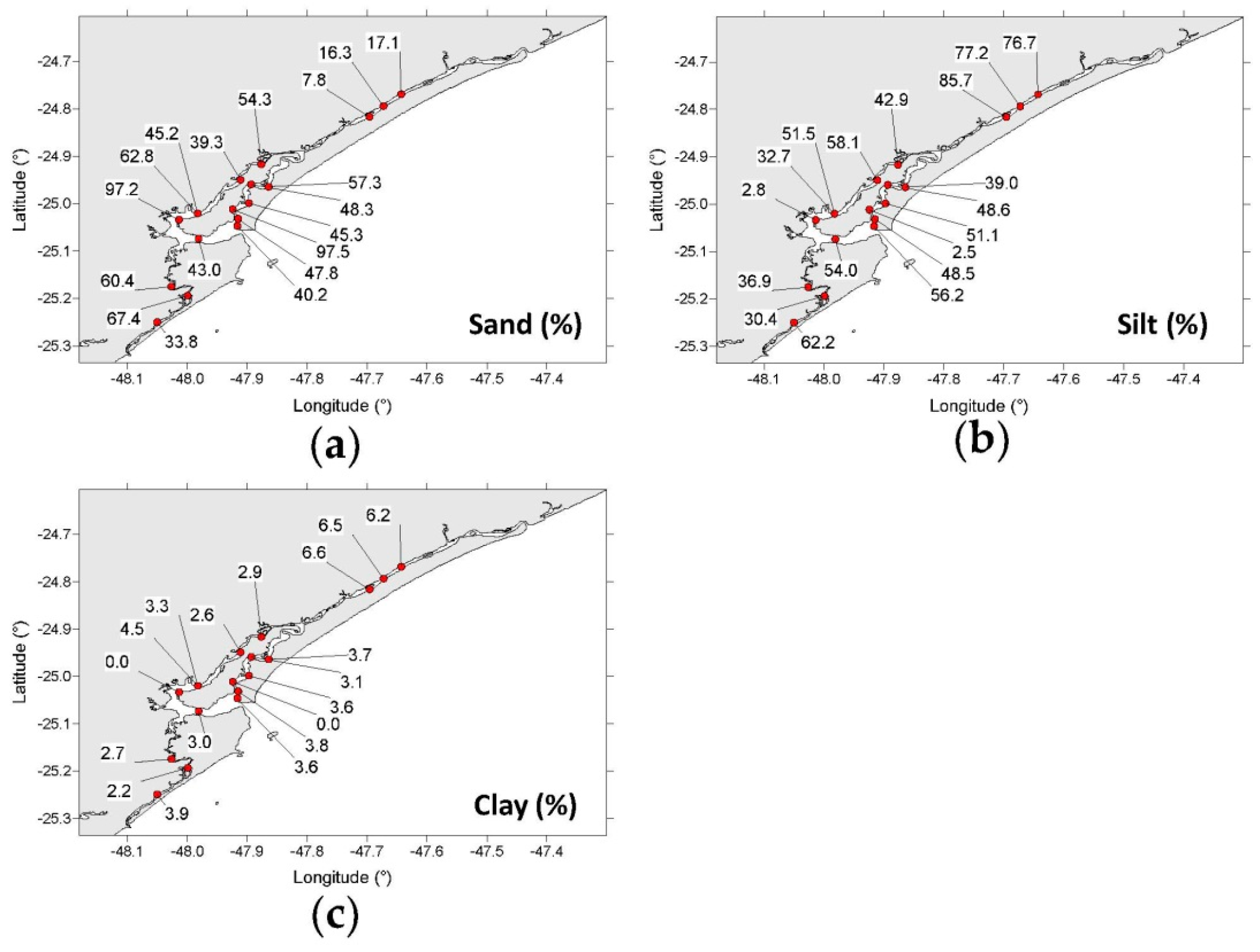
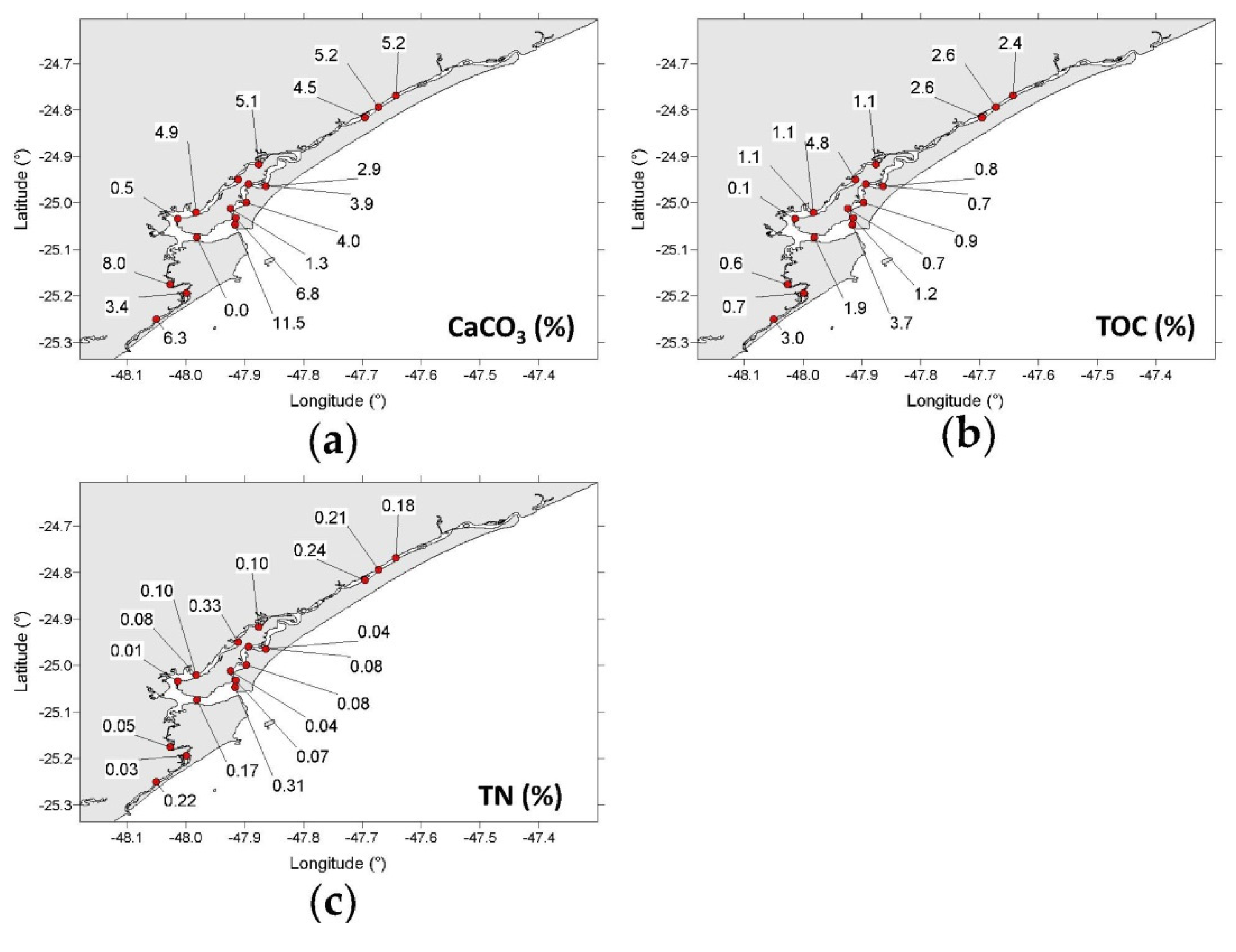
3.1. Grain Size, CaCO3, TOC, and TN Contents
3.2. Concentration of HS
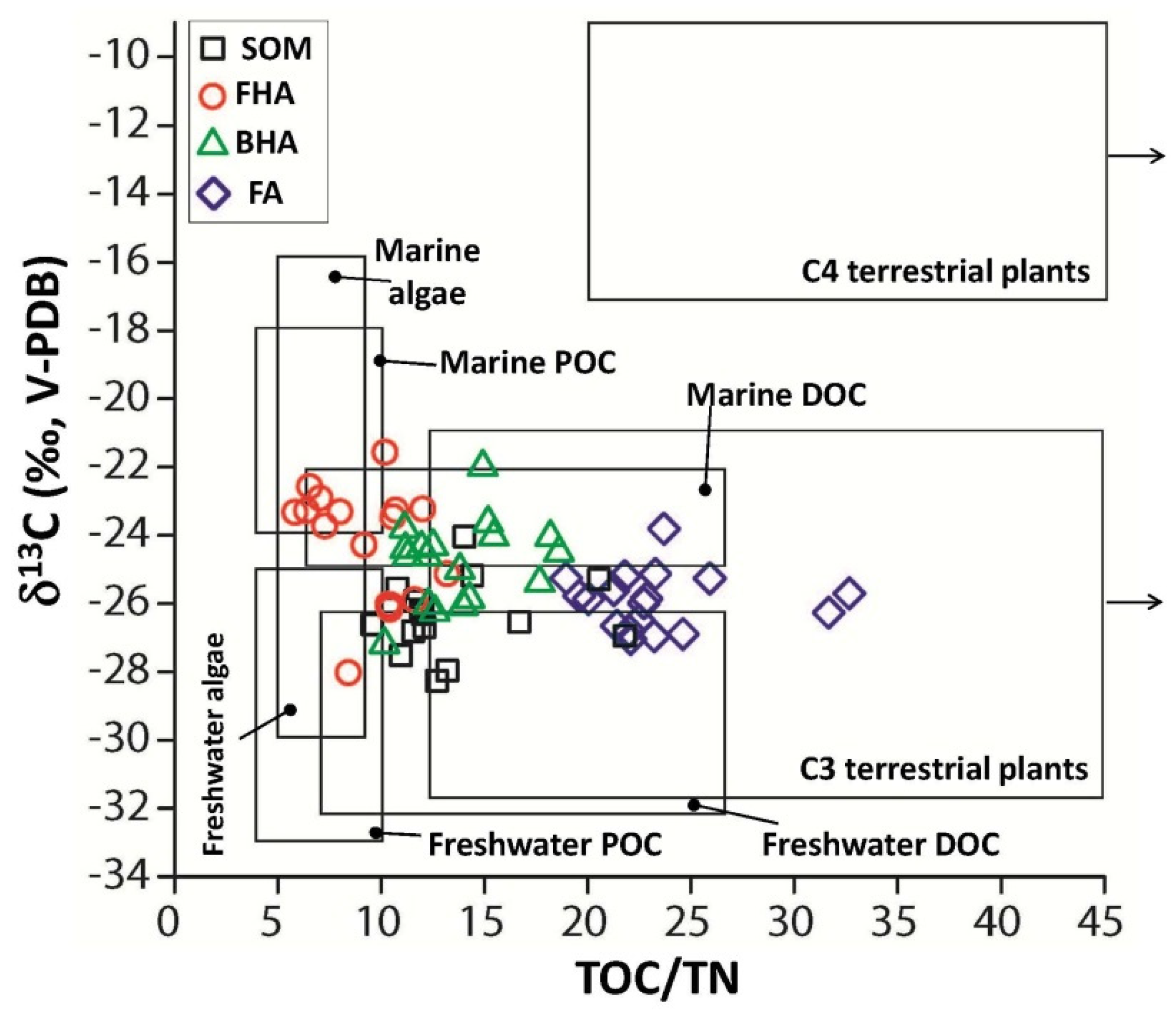
3.3. δ13C Values of SOM, FHA, BHA and FA
3.4. Metal and Metalloid Contents in FHA, BHA, and FA
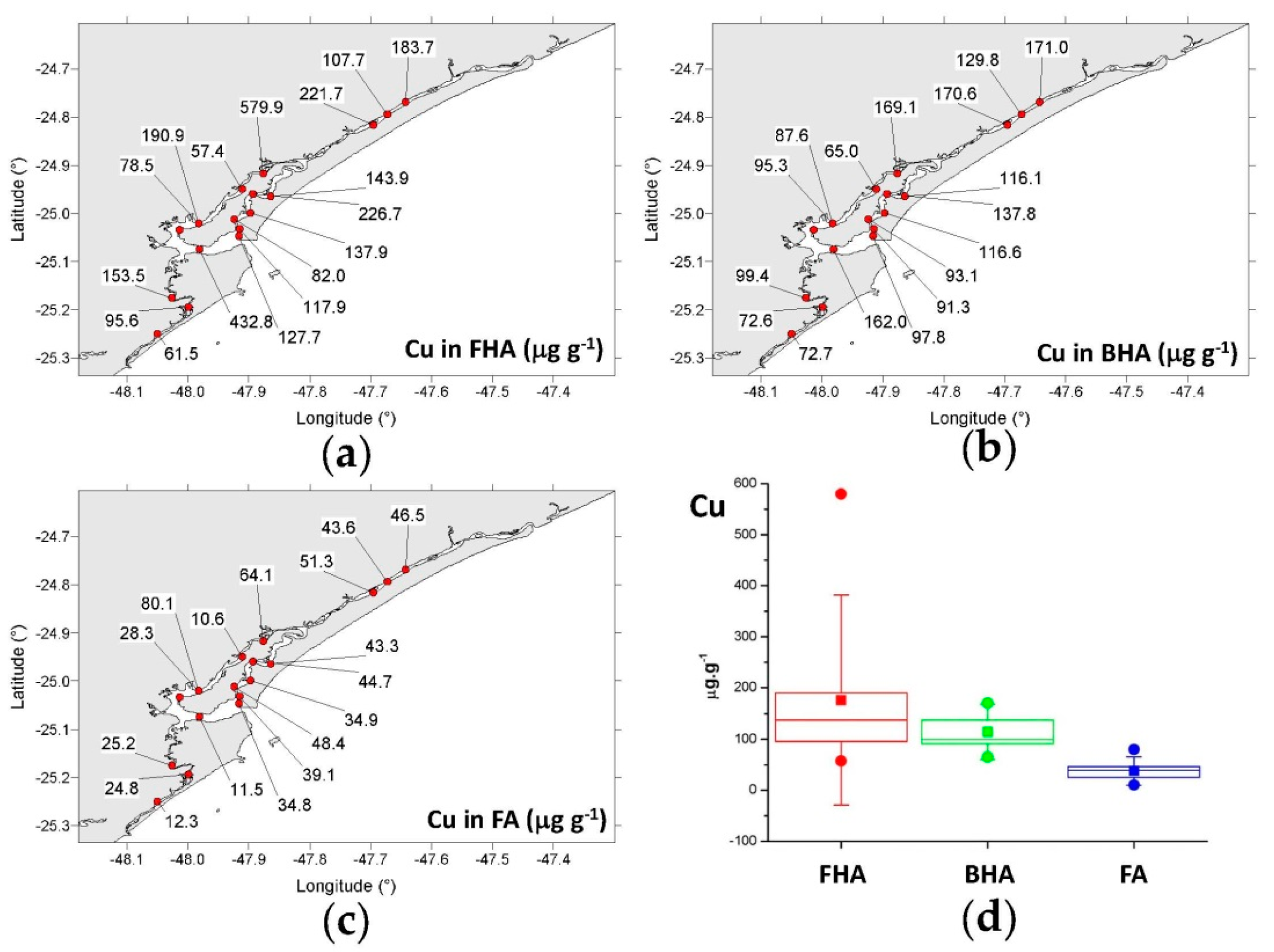
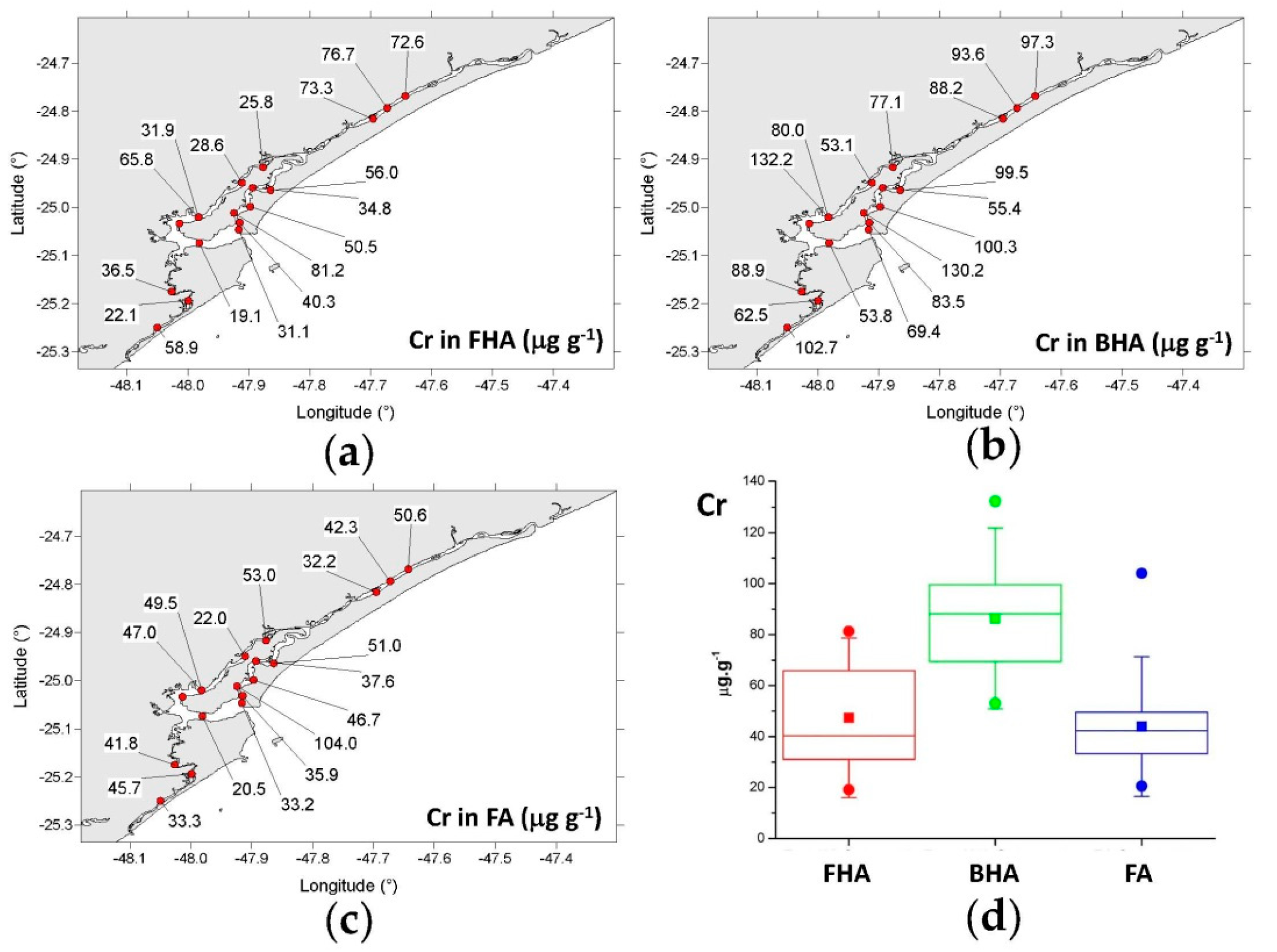
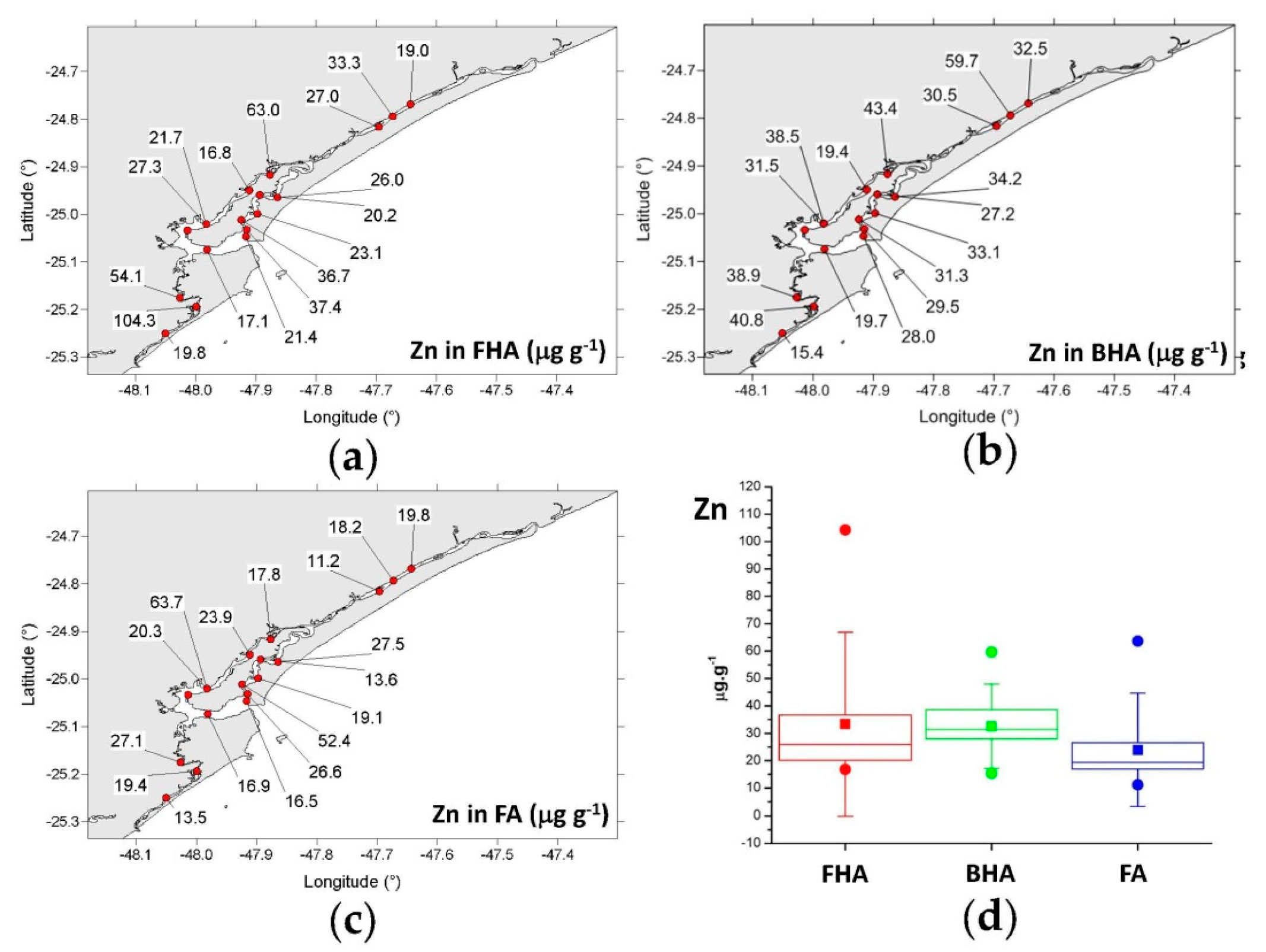
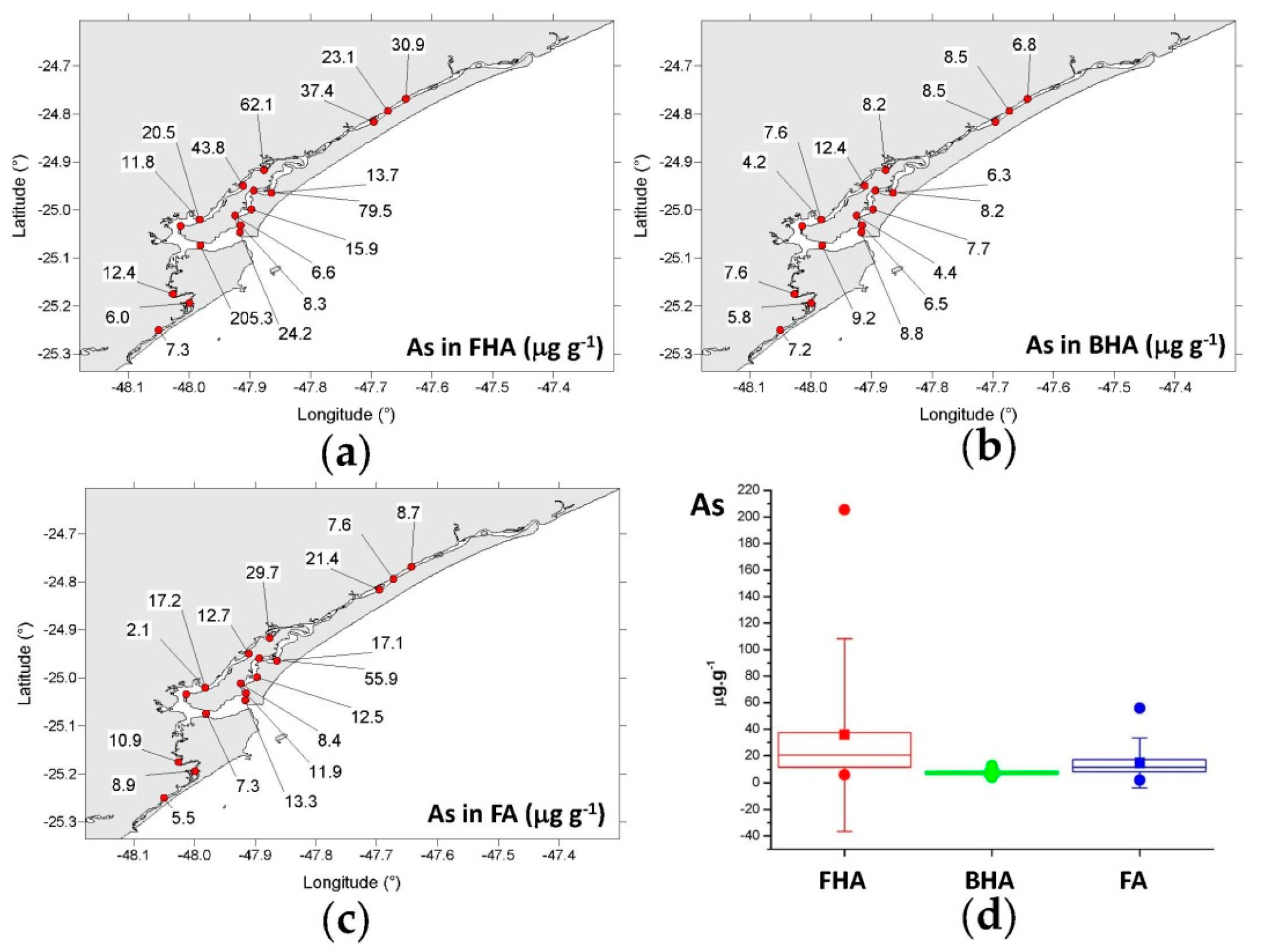
3.4.1. Copper, Chromium, Zinc, and Arsenic
- FHA: 57.4–580 μg g−1 (mean = 176 μg g−1);
- BHA: 65.0–171 μg g−1 (mean = 115 μg g−1);
