Microwave Planar Resonant Solutions for Glucose Concentration Sensing: A Systematic Review
Abstract
Featured Application
Abstract
1. Introduction
2. Microwave Planar Resonant Glucose Sensors: Fundamentals and Classification
2.1. Approach to Microwave Planar Resonant Glucose Sensors
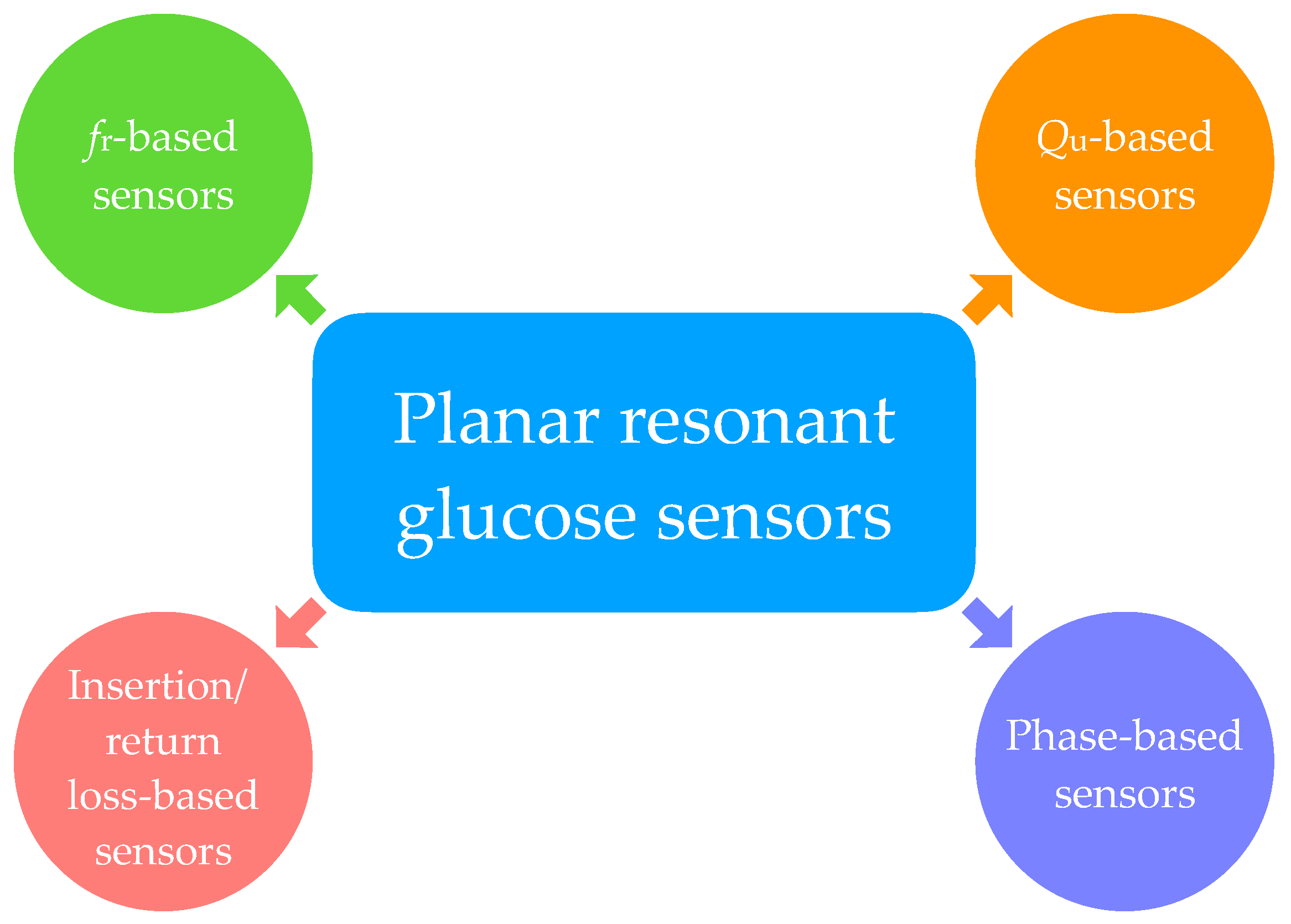
2.2. Proposal of Classification
3. Resonant Frequency-Based Sensors
4. Insertion/Return Loss-Based Sensors
5. Quality Factor-Based Sensors
6. Phase-Based Sensors
7. Discussion
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmud, M.Z.; Islam, M.T.; Misran, N.; Almutairi, A.F.; Cho, M. Ultra-Wideband (UWB) antenna sensor based microwave breast imaging: A review. Sensors 2018, 18, 2951. [Google Scholar] [CrossRef]
- Guha, S.; Warsinke, A.; Tientcheu, C.M.; Schmalz, K.; Meliani, C.; Wenger, C. Label free sensing of creatinine using a 6 GHz CMOS near-field dielectric immunosensor. Analyst 2015, 140, 3019–3027. [Google Scholar] [CrossRef]
- Greene, J.; Abdullah, B.; Cullen, J.; Korostynska, O.; Louis, J.; Mason, A. Non-invasive monitoring of glycogen in real-time using an electromagnetic sensor. In Modern Sensing Technologies. Smart Sensors, Measurement and Instrumentation; Mukhopadhyay, S., Jayasundera, K., Postolache, O., Eds.; Springer: Cham, Switzerland, 2019; Volume 29, pp. 1–15. [Google Scholar] [CrossRef]
- Amin, B.; Elahi, M.A.; Shahzad, A.; Porter, E.; McDermott, B.; O’Halloran, M. Dielectric properties of bones for the monitoring of osteoporosis. Med. Biol. Eng. Comput. 2019, 57, 1–13. [Google Scholar] [CrossRef]
- Mohammed, B.J.; Abbosh, A.M.; Mustafa, S.; Ireland, D. Microwave system for head imaging. IEEE Trans. Instrum. Meas. 2014, 63, 117–123. [Google Scholar] [CrossRef]
- Rowe, D.J.; Porch, A.; Barrow, D.A.; Allender, C.J. Microfluidic device for compositional analysis of solvent systems at microwave frequencies. Sens. Actuator B Chem. 2012, 169, 213–221. [Google Scholar] [CrossRef]
- Grenier, K.; Dubuc, D.; Chen, T.; Artis, F.; Chretiennot, T.; Poupot, M.; Fournié, J.-J. Recent advances in microwave-based dielectric spectroscopy at the cellular level for cancer investigations. IEEE Trans. Microw. Theory Tech. 2013, 61, 2023–2030. [Google Scholar] [CrossRef]
- Entesari, E.; Helmy, A.A.; Sekar, V. A review of frequency synthesizer-based microwave chemical sensors for dielectric detection of organic liquids. In Proceedings of the 2013 IEEE Annual Conference on Wireless and Microwave Technology (WAMICON), Orlando, FL, USA, 9–11 December 2013. [Google Scholar] [CrossRef]
- Guha, S.; Jamal, F.I.; Wenger, C. A review on passive and integrated near-field microwave biosensors. Biosensors 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Mata-Contreras, J.; Su, L.; Martín, F. Microwave sensors based on symmetry properties and metamaterial concepts: A review of some recent developments. In Proceedings of the 2017 IEEE 18th Wireless and Microwave Technology Conference (WAMICON), Cocoa Beach, FL, USA, 24–25 April 2017. [Google Scholar] [CrossRef]
- Naqui, J.; Martín, F. Microwave sensors based on symmetry properties of resonator-loaded transmission lines. J. Sens. 2015, 2015, 741853. [Google Scholar] [CrossRef]
- Bahar, A.A.M.; Zakaria, Z.; Isa, A.A.M.; Ruslan, E.; Alahnomi, R.A. A review of characterization techniques for materials’ properties measurement using microwave resonant sensor. J. Telecommun. Electron. Comput. Eng. 2015, 7, 1–6. [Google Scholar]
- Sekar, V.; Torke, W.J.; Palermo, S.; Entesari, K. A self-sustained microwave system for dielectric-constant measurement of lossy organic liquids. IEEE Trans. Microw. Theory Tech. 2012, 60, 1444–1455. [Google Scholar] [CrossRef]
- Rodboard, D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther. 2016, 18, S2-3–S2-13. [Google Scholar] [CrossRef]
- Tamune, H.; Takeya, H.; Suzuki, W.; Tagashira, Y.; Kuki, T.; Honda, H.; Nakamura, M. Cerebrospinal fluid/blood glucose ratio as an indicator for bacterial meningitis. Am. J. Emerg. Med. 2014, 32, 263–266. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Ichikawa, A.; Kikuchi, K.; Echida, Y.; Onuki, T.; Nitta, K. Glycated albumin is a better indicator of the glucose excursion than predialysis glucose and hemoglobin A1c in hemodialysis patients. Ren. Replace. Ther. 2016, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Årsand, E.; Walseth, O.A.; Andersson, N.; Fernando, R.; Granberg, O.; Bellika, J.G.; Hartvigsen, G. Using blood glucose data as an indicator for epidemic disease outbreaks. Stud. Health Technol. Inform. 2005, 116, 217–222. [Google Scholar] [PubMed]
- Tao, Y.; Yan, B.; Zhang, N.; Wang, M.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Microwave vacuum evaporation as a potential technology to concentrate sugar solutions: A study based on dielectric spectroscopy. J. Food Eng. 2021, 294, 110414. [Google Scholar] [CrossRef]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Guadarrama-Fernández, L.; Novell, M.; Blondeau, P.; Andrade, F.J. A disposable, simple, fast and low-cost paper-based biosensor and its application to the determination of glucose in commercial orange juices. Food Chem. 2018, 265, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhang, D.; Wu, D.; Wei, H.; Lu, G. Design of a breath analysis system for diabetes screening and blood glucose level prediction. IEEE Trans. Biomed. Eng. 2014, 61, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Xu, H.; Zhao, W.; Zhao, Y.; Zhang, X. Highly responsive and ultrasensitive non-enzymatic electrochemical glucose sensor based on Au foam. Sensors 2019, 19, 1203. [Google Scholar] [CrossRef]
