Abstract
At the Nalón River estuary (Asturias, Northern Spain), the occurrence of Hg is due to historical mining activity which has resulted in environmental issues of great concern. Although several studies have investigated the sediment compartment regarding Hg contamination, no information is currently available on the fate of Hg and MeHg in the water column. Considering different hydrodynamic/seasonal conditions, water samples were collected along the estuary to evaluate Hg and MeHg distribution and partitioning behaviour between solid and aqueous phases. The complementary effect of the river discharge and tidal currents contributed to the prevalence of the dissolved (4.02 ± 1.33 ng L−1) or particulate (8.37 ± 4.20 ng L−1) Hg under different conditions of discharge in summer and autumn, respectively. Conversely, particulate MeHg prevailed when the river flow was low, especially at the estuary mouth (25.8 ± 19.1 pg L−1) and most likely due to the resuspension of fine particles promoted by a stronger tidal current. In comparison with the total Hg concentration, extremely low amounts of dissolved and particulate MeHg were observed, and strong interactions between MeHg and organic carbon highlighted a negligible risk of increased mobility and potential bioaccumulation of MeHg.
1. Introduction
Mercury (Hg) distribution and fate in estuaries represent major issues of global concern [1,2,3,4,5] due to the toxicity and potential bioavailability of its organic form, methylmercury (MeHg), which can be readily bioaccumulated in the aquatic food chain [6,7]. Several anthropogenic activities as well as natural processes (e.g., erosion, river transport and atmospheric deposition) may contribute to increasing the total amount of Hg and other potentially toxic trace elements in estuaries and coastal areas [8,9] where water circulation dynamics strongly affect the sedimentary processes leading to the accumulation of contaminants, including Hg, in the sediment compartment.
Most of the MeHg in estuaries mainly originates from in situ sedimentary production mediated by bacteria and promoted by changes in physico-chemical boundary conditions [10]. Indeed, sediments are generally thought to act both as a sink and a secondary source of contamination due to the remobilisation of Hg and other potentially toxic compounds at the sediment–water interface (SWI) with the subsequent transfer to the water column [11,12,13]. Several mechanisms can be responsible for Hg and MeHg remobilisation from sediments, including diffusive/advective fluxes, desorption processes, mixing between different water masses and changes in the suspended particulate matter (SPM) composition [14]. Moreover, the behaviour of Hg and MeHg in estuarine environments may also be affected by resuspension events induced by natural (e.g., tidal currents, riverine flow conditions, wave action and bioturbation) [15,16,17] and anthropogenic factors (e.g., dredging, shipping and trawling) [18,19,20,21]. This is of relevant concern, especially in the case of shallow aquatic systems where sediment resuspension can be considered the main factor governing the redistribution of particles as well as potentially toxic trace elements adsorbed on their reactive surface [22].
The coastal area of Asturias (northern Spain) is one of the most economically productive regions due to historical extraction activity of coal and sulphide ores (mainly cinnabar, HgS). Indeed, Asturias is known as one of the largest Hg producers worldwide and the main Hg deposits are in Mieres and Pola de Lena (the La Peña-Terronal and La Sotteraña mining districts, respectively) [23,24]. Although this long-term mining activity has certainly promoted the economic growth of the region, it can also be identified as the primary source of several environmental issues. Indeed, several studies focused on different environmental matrices such as soils, mine tailings, vegetation, surface water and sediments in areas affected by Hg mining [25,26,27,28,29,30] highlighted the occurrence of notable inputs of Hg and other potentially toxic trace elements to the Nalón River drainage basin, which is the main hydrological system in Asturias, and its estuary [25,29,31,32].
At the Nalón River estuary, the behaviour of Hg has been investigated with respect to Hg contamination in estuarine sediments and salt marshes [33,34,35], Hg bioaccumulation in halophytes [36] and Hg mobility in resuspended contaminated estuarine sediments [37]. Conversely, there is a lack of information pertaining to the water column. Indeed, the primary aim of this research is to assess the occurrence of Hg and MeHg and their water column distribution along the Nalón River estuary focusing on the partitioning behaviour between solid (suspended particulate matter, SPM) and dissolved phases as well as on the effects of different seasonal conditions during low-normal river discharge.
Investigation into the mobility and fate of dissolved and particulate Hg and MeHg, as well as their potential mobility in the estuarine environment of the Nalón River, is of great importance with reference to the goals of the European Union’s Water Framework Directive (WFD, European Parliament, Council of the European Union, 2000) and the European Union’s Marine Strategy Framework Directive (MSFD, European Parliament, Council of the European Union, 2008) [38,39]. In this context, the present research provides useful scientific results to evaluate the chemical status of the investigated estuarine environment with the final aim of supporting policymakers in protecting the fragile aquatic ecosystem.
2. Materials and Methods
2.1. Environmental Setting
The Nalón River is the largest hydrographic system of the Asturias region (northern coastline of Spain) (Figure 1). The river basin presents a range of annual flows between 134 m3 s−1 to 171 m3 s−1, with an average annual flow of 143 m3 s−1. A marked variation in river flow occurs between the wet months (maximum average monthly flow 417 m3 s−1) and dry months (minimum average monthly flow 17.1 m3 s−1), with an approximate difference of 400 m3 s−1. This difference occurs due to the grouping of rain and snowfall in the upper sector of the river drainage basin during the autumn and winter months.
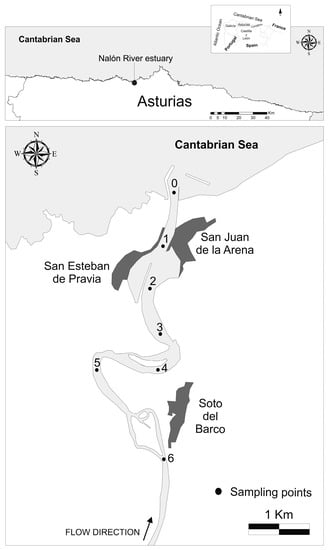
Figure 1.
Study area and sampling points along the Nalón River estuary.
The river freshwater input to the estuarine environment is high and mixing processes between river freshwater and marine saltwater may extend for several kilometres inland [40]. Indeed, the estuary is over 6 km long, and its inland limit is defined by the migration of saltwater in the upper sector of the estuary, according to the tidal range. The main estuarine channel reaches a depth higher than 2 m and tides are over 2 m for more than 70% of the year, showing an annual meso-tidal range fluctuating between 1.0 and 4.2 m [41].
From a morphological point of view, the upper sector of the estuary is characterised by natural riverbanks and meanders, whereas in the lowest sector of the estuary the natural morphology has been modified by the construction of two small regional ports (San Juan de la Arena and San Esteban de Pravia) with the subsequent alteration of the natural sedimentary dynamics [42].
The Nalón River and its estuarine system are affected by several anthropogenic activities including tourism, fishing and yachting, as well as by built-up areas and agricultural and mining-industrial settlements located inland along the Nalón River drainage basin. Among these potential sources of contamination, the mining activity is the greatest source of concern as it has been ongoing since 1850 and mainly focused on the extraction of cinnabar (HgS) followed by coal, gold and iron. Water draining from the decomissioned mining districts as well as the erosion of tailing deposits accumulated along the riverbanks affected the environmental quality of water and sediments downstream [23,33,43,44]. For this reason, the Hg contamination at the Nalón River estuary still represents an environmental issue of concern to be investigated.
2.2. Sampling and Analysis
Sampling in the estuary was carried out using a boat at seven previously selected stations (Figure 1) during three different river flow conditions in the range of low-normal flow in April (spring, 34.8 m3 s−1), July (summer, 21.6 m3 s−1) and October (autumn, 69.0 m3 s−1) 2015. At each sampling station, water samples and physico-chemical parameters were taken and measured, respectively. For obtaining physico-chemical parameters, a YSY 556 MPS multiprobe previously calibrated using pH (4.00, 7.00 and 9.28), conductivity (147 µS cm−1 and 12.88 mS cm−1) and redox (220 mV recalibrated with temperature) standards from Hach was used. A Niskin bottle (5 litre capacity) was used to collect water samples at different depths. One aliquot of the water samples was filtered and used for the determination of dissolved Hg, MeHg, Fe, Mn and DOC (Dissolved Organic Carbon). Another aliquot was vacuum-filtered in the laboratory to recover suspended particulate matter (SPM). This solid matrix was analysed for grain-size, Hg, MeHg, Fe, Mn, and POC (Particulate Organic Carbon).
