Radiation-Assisted Synthesis of Polymer-Based Nanomaterials
Abstract
:1. Introduction
- 1.
- Carbon-based nanomaterials;
- 2.
- Metal-based nanomaterials;
- 3.
- Polymer-based nanomaterials;
- 4.
- Composite nanomaterials.
2. Carbon-Based Nanomaterials
3. Metal-Based Nanomaterials
3.1. Polymer–Nanometal Composites
3.2. Quantum Dots
4. Polymer-Based Nanomaterials
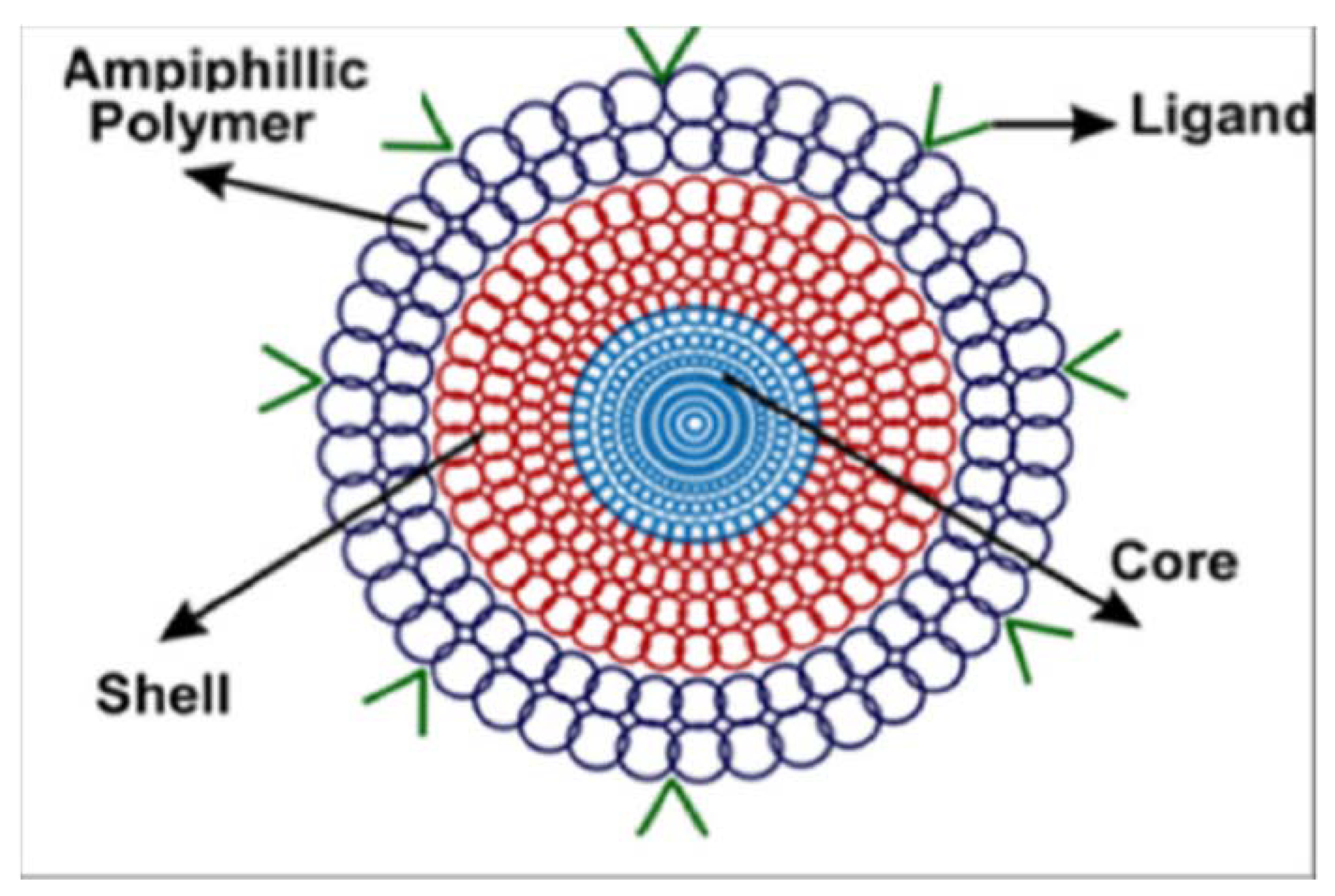
4.1. Polymer Nanoparticles
4.2. Protein-Based Nanoparticles
4.3. Nanogels
- PVP nanogels
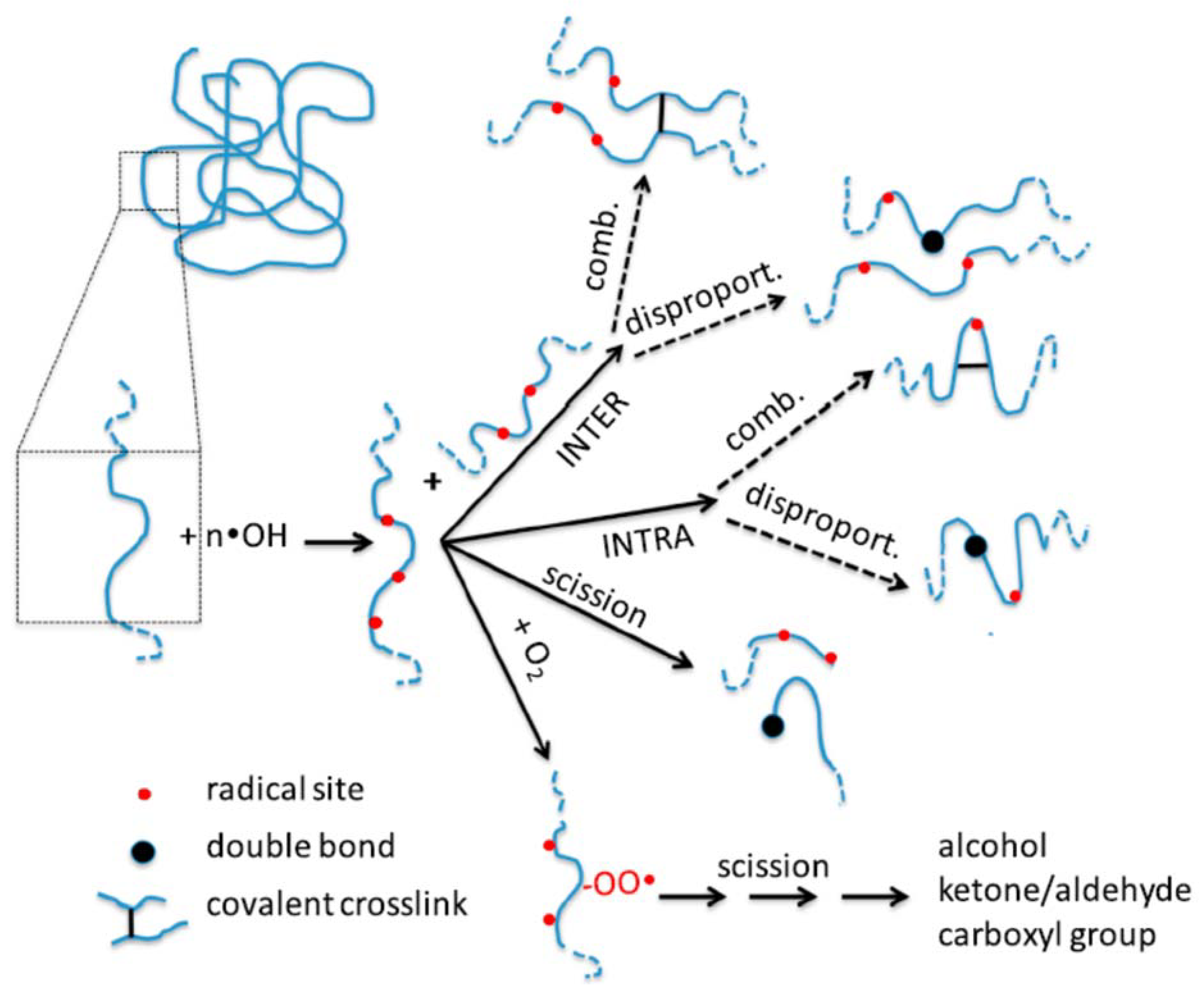
- PAA nanogels
- Synthesis of nanogels from interpolymer complexes
5. Composite Nanomaterials, Polymer Nanocomposites
6. Controlling Radiation-Induced Grafting on Nanoscale
6.1. Advanced Functional Track-Etched Membranes
6.2. Fuel Cell Membranes
6.3. Cell Sheet Harvesting
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Request
Conflicts of Interest
References
- Taniguchi, N. On the basic concept of ‘Nano-Technology’. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974; Japan Society of Precision Engineering: Tokyo, Japan, 1974; pp. 18–23. [Google Scholar]
- Huth, M. Radiation-induced nanostructures: Formation processes and applications. Bellstein J. Nanotechnol. 2012, 3, 533–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güven, O. Ionizing radiation: A versatile tool for nanostructuring of polymers. Pure Appl. Chem. 2016, 88, 1049–1061. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakan, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Saif, M.J.; Naveed, M.; Asif, H.M.; Akhtar, R. Irradiation applications for polymer nanocomposites: A state-of-the-art review. J. Ind. Eng. Chem. 2018, 60, 218–236. [Google Scholar] [CrossRef]
- Chung, D.J.; Seong, M.K.; Choi, S.H. Radiolytic sythesis of –OH group functionalized fullerene structures for biosensor applications. J. Appl. Polym. Scien. 2011, 122, 1785–1791. [Google Scholar] [CrossRef]
- Chen, S.; Wu, G.; Li, Y.; Long, D. Preparation of PAA grafted MWCNTs by a two-step irradiation technique. Macromolecules 2006, 39, 330–334. [Google Scholar] [CrossRef]
- Jung, C.H.; Hwang, I.T.; Sohn, J.Y.; Shin, J. Preparation and characterization of solution-processable polymer-grafted reduced graphene oxide by radiation technology. Radiat. Phys. Chem. 2020, 166, 108504. [Google Scholar] [CrossRef]