- Boubin, M.; Shrestha, S. Microcontroller implementation of support vector machine for detecting blood glucose levels using breath volatile organic compounds. Sensors 2019, 19, 2283. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Ghazaryan, A.; Ovsepian, S.V.; Ntziachristos, V. Extended near-infrared optoacoustic spectrometry for sensing physiological concentrations of glucose. Front. Endocrinol. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Schuler, B.; Kühner, L.; Hentschel, M.; Giessen, H.; Tarín, C. Adaptive method for quantitative estimation of glucose and fructose concentrations in aqueous solutions based on infrared nanoantenna optics. Sensors 2019, 19, 3053. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C. Exciting developments for microwave sensing and monitoring in biology and medicine. IEEE Micorw. Mag. 2017, 18, 20–30. [Google Scholar] [CrossRef]
- Yilmaz, T.; Foster, R.; Hao, Y. Radio-Frequency and microwave techniques for non-invasive measurement of blood glucose levels. Diagnostics 2019, 9, 6. [Google Scholar] [CrossRef]
- Jang, C.; Lee, H.J.; Yook, J.G. Radio-Frequency biosensors for real-time and continuous glucose detection. Sensors 2021, 21, 1843. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Simulating the effects of skin thickness and fingerprints to highlight problems with non-invasive RF blood glucose sensing from fingertips. IEEE Sens. J. 2017, 17, 7553–7560. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Sabater-Navarro, J.M. Glucose concentration measurement in human blood plasma solutions with microwave sensors. Sensors 2019, 19, 3779. [Google Scholar] [CrossRef]
- Zidane, M.A.; Amar, H.; Rouane, A. Study of two constraints impacting measurements of human glycemia using a microwave sensor. Biosensors 2021, 11, 83. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Thundat, T.; Daneshmand, M. High resolution microwave microstrip resonator for sensing applications. Sens. Actuator A Phys. 2015, 233, 224–230. [Google Scholar] [CrossRef]
- Cui, Y.; Ge, A. A tunable high-Q microwave detector for on-column capillary liquid chromatography. IEEE Trans. Instrum. Meas. 2020, 69, 5978–5980. [Google Scholar] [CrossRef]
- Babay, M.; Hallepee, C.; Dalmay, C.; Barelaud, B.; Durmaz, E.C.; Kaynak, C.B.; Kaynak, M.; Cordeau, D.; Pothier, A. Highly sensitive capacitive sensor based on injection locked oscillators with ppm sensing resolution. In Proceedings of the 2020 IEEE/MTT-S International Microwave Symposium (IMS), Los Angeles, CA, USA, 4–6 August 2020. [Google Scholar] [CrossRef]
- Abdolrazzaghi, M.; Daneshmand, M. Exploiting sensitivity enhancement in micro-wave planar sensors using intermodulation products with phase noise analysis. IEEE Trans. Circuits Syst. I Regul. Pap. 2020, 67, 4382–4395. [Google Scholar] [CrossRef]
- Caduff, A.; Mueller, M.; Megej, A.; Dewarrat, F.; Suri, R.E.; Klisic, J.; Donath, M.; Zakharov, P.; Schaub, D.; Stahel, W.A.; et al. Characteristics of a multisensor system for non invasive glucose monitoring with external validation and prospective evaluation. Biosens. Bioelectron. 2011, 26, 3794–3800. [Google Scholar] [CrossRef]
- Acciaroli, G.; Zanon, M.; Facchinetti, A.; Caduff, A.; Sparacino, G. Retrospective continuous-time blood glucose estimation in free living conditions with a non-invasive multisensor device. Sensors 2019, 19, 3677. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.G.; García, H.; Ávila-Navarro, E.; Bronchalo, E.; Galiano, V.; Moreno, O.; Orozco, D.; Sabater-Navarro, J.M. Feasibility study of portable microwave microstrip open-loop resonator for noninvasive blood glucose level sensing: Proof of concept. Med. Biol. Eng. Comput. 2019, 57, 2389–2405. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 15200. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Shubair, R.M.; Ngo, K.; Alquié, G.; Deshours, F.; Kokabi, H. Multiple-cell microfluidic dielectric resonator for liquid sensing applications. IEEE Sens. J. 2021, 21, 6094–6104. [Google Scholar] [CrossRef]
- Tripathi, P.; Kumar, P.; Raj, S.; Tripathi, S.S.; Tripathi, V.S. ANN based design of SRR loaded patch antenna for non-invasive blood glucose monitoring. In Proceedings of the 2019 4th International Conference on Information Systems and Computer Networks (ISCON), Mathura, India, 21–22 November 2019; pp. 279–283. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S. Portable radar-driven microwave sensor for intermittent glucose levels monitoring. IEEE Sens. Lett. 2020, 4, 3500604. [Google Scholar] [CrossRef]
- Rui, F.; Zhanxiao, G.; Ang, L.; Yao, C.; Chenyang, W.; Ning, Z.; Xiaohui, G.; Junqing, Z.; Xiaohao, W.; Fei, T. Noninvasive blood glucose monitor via multi-sensor fusion and its clinical evaluation. Sens. Actuator B Chem. 2021, 332, 129445. [Google Scholar] [CrossRef]
- Bteich, M.; Hanna, J.; Costantine, J.; Kanj, R.; Tawk, Y.; Ramadan, A.H.; Eid, A.A. A non-invasive flexible glucose monitoring sensor using a broadband reject filter. IEEE J. Electromagn. RF Microw. Med. Biol. 2020. [Google Scholar] [CrossRef]
- Obaid, S.M.; Elwi, T.A.; Ilyas, M. Fractal Minkowski-shaped resonator for noninvasive biomedical measurements: Blood glucose test. Prog. Electromagn. Res. C 2021, 107, 143–156. [Google Scholar] [CrossRef]