The measurement of Hg species was carried out via gas chromatography combined with inductively coupled plasma mass spectrometry (7890A Agilent GC and HP 7500c Agilent ICP-MS). The determination of the Hg elemental species concentration was carried out by species-specific isotope dilution mass spectrometry. All sample preparation procedures and methods for water [45,46,47], sediments/SPM [48,49] and the mathematical equations [50] are described in detail in the cited documents.
Total Fe and Mn in dissolved and solid (SPM) phases were determined by ICP-MS (HP 7500c Agilent). The SPM phase was previously digested by aqua regia+ HF in a microwave vessel [51]. Grain-size analyses on SPM were performed following the method outlined by García-Ordiales et al. [34] by means of a laser diffractometer (Fritsch Anaysette 22 Laser-Particle Sizer Microtec). The DOC and POC were determined by direct combustion using a TOC-V CSH (Shimadzu) instrument.
2.3. Exploratory Multivariate Data Analysis
A 3-way principal component analysis (3W-PCA) obtained using the Tucker-3 algorithm [52] was used as an unsupervised exploratory chemometric tool for the identification of relationships within objects (surface and bottom water samples collected at the seven investigated sites), variables and conditions (spring, summer, autumn) [53]. Prior to multivariate analysis, a log-transform was applied to those variables which did not show a normal distribution. Systematic differences between the experimental variables were minimised by applying column autoscaling (j-scaling) to data matrices [52]. Multivariate data processing was performed using the CAT (Chemometric Agile Tool) package, based on the R platform (The R Foundation for Statistical Computing, Vienna, Austria) and freely distributed by Gruppo Italiano di Chemiometria (Italy) [54].
3. Results and Discussion
3.1. Physico-Chemical Parameters of the Water Column
The Nalón River discharge was found to be quite different among the three sampling campaigns: the highest river flow was observed in October (autumn, 69.0 m3 s−1) followed by the sampling performed in April (spring, 34.8 m3 s−1) and July (summer, 21.6 m3 s−1).
Salinity distribution varied slightly among different seasonal conditions of river discharge as well as along the whole estuary (Figure 2). Indeed, variations in the vertical salinity gradient were limited and the estuary appeared to be dominated by partially mixed estuarine circulation. In this context, conditions of turbulent mixing between different water masses as well as the effect of strong tidal currents at the bottom may inhibit vertical salinity stratification [55]. Moreover, the interaction among the wave motion, tidal currents and river inputs represented the main factors regulating the energy pattern in this estuary [33,41] and the mixing zone between freshwater and saltwater reached a different extension along the river axis. The maximum extension of saltwater (at the bottom of site 3) was observed in summer, when the river discharge was lowest. When the river flow became higher, the extension of the mixing zone increased and brackish water dominated throughout the water column along the Nalón River estuary (from site 1 to site 4). In autumn, when the river discharge was highest, the extension of the mixing zone was comparable to that observed in spring with the only difference being that river freshwater dominated the surface water layer at site 3 and slightly lower values of salinity were observed at a higher depth at site 3 (7.14 ± 0.93) and at site 4 (8.89 ± 1.65).
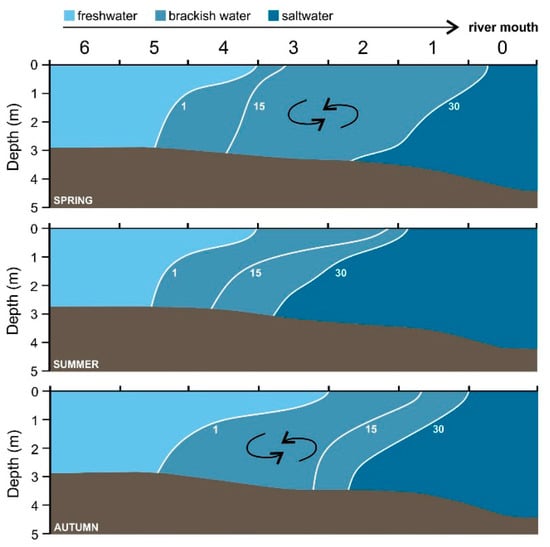
Figure 2.
Schematic areal distribution of salinity measured along the water column at the seven investigated sites along the Nalón River estuary under different seasonal conditions (spring, summer, autumn).
Commonly, saltwater prevailed at the estuary mouth (site 0) and in the lower estuary (sites 1 and 2) especially at the bottom. The central sector of the estuary (sites 3 and 4) was usually dominated by the occurrence of brackish water whereas the upper estuary was characterised by river freshwater (sites 5 and 6) (Figure 2). Saltwater always prevailed at the estuary mouth (site 0, always >31) and at site 1 under conditions of low discharge observed in summer (avg. 31.1 ± 0.9) whereas brackish water was found in the surface layer in spring (25.6) and autumn (18.6) when the river flow was highest. The occurrence of brackish water along the water column was evident at site 2 (avg. 23.5 ± 6.8, 25.5 ± 10.6, and 21.7 ± 8.7 in spring, summer and autumn, respectively) and site 3 in summer (avg. 20.5 ± 8.8) and spring (avg. 18.6 ± 1.5) whereas freshwater prevailed in the surface layer in autumn (0.49) where salinity increased with depth. Similar conditions were also evident at site 4 where brackish water was observed at the intermediate depth whereas freshwater always occurred in the surface layer (avg. 0.26 ± 0.03). The upper sector of the estuary (sites 5 and 6) was always dominated by freshwater along the water column showing an average salinity of 0.21 ± 0.02 during the year.
Notable disparities in temperature were not observed although the bottom water displayed slightly lower values (13.7 ± 0.5, 16.6 ± 0.5, 12.7 ± 0.4 °C in spring, summer and autumn, respectively) compared to those found in the surface layer (14.1 ± 0.6, 18.4 ± 0.6, and 13.8 ± 0.3 °C in spring, summer and autumn, respectively) especially in July (Table S1). Slight variations in pH were measured among the different sampling campaigns (7.64 ± 0.06, 7.14 ± 0.29 and 7.87 ± 0.17 in spring, summer and autumn, respectively) and the maximum values were observed at the estuary mouth (site 0), both in the surface saltwater (8.12) and at the bottom (8.09) (Table S1).
The redox potential (Eh) pointed to oxidative conditions (444 ± 28, 436 ± 43, and 472 ± 18 mV in spring, summer and autumn, respectively) (Table S1). This is consistent with the dissolved oxygen (DO) which varied slightly among different sampling campaigns (7.93 ± 0.12, 7.44 ± 0.27, and 8.02 ± 0.06 mg L−1 in spring, summer and autumn, respectively). The highest values were measured in saltwater in particular at the estuary mouth under low river flow in summer (Table S1).
3.2. Suspended Particulate Matter: Distribution, Grain Size and Sources
The maximum values of SPM were found in autumn, when the river discharge was highest (7.64 ± 3.05 mg L−1) whereas lower values were observed during the sampling campaigns performed under conditions of low discharge both in spring (3.48 ± 0.95 mg L−1) and summer (2.81 ± 0.68 mg L−1) (Table S1). At the seven investigated sites along the Nalón River estuary, notable differences in the SPM values were not observed with increasing depth, suggesting that the water column was vertically well-mixed most likely due to the shallow depth of the water. This was consistent with the salinity distribution and confirmed by the surface-bottom SPM ratios which were generally low (0.52–0.88, 0.60–1.34 and 0.36–0.92 in spring, summer and autumn, respectively).