- Abd El-Rehim, H.A.; Tartour, A.R. Green synthesis of water-dispersed graphene nanosheets using gamma irradiation and natural capping agents. Radiat. Phys. Chem. 2018, 153, 208–213. [Google Scholar] [CrossRef]
- Yang, D.-S.; Jung, D.-J.; Choi, S.-H. One-step functionalization of MWCNTs by radiation-induced graft polymerization and their applications as enzyme-free biosensors. Radiat. Phys. Chem. 2010, 79, 434–440. [Google Scholar] [CrossRef]
- Li, B.; Zhou, L.I.; Hua, L.U. Radiation curing behavior of MWCNTs. J. Func. Polym. 2009, 2, 159–165. [Google Scholar]
- Ngahdi, M.; Aheran, M.; Brar, S.K.; Verma, M.; Surampulli, R.Y.; Valero, J.R. Green and energy-efficient methods for the production of metallic nanoparticles. Bellstein J. Nanotechnol. 2015, 6, 2354–2376. [Google Scholar]
- Belloni, J.; Marignier, J.-L.; Mostafavi, M. Mechanisms of metal nanoparticles nucleation and growth studied by radiolysis. Radiat. Phys. Chem. 2020, 169, 107952. [Google Scholar] [CrossRef]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemical study and application to catalysis. Cat. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Bakar, A.; Güven, O.; Zezin, A.A.; Feldman, V.I. Controlling the size and distribution of copper nanoparticles in double and triple polymer metal complexes by X-ray irradiation. Radiat. Phys. Chem. 2014, 94, 62–66. [Google Scholar] [CrossRef]
- Zezina, E.A.; Emelyanov, A.I.; Pozdnyakov, A.S.; Prozorova, G.F.; Abramchuk, S.S.; Feldman, V.I.; Zezin, A.A. Radiation-induced synthesis of copper nanoparticles in the films of interpolymer complexes. Radiat. Phys. Chem. 2019, 158, 115–121. [Google Scholar] [CrossRef]
- Klimov, D.I.; Zezina, E.A.; Zezin, S.B.; Yang, M.; Weng, F.; Shvedinov, V.I.; Feldman, V.I.; Zezin, A.A. Radiation-induced preparation of bimetallic nanoparticles in the films of interpolymer complexes. Radiat. Phys. Chem. 2018, 142, 65–69. [Google Scholar] [CrossRef]
- Cubova, K.; Cuba, V. Synthesis of inorganic nanoparticles by irradiation—A review. Radiat. Phys. Chem. 2020, 169, 108774. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Scien. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Kang, B.; Chang, S.Q.; Dai, Y.D.; Chen, D. Synthesis of green CdSe/chitosan quantum dots using a polymer-assisted gamma radiation route. Radiat. Phys. Chem. 2008, 77, 859–863. [Google Scholar] [CrossRef]