- Su, L.; Mata-Contreras, J.; Vélez, P.; Martín, F. A review of sensing strategies for microwave sensors based on metamaterial-inspired resonators: Dielectric characterization, displacement, and angular velocity measurements for health diagnosis, telecommunication, and space applications. Int. J. Antennas Propag. 2017, 2017, 5619728. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Vélez, P.; Gil, M.; Martín, F. Planar microwave resonant sensors: A review and recent developments. Appl. Sci. 2020, 10, 2615. [Google Scholar] [CrossRef]
- Alahnomi, R.A.; Zakaria, Z.; Yussof, Z.M.; Althuwayb, A.A.; Alhegazi, A.; Alsariera, H.; Rahman, N.A. Review of recent microwave planar resonator-based sensors: Techniques of complex permittivity extraction, applications, open challenges and future research directions. Sensors 2021, 21, 2267. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, R.; Zheng, Z.; Zheng, Y. Electromagnetic—Acoustic sensing for biomedical applications. Sensors 2018, 18, 3203. [Google Scholar] [CrossRef]
- Mehrotra, P.; Chatterjee, B.; Sen, S. EM-wave biosensors: A review of RF, microwave, mm-wave and optical sensing. Sensors 2019, 19, 1013. [Google Scholar] [CrossRef] [PubMed]
- Gholami Mayani, M.; Herraiz-Martínez, F.J.; Matanza Domingo, J.; Giannetti, R. Resonator-based microwave metamaterial sensors for instrumentation: Survey, classification, and performance comparison. IEEE Trans. Instrum. Meas. 2021, 70, 9503414. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive electromagnetic wave sensing of glucose. Sensors 2019, 19, 1151. [Google Scholar] [CrossRef]
- Yunos, M.F.A.M.; Nordin, A.N. Non-invasive glucose monitoring devices: A review. Bull. Electr. Eng. Inform. 2020, 9, 2609–2618. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Arduini, F. The technology tree in the design of glucose biosensors. Trends Anal. Chem. 2019, 120, 115642. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Juan, C.G. Designing Microwave Sensors for Glucose Concentration Detection in Aqueous and Biological Solutions: Towards Non-invasive Glucose Sensing; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Fuchs, K.; Kaatze, U. Molecular dynamics of carbohydrate aqueous solutions. Dielectric relaxation as a function of glucose and fructose concentration. J. Phys. Chem. B 2001, 105, 2036–2042. [Google Scholar] [CrossRef]
- Kaatze, U. Dielectric relaxation of water. In Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Application; Raicu, V., Feldman, Y., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 189–227. [Google Scholar] [CrossRef]
- Shiraga, K.; Suzuki, T.; Kondo, N.; Tajima, T.; Nakamura, M.; Togo, H.; Hirata, A.; Ogawa, Y. Broadband dielectric spectroscopy of glucose aqueous solution: Analysis of the hydration state and the hydrogen bond network. J. Chem. Phys. 2015, 142, 234504. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G. Dielectric properties of glucose in bulk aqueous solutions: Influence of electrode polarization and modeling. Biosens. Bioelectron. 2011, 26, 2347–2353. [Google Scholar] [CrossRef]
- Potelon, B.; Quendo, C.; Carré, J.-L.; Chevalier, A.; Person, C.; Queffelec, P. Electromagnetic signature of glucose in aqueous solutions and human blood. In Proceedings of the MEMSWAVE Conference, La Rochelle, France, 30 June–2 July 2014. [Google Scholar]
- Juan, C.G.; Bronchalo, E.; Torregrosa, G.; Ávila, E.; García, N.; Sabater-Navarro, J.M. Dielectric characterization of water glucose solutions using a transmission/reflection line method. Biomed. Signal Process. Control 2017, 31, 139–147. [Google Scholar] [CrossRef]
- Lin, T.; Gu, S.; Lasri, T. Highly sensitive characterization of glucose aqueous solution with low concentration: Application to broadband dielectric spectroscopy. Sens. Actuator A Phys. 2017, 267, 318–326. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Permittivity extraction of glucose solutions through artificial neural networks and non-invasive microwave glucose sensing. Sens. Actuator A Phys. 2018, 277, 65–72. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, C.-S.; Choi, B.-C.; Ham, K.-Y. The correlation of the complex dielectric constant and blood glucose at low frequency. Biosens. Bioelectron. 2003, 19, 321–324. [Google Scholar] [CrossRef]
- Hayashi, Y.; Livshits, L.; Caduff, A.; Feldman, Y. Dielectric spectroscopy study of specific glucose influence on human erythrocyte membranes. J. Phys. D Appl. Phys. 2003, 36, 369–374. [Google Scholar] [CrossRef]
- Karacolak, T.; Moreland, E.C.; Topsakal, E. Cole–cole model for glucose-dependent dielectric properties of blood plasma for continuous glucose monitoring. Microw. Opt. Technol. Lett. 2013, 55, 1160–1164. [Google Scholar] [CrossRef]
- Caduff, A.; Ben Ishai, P.; Feldman, Y. Continuous noninvasive glucose monitoring; water as a relevant marker of glucose uptake in vivo. Biophys. Rev. 2019, 11, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, K.S.; Eremenko, Z.E.; Shubnyi, A.I.; Glamazdin, V.V.; Sklyar, N.I. Glucose minimal concentration limit determination using waveguide-differential dielectrometer at microwaves. In Proceedings of the IEEE 38th International Conference on Electronics and Nanotechnology (ELNANO), Kiev, Ukraine, 24–28 April 2018; pp. 245–248. [Google Scholar] [CrossRef]