- FA: 10.6–80.1 μg g−1 (mean = 37.9 μg g−1).
- FHA: 19.1–81.2 μg g−1 (mean = 47.4 μg g−1);
- BHA: 53.1–132 μg g−1 (mean = 86.3 μg g−1);
- FA: 20.5–104 μg g−1 (mean = 43.9 μg g−1).
- FHA: 16.8–104 μg g−1 (mean = 33.4 μg g−1);
- BHA: 15.4–59.7 μg g−1 (mean = 32.6 μg g−1);
- FA: 11.2–63.7 μg g−1 (mean = 24.0 μg g−1).
- FHA: 6.00–205 μg g−1 (mean = 35.8 μg g−1);
- BHA: 4.22–12.4 μg g−1 (mean = 7.53 μg g−1);
- FA: 2.11–55.9 μg g−1 (mean = 14.8 μg g−1).
3.4.2. Nickel, Manganese, Vanadium, and Lead
- FHA: 9.65–38.0 μg g−1 (mean = 23.0 μg g−1);
- BHA: 14.8–45.5 μg g−1 (mean = 30.5 μg g−1);
- FA: 2.26–10.6 μg g−1 (mean = 5.98 μg g−1).
- FHA: 6.98–50.0 μg g−1 (mean = 19.7 μg g−1);
- BHA: 2.98–88.1 μg g−1 (mean = 20.9 μg g−1);
- FA: 3.32–18.4 μg g−1 (mean = 7.90 μg g−1).
- FHA: 8.13–69.8 μg g−1 (mean = 23.2 μg g−1);
- BHA: 6.01–71.9 μg g−1 (mean = 22.1 μg g−1);
- FA: 1.16–10.2 μg g−1 (mean = 5.44 μg g−1).
- FHA: 1.30–18.7–1.3 μg g−1 (mean = 4.10 μg g−1);
- BHA: 1.85–12.2 μg g−1 (mean = 5.76 μg g−1);
- FA: 0.66–28.3 μg g−1 (mean = 3.82 μg g−1).
4. Discussion