- Biswal, J.; Singh, S.; Rath, M.C.; Ramnani, S.P.; Sarkar, S.K.; Sabharwal, S. Synthesis of CdSe quantum dots in PVA matrix by radiolytic methods. Int. J. Nanotechnol. 2010, 7, 9–12. [Google Scholar] [CrossRef]
- Singh, A.; Guleria, A.; Kunwar, A.; Neogy, S.; Rath, M.C. Saccharide capped CdSe quantum dots grown via e-beam irradiation. Mater. Chem. Phys. 2017, 199, 609–615. [Google Scholar] [CrossRef]
- Li, Z.; Peng, L.; Fang, Y.; Chen, Z.; Pan, D.; Nu, M. Synthesis of colloidal SnSe quantum dots by E-beam irradiation. Radiat. Phys. Chem. 2011, 80, 1333–1336. [Google Scholar] [CrossRef]
- Rao, Y.N.; Datta, A.; Das, S.K.; Saha, A. Irradiation route to aqueous synthesis of highly luminescent ZnSe quantum dots. Mater. Res. Bull. 2016, 80, 180–187. [Google Scholar] [CrossRef]
- Bekasova, O.D.; Revina, A.A.; Rusanov, A.L.; Kornienko, E.S.; Kurganov, B.I. Effect of gamma irradiation on the size and properties of CdS quantum dots in reverse micelles. Radiat. Phys. Chem. 2013, 92, 87–92. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Pan, D.; Wu, M. Radiation-induced synthesis of graphene quantum dots via E-beam irradiation and their application in cell imaging. Chem. Eng. J. 2017, 309, 374–380. [Google Scholar] [CrossRef]
- Wang, M.; Ge, X.; Zhang, Z. Radiation Emulsion Polymerization. In Radiation Technology for Advanced Materials; Wu, G., Zhai, M., Wang, M., Eds.; Jiao Tong University Press: Shanghai, China, 2019; pp. 183–205. [Google Scholar]
- Nagarajan, R.; Ganesh, K. Block copolymers self-assembly in selective solvents. Macromolecules 1989, 22, 4312–4325. [Google Scholar] [CrossRef]
- Zhang, G.; Niu, A.; Peng, S.; Jiang, M.; Tu, Y.; Li, M.; Wu, C. Formation of novel polymeric nanoparticles. Acc. Chem. Res. 2001, 34, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Ge, X.; Zhang, Z. Formation of monodisperse PMMA particles by radiation-induced dispersion polymerization. Radiat. Phys. Chem. 2003, 66, 11–16. [Google Scholar] [CrossRef]
- Ikeda, S.; Tabata, Y.; Suzuki, H.; Miyoshi, T.; Kudo, H.; Katsumura, Y. Radiation-induced synthesis of low molecular weight PTFE and their crosslinking in acetone medium. Radiat. Phys. Chem. 2008, 77, 1050–1056. [Google Scholar] [CrossRef]