- Srour, M.; Potelon, B.; Quendo, C.; Person, C.; Carré, J.-L. Biosensor based on a resonant technique for aqueous glucose monitoring using standardized medical test tubes. In Proceedings of the 2020 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Toulouse, France, 14–17 December 2020. [Google Scholar] [CrossRef]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-based noninvasive concentration measurements for biomedical applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; Mackenzie, R.; Reeve, T.; Kosmas, P.; et al. A glucose sensing system based on transmission measurements at millimetre waves using micro strip patch antennas. Sci. Rep. 2017, 7, 6855. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Fersch, T.; Weigel, R.; Fischer, G.; Kissinger, D. A novel approach to non-invasive blood glucose measurement based on RF transmission. In Proceedings of the 2011 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 30–31 May 2011; pp. 39–42. [Google Scholar] [CrossRef]
- Kandwal, A.; Nie, Z.; Igbe, T.; Li, J.; Liu, Y.; Liu, L.W.; Hao, Y. Surface plasmonic feature microwave sensor with highly confined fields for aqueous-glucose and blood-glucose measurements. IEEE Trans. Instrum. Meas. 2021, 70, 8000309. [Google Scholar] [CrossRef]
- Bernard, P.A.; Gautray, J.M. Measurement of dielectric constant using a microstrip ring resonator. IEEE Trans. Microw. Theory Tech. 1991, 39, 592–595. [Google Scholar] [CrossRef]
- Pozar, D.M. Microwave resonators. In Microwave Engineering, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 272–316. [Google Scholar]
- Jean, B.R.; Green, E.C.; McClung, M.J. A microwave frequency sensor for non-invasive blood-glucose measurement. In Proceedings of the 2008 IEEE Sensors Applications Symposium (SAS), Atlanta, GA, USA, 12–14 February 2008; pp. 12–14. [Google Scholar] [CrossRef]
- Saeed, K.; Guyette, A.C.; Hunter, I.C.; Pollard, R.D. Microstrip resonator technique for measuring dielectric permittivity of liquid solvents and for solution sensing. In Proceedings of the 2007 IEEE/MTT-S International Microwave Symposium (IMS), Honolulu, HI, USA, 3–8 June 2007; pp. 1185–1188. [Google Scholar] [CrossRef]
- Yilmaz, T.; Hao, Y. Electrical property characterization of blood glucose for on–body sensors. In Proceedings of the 5th European Conference on Antennas and Propagation (EUCAP), Rome, Italy, 11–15 April 2011. [Google Scholar]
- Souza, M.I.O.; Mota, A.F.; Pepino, V.M.; Carmo, J.P.; Borges, B.-H.V. Multi-purpose microwave biosensor based on signal encoding technique and microfluidics for improved sensitivity. IEEE Sens. J. 2021, 21, 4571–4581. [Google Scholar] [CrossRef]
- Yilmaz, T.; Foster, R.; Hao, Y. Towards accurate dielectric property retrieval of biological tissues for blood glucose monitoring. IEEE Trans. Microw. Theory Tech. 2014, 62, 3193–3204. [Google Scholar] [CrossRef]
- Yilmaz, T.; Hao, Y. Compact resonators for permittivity reconstruction of biological tissues. In Proceedings of the 2011 XXXth URSI General Assembly and Scientific Symposium, Istanbul, Turkey, 13–20 August 2011. [Google Scholar] [CrossRef]
- Huang, S.Y.; Yoshida, Y.; Garcia, A.; Chia, X.; Mu, W.C.; Meng, Y.S.; Yu, W. Microstrip line-based glucose sensor for noninvasive continuous monitoring using the main field for sensing and multivariable crosschecking. IEEE Sens. J. 2019, 19, 535–547. [Google Scholar] [CrossRef]
- Schwerthoeffer, U.; Warter, C.; Weigel, R.; Kissinger, D. A microstrip resonant biosensor for aqueous glucose detection in microfluidic medical applications. In Proceedings of the 2014 IEEE Topical Conference on Biomedical Wireless Technologies Networks, and Sensing Systems (BioWireleSS), Newport Beach, CA, USA, 19–23 January 2014. [Google Scholar] [CrossRef]
- Schwerthoeffer, U.; Weigel, R.; Kissinger, D. A highly sensitive glucose biosensor based on a microstrip ring resonator. In Proceedings of the 2013 IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-BIO), Singapore, 9–11 December 2013. [Google Scholar] [CrossRef]
- Rowe, D.J.; al-Malki, S.; Abduljabar, A.A.; Porch, A.; Barrow, D.A.; Allender, C.J. Improved split-ring Resonator for microfluidic sensing. IEEE Trans. Microw. Theory Tech. 2014, 62, 689–699. [Google Scholar] [CrossRef]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in vitro interference test of microwave noninvasive blood glucose monitoring sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef]
- Camli, B.; Kusakci, E.; Lafci, B.; Salman, S.; Torun, H.; Yalcinkaya, A.D. Cost-effective, microstrip antenna driven ring resonator microwave biosensor for biospecific detection of glucose. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 6900706. [Google Scholar] [CrossRef]
- Vélez, P.; Mata-Contreras, J.; Dubuc, D.; Grenier, K.; Martín, F. Solute concentration measurements in diluted solutions by means of Split Ring Resonators. In Proceedings of the 48th European Microwave Conference (EuMC), Madrid, Spain, 23–27 September 2018; pp. 231–234. [Google Scholar] [CrossRef]
- Odabashyan, L.; Babajanyan, A.; Baghdasaryan, Z.; Kim, S.; Kim, J.; Friedman, B.; Lee, J.-H.; Lee, K. Real-time noninvasive measurement of glucose concentration using a modified Hilbert shaped microwave sensor. Sensors 2019, 19, 5525. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Ávila-Navarro, E.; Sabater-Navarro, J.M. Concentration measurement of microliter-volume water–glucose solutions using Q factor of microwave sensors. IEEE Trans. Instrum. Meas. 2019, 68, 2621–2634. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Alquié, G.; Deshours, F.; Kokabi, H.; Shubair, R.M. Non-invasive real-time monitoring of glucose level using novel microwave biosensor based on triple-pole CSRR. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1407–1420. [Google Scholar] [CrossRef]