- FHA: Cu (57.4–579.9 μg g−1) > As (6.0–205.3 μg g−1) > Zn (16.8–104.3 μg g−1) > Cr (19.1–81.2 μg g−1) > V (8.1–69.8 μg g−1) > Mn (7.0–50.0 μg g−1) > Ni (9.6–38.0 μg g−1) > Pb (1.3–18.7 μg g−1).
- BHA: Cu (65.0–171.0 μg g−1) > Cr (53.1–132.2 μg g−1) > Mn (31.0–88.1 μg g−1) > V (6.0–71.9 μg g−1) > Zn (15.4–59.7 μg g−1) > Ni (14.0–45.5 μg g−1) > As (4.2–12.4 μg g−1) > Pb (1.9–12.2- μg g−1).
- FA: Cr (20.5–104.0 μg g−1) > Cu (10.6–80.1 μg g−1) > Zn (11.2–63.7 μg g−1) > As (2.1–55.9 μg g−1) > Pb (0.7–28.3 μg g−1) > Mn (3.3–18.4 μg g−1) > Ni (2.3–10.6 μg g−1) > V (1.2–10.2 μg g−1).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coates, J.D.; Cole, K.A.; Chakraborty, R.; O’Connor, S.M.; Achenbach, L.A. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl. Environ. Microbiol. 2002, 68, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Powlson, D.; Smith, P.; De Nobili, M. Soil organic matter. In Soil Conditions and Plant Growth; Gregory, P.J., Nortcliff, S., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2013; pp. 86–131. [Google Scholar]
- International Humic Substances Society (IHSS). Available online: https://humic-substances.org (accessed on 3 July 2021).