- Pasanphan, W.; Rattanawongwiboon, T.; Choofong, S.; Güven, O.; Katti, K.K. Irradiated chitosan nanoparticle as water based antioxidant and reducing agent for green synthesis of gold nanoplatforms. Radiat. Phys. Chem. 2015, 106, 360–370. [Google Scholar] [CrossRef]
- Espinoza, S.C.S.; Sanchez, M.L.; Risso, V.; Smolko, E.E.; Graselli, M. Radiation synthesis of seroalbumin nanoparticles. Radiat. Phys. Chem. 2012, 81, 1417–1421. [Google Scholar] [CrossRef]
- Queiroz, R.G.; Varca, G.H.C.; Kadlubowski, S.; Ulanski, P.; Lugao, A.B. Radiation-synthesized protein-based drug carriers: Size controlled BSA nanoparticles. Int. J. Biol. Macromol. 2016, 85, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fazolin, G.N.; Varca, G.H.C.; de Freitas, L.F.; Rokita, B.; Kadlubowski, S.; Lugao, A.B. Simultaneous intramolecular crossslinking and sterilization of papain nanoparticles by gamma radiation. Radiat. Phys. Chem. 2020, 171, 108697. [Google Scholar] [CrossRef]
- Kimura, A.; Ueno, M.; Arai, T.; Oyama, K.; Taguchi, M. Radiation crosslinked smart peptide nanoparticles: A new platform for tumor imaging. Nanomaterials 2021, 11, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks, infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulanski, P.; Janik, I.; Rosiak, J.M. Radiation formation of polymeric nanogels. Radiat. Phys. Chem. 1998, 52, 289–294. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M. The use of radiation technique in the synthesis of polymeric nanogels. Nucl. Instrum. Methods Phys. Res. B 1999, 15, 356–360. [Google Scholar] [CrossRef]
- Galia, A.; Lanzalaco, S.; Sabatino, M.A.; Dispenza, C.; Scialdone, O.; Sires, I. Crosslinking of PVP activated by electrogenerated hydroxyl radicals. Electrochem. Commun. 2016, 62, 64–68. [Google Scholar] [CrossRef]
- Barros, J.A.G.; Fechine, G.J.M.; Alcantra, M.R.; Catalani, L.H. PVP hydrogels produced by Fenton reaction. Polymer 2006, 47, 8414–8419. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hypertermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Sütekin, S.D.; Güven, O.; Şahiner, N. Nanogel Synthesis by Irradiation of Aqueous Polymer Solutions. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer-Nature: Cham, Switzerland, 2021; pp. 167–202. [Google Scholar]