The SPM grain size spectra did not notably change among the three different sampling campaigns, whereas the SPM was found to have a different grain size distribution along the Nalón River estuary (Figure S1). At the estuary mouth (site 0) as well as in the lower and central sectors (sites 1, 2, 3 and 4) surface SPM samples were skewed and sorted whereas a poorly sorted distribution was usually observed at the bottom testifying to the marine origin of the suspended particles. The only exception is represented by site 3 where skewed and sorted spectra were observed throughout the water column most likely due to low hydrodynamics at this site where a clear dominance of riverine or marine particles did not appear to occur.
Conversely, the SPM samples collected in the upper sector of the estuary (sites 5 and 6) appeared to be poorly sorted both in the surface layer and at the bottom. There, the grain size distribution and selection appeared to be inhibited by the shallow water depth and the absence of a relevant freshwater flow.
Marked differences in PHg, PFe and PMn concentrations along the water column were not generally observed at the different investigated sites suggesting that mixing processes between different water masses as well as resuspension of bottom sediments were the main factors governing trace element distribution along the Nalón River estuary.
Particulate Hg was found to be quite constant by comparing the results relating to spring (1.59 ± 0.53 µg g−1), summer (1.80 ± 0.47 µg g−1) and autumn (1.20 ± 0.66 µg g−1) conditions (Table S2). Particulate Hg appeared to be mainly associated with fine particles (2–8 µm) especially in autumn when a significant correlation was found (N = 17, r = 0.787, p < 0.01). This is consistent with previous research which highlighted that Hg distribution along the Nalón River estuary was strictly correlated to decreasing grain size leading to Hg accumulation in fine sediments restricted to the port areas of San Esteban de Pravia and San Juan de la Arena [33]. Moreover, the amount of PHg was found to be comparable to the total Hg concentration in the surface sediments ranging between 0.10 and 1.33 µg g−1 [33].
From the comparison of several aquatic systems affected by anthropogenic activities it can be seen that the PHg concentrations obtained from this research were one order of magnitude lower than those observed at the Isonzo River mouth (0.09–37.3 µg g−1, Gulf of Trieste, Italy [56]; 12.1 µg g−1, [57]) and at the estuarine systems along the Portuguese coast (0.80–15.3 µg g−1, [58]). Conversely, comparable PHg contents were measured at the Tagus estuary in Portugal (0.36–8.63 µg g−1, [14]) as well as at the Tinto (0.06–1.12 µg g−1) and Odiel (0.14–6.52 µg g−1) estuaries along the SW Spanish coast [1].
Particulate MeHg was found to be notably lower with respect to the total amount of Hg (Table S2). As already stated for PHg, in the case of PMeHg a strong correlation with fine particles (2–8 µm) was observed in autumn when the river discharge was highest (N = 17, r = 0.779, p < 0.01). Although the PMeHg concentrations were extremely low, a significant correlation with PHg was found in the case of a high river flow observed in autumn (N = 17, r = 0.943, p < 0.01) most likely due to a vertically well-mixed water column along the Nalón River estuary. Conversely, a weaker correlation was observed in spring, when the river discharge was lower (N = 17, r = 0.772, p < 0.01) and no correlation was observed in summer. However, a slight increase in the PMeHg concentration was found in saltwater from autumn (≤0.005 µg g−1), to spring (0.006–0.015 µg g−1) and summer (0.015–0.022 µg g−1) following a decrease in the river discharge. This can be related to the role of tidal currents which were stronger when the river discharge was low (summer), thus promoting the resuspension of fine particles which were more enriched in MeHg with respect to those transported by the river freshwater.
Nevertheless, MeHg percentages in the SPM were found to be extremely low and generally below 1% (0.12–0.65%, 0.09–1.56% and 0.09–0.27% in spring, summer and autumn, respectively). This is in agreement with the results of previous investigations focused on estuarine sediments thus demonstrating that only low amounts of the total Hg are in methylated form (from 0.003 to 0.009 µg g−1 of MeHg in the surface sediments) [33] and potentially resuspended in the water column [37].
Iron and Mn were found to be notably present in the SPM and their average concentrations increased from summer (6.75 ± 1.72% for Fe and 4.60 ± 1.29% for Mn) to spring (8.17 ± 1.33% for Fe and 4.98 ± 1.19% for Mn) following the increase in the river flow showing the maximum values when the river discharge was higher in autumn (10.9 ± 1.38% for Fe and 5.86 ± 0.96% for Mn) (Table S2). The occurrence of such elevated concentrations of PFe and PMn could be the result of the combined effect of riverine suspended particles together with the resuspension of bottom sediments. In addition, Asturias is known as one of the main Hg mining districts worldwide and anthropogenic inputs of Hg and As in the Nalón River estuary are mainly due to the long-term mining activity in the river basin [25,29,31,33]. In detail, the physico-chemical alteration of tailing deposits at La Soterraña, El Terronal and Los Rueldos decommissioned mine sites can lead to an increasing mobility of Hg, As and other potentially toxic trace elements in the Caudal River freshwater, which is the second most important tributary of the Nalón River [59]. This can also be a possible explanation for the elevated PFe and PMn concentrations since iron sulphides (pyrite and marcasite) and iron oxides were found to be the most abundant mineral phases in mine tailings [24].
3.3. Dissolved and Particulate Hg and MeHg: The Effect of the River Discharge
3.3.1. Occurrence and Distribution of Dissolved and Particulate Hg and MeHg
River discharge can seriously affect the occurrence and distribution of trace elements as well as their partitioning between solid and dissolved phases in estuarine environments. Moreover, trace element distribution in estuaries can be notably affected by sediment resuspension events [19,60,61] which control both the movement and redistribution of particles in shallow aquatic systems playing a crucial role in regulating trace element mobility and bioavailability [22].
In this research, a 3W-PCA (59.4% of explained variance) was used as an additional chemometric tool to highlight the effects of different seasonal conditions of river flow (spring, summer and autumn) on the behaviour of dissolved and particulate variables (Hg, MeHg, Fe, Mn, DOC and POC) as well as the physico-chemical parameters along the Nalón River estuary (Figure 3). The seven investigated sites along the Nalón River estuary are clearly represented by the multivariate model and their position in the 3W-PCA output appears to be regulated by salinity which varies along Axis 2 as seen in Figure 3.
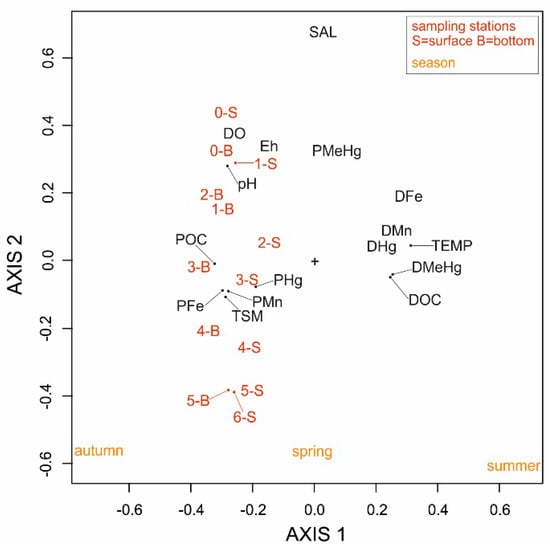
Figure 3.
Plot summarising the results of 3W-PCA performed on dissolved and particulate Hg, MeHg, Fe and Mn as well as DOC, POC and physico-chemical parameters measured in the water column along the Nalón River estuary under different seasonal conditions (spring, summer and autumn).