- Olk, D.C.; Cassman, K.G.; Fan, T.W.M. Characterization of two humic acid fractions from a calcareous vermiculitic soil: Implications for the humification process. Geoderma 1995, 65, 195–208. [Google Scholar] [CrossRef]
- De Nobili, M.; Contin, M.; Mahieu, N.; Randall, E.W.; Brookes, P.C. Assessment of chemical and biochemical stabilization of organic C in soils from the long-term experiments at Rothamsted (UK). Waste Manag. 2008, 28, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Bravo, C.; Millo, C.; Covelli, S.; Contin, M.; De Nobili, M. Terrestrial-marine continuum of sedimentary natural organic matter in a mid-latitude estuarine system. J. Soils Sediments 2020, 20, 1074–1086. [Google Scholar] [CrossRef]
- Yang, R.; van den Berg, C.M.G. Metal Complexation by Humic Substances in Seawater. Environ. Sci. Technol. 2009, 43, 7192–7197. [Google Scholar] [CrossRef]
- Ashley, J.T.F. Adsorption of Cu(II) and Zn(II) by estuarine, riverine and terrestrial humic acids. Chemosphere 1996, 33, 2175–2187. [Google Scholar] [CrossRef]
- Orsetti, S.; Andrade, E.M.; Molina, F.V. Application of a constrained regularization method to extraction of affinity distributions: Proton and metal binding to humic substances. J. Colloid Interface Sci. 2009, 336, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.J.; Filip, Z. Metal Binding in Estuarine Humic and Fulvic Acids: FTIR Analysis of Humic Acid-Metal Complexes. Environ. Technol. 1998, 19, 923–931. [Google Scholar] [CrossRef]
- Botero, W.G.; Souza, S.d.O.; Santos, O.S.; Oliveira, L.C.d.; Amarante, C.B.d. Influência das substâncias húmicas de sedimentos na biodisponibilidade de metais para o sistema aquático. Química Nova 2014, 37, 943–949. [Google Scholar] [CrossRef]
- Kinniburgh, D.G.; van Riemsdijk, W.H.; Koopal, L.K.; Borkovec, M.; Benedetti, M.F.; Avena, M.J. Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 147–166. [Google Scholar] [CrossRef]
- Fengler, G.; Grossman, D.; Kersten, M.; Liebezeit, G. Trace metals in humic acids from recent Skagerrak sediments. Mar. Pollut. Bull. 1994, 28, 143–147. [Google Scholar] [CrossRef]
- Nissenbaum, A.; Swaine, D.J. Organic matter-metal interactions in Recent sediments: The role of humic substances. Geochim. Cosmochim. Acta 1976, 40, 809–816. [Google Scholar] [CrossRef]
- Wasserman, J.C.; Oliveira, F.B.L.; Bidarra, M. Cu and Fe associated with humic acids in sediments of a tropical coastal lagoon. Org. Geochem. 1998, 28, 813–822. [Google Scholar] [CrossRef]
- Schaeffer-Novelli, Y.; Mesquita, H.d.S.L.; Cintrón-Molero, G. The Cananéia Lagoon estuarine system, São Paulo, Brazil. Estuaries 1990, 13, 193–203. [Google Scholar] [CrossRef]
- Megens, L.; van der Plicht, J.; de Leeuw, J.W.; Smedes, F. Stable carbon and radiocarbon isotope compositions of particle size fractions to determine origins of sedimentary organic matter in an estuary. Org. Geochem. 2002, 33, 945–952. [Google Scholar] [CrossRef][Green Version]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Keeling, C.D.; Whorf, T.P.; Wahlen, M.; van der Plichtt, J. Interannual extremes in the rate of rise of atmospheric carbon dioxide since 1980. Nature 1995, 375, 666–670. [Google Scholar] [CrossRef]
- Osmond, C.B.; Valaane, N.; Haslam, S.M.; Uotila, P.; Roksandic, Z. Comparisons of δ13C values in leaves of aquatic macrophytes from different habitats in Britain and Finland; some implications for photosynthetic processes in aquatic plants. Oecologia 1981, 50, 117–124. [Google Scholar] [CrossRef]
- Keeley, J.E.; Sandquist, D.R. Carbon: Freshwater plants. Plant Cell Environ. 1992, 15, 1021–1035. [Google Scholar] [CrossRef]
- Deines, P. Chapter 9—The Isotopic Composition of Reduced Organic Carbon. In The Terrestrial Environment, A; Fritz, P., Fontes, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; pp. 329–406. [Google Scholar]
- Meyers, P.A. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol. 1994, 114, 289–302. [Google Scholar] [CrossRef]
- Haines, E.B. Stable carbon isotope ratios in the biota, soils and tidal water of a Georgia salt marsh. Estuar. Coast. Mar. Sci. 1976, 4, 609–616. [Google Scholar] [CrossRef]
- Salomons, W.; Mook, W.G. Field observations of the isotopic composition of particulate organic carbon in the southern North Sea and adjacent estuaries. Mar. Geol. 1981, 41, M11–M20. [Google Scholar] [CrossRef]
- Barth, J.A.C.; Veizer, J.; Mayer, B. Origin of particulate organic carbon in the upper St. Lawrence: Isotopic constraints. Earth Planet. Sci. Lett. 1998, 162, 111–121. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J. Carbon and nitrogen stable isotopes in suspended matter and sediments from the Schelde Estuary. Mar. Chem. 1998, 60, 217–225. [Google Scholar] [CrossRef]
- Peters, K.E.; Sweeney, R.E.; Kaplan, I.R. Correlation of carbon and nitrogen stable isotope ratios in sedimentary organic matter 1. Limnol. Oceanogr. 1978, 23, 598–604. [Google Scholar] [CrossRef]