- Mauri, E.; Giantelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of nanogels: Curent trends and future Outlook. Gels 2021, 7, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Kadlubowski, S. Radiation-induced synthesis of nanogels based on PVP—A review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Dispenza, C.; Sabatino, M.A.; Grimaldi, N.; Mangione, M.R.; Walo, M.; Murugan, E.; Jonsson, M. On the origin of functionalization of one pot radiation synthesis nanaogels from aqueous polymer solutions. RSC Adv. 2016, 6, 2582–2591. [Google Scholar] [CrossRef]
- Ditta, C.A.; Dahlgren, B.; Sabatino, M.A.; Dispenza, C.; Jonsson, M. The role of oxygen in the formation of radiation engineered multifunctional nanogels. Eur. Polym. J. 2019, 114, 164–175. [Google Scholar] [CrossRef]
- An, J.-C.; Weaver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of PVP nanogels. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- Sütekin, S.D.; Güven, O. Application of radiation for the synthesis of PVP nanogels with controlled sizes from aqueous solutions. Appl. Radiat. Isot. 2019, 145, 161–169. [Google Scholar] [CrossRef]
- Ulanski, P.; Kadlubowski, S.; Rosiak, J.M. Synthesis of PAA nanogels by preparative pulse radiolysis. Radiat. Phys. Chem. 2002, 63, 533–537. [Google Scholar] [CrossRef]
- Kadlubowski, S.; Grobelny, J.; Olejniczak, W.; Cichomski, M.; Ulanski, P. Pulses of fast electrons as a tool to synthesize PAA nanogels. Macromolecules 2009, 36, 2484–2492. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Ulanski, P. Radiation-induced synthesis of PAA nanogels. Radiat. Phys. Chem. 2018, 142, 125–129. [Google Scholar] [CrossRef]
- Abd El-Rehim, H.A.; Hegazy, E.-S.A.; Hamed, A.A.; Swilem, A.E. Controlling the size and swellability of PVP-PAA nanogels synthesized by gamma radiation induced template polymerization. Eur. Polym. J. 2013, 49, 601–612. [Google Scholar] [CrossRef]
- Swilem, A.E.; Elshazly, A.H.M.; Hamed, A.A.; Hegazy, E.-S.A.; Abd El-Rehim, H.A. Nanoscale PAA based hydrogels prepared via green single-step approach for application as biomimetic fluid tears. Mater. Sci. Eng. 2020, 110, 110726. [Google Scholar] [CrossRef]
