Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Solvents
2.3. Controlled Atmosphere Conditions during Apple Storage
2.4. The Evaluation of Sugars
2.5. The Determination of Soluble Solids
2.6. The Evaluation of Titratable Acidity
2.7. The Determination of Ascorbic Acid
2.8. The Determination of the Content of Soluble Solids
2.9. Fruit Color Measurement
2.10. Fruit Firmness Measurement
2.11. Statistical Analysis
3. Results and Discussion
3.1. Changes in Chemical Composition Before and after Storage in CA
3.1.1. Variability of the Composition of Sugars, Sucrose, and Ascorbic Acid
3.1.2. Evaluation of Soluble Solids and Titratable Acidity
3.2. Changes in Organoleptic Characteristics of Apple before and after Storage in CA
3.2.1. Changes in Apple Peel Color
3.2.2. Changes in Apple Peel and Flesh Firmness
3.3. Evaluation of Apple Weight Loss and Quality during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanrong, L. Triterpenes and phenolic compounds in apple fruit (Malus domestica Borkh). Acta Univ. Agric. Suec. Agrar. 2016, 5, 13–24. [Google Scholar]
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 23 January 2021).
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hotric. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Bi, J.; Liu, X.; Li, X.; Guo, C. Polyphenols accumulation effects on surface color variation in apple slices hot airdrying process. LWT—Food Sci. Technol. 2019, 108, 421–428. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverrı, G.; Soria, Y. Differences in fruit colour development, anthocyanin content, fruit quality and consumer acceptability of eight ‘Gala’ apple strains. Sci. Hortic. 2008, 119, 32–40. [Google Scholar] [CrossRef]
- Mditshwa, A. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kviklys, D.; Liaudanskas, M.; Janulis, V.; Uselis, N.; Viškelis, J.; Viškelis, P. Lower nitrogen nutrition determines higher phenolic content of organic apples. Hortic. Sci. 2017, 44, 113–119. [Google Scholar]
- Raudonė, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viškelis, P. Phenolic antioxidant profiles in the whole fruit, flesh and peel of apple cultivars grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2017, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Cantini, C.; Guarnieri, M.; Nepi, M.; Hausman, J.-F.; Guerriero, G.; Romi, M.; Cai, G. Nutraceutical characteristics of ancient Malus × domestica Borkh. fruits recovered across Siena in Tuscany. Medicines 2019, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Oszmianski, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2019, 246, 437–448. [Google Scholar] [CrossRef]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a source of dietary phytonutrients: Bioavailability and evidence of protective effects against human cardiovascular disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Nour, V.; Trandafir, I.; Ionica, M.E. Compositional characteristics of fruits of several apple (Malus domestica Borkh.) cultivars. Not. Bot. Hort. Agrobot. 2010, 38, 228–233. [Google Scholar]
- Morresi, C.; Cianfruglia, L.; Armeni, T.; Mancini, F.; Tenore, G.C.; D’Urso, E.; Micheletti, A.; Ferretti, G.; Bacchetti, T. Polyphenolic compounds and nutraceutical properties of old and new apple cultivars. J. Food Biochem. 2018, 42, e12641. [Google Scholar] [CrossRef]
- Hoang, N.T.T.; Golding, J.B.; Wilkes, M.A. The effect of postharvest 1-MCP treatment and storage atmosphere on ‘Cripps Pink’ apple phenolics and antioxidant activity. Food Chem. 2011, 127, 1249–1256. [Google Scholar] [CrossRef]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Zanella, A.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Klein, B.; Falk, R.B.; Thewes, F.R.; Anese, R.O.; Santos, I.D.; Ribeiro, S.R.; Donadel, J.Z.; Brackmann, A.; Barin, J.S.; Cichoski, A.J.; et al. Dynamic controlled atmosphere: Effects on the chemical composition of cuticular wax of ‘Cripps pink’ apples after long-term storage. Postharvest Biol. Technol. 2020, 164, 111170. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.; Soethe, C.; Moreira, M.A.; Amarante, C.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- de Oliveira Anese, R.; Brackmann, A.; Wendt, L.M.; Thewes, F.R.; Schultz, E.E.; Ludwig, V.; Berghetti, M.R.P. Interaction of 1-methylcyclopropene, temperature and dynamic controlled atmosphere by respiratory quotient on ‘Galaxy’ apples storage. Food Packag. Shelf Life 2019, 20, 100246. [Google Scholar] [CrossRef]
- Vondráková, Z.; Trávníčková, A.; Malbeck, J.; Haisel, D.; Černý, R.; Cvikrová, M. The effect of storage conditions on the carotenoid and phenolic acid contents of selected apple cultivars. Eur. Food Res. Technol. 2020, 246, 1783–1794. [Google Scholar] [CrossRef]
- Anese, R.D.O.; Thewes, F.R.; Brackmann, A.; Schultz, E.E.; Wagner, R.; Klein, B.; Berghetti, M.R.P.; Wendt, L.M. Growth regulators on quality traits and volatile organic compounds profile of ‘Royal Gala’ apple at harvest and after dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2020, 164, 111158. [Google Scholar] [CrossRef]