- Wada, E.; Minagawa, M.; Mizutani, H.; Tsuji, T.; Imaizumi, R.; Karasawa, K. Biogeochemical studies on the transport of organic matter along the Otsuchi River watershed, Japan. Estuar. Coast. Shelf Sci. 1987, 25, 321–336. [Google Scholar] [CrossRef]
- Bordovskiy, O.K. Sources of organic matter in marine basins. Mar. Geol. 1965, 3, 5–31. [Google Scholar] [CrossRef]
- Goñi, M.A.; Teixeira, M.J.; Perkey, D.W. Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuar. Coast. Shelf Sci. 2003, 57, 1023–1048. [Google Scholar] [CrossRef]
- Otero, E.; Culp, R.; Noakes, J.E.; Hodson, R.E. The distribution and δ13C of dissolved organic carbon and its humic fraction in estuaries of southeastern USA. Estuar. Coast. Shelf Sci. 2003, 56, 1187–1194. [Google Scholar] [CrossRef]
- Rashid, M.A. Geochemistry of Marine Humic Compounds; Springer: New York, NY, USA, 1985; pp. 1–300. [Google Scholar]
- Abessa, D.M.d.S.; Morais, L.G.; Perina, F.C.; Davanso, M.B.; Rodrigues, V.G.S.; Martins, L.M.d.P.; Sígolo, J.B. Sediment geochemistry and climatic influences in a river influenced by former mining activities: The case of Ribeira de Iguape River, SP-PR, Brazil. Open J. Water Pollut. Treat. 2014, 1, 43–53. [Google Scholar] [CrossRef]
- Mahiques, M.M.d.; Burone, L.; Figueira, R.C.L.; Lavenére-Wanderley, A.A.d.O.; Capellari, B.; Rogacheski, C.E.; Barroso, C.P.; Samaritano dos Santos, L.A.; Cordero, L.M.; Cussioli, M.C. Anthropogenic influences in a lagoonal environment: A multiproxy approach at the valo grande mouth, Cananéia-Iguape system (SE Brazil). Braz. J. Oceanogr. 2009, 57, 325–337. [Google Scholar] [CrossRef]
- De Mahiques, M.M.; Figueira, R.C.L.; Salaroli, A.B.; Alves, D.P.V.; Gonçalves, C. 150 years of anthropogenic metal input in a Biosphere Reserve: The case study of the Cananéia–Iguape coastal system, Southeastern Brazil. Environ. Earth Sci. 2013, 68, 1073–1087. [Google Scholar] [CrossRef]
- Cruz, A.C.F.; Davanso, M.B.; Araujo, G.S.; Buruaem, L.M.; Santaella, S.T.; de Morais, R.D.; Abessa, D.M.S. Cumulative influences of a small city and former mining activities on the sediment quality of a subtropical estuarine protected area. Environ. Monit. Assess. 2014, 186, 7035–7046. [Google Scholar] [CrossRef]
- Mesquita, A.R.; Harari, J. Tides and Tide gauges of Cananeia and Ubatuba. Relat. Interno Inst. Oceanogr. USP 1983, 1, 14. [Google Scholar]
- Bérgamo, A.L. Caraterística da Hidrografia, Circulação e Transporte de Sal: Barra de Cananéia, Sul do Mar de Cananéia e Baia do Trapandé. Master Thesis, University of São Paulo, São Paulo, Brazil, 2000. [Google Scholar]
- De Mahiques, M.M.; Figueira, R.C.; Alves, D.P.; Italiani, D.M.; Martins, C.C.; Dias, J.M. Coastline changes and sedimentation related with the opening of an artificial channel: The Valo Grande Delta, SE Brazil. Anais Acad. Bras. Cienc. 2014, 86, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Amorim, E.P.; Fávaro, D.I.T.; Berbel, G.B.B.; Braga, E.S. Assessment of metal and trace element concentrations in the Cananéia estuary, Brazil, by neutron activation and atomic absorption techniques. J. Radioanal. Nucl. Chem. 2008, 278, 485–489. [Google Scholar] [CrossRef]
- Tramonte, K.M.; Figueira, R.C.L.; de Lima Ferreira, P.A.; Ribeiro, A.P.; Batista, M.F.; de Mahiques, M.M. Environmental availability of potentially toxic elements in estuarine sediments of the Cananéia–Iguape coastal system, Southeastern Brazil. Mar. Pollut. Bull. 2016, 103, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Tramonte, K.M.; Figueira, R.C.L.; Majer, A.P.; de Lima Ferreira, P.A.; Batista, M.F.; Ribeiro, A.P.; de Mahiques, M.M. Geochemical behavior, environmental availability, and reconstruction of historical trends of Cu, Pb, and Zn in sediment cores of the Cananéia-Iguape coastal system, Southeastern Brazil. Mar. Pollut. Bull. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Azevedo, J.S.; Braga, E.S.; Favaro, D.T.; Perretti, A.R.; Rezende, C.E.; Souza, C.M.M. Total mercury in sediments and in Brazilian Ariidae catfish from two estuaries under different anthropogenic influence. Mar. Pollut. Bull. 2011, 62, 2724–2731. [Google Scholar] [CrossRef]
- Fernandez, W.S.; Dias, J.F.; Boufleur, L.A.; Amaral, L.; Yoneama, M.L.; Dias, J.F. Bioacumulation of trace elements in hepatic and renal tissues of the white mullet Mugil curema Valenciennes, 1836 (Actinopterygii, Mugilidae) in two coastal systems in southeastern Brazil. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 318, 94–98. [Google Scholar] [CrossRef]
- Barbieri, E. Concentration of heavy metals in tissues of green turtles (Chelonia mydas) sampled in the Cananéia estuary, Brazil. Braz. J. Oceanogr. 2009, 57, 243–248. [Google Scholar] [CrossRef]
- Shepard, F.P. Nomenclature based on sand-silt-clay ratios. J. Sediment. Res. 1954, 24, 151–158. [Google Scholar]