- Henke, A.; Kadlubowski, S.; Ulanski, P.; Rosiak, J.M.; Arndt, K.-F. Radiation-induced crosslinking of PVP-PAA complexes. NIM B 2005, 236, 391–398. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Sütekin, S.D.; Güven, O. Preparation of nanogels by radiation –induced crosslinking of interpolymer complexes of PVP with PAA in aqueous medium. Radiat. Phys. Chem. 2018, 142, 130–136. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Ghaffarlou, M.; Sütekin, S.D.; Pasanphan, W.; Güven, O. Preparation of multifunctional PAA-PEO nanogels from their interpolymer complexes by radiation induced intramolecular crosslinking. Colloid Polym. Sci. 2018, 296, 1599–1608. [Google Scholar] [CrossRef]
- Ghorbaniazar, P.; Sepehrianazar, A.; Eskandani, M.; Nabi-Mehbodi, M.; Kouhsoltani, M.; Hamishehkar, H. Preparation of PAA-PAAm composite nanogels by radiation technique. Adv. Pharm. Bull. 2015, 5, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.; Patra, S.; Gupta, B. Preparation and bactericidal action of biofunctional PAAm nanogels. Adv. Mater. Lett. 2017, 8, 13–18. [Google Scholar] [CrossRef]
- Zschoche, S.; Rueda, J.; Boyko, V.; Krahl, F.; Arndt, K.-F.; Voit, B. Thermo-responsive nanogels based on poly[NiPAAm-graft-(2-alkyl-2-oxazoline)]s crosslinked in the miceller state. Macromol. Chem. Phys. 2010, 211, 1035–1042. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Rosiak, J.M. Nanogels synthesized by radiation-induced intramolecular crosslinking of water soluble polymers. Radiat. Phys. Chem. 2020, 169, 108099. [Google Scholar] [CrossRef]
- Sabatino, M.A.; Ditta, L.A.; Conigliano, A.; Dispenza, C. A multifunctional platform for drug targeted delivery based on radiation engineered nanogels. Radiat. Phys. Chem. 2020, 169, 108059. [Google Scholar] [CrossRef]
- Thakur, V.; Gohs, U.; Wagenknecht, U.; Heinrich, G. In situ modification of unmodified MMT in PP by electron induced reactive processing. Macromol. Chem. Phys. 2012, 213, 729–737. [Google Scholar] [CrossRef]
- Çağlayan, T.; Güven, O. Preparation and characterization of EVA based nanocomposites using radiation-modified MMT. Radiat. Phys. Chem. 2020, 169, 107844. [Google Scholar] [CrossRef]