- Camli, B.; Kusakci, E.; Lafci, B.; Salman, S.; Torun, H.; Yalcinkaya, A. A microwave ring resonator based glucose sensor. Procedia Eng. 2016, 168, 465–468. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F. Compact honey-cell CSRR-based microwave biosensor for monitoring glucose levels. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020. [Google Scholar] [CrossRef]
- Verma, A.; Bhushan, S.; Tripathi, P.N.; Goswami, M.; Singh, B.R. A defected ground split ring resonator for an ultra-fast, selective sensing of glucose content in blood plasma. J. Electromagn. Waves Appl. 2017, 31, 1049–1061. [Google Scholar] [CrossRef]
- Choi, H.; Luzio, S.; Beutler, J.; Porch, A. Microwave noninvasive blood glucose monitoring sensor: Human clinical trial results. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium (IMS), Honolulu, HI, USA, 4–9 June 2017; pp. 876–879. [Google Scholar] [CrossRef]
- García, H.; Juan, C.G.; Ávila-Navarro, E.; Bronchalo, E.; Sabater-Navarro, J.M. Portable device based on microwave resonator for noninvasive blood glucose monitoring. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1115–1118. [Google Scholar] [CrossRef]
- Kandwal, A.; Igbe, T.; Li, J.; Liu, Y.; Li, S.; Liu, L.W.Y.; Nie, Z. Highly sensitive closed loop enclosed split ring biosensor with high field confinement for aqueous and blood-glucose measurements. Sci. Rep. 2020, 10, 4081. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Ultrahigh-sensitivity microwave sensor for microfluidic complex permittivity measurement. IEEE Trans. Microw. Theory Tech. 2019, 67, 4269–4277. [Google Scholar] [CrossRef]
- Su, L.; Muñoz-Enano, J.; Vélez, P.; Gil-Barba, M.; Casacuberta, P.; Martín, F. Highly sensitive reflective-mode phase-variation permittivity sensor based on a coplanar waveguide terminated with an open complementary split ring resonator (OCSRR). IEEE Access 2021, 9, 27928–27944. [Google Scholar] [CrossRef]
- Su, L.; Mata-Contreras, J.; Vélez, P.; Martín, F. Splitter/combiner microstrip sections loaded with pairs of complementary split ring resonators (CSRRs): Modeling and optimization for differential sensing applications. IEEE Trans. Microw. Theory Tech. 2016, 64, 4362–4370. [Google Scholar] [CrossRef]
- Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E.; Sabater-Navarro, J.M. Highly-sensitive glucose concentration sensor exploiting inter-resonators couplings. In Proceedings of the 49th European Microwave Conference (EuMC), Paris, France, 1–3 October 2019; pp. 662–665. [Google Scholar] [CrossRef]
- Naqui, J.; Durán-Sindreu, M.; Martín, F. Novel sensors based on the symmetry properties of split ring resonators (SRRs). Sensors 2011, 11, 7545–7553. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterization. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. Microwave microfluidic sensor for determination of glucose concentration in water. In Proceedings of the 2015 IEEE 15th Mediterranean Microwave Symposium (MMS), Lecce, Italy, 30 November–2 December 2015. [Google Scholar] [CrossRef]
- Vélez, P.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Highly-sensitive microwave sensors based on open complementary split ring resonators (OCSRRs) for dielectric characterization and solute concentration measurement in liquids. IEEE Access 2018, 6, 48324–48338. [Google Scholar] [CrossRef]
- Kiani, S.; Rezaei, P.; Navaei, M. Dual-sensing and dual-frequency microwave SRR sensor for liquid samples permittivity detection. Measurement 2020, 160, 107805. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, F.; Feng, X.; Liu, S.; Kishor, R.; Luo, Y.; Zheng, Y. Noninvasive photoacoustic measurement of glucose by data fusion. Analyst 2017, 142, 2892–2896. [Google Scholar] [CrossRef]
- Benkhaoua, L.; Benhabiles, M.T.; Mouissat, S.; Riabi, M.L. Miniaturized quasi-lumped resonator for dielectric characterization of liquid mixtures. IEEE Sens. J. 2016, 16, 1603–1610. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Sensitivity of non-invasive RF/microwave glucose sensors and fundamental factors and challenges affecting measurement accuracy. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018. [Google Scholar] [CrossRef]
- Camli, B.; Yalcinkaya, A.D. Resonant type RF glucose biosensors. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Sharafadinzadeh, N.; Abdolrazzaghi, M.; Daneshmand, M. Highly sensitive microwave split ring resonator sensor using gap extension for glucose sensing. In Proceedings of the IEEE MTT-S International Microwave Workshop Series on Advanced Materials and Processes (IMWS-AMP 2017), Pavia, Italy, 20–22 September 2017. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Microwave reflective biosensor for glucose level detection in aqueous solutions. Sens. Actuator A Phys. 2020, 301, 111662. [Google Scholar] [CrossRef]
- Abir, M.A.I.; Ahmed, S.; Alam, M.S.; Islam, M.A. Application of a complementary split ring resonator based biosensor for detection of micromolar glucose concentrations in aqueous solution. In Proceedings of the 2020 11th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 17–19 December 2020. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.-K.; Lee, H.-J.; Yun, G.-H.; Yook, J.-G. Non-invasive fluidic glucose detection based on dual microwave complementary split ring resonators with a switching circuit for environmental effect elimination. IEEE Sens. J. 2020, 20, 8520–8527. [Google Scholar] [CrossRef]
- Bahar, A.A.M.; Zakaria, Z.; Arshad, M.K.M.; Isa, A.A.M.; Dasril, Y.; Alahnomi, R.A. Real time microwave biochemical sensor based on circular SIW approach for aqueous dielectric detection. Sci. Rep. 2019, 9, 5467. [Google Scholar] [CrossRef]