Notable differences in the dissolved and particulate concentrations were not observed among the seven investigated sites along the Nalón River estuary (Figure 4 and Figure 5, Table S2). This is also evident in the 3W-PCA (Figure 3) output and most likely due to the fact that the water column was generally vertically well-mixed at each site as the result of the relatively shallow water depth along the river axis. Moreover, the 3W-PCA output also clearly denotes the difference among the three levels of river flow during the year and especially between summer and autumn (Figure 3). Generally, concentrations of DHg (4.02 ± 1.33 ng L−1), DMeHg (18.1 ± 6.8 pg L−1), DFe (7.56 ± 2.39 µg L−1), DMn (2.86 ± 0.62 µg L−1) and DOC (0.64 ± 0.18 mg L−1) prevailed in summer under conditions of low discharge. Conversely, they reached minimum values during high river discharge in autumn (DHg = 2.13 ± 0.70 ng L−1, DMeHg = 5.69 ± 2.10 pg L−1, DFe = 2.50 ± 0.63 µg L−1, DMn = 0.81 ± 0.27 µg L−1, DOC = 0.37 ± 0.08 mg L−1) most likely due to dilution effects related to higher river discharge (Figure S2).
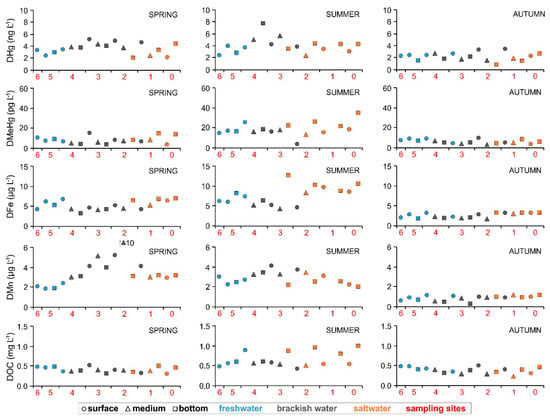
Figure 4.
Distribution of DHg, DMeHg, DFe, DMn and DOC in the water column from the upper sector of the estuary of the Nalón River to its mouth (from site 6 to site 0) under different seasonal conditions (spring, summer and autumn).
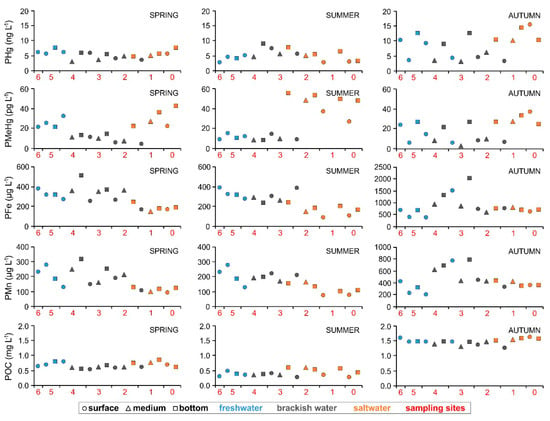
Figure 5.
Distribution of PHg, PMeHg, PFe, PMn and POC in the water column from the upper sector of the estuary of the Nalón River to its mouth (from site 6 to site 0) under different seasonal conditions (spring, summer and autumn).
Dissolved Hg was found to be one order of magnitude lower compared to other aquatic systems in the Iberian Peninsula such as the Tagus estuary (3.61–65.4 ng L−1, [14]), the Portuguese coast (1.00–28.1 ng L−1, [58]), the Tinto estuary (1.66–19.8 ng L−1, [1]) and the Odiel estuary (3.29–40.0 ng L−1, [1]). In contrast, slightly lower concentrations of DHg were observed in the Gulf of Cadíz (0.42–0.76 ng L−1, [1]).
The most toxic and bioavailable DMeHg reached concentrations in the range between 2.48 and 34.5 pg L−1 which were higher than those measured in the Tagus estuary (0.06–6.90 pg L−1, [14]) but notably lower if compared to other estuarine systems affected by the legacy of mining activities, such as the Isonzo River mouth (Gulf of Trieste, Italy, <LOD–1323 pg L−1, [56]), or chlor-alkali plants as in the case of the Wuli estuary and Jinzhou Bay in NE China (640 and 45–280 pg L−1, respectively, [62]).
The amount of DMeHg was also found to be significantly low if compared to the total concentration of Hg in the dissolved fraction. Indeed, the percentage of DMeHg was always <1% (0.07–0.43%, 0.08–0.81%, and 0.15–0.58% in spring, summer and autumn, respectively). This would testify to the minimal risk of MeHg bioaccumulation since the amount of MeHg in the water column can be seen as a strong predictor of its concentration in the biota [63]. Moreover, the bioavailability of MeHg depends on several factors and its dissolved concentration is usually the result of the balance between methylation and demethylation processes [64]. In aquatic systems, most of the MeHg originates from in situ production in the sediment compartment [2,11,12,65] near the SWI [66] and methylation is not simply influenced by the total amount of Hg [67,68] since several parameters (e.g., temperature, pH, Eh, inorganic and/or organic complexing agents) contribute to MeHg production, mobility and fate [69,70,71]. Once released in the water column, the amount of DMeHg also depends on demethylation processes due to sunlight [72] which could be enhanced at the Nalón River estuary where the water depth is generally shallow.
Regarding the SPM, the particulate forms of the investigated variables reached the maximum values on average in autumn under higher river discharge (PHg = 8.37 ± 4.20 ng L−1, PFe = 848 ± 413 µg L−1, PMn = 445 ± 175 µg L−1, POC = 1.48 ± 0.10 mg L−1) and were found to be notably lower and quite constant in summer (PHg = 5.05 ± 1.84 ng L−1, PFe = 195 ± 83 µg L−1, PMn = 131 ± 54 µg L−1, POC = 0.40 ± 0.10 mg L−1) (Figure S2 and Table S2).
The only exception is represented by PMeHg which appeared to be mostly present in the saltwater in the lower sector of the estuary as well as at the estuary mouth, especially in summer under conditions of low river discharge (25.8 ± 19.1 pg L−1) rather than in autumn (18.2 ± 11.1 pg L−1) when the river flow was highest (Figure 3, Figure 5 and Figure S2).
In this context, the surface sediments can be considered a secondary source of MeHg to the overlying water column, most likely due to sediment resuspension induced by natural and/or anthropogenic events [22,37,64,68,73]. Indeed, it has been demonstrated that the mobilisation of sediment particles during a tidal resuspension event can notably affect the PMeHg concentrations in the water column [74]. In the case of the Nalón River, the tidal currents prevailing under conditions of low discharge could be responsible for perturbed conditions at the bottom of the estuary mouth. This could enhance the resuspension of fine particles which may be a valid explanation for the highest values of PMeHg in the lower sector of the estuary.
Although slightly lower values were observed during resuspension experiments, it has been demonstrated that PMeHg concentrations sharply decreased after 24 h [37]. In addition, Kim et al. [22] stated that the dynamics between the dissolved and the particulate phases of Hg and MeHg during resuspension experiments also depended on the type of particles involved which can accumulate and may or may not release Hg and MeHg.
Moreover, as previously mentioned for the dissolved fraction, the percentages of PMeHg with respect to the total amount of PHg were generally low (0.12–0.65%, 0.09–1.56% and 0.61–3.47% in spring, summer and autumn, respectively) and did not seem to represent a relevant environmental issue in terms of potential MeHg bioaccumulation.
A strong correlation (N = 50, r = 0.934, p < 0.01) occurred between DMeHg and DOC, especially in summer as also evident from the 3W-PCA output (Figure 3), as well as between PMeHg and POC (N = 17, r = 0.651, 0.01 < p<0.1; N = 16, r = 0.768, p < 0.01; N = 17, r = 0.784, p < 0.01 for spring, summer and autumn) (Figure 6) thus suggesting that organic carbon strongly interacted with MeHg playing a crucial role in MeHg binding, distribution and fate in the water column. Indeed, it has been demonstrated that complexation with DOC can limit the solubility of MeHg and subsequently its bioaccumulation [75,76,77].
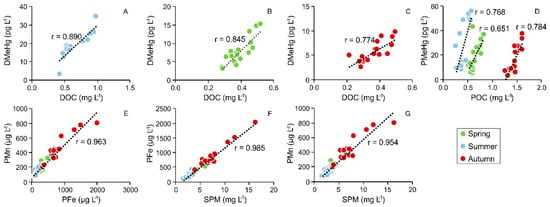
Figure 6.