- Barcellos, R.L.; Camargo, P.B.; Galvão, A.; Weber, R.R. Sedimentary Organic Matter in Cores of the Cananéia-Iguape Lagoonal Estuarine System, São Paulo State, Brazil. J. Coast. Res. 2009, SI 56, 1335–1339. [Google Scholar]
- Hem, J.D. Chemical equilibria affecting the behavior of Manganese in natural water. Int. Assoc. Sci. Hydrol. Bull. 1963, 8, 30–37. [Google Scholar] [CrossRef]
- Manunza, B.; Deiana, S.; Maddau, V.; Gessa, C.; Seeber, R. Stability Constants of Metal-Humate Complexes: Titration Data Analyzed by Bimodal Gaussian Distribution. Soil Sci. Soc. Am. J. 1995, 59, 1570–1574. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Santos, M.; Araújo, M.F. Metal binding by humic acids in recent sediments from the SW Iberian coastal area. Estuar. Coast. Shelf Sci. 2011, 93, 478–485. [Google Scholar] [CrossRef]
- Aguiar, V.M.C.; Braga, E.S.; Baptista Neto, J.A. Heavy metal assessment in two subtropical estuarine systems in the state of São Paulo, Brazil. In Marine Pollution: New Research, 1st ed.; Hofer, T.N., Ed.; Nova Publishers: New York, NY, USA, 2008; pp. 379–397. [Google Scholar]


| CaCO3 | TOC | TN | δ13CSOM | Sand | Silt | Clay | δ13CFHA | δ13CBHA | δ13CFA | [FHA] | [BHA] | [FA] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCO3 | 1.00 | 0.55 | 0.51 | −0.02 | −0.17 | 0.17 | 0.16 | −0.03 | 0.04 | −0.01 | 0.13 | 0.09 | 0.24 |
| TOC | 1.00 | 0.98 | −0.07 | −0.69 | 0.70 | 0.54 | −0.19 | −0.07 | −0.05 | 0.60 | 0.76 | 0.89 | |
| TN | 1.00 | −0.13 | −0.72 | 0.74 | 0.55 | −0.26 | −0.16 | −0.16 | 0.65 | 0.73 | 0.92 | ||
| δ13CSOM | 1.00 | 0.44 | −0.43 | −0.56 | 0.89 | 0.91 | 0.91 | −0.61 | 0.35 | 0.04 | |||
| Sand | 1.00 | −1.00 | −0.94 | 0.69 | 0.51 | 0.43 | −0.92 | −0.44 | −0.60 | ||||
| Silt | 1.00 | 0.93 | −0.68 | −0.50 | −0.42 | 0.92 | 0.46 | 0.62 | |||||
| Clay | 1.00 | −0.82 | −0.63 | −0.53 | 0.92 | 0.20 | 0.37 | ||||||
| δ13CFHA | 1.00 | 0.94 | 0.90 | −0.83 | 0.21 | −0.09 | |||||||
| δ13CBHA | 1.00 | 0.98 | −0.71 | 0.33 | −0.02 | ||||||||
| δ13CFA | 1.00 | −0.65 | 0.32 | −0.03 | |||||||||
| [FHA] | 1.00 | 0.28 | 0.53 | ||||||||||
| [BHA] | 1.00 | 0.88 | |||||||||||
| [FA] | 1.00 |
| Pb | V | Cr | Mn | Ni | Cu | Zn | As | |
|---|---|---|---|---|---|---|---|---|
| Pb | 1.00 | −0.19 | −0.25 | −0.27 | −0.07 | 0.77 | 0.39 | 0.12 |
| V | 1.00 | 0.76 | 0.86 | −0.62 | −0.10 | −0.32 | −0.06 | |
| Cr | 1.00 | 0.67 | −0.35 | −0.43 | −0.37 | −0.41 | ||
| Mn | 1.00 | −0.58 | −0.22 | −0.21 | −0.10 | |||
| Ni | 1.00 | −0.14 | −0.27 | −0.08 | ||||
| Cu | 1.00 | 0.08 | 0.68 | |||||
| Zn | 1.00 | −0.23 | ||||||
| As | 1.00 |
| Pb | V | Cr | Mn | Ni | Cu | Zn | As | |
|---|---|---|---|---|---|---|---|---|
| Pb | 1.00 | 0.53 | 0.47 | 0.46 | 0.39 | −0.05 | 0.54 | −0.03 |
| V | 1.00 | 0.34 | 0.97 | −0.44 | 0.19 | 0.69 | 0.27 | |
| Cr | 1.00 | 0.19 | 0.13 | −0.07 | 0.14 | −0.32 | ||
| Mn | 1.00 | −0.44 | 0.15 | 0.68 | 0.33 | |||
| Ni | 1.00 | −0.44 | −0.37 | −0.18 | ||||
| Cu | 1.00 | 0.09 | 0.49 | |||||
| Zn | 1.00 | −0.04 | ||||||
| As | 1.00 |
| Pb | V | Cr | Mn | Ni | Cu | Zn | As | |
|---|---|---|---|---|---|---|---|---|
| Pb | 1.00 | −0.12 | 0.23 | 0.27 | 0.37 | 0.04 | 0.23 | 0.00 |
| V | 1.00 | 0.15 | −0.02 | −0.22 | 0.40 | −0.19 | 0.35 | |
| Cr | 1.00 | 0.17 | 0.11 | 0.62 | 0.40 | 0.11 | ||
| Mn | 1.00 | 0.45 | 0.06 | 0.20 | −0.24 | |||
| Ni | 1.00 | −0.02 | 0.08 | −0.03 | ||||
| Cu | 1.00 | 0.61 | 0.40 | |||||
| Zn | 1.00 | −0.09 | ||||||
| As | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millo, C.; Bravo, C.; Covelli, S.; Pavoni, E.; Petranich, E.; Contin, M.; De Nobili, M.; Crosera, M.; Otero Sutti, B.; das Mercês Silva, C.; et al. Metal Binding and Sources of Humic Substances in Recent Sediments from the Cananéia-Iguape Estuarine-Lagoon Complex (South-Eastern Brazil). Appl. Sci. 2021, 11, 8466. https://doi.org/10.3390/app11188466
Millo C, Bravo C, Covelli S, Pavoni E, Petranich E, Contin M, De Nobili M, Crosera M, Otero Sutti B, das Mercês Silva C, et al. Metal Binding and Sources of Humic Substances in Recent Sediments from the Cananéia-Iguape Estuarine-Lagoon Complex (South-Eastern Brazil). Applied Sciences. 2021; 11(18):8466. https://doi.org/10.3390/app11188466
Chicago/Turabian StyleMillo, Christian, Carlo Bravo, Stefano Covelli, Elena Pavoni, Elisa Petranich, Marco Contin, Maria De Nobili, Matteo Crosera, Bruno Otero Sutti, Camila das Mercês Silva, and et al. 2021. "Metal Binding and Sources of Humic Substances in Recent Sediments from the Cananéia-Iguape Estuarine-Lagoon Complex (South-Eastern Brazil)" Applied Sciences 11, no. 18: 8466. https://doi.org/10.3390/app11188466
APA StyleMillo, C., Bravo, C., Covelli, S., Pavoni, E., Petranich, E., Contin, M., De Nobili, M., Crosera, M., Otero Sutti, B., das Mercês Silva, C., & de Santis Braga, E. (2021). Metal Binding and Sources of Humic Substances in Recent Sediments from the Cananéia-Iguape Estuarine-Lagoon Complex (South-Eastern Brazil). Applied Sciences, 11(18), 8466. https://doi.org/10.3390/app11188466