- Zidane, M.A.; Rouane, A.; Hamouda, C.; Amar, H. Hyper-sensitive microwave sensor based on split ring resonator (SRR) for glucose measurement in water. Sens. Actuator A Phys. 2021, 321, 112601. [Google Scholar] [CrossRef]
- Baghelani, M.; Abbasi, Z.; Daneshmand, M.; Light, P.E. Non-invasive continuous-time glucose monitoring system using a chipless printable sensor based on split ring microwave resonators. Sci. Rep. 2020, 10, 12980. [Google Scholar] [CrossRef] [PubMed]
- Camli, B.; Altinagac, E.; Kizil, H.; Torun, H.; Dundar, G.; Yalcinkaya, A.D. Gold-on-glass microwave split-ring resonators with PDMS microchannels for differential measurement in microfluidic sensing. Biomicrofluidics 2020, 14, 054102. [Google Scholar] [CrossRef] [PubMed]
- Byford, J.A.; Park, K.Y.; Chahal, P. Metamaterial inspired periodic structure used for microfluidic sensing. In Proceedings of the 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015; pp. 1997–2002. [Google Scholar] [CrossRef]
- Tiwari, N.K.; Singh, S.P.; Mondal, D.; Akhtar, M.J. Flexible biomedical RF sensors to quantify the purity of medical grade glycerol and glucose concentrations. Int. J. Microw. Wirel. Technol. 2020, 12, 120–130. [Google Scholar] [CrossRef]
- Govind, G.; Akhtar, M.J. Metamaterial-inspired microwave microfluidic sensor for glucose monitoring in aqueous solutions. IEEE Sens. J. 2019, 19, 11900–11907. [Google Scholar] [CrossRef]
- Govind, G.; Akhtar, M.J. Design of an ELC resonator-based reusable RF microfluidic sensor for blood glucose estimation. Sci. Rep. 2020, 10, 18842. [Google Scholar] [CrossRef]
- Schwerthoeffer, U.; Weigel, R.; Kissinger, D. Microwave sensor for precise permittivity characterization of liquids used for aqueous glucose detection in medical applications. In Proceedings of the German Microwave Conference (GeMiC) 2014, Aachen, Germany, 10–12 March 2014. [Google Scholar]
- Jha, A.K.; Akhter, Z.; Tiwari, N.; Shafi, K.T.M.; Samant, H.; Akhtar, M.J.; Cifra, M. Broadband wireless sensing system for non-invasive testing of biological samples. IEEE J. Emer. Select. Top. Circuits Syst. 2018, 8, 251–259. [Google Scholar] [CrossRef]
- Abedeen, Z.; Agarwal, P. Microwave sensing technique based label-free and real-time planar glucose analyzer fabricated on FR4. Sens. Actuator A Phys. 2018, 279, 132–139. [Google Scholar] [CrossRef]
- Kumari, R.; Patel, P.N.; Yadav, R. An ENG resonator-based microwave sensor for the characterization of aqueous glucose. J. Phys. D Appl. Phys. 2018, 51, 075601. [Google Scholar] [CrossRef]
- Pandit, N.; Jaiswal, R.K.; Pathak, N.P. Plasmonic metamaterial-based label-free microfluidic microwave sensor for aqueous biological applications. IEEE Sens. J. 2020, 20, 10582–10590. [Google Scholar] [CrossRef]
- Abdulkarim, Y.I.; Muhammadsharif, F.F.; Bakır, M.; Awl, H.N.; Karaaslan, M.; Deng, L.; Huang, S. Hypersensitized metamaterials based on a corona-shaped resonator for efficient detection of glucose. Appl. Sci. 2021, 11, 103. [Google Scholar] [CrossRef]
- Yang, C.-L.; Lee, C.-S.; Chen, K.-W.; Chen, K.-Z. Noncontact measurement of complex permittivity and thickness by using planar resonators. IEEE Trans. Microw. Theory Tech. 2016, 64, 247–257. [Google Scholar] [CrossRef]
- Kim, J.; Babajanyan, A.; Hovsepyan, A.; Lee, K.; Friedman, B. Microwave dielectric resonator biosensor for aqueous glucose solution. Rev. Sci. Instrum. 2008, 79, 086107. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Park, J.-K.; Lee, H.-J.; Yun, G.-H.; Yook, J.-G. Temperature-corrected fluidic glucose sensor based on microwave resonator. Sensors 2018, 18, 3850. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Charoen-In, U.; Wanthong, A. Determination of glucose concentration with resonant coplanar microwave sensor. In Proceedings of the 2017 International Electrical Engineering Congress (iEECON), Pattaya, Thailand, 8–10 March 2017. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Coplanar waveguides loaded with a split ring resonator-based microwave sensor for aqueous sucrose solutions. Meas. Sci. Technol. 2016, 27, 015103. [Google Scholar] [CrossRef]
- Mohammadi, S.; Wiltshire, B.; Jain, M.C.; Nadaraja, A.V.; Clements, A.; Golovin, K.; Roberts, D.J.; Johnson, T.; Foulds, I.; Zarifi, M.H. Gold coplanar waveguide resonator integrated with a microfluidic channel for aqueous dielectric detection. IEEE Sens. J. 2020, 17, 9825–9833. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Coplanar waveguide transmission line loaded with electric-LC resonator for determination of glucose concentration sensing. IEEE Sens. J. 2017, 17, 1635–1640. [Google Scholar] [CrossRef]
- Yu, W.; Huang, S.Y. T-shaped patterned microstrip line for noninvasive continuous glucose sensing. IEEE Microw. Wirel. Compon. Lett. 2018, 28, 942–944. [Google Scholar] [CrossRef]
- Chretiennot, T.; Dubuc, D.; Grenier, K. Microwave-based microfluidic sensor for non-destructive and quantitative glucose monitoring in aqueous solution. Sensors 2016, 16, 1733. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Jaruwongrungsee, K.; Tuantranont, A.; Fumeaux, C.; Abbott, D. Metamaterial-based microfluidic sensor for dielectric characterization. Sens. Actuator A Phys. 2013, 189, 233–237. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Planar microwave sensor for detection and discrimination of aqueous organic and inorganic solutions. Sens. Actuator B Chem. 2018, 271, 300–305. [Google Scholar] [CrossRef]