- Karslı, N.G.; Aytaç, A.; Akbulut, M.; Deniz, V.; Güven, O. Effect of irradiated PP compatibilizer on the properties of short carbon fiber reinforced PP composites. Radiat. Phys. Chem. 2013, 84, 74–78. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Chapiro, A.; Stannett, V. Direct radiation grafting on hydrophilic polymers. Int. J. Appl. Radiat. Isot. 1960, 8, 164–167. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Nanostructuring of polymers by controlling radiation-induced free radical polymerization, copolymerization, grafting and crosslinking by RAFT mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Apel, P.Y. Fabrication of functional micro- and nanoporous materials from polymers modified by swift heavy ions. Radiat. Phys. Chem. 2019, 159, 25–34. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Grafting in confined spaces: Functionalization of nanochannels of track-etched membranes. Radiat. Phys. Chem. 2014, 105, 26–30. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O.; Bessbousse, H.; Wade, T.L.; Beuneu, F.; Clochard, M.-C. Nanopore size tuning of polymeric membranes using RAFT-mediated radical polymerization. J. Membr. Sci. 2013, 445, 135–145. [Google Scholar] [CrossRef]
- Bessbousse, H.; Zran, N.; Fauleau, J.; Godin, B.; Lemee, V.; Wade, T.; Clochard, M.-C. Poly(4-vinyl pyriidine) radiografted PVDF track-etched membranes as sensors for monitoring trace mercury in water. Radiat. Phys. Chem. 2016, 118, 48–54. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Güven, O.; Mashentseva, A.; Bakar, A.; Gorin, Y.G.; Zdorovets, M.V.; Taltenov, A.A. Radiation-induced deposition of copper nanoparticles inside the nanochannels of PAA grafted PET track-etched membranes. Radiat. Phys. Chem. 2017, 130, 480–487. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.; Güven, O.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.; Zhidkov, I.; Cholach, S.O. Electron/gamma radiation induced synthesis and catalytic activity gold nanoparticles ontrack-etched PET membranes. Mater. Chem. Phys. 2018, 217, 31–39. [Google Scholar] [CrossRef]
- Ma, T.; Janot, J.-M.; Balme, S. Track-etched nanopore/membranes: From Fundamentals to applications. Small Methods 2020, 4, 2000366. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Prog. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Yoshida, M.; Kimura, Y.; Chen, J.; Asano, M.; Maekawa, Y. Preparation of PTFE-based fuel cell formation technique by combining latent track formation technique with graft polymerization. Radiat. Phys. Chem. 2013, 78, 1060–1066. [Google Scholar] [CrossRef]