Relationships between DMeHg and DOC (A–C), between PMeHg and POC (D), between PMn and PFe (E) and between PFe, PMn and SPM (F,G) along the water column at the Nalón River estuary under different seasonal conditions (spring, summer and autumn).
Particulate Fe and Mn were found to be notably present at the Nalón River estuary and a clear increase of the amount of these elements in the SPM as a function of the three different levels of river discharge (summer < spring < autumn) was evident (Figure S2). Indeed, the highest concentrations were observed in autumn (358–2022 and 192–792 µg L−1 of PFe and PMn, respectively) followed by spring (152–516 and 90–319 µg L−1 of PFe and PMn, respectively) and summer (86–389 and 66–221 µg L−1 of PFe and PMn, respectively) conditions of river discharge (Table S2). Generally, PFe and PMn were found to be slightly higher in freshwater and brackish water with respect to saltwater as the result of dilution effects involving riverine and marine particles [78,79].
Moreover, the significant correlation between PFe and PMn (N = 50, r = 0.963, p < 0.01) testified to a common source and behaviour of these elements in the SPM which is also confirmed by the fact that both PFe and PMn show strong correlations with SPM (N = 50, r = 0.985, p < 0.01 and N = 50, r = 0.954, p < 0.01 for PFe and PMn, respectively) (Figure 3 and Figure 6).
3.3.2. Distribution Coefficients (KD)
In estuarine systems, a chemical constituent can be partitioned in the solid or dissolved fraction reflecting the predominance of adsorption/precipitation or desorption/dissolution processes [80,81,82]. In this research, the partitioning behaviour of Hg and MeHg as well as that of Fe and Mn among different environmental conditions at the Nalón River estuary was evaluated by means of distribution coefficients (KD, L kg−1) which were calculated according to the following equation and expressed on a logarithmic scale [80] (Table 1):
where MeSPM and MeDISS are the metal concentrations in the SPM and in the dissolved fraction, respectively. Although information on the precise chemical form is not provided by logKD, this index can be useful in the evaluation of the behaviour of contaminants among different phases in aquatic systems [82,83].
LogKD = [MeSPM]/[MeDISS]

Table 1.
Minimum, maximum and average values of logKD (L Kg−1) for Hg, MeHg, Fe and Mn along the Nalón River estuary under low, normal and high river discharge.
The logKD values were rather constant among different seasonal conditions of the river discharge and generally high suggesting that the investigated elements were preferentially partitioned in the suspended particles (Table 1).
This is in agreement with the Eh values (always >400 mV) indicating oxidative conditions which can promote precipitation and/or adsorption processes. Mercury showed an average logKD value ranging between 5.66 ± 0.16 and 6.65 ± 0.21 (Table 1) which was comparable to that observed at the NY/NJ Harbor (5.3–6.5, [2]) as well as at the Isonzo River mouth (Italy, avg. 6.19) where the element was found to be almost completely partitioned in the SPM [57].
In the case of MeHg, the logKD values were found to be comparable with those observed for Hg and similar to those observed in the Adour River estuary (SW France, 4.8–6.9, [84]) and in the waters of the NY/NJ Harbor (4.5–5.6, [2]). Conversely, slightly lower values were reported by Cesário et al. [14] at the Tagus estuary (2.1–5.5) and by Gosnell et al. [73] at the Delaware River estuary (3.4–5.1).
The highest logKD values were reached by Fe and Mn, especially in autumn when the river flow was highest, confirming their affinity for SPM which was often the main vehicle for trace elements from inland areas to estuaries and coastal environments [85,86]. Although significant disparities in the logKD values were not observed among the different sampling campaigns, a clear relationship describes the partitioning behaviour of Fe and Mn which appeared to be governed by the partially mixed estuarine circulation at the Nalón River estuary and, as a result, by the increase in the SPM content from low to high river flow (Figure S3). Adsorption on the surface of the suspended particles as well as precipitation of Fe and Mn oxy-hydroxides promoted by the oxidative conditions along the water column (Eh always >400 mV) appeared to be responsible for the increase in PFe and PMn from low (summer) to high (autumn) river flow (Figure S3). This was consistent with the behaviour of Fe and Mn in the dissolved fraction since the lowest DFe and DMn concentrations were observed when the river flow was high most likely due to stronger dilution effects.
4. Conclusions
The occurrence of Hg and MeHg at the Nalón River estuary still represents an issue of environmental concern due to the legacy of long-term mining activity along the river drainage basin.
The interaction between the river freshwater inputs and the tidal currents, as well as the relatively shallow water depth, appeared to be the primary environmental agents regulating the distribution of both dissolved and particulate Hg and MeHg.
The estuary was dominated by oxidative conditions along the water column which promoted the precipitation and/or adsorption processes responsible for Hg and MeHg preferential partitioning in the suspended particles. However, the partitioning behaviour of Hg and MeHg was also affected by different seasonal hydrodynamic conditions. Indeed, results suggested that the dissolved fraction commonly prevailed under conditions of low river discharge (summer), whereas the particulate fraction dominated the water column when the river flow was high (autumn). Particulate MeHg was found to be the only exception since the effects of the tidal currents, which were prevailing when the river discharge was low, promoted resuspension of MeHg enriched particles from bottom sediment thus increasing PMeHg concentrations in the lower sector of the estuary and at the estuary mouth.
Although resuspension may increase the mobility of MeHg at the estuary mouth, its concentration both in the dissolved and the SPM was found to be extremely low with respect to the total amount of Hg thus suggesting a minimal risk of MeHg bioaccumulation in the aquatic trophic chain. Moreover, organic carbon was found to be one of the main factors governing MeHg distribution and fate at the Nalón River estuary. This is of relevant concern since complexation with organic matter appeared to play a large role in inhibiting MeHg mobility and potential bioaccumulation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11104396/s1, Figure S1: Grain size spectra of SPM along the water column at the seven investigated sites (from site 6 to site 0) along the Nalón River estuary in spring, Figure S2: Minimum (yellow circle), maximum (black square) and average (red triangle) values of DHg, DMeHg, DFe, DMn, DOC, PHg, PMeHg, PFe, PMn and POC in freshwater, brackish water and saltwater collected along the Nalón River estuary under different seasonal conditions (spring, summer and autumn), Figure S3: Relationship between logKD and dissolved Fe and Mn (A, B) as well as between logKD and particulate Fe and Mn (C, D) along the water column at the Nalón River estuary under different seasonal conditions (spring, summer and autumn), Table S1: Physico-chemical parameters observed along the water column at the Nalón River estuary under different seasonal conditions (spring, summer and autumn), Table S2: Concentrations of PHg, PMeHg, PFe and PMn along the water column at the Nalón River estuary under different seasonal conditions (spring, summer and autumn).