- Verma, A.K.; Omar, A.S. Microstrip resonator sensors for determination of complex permittivity of materials in sheet, liquid and paste forms. IEE Proc. Microw. Antennas Propag. 2005, 152, 47–54. [Google Scholar] [CrossRef]
- Dawsmith, W.; Ohtani, N.; Donnan, R.; Naftaly, M.; Dudley, R.A.; Chowdhury, T.T. Microwave frequency dependent dielectric properties of blood as a potential technique to measure hydration. IEEE Access 2020. [Google Scholar] [CrossRef]
- Costanzo, S. Loss tangent effect on the accurate design of microwave sensors for blood glucose monitoring. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 661–663. [Google Scholar] [CrossRef]
- Gubin, A.I.; Barannik, A.A.; Cherpak, N.T.; Protsenko, I.A.; Pud, S.; Offenhäusser, A.; Vitusevich, S.A. Whispering-gallery-mode resonator technique with microfluidic channel for permittivity measurement of liquids. IEEE Trans. Microw. Theory Tech. 2015, 63, 2003–2009. [Google Scholar] [CrossRef]
- Gubin, A.I.; Protsenko, I.A.; Barannik, A.A.; Vitusevich, S.; Lavrinovich, A.A.; Cherpak, N.T. Quartz whispering-gallery-mode resonator with microfluidic chip as sensor for permittivity measurement of liquids. IEEE Sens. J. 2019, 19, 7976–7982. [Google Scholar] [CrossRef]
- He, X.; Hao, X.; Yan, S.; Wu, F.; Jiang, J. Biosensing using an asymmetric split-ring resonator at microwave frequency. Integr. Ferroelectr. 2016, 172, 142–146. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Álvarez-Pastor, J.; Sabater-Navarro, J.M. Use of coplanar quarter-wave resonators for glucose sensing in aqueous solutions. In Proceedings of the 2020 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Toulouse, France, 14–17 December 2020. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Coromina, J.; Vélez, P.; Su, L.; Gil, M.; Casacuberta, P.; Martín, F. Planar phase-variation microwave sensors for material characterization: A review and comparison of various approaches. Sensors 2021, 21, 1542. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Vélez, P.; Gil, M.; Mata-Contreras, J.; Martín, F. Differential-mode to common-mode conversion detector based on rat-race hybrid couplers: Analysis and application to differential sensors and comparators. IEEE Trans. Microw. Theory Tech. 2020, 68, 1312–1325. [Google Scholar] [CrossRef]
- Zeising, S.; Kirchner, J.; Khalili, H.F.; Ahmed, D.; Lübke, M.; Thalmayer, A.; Fischer, G. Towards realisation of a non-invasive blood glucose sensor using microstripline. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020. [Google Scholar] [CrossRef]
- Guariti, G.; Hofmann, M.; Weigel, R.; Fischer, G.; Kissinger, D. Determination of sugar concentration in aqueous solutions using ultra-wideband microwave impedance spectroscopy. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (IMS), Seattle, WA, USA, 2–7 June 2013. [Google Scholar] [CrossRef]
- Su, L.; Muñoz-Enano, J.; Vélez, P.; Casacuberta, P.; Gil, M.; Martín, F. Phase-variation microwave sensor for permittivity measurements based on a high-impedance half-wavelength transmission line. IEEE Sens. J. 2021, 21, 10647–10656. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Vélez, P.; Gil, M.; Martín, F. An analytical method to implement high-sensitivity transmission line differential sensors for dielectric constant measurements. IEEE Sens. J. 2020, 20, 178–184. [Google Scholar] [CrossRef]
- Coromina, J.; Muñoz-Enano, J.; Vélez, P.; Ebrahimi, A.; Scott, J.; Ghorbani, K.; Martín, F. Capacitively-loaded slow-wave transmission lines for sensitivity improvement in phase-variation permittivity sensors. In Proceedings of the 50th European Microwave Conference (EuMC), Utrecht, Netherlands, 12–14 January 2021; pp. 491–494. [Google Scholar] [CrossRef]
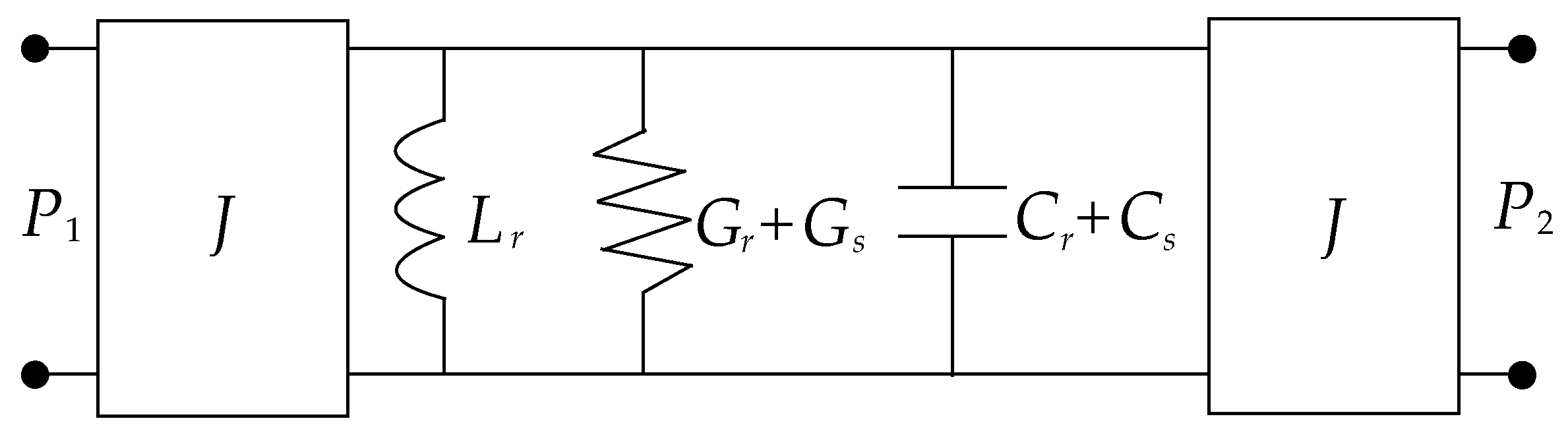
| Ref. | Sensor Type | Sample (Volume) | 𝜌g Range (wt%) | Bare fr (GHz) | Operating fr (GHz) | Sfr (KHz per mg/dL) | RS(G) (%/%) | Remarks |
|---|---|---|---|---|---|---|---|---|
| [124] | IDC-based resonator | AG (2 μL) | 0–8 | 2.46 | 0.64 | — | 0.556 | Biodegradable flexible substrate |
| [108] | Single CELC | AG (MF) | 0–10 | 1.40 | 1.16 | — | 0.408 | MF channel |
| [125] | IDC SRR | AG (MF) | 0–5 | 4.18 | 1.32 | — | 0.622 | MF channel |
| [122] | Differential double SRR | AG (MF) | 0.05–0.3 | 1.74 | 1.44 | 8.9 | 0.510 | Gold-on-glass substrate, MF |
| [91] | Single SRR | AG (90 μL) | 0–50 | 1.83 | 1.49 | — | 0.072 | Enzyme-coated |
| [96] | Single SRR | AG (90 μL) | 0–40 | 1.82 | 1.59 | — | 0.099 | Enzyme-coated |
| [41] | Triple CSRR | AG (1.2 mL) | 0.07–0.11 | 2.30 | 1.60 | 67.0 | 2.188 | MF channel |
| [126] | Single CELC | AG (MF) | 0.1–0.5 | 1.71 | 1.64 | 18.5 | 1.128 | Reusable, MF channel |
| [130] | ENG unit-cell resonator | AG (2 μL) | 0–10 | 2.09 | 1.91 | — | 0.895 | SRR- and horn-shaped elements |
| [127] | MLIN res. | AG (—) | 0–0.3 | — | 2.00 | 2.5 | 0.125 | — |
| [115] | Modified SRR | AG (MF) | 0–6 | — | 2.00 | — | 0.011 | Capacitive gap |
| [116] | Single CSRR | AG (MF) | 0–0.5; 0–8 | — | 2.48 | 5.0 | 0.202 | MF channel |
| [128] | Coplanar TX line | AG (50 μL) | 0–70 | 5.81 | 2.88 | — | 0.109 | Wireless system |
| [131] | Plasmonic, planar WGM | AG (MF) | 0–20 | 4.15 | 3.39 | — | 0.228 | MF channel |
| [30] | IDC resonator | AG (125 μL) | 0–1 | 4.80 | 3.43 | 14.0 | 0.408 | Pressure correction |
| [129] | Coplanar IDC | AG (15 μL) | 0–100 | 4.8 | 3.9 | — | 0.060 | Non-reciprocal |
| [119] | Circular SIW | AG (2.5 μL) | 0–30 | 4.40 | 4.33 | — | 0.089 | — |
| [117] | CSRR patch | AG (2.3 μL) | 0.3–0.7 | 5.00 | 4.39 | 6.8 | 0.155 | — |
| [132] | Corona res. | AG (—) | 0.1–0.5 | 6.25 | 7.01 | 7.25 | 0.103 | — |