- Clochard, M.-C.; Berthelot, T.; Baudin, C.; Betz, N.; Balanzat, E.; Gebel, G.; Morin, A. Ion-track grafting: A way of producing low cost and highly conductive membranes for fuel cell applications. J. Power Sour. 2010, 195, 223–231. [Google Scholar] [CrossRef]
- Çelik, G.; Barsbay, M.; Güven, O. Toward new proton exchange membrane maerials with enhanced performance via RAFT polymerization. Polym. Chem. 2016, 7, 701–714. [Google Scholar] [CrossRef]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Samasti, Y.; Sakurai, Y. Thermoresponsive polymeric surfaces in control of attachment and detachment of cultured cells. Macromol. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma treated polystyrene dishes grafted with PNiPAAm. J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Kamashiro, Y.; Kobayashi, J.; Yamato, M.; Sakai, K.; Okano, T. Characterization of ultrathin temperature responsive polymer layer and its polymer thickness on cell attachment/detachment properties. Macromol. Biosci. 2010, 10, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell sheet recovery from a surface successively grafted with PAAm and PNiPAAm. Acta Biomater. 2014, 10, 3398–3408. [Google Scholar] [CrossRef]
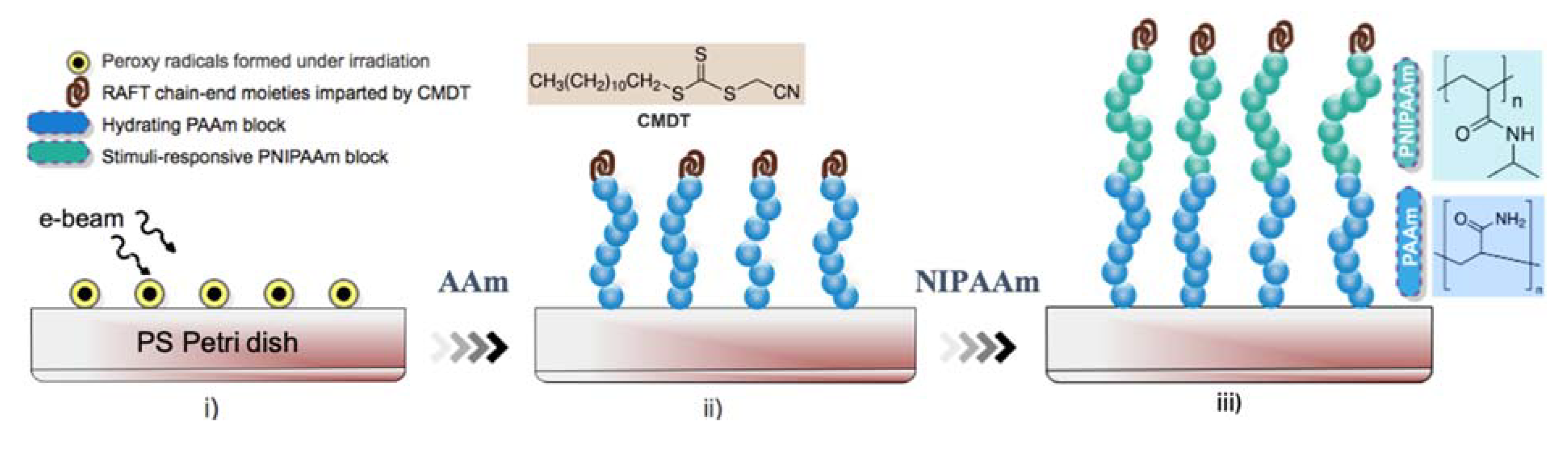
- Barsbay, M.; Güven, O. Modification of polystyrene cell culture dish surfaces by consecutive grafting of PAAm and PNiPAAm via RAFT-mediated polymerization. Eur. Polym. J. 2021, 143, 110330. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. PNiPAAm-based thermoresponsive surfaces provide new biomadical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güven, O. Radiation-Assisted Synthesis of Polymer-Based Nanomaterials. Appl. Sci. 2021, 11, 7913. https://doi.org/10.3390/app11177913
Güven O. Radiation-Assisted Synthesis of Polymer-Based Nanomaterials. Applied Sciences. 2021; 11(17):7913. https://doi.org/10.3390/app11177913
Chicago/Turabian StyleGüven, Olgun. 2021. "Radiation-Assisted Synthesis of Polymer-Based Nanomaterials" Applied Sciences 11, no. 17: 7913. https://doi.org/10.3390/app11177913
APA StyleGüven, O. (2021). Radiation-Assisted Synthesis of Polymer-Based Nanomaterials. Applied Sciences, 11(17), 7913. https://doi.org/10.3390/app11177913