Author Contributions
Conceptualisation, data curation, writing—original draft preparation, visualization E.P., S.C. and E.G.-O. Methodology, analyses, data survey S.C., E.G.-O., P.C. and N.R. Data processing, software E.P. Project administration, funding acquisition, supervision E.G.-O. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-supported by the Spanish Ministry of Economy, Industry and Competitiveness through the Research Projects METRAMER (grant number: MINECO-13-CGL2013-44980-R) and ECOMER (grant number: MINECO-18-CGL2017-84268-R) and the Asturias Ministry of Education and Science (grant number: FC-15-GRUPIN14-067).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Karry Close is warmly acknowledged for proofreading the manuscript. The anonymous reviewers are greatly acknowledged for their critical reviews and useful suggestions, which improved the quality of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cossa, D.; Elbaz-Poulichet, F.; Nieto, J.M. Mercury in the Tinto-Odiel Estuarine System (Gulf of Cádiz, Spain): Sources and Dispersion. Aquat. Geochem. 2001, 7, 1–12. [Google Scholar] [CrossRef]
- Balcom, P.H.; Hammerschmidt, C.R.; Fitzgerald, W.F.; Lamborg, C.H.; O’Connor, J.S. Seasonal Distributions and Cycling of Mercury and Methylmercury in the Waters of New York/New Jersey Harbor Estuary. Mar. Chem. 2008, 109, 1–17. [Google Scholar] [CrossRef]
- Schäfer, J.; Castelle, S.; Blanc, G.; Dabrin, A.; Masson, M.; Lanceleur, L.; Bossy, C. Mercury Methylation in the Sediments of a Macrotidal Estuary (Gironde Estuary, South-West France). Estuar. Coast. Shelf Sci. 2010, 90, 80–92. [Google Scholar] [CrossRef]
- Mao, L.; Liu, X.; Wang, B.; Lin, C.; Xin, M.; Zhang, B.-T.; Wu, T.; He, M.; Ouyang, W. Occurrence and Risk Assessment of Total Mercury and Methylmercury in Surface Seawater and Sediments from the Jiaozhou Bay, Yellow Sea. Sci. Total Environ. 2020, 714, 136539. [Google Scholar] [CrossRef]
- Covelli, S.; Petranich, E.; Pavoni, E.; Signore, S. Can Sediments Contaminated by Mining Be a Source of Mercury in the Coastal Environment Due to Dredging? Evidence from Thermo-Desorption and Chemical Speciation. Bull. Environ. Contam. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Cech, J.J.; Lagunas-Solar, M.C. Bioavailability of Methylmercury to Sacramento Blackfish (Orthodon Microlepidotus): Dissolved Organic Carbon Effects. Environ. Toxicol. Chem. 1998, 17, 695–701. [Google Scholar] [CrossRef]
- Bocchetti, R.; Fattorini, D.; Pisanelli, B.; Macchia, S.; Oliviero, L.; Pilato, F.; Pellegrini, D.; Regoli, F. Contaminant Accumulation and Biomarker Responses in Caged Mussels, Mytilus Galloprovincialis, to Evaluate Bioavailability and Toxicological Effects of Remobilized Chemicals during Dredging and Disposal Operations in Harbour Areas. Aquat. Toxicol. 2008, 89, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Covelli, S.; Acquavita, A.; Piani, R.; Predonzani, S.; De Vittor, C. Recent Contamination of Mercury in an Estuarine Environment (Marano Lagoon, Northern Adriatic, Italy). Estuar. Coast. Shelf Sci. 2009, 82, 273–284. [Google Scholar] [CrossRef]
- Cusack, M.; Arrieta, J.M.; Duarte, C.M. Source Apportionment and Elemental Composition of Atmospheric Total Suspended Particulates (TSP) Over the Red Sea Coast of Saudi Arabia. Earth Syst. Environ. 2020, 4, 777–788. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms Regulating Mercury Bioavailability for Methylating Microorganisms in the Aquatic Environment: A Critical Review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef] [PubMed]
- Cesário, R.; Monteiro, C.E.; Nogueira, M.; O’Driscoll, N.J.; Caetano, M.; Hintelmann, H.; Mota, A.M.; Canário, J. Mercury and Methylmercury Dynamics in Sediments on a Protected Area of Tagus Estuary (Portugal). Water Air Soil Pollut. 2016, 227, 475. [Google Scholar] [CrossRef]
- Cesário, R.; Hintelmann, H.; O’Driscoll, N.J.; Monteiro, C.E.; Caetano, M.; Nogueira, M.; Mota, A.M.; Canário, J. Biogeochemical Cycle of Mercury and Methylmercury in Two Highly Contaminated Areas of Tagus Estuary (Portugal). Water Air Soil Pollut. 2017, 228, 257. [Google Scholar] [CrossRef]
- Petranich, E.; Croce, S.; Crosera, M.; Pavoni, E.; Faganeli, J.; Adami, G.; Covelli, S. Mobility of Metal(Loid)s at the Sediment-Water Interface in Two Tourist Port Areas of the Gulf of Trieste (Northern Adriatic Sea). Environ. Sci. Pollut. Res. 2018, 25, 26887–26902. [Google Scholar] [CrossRef] [PubMed]
- Cesário, R.; Mota, A.M.; Caetano, M.; Nogueira, M.; Canário, J. Mercury and Methylmercury Transport and Fate in the Water Column of Tagus Estuary (Portugal). Mar. Pollut. Bull. 2018, 127, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Sanford, L.P.; Panageotou, W.; Halka, J.P. Tidal Resuspension of Sediments in Northern Chesapeake Bay. Mar. Geol. 1991, 97, 87–103. [Google Scholar] [CrossRef]
- Arfi, R.; Guiral, D.; Bouvy, M. Wind Induced Resuspension in a Shallow Tropical Lagoon. Estuar. Coast. Shelf Sci. 1993, 36, 587–604. [Google Scholar] [CrossRef]
- Kalnejais, L.H.; Martin, W.R.; Signell, R.P.; Bothner, M.H. Role of Sediment Resuspension in the Remobilization of Particulate-Phase Metals from Coastal Sediments. Environ. Sci. Technol. 2007, 41, 2282–2288. [Google Scholar] [CrossRef]
- Schoellhamer, D.H. Anthropogenic Sediment Resuspension Mechanisms in a Shallow Microtidal Estuary. Estuar. Coast. Shelf Sci. 1996, 43, 533–548. [Google Scholar] [CrossRef]
- Bloom, N.S.; Lasorsa, B.K. Changes in Mercury Speciation and the Release of Methyl Mercury as a Result of Marine Sediment Dredging Activities. Sci. Total Environ. 1999, 237–238, 379–385. [Google Scholar] [CrossRef]
- Lewis, M.A.; Weber, D.E.; Stanley, R.S.; Moore, J.C. Dredging Impact on an Urbanized Florida Bayou: Effects on Benthos and Algal-Periphyton. Environ. Pollut. 2001, 115, 161–171. [Google Scholar] [CrossRef]
- Moog, O.; Stubauer, I.; Haimann, M.; Habersack, H.; Leitner, P. Effects of Harbour Excavating and Dredged Sediment Disposal on the Benthic Invertebrate Fauna of River Danube (Austria). Hydrobiologia 2018, 814, 109–120. [Google Scholar] [CrossRef]
- Kim, E.H.; Mason, R.P.; Porter, E.T.; Soulen, H.L. The Effect of Resuspension on the Fate of Total Mercury and Methyl Mercury in a Shallow Estuarine Ecosystem: A Mesocosm Study. Mar. Chem. 2004, 86, 121–137. [Google Scholar] [CrossRef]
- Ordóñez, A.; Álvarez, R.; Loredo, J. Asturian Mercury Mining District (Spain) and the Environment: A Review. Environ. Sci. Pollut. Res. 2013, 20, 7490–7508. [Google Scholar] [CrossRef]
- Silva, V.; Loredo, J.; Fernández-Martínez, R.; Larios, R.; Ordóñez, A.; Gómez, B.; Rucandio, I. Arsenic Partitioning among Particle-Size Fractions of Mine Wastes and Stream Sediments from Cinnabar Mining Districts. Environ. Geochem. Health 2014, 36, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Loredo, J.; Ordóñez, A.; Gallego, J.R.; Baldo, C.; García-Iglesias, J. Geochemical Characterisation of Mercury Mining Spoil Heaps in the Area of Mieres (Asturias, Northern Spain). J. Geochem. Explor. 1999, 67, 377–390. [Google Scholar] [CrossRef]