| [123] | Triple open SRR | AG (MF) | 0–40 | 6.50 | 5.40 | — | 0.035 | MF channel |
| [126] | Single CELC | Goat blood (MF) | 0.1–0.5 | 1.71 | 1.64 | 56.0 | 3.415 | Reusable, MF channel |
| [121] | Tag single SRR | Mimicked ISF (200 μL) | 0–0.5 | 4.35 | 3.76 | 2.11 | 0.056 | RF tag sensor |
| [132] | Corona res. | Blood (—) | 0.1–0.5 | 6.25 | 7.01 | 3.50 | 0.050 | — |
| [98] | Single SRR | Blood plasma (—) | 0.09–0.15 | 8.33 | 8.32 | 123.08 | 1.479 | Defected ground |
| Ref. | Sensor Type | Sample (Volume) | 𝜌g Range (wt%) | Op. f (GHz) | Bare S11/S21 (dB) | Operating S11/S21 (dB) | SS11/SS21 (dB Per mg/dL) | RS(G) (%/%) | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| [92] | Differential double SRR | AG (MF) | 0–10 | 0.75 | — | −12.75 | 0.55 × 10−3 | 4.314 | MF channel, differential |
| [120] | Four SRR structure | AG (7.5 mL) | 0–0.3 | 1.80 | −12.65 | −10.75 | 0.42 × 10−3 | 3.923 | Portable solution |
| [136] | Single SRR | AG (20 μL) | 0–20 | 1.96 | −17.20 | −16.40 | — | 0.264 | — |
| [127] | MLIN res. | AG (—) | 0–0.3 | 2.00 | — | −11.17 | 0.12 × 10−3 | 1.044 | — |
| [130] | ENG unit-cell resonator | AG (2 μL) | 0–10 | 2.09 | −21.00 | −19.60 | — | 0.506 | SRR- and horn-shaped elements |
| [137] | Single SRR | AG (20 μL) | 0–100 | 2.41 | −12.80 | −13.20 | — | 0.386 | — |
| [135] | Single CSRR | AG (MF) | 0–0.4 | 2.42 | −28.00 | −22.00 | 0.08 × 10−3 | 0.341 | Temp. corrected, MF channel |
| [118] | Double CSRR | AG (MF) | 0–0.4 | 2.42 | −28.00 | −16.80 | 0.08 × 10−3 | 0.446 | Temp. and humidity corr., MF |
| [139] | Coplanar ELC | AG (20 μL) | 4–20 | 3.41 | −19.50 | −4.80 | — | 0.651 | — |
| [129] | Coplanar IDC | AG (15 μL) | 0–100 | 3.90 | −27.50 | −18.00 | — | 0.850 | Non-reciprocal |
| [105] | Two coupled SRR | AG (5 μL) | 0–10 | 4.23 | −7.21 | −10.48 | — | 3.244 | Inter-resonators coupling |
| [119] | Circular SIW | AG (2.5 μL) | 0–30 | 4.33 | −4.63 | −9.16 | — | 1.380 | — |
| [86] | MLIN res. | AG (7.5 mL) | 0.08–5 | 4.88 | — | −22.1 | 0.52 × 10−3 | 2.344 | — |
| [94] | Single SRR | AG (25 μL) | 0–10 | 5.16 | −14.27 | −25.50 | — | 0.329 | — |
| [140] | T-shaped line | AG (0.6 mL) | 0–0.6 | 6.00 | — | −15.00 | 0.54 × 10−3 | 3.600 | Finger shaped |
| [141] | λ/4 stub with IDC structure | AG (MF) | 0–8 | 7.50 | −17.80 | −9.00 | 0.72 × 10−3 | 4.889 | MF channel |
| [138] | Coplanar single SRR | AG (MF) | 0–1.2 | 18.63 | −24.85 | −19.84 | 0.23 × 10−3 | 1.159 | Chromium-gold layer, MF |
| [139] | Coplanar ELC | PBS (20 μL) | 4–20 | 3.41 | −19.5 | −3.90 | — | 0.321 | — |
| [121] | Tag single SRR | Mimicked ISF (200 μL) | 0–0.5 | 3.76 | −41.00 | −56.00 | 0.83 × 10−3 | 1.486 | RF tag sensor |
| [31] | Single SRR | Blood plasma (25 μL) | 0–10 | 5.17 | −14.27 | −25.08 | — | 0.163 | Multicomponent solutions study |
| [132] | Corona res. | Blood (—) | 0.1–0.5 | 7.01 | — | −27.50 | 0.57 × 10−3 | 2.045 | — |
| Ref. | Sensor Type | Sample (Volume) | 𝜌g Range (wt%) | Op. f (GHz) | Bare Qu | Operating Qu | SQu (Per mg/dL) | RS(G) (%/%) | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| [81] | Open-loop line resonator | AG (5 μL) | 0–4 | 1.11 | — | 187.14 | — | 0.186 | — |
| [143] | Single SRR | AG (200 μL) | 0–20 | 2.45 | — | 60.00 | — | 0.948 | Coplanar WG |
| [149] | Asymmetric single SRR | AG (—) | 0–5 | 4.50 | 118.78 | 116.18 | — | 1.511 | — |
| [150] | Coplanar λ/4 resonator | AG (5 μL) | 0–10 | 4.79 | 96.80 | 85.46 | — | 0.901 | Temperature effect analysis |
| [94] | Single SRR | AG (25 μL) | 0–10 | 5.16 | 112.31 | 60.65 | — | 0.978 | — |
| [94] | Single SRR | AG (5 μL) | 0–10 | 7.16 | 109.27 | 72.68 | — | 0.584 | — |
| [31] | Single SRR | Blood plasma (25 μL) | 0–10 | 5.17 | 112.31 | 58.16 | — | 0.829 | Multicomponent solutions study |
| [31] | Single SRR | Blood plasma (5 μL) | 0–10 | 7.17 | 109.27 | 64.99 | — | 0.571 | Multicomponent solutions study |
| [105] | Two coupled SRR | AG (5 μL) | 0–10 | 4.23 | 89.85 | 47.63 | — | 4.115 | Inter-resonators coupling |
| Ref. | Sensor Type | Sample (Volume) | 𝜌g Range (wt%) | Op. f (GHz) | Operating ϕS11/ϕS21 (°) | SϕS11/SϕS21 (° per mg/dL) | SϕS11/SϕS21 (° per wt%) | Remarks |
|---|---|---|---|---|---|---|---|---|
| [88] | Closed-loop ring | AG (—) | 0–0.25 | 4.02 | −66.00 | 4.694 × 10−3 | 4.694 | — |
| [86] | MLIN res. | AG (7.5 mL) | 0.08–5 | 4.88 | −111.43 | 1.510 × 10−3 | 1.510 | — |
| [123] | Triple open SRR | AG (MF) | 0–40 | 5.40 | 134.38 | — | 7.813 | MF channel |
| [154] | MLIN-based sensor | AG (—) | 0–5 | 7.81 | — | 0.037 × 10−3 | 0.037 | Portable, PRN-driven system |
| [153] | MLIN res. | AG (—) | 0–0.11 | 19.04 | −82.79 | 3.182 × 10−3 | 3.182 | Only simulations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E. Microwave Planar Resonant Solutions for Glucose Concentration Sensing: A Systematic Review. Appl. Sci. 2021, 11, 7018. https://doi.org/10.3390/app11157018
Juan CG, Potelon B, Quendo C, Bronchalo E. Microwave Planar Resonant Solutions for Glucose Concentration Sensing: A Systematic Review. Applied Sciences. 2021; 11(15):7018. https://doi.org/10.3390/app11157018
Chicago/Turabian StyleJuan, Carlos G., Benjamin Potelon, Cédric Quendo, and Enrique Bronchalo. 2021. "Microwave Planar Resonant Solutions for Glucose Concentration Sensing: A Systematic Review" Applied Sciences 11, no. 15: 7018. https://doi.org/10.3390/app11157018
APA StyleJuan, C. G., Potelon, B., Quendo, C., & Bronchalo, E. (2021). Microwave Planar Resonant Solutions for Glucose Concentration Sensing: A Systematic Review. Applied Sciences, 11(15), 7018. https://doi.org/10.3390/app11157018