- Loredo, J.; Ordóñez, A.; Pendás, F. Hydrogeological and Geochemical Interactions of Adjoining Mercury and Coal Mine Spoil Heaps in the Morgao Catchment (Mieres, North-Western Spain). Geol. Soc. Spec. Publ. 2002, 198, 327–336. [Google Scholar] [CrossRef]
- Loredo, J.; Alvarez, R.; Ordonez, A. Release of Toxic Metals and Metalloids from Los Rueldos Mercury Mine (Asturias, Spain). Sci. Total Environ. 2005, 340, 247–260. [Google Scholar] [CrossRef]
- Loredo, J.; Ordonez, A.; Alvarez, R. Environmental Impact of Toxic Metals and Metalloids from the Muñón Cimero Mercury-Mining Area (Asturias, Spain). J. Hazard. Mater. 2006, 136, 455–467. [Google Scholar] [CrossRef]
- Fernández-Martínez, R.; Loredo, J.; Ordóñez, A.; Rucandio, M.I. Distribution and Mobility of Mercury in Soils from an Old Mining Area in Mieres, Asturias (Spain). Sci. Total Environ. 2005, 346, 200–212. [Google Scholar] [CrossRef]
- Fernández-Martínez, R.; Loredo, J.; Ordóñez, A.; Rucandio, M.I. Physicochemical Characterization and Mercury Speciation of Particle-Size Soil Fractions from an Abandoned Mining Area in Mieres, Asturias (Spain). Environ. Pollut. 2006, 142, 217–226. [Google Scholar] [CrossRef]
- Loredo, J. Historic Unreclaimed Mercury Mines in Asturias (Northwestern Spain): Environmental Approaches. In Assessing and Managing Mercury from Historic and Current Mining Activities; U.S. Environmental Protection Agency: San Francisco, CA, USA, 2000; pp. 175–180. [Google Scholar]
- Loredo, J.; Ordóñez, A.; Álvarez, R. The Problem of Hg Contamination in Asturias (Spain). RMZ Mater. Geoenviron. 2004, 51, 133–136. [Google Scholar]
- Garcia-Ordiales, E.; Covelli, S.; Rico, J.M.; Roqueñí, N.; Fontolan, G.; Flor-Blanco, G.; Cienfuegos, P.; Loredo, J. Occurrence and Speciation of Arsenic and Mercury in Estuarine Sediments Affected by Mining Activities (Asturias, Northern Spain). Chemosphere 2018, 198, 281–289. [Google Scholar] [CrossRef]
- Garcia-Ordiales, E.; Cienfuegos, P.; Roqueñí, N.; Covelli, S.; Flor-Blanco, G.; Fontolan, G.; Loredo, J. Historical Accumulation of Potentially Toxic Trace Elements Resulting from Mining Activities in Estuarine Salt Marshes Sediments of the Asturias Coastline (Northern Spain). Environ. Sci. Pollut. Res. 2019, 26, 3115–3128. [Google Scholar] [CrossRef]
- García-Ordiales, E.; Flor-Blanco, G.; Roqueñí, N.; Covelli, S.; Cienfuegos, P.; Álvarez, R.; Fontolan, G.; Loredo, J. Anthropocene Footprint in the Nalón Estuarine Sediments (Northern Spain). Mar. Geol. 2020, 424, 106167. [Google Scholar] [CrossRef]
- Garcia-Ordiales, E.; Roqueñí, N.; Loredo, J. Mercury Bioaccumulation by Juncus Maritimus Grown in a Hg Contaminated Salt Marsh (Northern Spain). Mar. Chem. 2020, 226, 103859. [Google Scholar] [CrossRef]
- García-Ordiales, E.; Covelli, S.; Braidotti, G.; Petranich, E.; Pavoni, E.; Acquavita, A.; Sanz-Prada, L.; Roqueñí, N.; Loredo, J. Mercury and Arsenic Mobility in Resuspended Contaminated Estuarine Sediments (Asturias, Spain): A Laboratory-Based Study. Sci. Total Environ. 2020, 744, 140870. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. Water Framework Directive; 2000/60/EC; European Union: Brussels, Belgium, 2000. [Google Scholar]
- European Parliament, Council of the European Union. Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive); 2008/56/EC; European Union: Brussels, Belgium, 2008. [Google Scholar]
- Ceñal, R.C.; Flor, G. Evolución Reciente Del Estuario Del Nalón (Asturias). Cuaternario Geomorfol. 1993, 7, 23–34. [Google Scholar]
- Flor, G.; Ceñal, R.C.; González, M.S.; Ortega, M.I. Aspectos Morfológicos, Dinámicos y Sedimentológicos Del Estuario Del Nalón (Asturias, Noroeste de España). Trab. Geol. 1998, 20, 3–39. [Google Scholar]
- Flor-Blanco, G.; Pando, L.; Morales, J.A.; Flor, G. Evolution of Beach–Dune Fields Systems Following the Construction of Jetties in Estuarine Mouths (Cantabrian Coast, NW Spain). Environ. Earth Sci. 2015, 73, 1317–1330. [Google Scholar] [CrossRef]
- Loredo, J.; Pereira, A.; Ordóñez, A. Untreated Abandoned Mercury Mining Works in a Scenic Area of Asturias (Spain). Environ. Int. 2003, 29, 481–491. [Google Scholar] [CrossRef]
- Ordoñez, A.; Silva, V.; Galán, P.; Loredo, J.; Rucandio, I. Arsenic Input into the Catchment of the River Caudal (Northwestern Spain) from Abandoned Hg Mining Works: Effect on Water Quality. Environ. Geochem. Health 2014, 36, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Amouroux, D.; Tessier, E.; Pécheyran, C.; Donard, O.F.X. Sampling and Probing Volatile Metal(Loid) Species in Natural Waters by in-Situ Purge and Cryogenic Trapping Followed by Gas Chromatography and Inductively Coupled Plasma Mass Spectrometry (P-CT–GC–ICP/MS). Anal. Chim. Acta 1998, 377, 241–254. [Google Scholar] [CrossRef]
- Rodríguez-González, P.; Ruiz Encinar, J.; García Alonso, J.I.; Sanz-Medel, A. Determination of Butyltin Compounds in Coastal Sea-Water Samples Using Isotope Dilution GC-ICP-MS. J. Anal. At. Spectrom. 2002, 17, 824–830. [Google Scholar] [CrossRef]
- Bouchet, S.; Tessier, E.; Monperrus, M.; Bridou, R.; Clavier, J.; Thouzeau, G.; Amouroux, D. Measurements of Gaseous Mercury Exchanges at the Sediment–Water, Water–Atmosphere and Sediment–Atmosphere Interfaces of a Tidal Environment (Arcachon Bay, France). J. Environ. Monit. 2011, 13, 1351–1359. [Google Scholar] [CrossRef]
- Rodriguez Martin-Doimeadios, R.C.; Monperrus, M.; Krupp, E.; Amouroux, D.; Donard, O.F.X. Using Speciated Isotope Dilution with GC− Inductively Coupled Plasma MS to Determine and Unravel the Artificial Formation of Monomethylmercury in Certified Reference Sediments. Anal. Chem. 2003, 75, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, P.; Monperrus, M.; García Alonso, J.I.; Amouroux, D.; Donard, O.F.X. Comparison of Different Numerical Approaches for Multiple Spiking Species-Specific Isotope Dilution Analysis Exemplified by the Determination of Butyltin Species in Sediments. J. Anal. At. Spectrom. 2007, 22, 1373–1382. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, P.; Bouchet, S.; Monperrus, M.; Tessier, E.; Amouroux, D. In Situ Experiments for Element Species-Specific Environmental Reactivity of Tin and Mercury Compounds Using Isotopic Tracers and Multiple Linear Regression. Environ. Sci. Pollut. Res. 2013, 20, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- EPA. EPA Method 3052. Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; U.S. Environmental Protection Agency: Washington, DC, USA, 1996.
- Smilde, A.; Bro, R.; Geladi, P. Multi-Way Analysis with Applications in the Chemical Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0-470-01211-0. [Google Scholar]
- Oliveri, P.; Malegori, C.; Casale, M. Chemometrics: Multivariate Analysis of Chemical Data. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-813266-1. [Google Scholar]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). 2019. Available online: http://Gruppochemiometria.It/Index.Php/Software (accessed on 3 September 2019).
- Akter, A.; Tanim, A.H. Salinity Distribution in River Network of a Partially Mixed Estuary. J. Waterw. Port. C 2021, 147, 04020055. [Google Scholar] [CrossRef]
- Covelli, S.; Piani, R.; Kotnik, J.; Horvat, M.; Faganeli, J.; Brambati, A. Behaviour of Hg Species in a Microtidal Deltaic System: The Isonzo River Mouth (Northern Adriatic Sea). Sci. Total Environ. 2006, 368, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, E.; Crosera, M.; Petranich, E.; Adami, G.; Faganeli, J.; Covelli, S. Partitioning and Mixing Behaviour of Trace Elements at the Isonzo/Soča River Mouth (Gulf of Trieste, Northern Adriatic Sea). Mar. Chem. 2020, 223, 103800. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Caetano, M.; Brito, P.; Canario, J.; Vale, C. The Relevance of Defining Trace Metal Baselines in Coastal Waters at a Regional Scale: The Case of the Portuguese Coast (SW Europe). Mar. Environ. Res. 2012, 79, 86–99. [Google Scholar] [CrossRef]
- Loredo, J.; Petit-Domínguez, M.D.; Fernández-Martínez, R.; Alvarez, R.; Rucandio, M.I. Surface Water Monitoring in the Mercury Mining District of Asturias (Spain). J. Hazard. Mater. 2010, 176, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Seelen, E.A.; Massey, G.M.; Mason, R.P. Role of Sediment Resuspension on Estuarine Suspended Particulate Mercury Dynamics. Environ. Sci. Technol. 2018, 52, 7736–7744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Song, Y.; Adediran, G.A.; Jiang, T.; Reis, A.T.; Pereira, E.; Skyllberg, U.; Björn, E. Mercury Transformations in Resuspended Contaminated Sediment Controlled by Redox Conditions, Chemical Speciation and Sources of Organic Matter. Geochim. Cosmochim. Acta 2018, 220, 158–179. [Google Scholar] [CrossRef]
- Wang, S.; Jia, Y.; Wang, S.; Wang, X.; Wang, H.; Zhao, Z.; Liu, B. Total Mercury and Monomethylmercury in Water, Sediments, and Hydrophytes from the Rivers, Estuary, and Bay along the Bohai Sea Coast, Northeastern China. Appl. Geochem. 2009, 24, 1702–1711. [Google Scholar] [CrossRef]
- Taylor, V.F.; Buckman, K.L.; Seelen, E.A.; Mazrui, N.M.; Balcom, P.H.; Mason, R.P.; Chen, C.Y. Organic Carbon Content Drives Methylmercury Levels in the Water Column and in Estuarine Food Webs across Latitudes in the Northeast United States. Environ. Pollut. 2019, 246, 639–649. [Google Scholar] [CrossRef]
- Kim, E.-H.; Mason, R.P.; Porter, E.T.; Soulen, H.L. The Impact of Resuspension on Sediment Mercury Dynamics, and Methylmercury Production and Fate: A Mesocosm Study. Mar. Chem. 2006, 102, 300–315. [Google Scholar] [CrossRef]
- Mason, R.P.; Benoit, J.M. Organomercury compounds in the environment. In Organometallics in the Environment; Craig, P., Ed.; John Wiley & Sons: New York, NY, USA, 2003; pp. 57–99. [Google Scholar]
- Heyes, A.; Mason, R.P.; Kim, E.H.; Sunderland, E. Mercury Methylation in Estuaries: Insights from Using Measuring Rates Using Stable Mercury Isotopes. Mar. Chem. 2006, 102, 134–147. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury Contamination of Coastal Sediments as the Result of Long-Term Cinnabar Mining Activity (Gulf of Trieste, Northern Adriatic Sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Acquavita, A.; Emili, A.; Covelli, S.; Faganeli, J.; Predonzani, S.; Koron, N.; Carrasco, L. The Effects of Resuspension on the Fate of Hg in Contaminated Sediments (Marano and Grado Lagoon, Italy): Short-Term Simulation Experiments. Estuar. Coast. Shelf Sci. 2012, 113, 32–40. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Ravichandran, M. Interactions between Mercury and Dissolved Organic Matter—A Review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Whalin, L.; Kim, E.-H.; Mason, R. Factors Influencing the Oxidation, Reduction, Methylation and Demethylation of Mercury Species in Coastal Waters. Mar. Chem. 2007, 107, 278–294. [Google Scholar] [CrossRef]
- Hines, M.E.; Faganeli, J.; Adatto, I.; Horvat, M. Microbial Mercury Transformations in Marine, Estuarine and Freshwater Sediment Downstream of the Idrija Mercury Mine, Slovenia. Appl. Geochem. 2006, 21, 1924–1939. [Google Scholar] [CrossRef]
- Gosnell, K.; Balcom, P.; Ortiz, V.; DiMento, B.; Schartup, A.; Greene, R.; Mason, R. Seasonal Cycling and Transport of Mercury and Methylmercury in the Turbidity Maximum of the Delaware Estuary. Aquat. Geochem. 2016, 22, 313–336. [Google Scholar] [CrossRef]
- Heyes, A.; Miller, C.; Mason, R.P. Mercury and Methylmercury in Hudson River Sediment: Impact of Tidal Resuspension on Partitioning and Methylation. Mar. Chem. 2004, 90, 75–89. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Blette, V.; Yan, C.; Schofield, C.L.; Munson, R.; Holsapple, J. The Role of Dissolved Organic Carbon in the Chemistry and Bioavailability of Mercury in Remote Adirondack Lakes. Water Air Soil Pollut. 1995, 80, 499–508. [Google Scholar] [CrossRef]
- Luengen, A.C.; Fisher, N.S.; Bergamaschi, B.A. Dissolved Organic Matter Reduces Algal Accumulation of Methylmercury. Environ. Toxicol. Chem. 2012, 31, 1712–1719. [Google Scholar] [CrossRef]
- Lee, C.S.; Fisher, N.S. Bioaccumulation of Methylmercury in a Marine Diatom and the Influence of Dissolved Organic Matter. Mar. Chem. 2017, 197, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Turner, A. Diagnosis of Chemical Reactivity and Pollution Sources from Particulate Trace Metal Distributions in Estuaries. Estuar. Coast. Shelf Sci. 1999, 48, 177–191. [Google Scholar] [CrossRef]
- Hatje, V.; Birch, G.F.; Hill, D.M. Spatial and Temporal Variability of Particulate Trace Metals in Port Jackson Estuary, Australia. Estuar. Coast. Shelf Sci. 2001, 53, 63–77. [Google Scholar] [CrossRef]
- Turner, A. Trace-Metal Partitioning in Estuaries: Importance of Salinity and Particle Concentration. Mar. Chem. 1996, 54, 27–39. [Google Scholar] [CrossRef]
- EPA. Understanding Variation in Partition Coefficient, Kd, Values; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- Gagnon, C.; Saulnier, I. Distribution and Fate of Metals in the Dispersion Plume of a Major Municipal Effluent. Environ. Pollut. 2003, 124, 47–55. [Google Scholar] [CrossRef]
- La Colla, N.S.; Negrin, V.L.; Marcovecchio, J.E.; Botté, S.E. Dissolved and Particulate Metals Dynamics in a Human Impacted Estuary from the SW Atlantic. Estuar. Coast. Shelf Sci. 2015, 166, 45–55. [Google Scholar] [CrossRef]
- Stoichev, T.; Amouroux, D.; Monperrus, M.; Point, D.; Tessier, E.; Bareille, G.; Donard, O.F.X. Mercury in Surface Waters of a Macrotidal Urban Estuary (River Adour, South-West France). Chem. Ecol. 2006, 22, 137–148. [Google Scholar] [CrossRef]
- Turner, A.; Millward, G.E. Particle Dynamics and Trace Metal Reactivity in Estuarine Plumes. Estuar. Coast. Shelf Sci. 2000, 50, 761–774. [Google Scholar] [CrossRef]
- Vignati, D.; Dominik, J. The Role of Coarse Colloids as a Carrier Phase for Trace Metals in Riverine Systems. Aquat. Sci. 2003, 65, 129–142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).