- Santos, I.D.; Pizzutti, I.R.; Dias, J.V.; Fontana, M.; Brackmann, A.; Anese, R.O.; Thewes, F.R.; Marques, L.N.; Cardoso, C.D. Patulin accumulation in apples under dynamic controlled atmosphere storage. Food Chem. 2018, 255, 275–281. [Google Scholar] [CrossRef]
- Thewes, F.R.; Brackmann, A.; Neuwald, D.A. Dynamics of sugars, anaerobic metabolism enzymes and metabolites in apples stored under dynamic controlled atmosphere. Sci. Hortic. 2019, 255, 145–152. [Google Scholar] [CrossRef]
- Bessemans, N.; Verboven, P.; Verlinden, B.E.; Nicolaï, B.M. A novel type of dynamic controlled atmosphere storage based on the respiratory quotient (RQ-DCA). Postharvest Biol. Technol. 2016, 115, 91–102. [Google Scholar] [CrossRef]
- Konopacka, D.; Plocharski, W.J. Effect of storage conditions on the relationship between apple firmness and texture acceptability. Postharvest Biol. Technol. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Hertog, M.; Nicolai, B.; Verlinden, B.E.; Impe, J.F.M.; Geeraerd, A. Towards flexible management of postharvest variation in fruit firmness of three apple cultivars. Postharvest Biol. Technol. 2013, 85, 18–29. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Sun, Y.; Xu, N.; Leng, Y. Prediction of firmness and pH for ‘Golden delicious’ apple based on elasticity index from modal analysis. J. Food Sci. 2018, 83, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Cappellina, L.; Fontanaria, M.; Longhia, S.; Guerrab, W.; Magnagoa, P.; Gasperia, F.; Biasioli, F. Texture dynamics during postharvest cold storage ripening in apple (Malus × domestica Borkh.). Postharvest Biol. Technol. 2012, 69, 54–63. [Google Scholar] [CrossRef]
- Liu, Y.; Che, F.; Wang, L.; Meng, R.; Zhang, X.; Zhao, Z. Fruit coloration and anthocyanin biosynthesis after bag removal in non-red and red apples (Malus × domestica Borkh.). Molecules. 2013, 18, 1549–1563. [Google Scholar] [CrossRef]
- Wang, W.; Celton, J.M.; Sorlin, G.B.; Balzergue, S.; Bucher, E.; Laurens, F. Skin color in apple fruit (Malus domestica): Genetic and epigenetic insights. Epigenomes 2020, 4, 13. [Google Scholar] [CrossRef]
- Guan, Y.; Peace, C.; Rudell, D.; Verma, S.; Evans, K. QTLs detected for individual sugars and soluble solids content in apple. Mol. Breed. 2015, 35, 135. [Google Scholar] [CrossRef]
- Cichowska, J.; Woźniak, L.; Figiel, A.; Rajchert, D.W. The influence of osmotic dehydration in polyols solutions on sugar profiles and color changes of apple tissue. Period. Polytech. Chem. Eng. 2020, 64, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 4950. [Google Scholar] [CrossRef] [PubMed]
- Dobrzański, B.; Rabcewicz, J.; Rybczyński, R.; Bylica, T. Handling of Apple: Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality; Institute of Agrophysics Polish Academy of Sciences: Lublin, Poland, 2006; pp. 33–177. [Google Scholar]
- Helrich, K.E. the Association of Official Analytical Chemists. AOACa in AOAC Official Methods of Analysis; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Helrich, K.E. Vitamin C (Ascorbic Acid) in Vitamin Preparations and Juices. AOACb in AOAC Official Methods of Analysis; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Luksiene, Z.; Buchovec, I.; Kairyte, K.; Paskeviciute, E.; Viskelis, P. High-power pulsed light for microbial decontamination of some fruits and vegetables with different surfaces. J. Food Agric. Environ. 2012, 10, 162–167. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. Hortic. Sci. 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Watkins, C.B. Dynamic Controlled atmosphere storage—A new technology for the New York storage industry. N. Y. Fruit Q. 2008, 16, 23–27. [Google Scholar]
- Jan, I.; Rab, A. Influence of storage duration on physico-chemical changes in fruit of apple cultivars. J. Anim. Plant Sci. 2012, 22, 708–714. [Google Scholar]
- Zhu, Z.; Liu, R.; Li, B.; Tian, S. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013, 141, 3323–3328. [Google Scholar] [CrossRef]
- Gorkom, G.N.Y.; Lookermans, E.L.; Elssen, C.H.M.J.; Bos, G.M.J. The effect of vitamin C (ascorbic acid) in the treatment of patients with cancer: A systematic review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [Green Version]
- Oyetade, O.A.; Oyeleke, G.O.; Adegoke, B.M.; Akintunde, A.O. Stability studies on ascorbic acid (vitamin c) from different sources. IOSR-JAC 2012, 2, 20–24. [Google Scholar]
- Łysiak, G.P.; Ciechanowska, A.M.; Wojdyło, A. Postharvest changes in phenolic compounds and antioxidant capacity of apples cv. Jonagold growing in different locations in Europe. Food Chem. 2020, 310, 125912. [Google Scholar] [CrossRef] [PubMed]
- Riveria, J. Cutting shape and storage temperature affect overall quality of fresh cut papaya cv. Maradol. J. Food Sci. 2005, 70, 488–489. [Google Scholar]
- Supapvanich, S.; Pimsaga, J.; Srisujan, P. Physicochemical changes in fresh-cut wax apple (Syzygium samarangenese [Blume] Merrill & L.M. Perry) during storage. Food Chem. 2011, 127, 912–917. [Google Scholar]
- Ghafir, S.A.M.; Gadalla, S.O.; Murajei, B.N.; El-Nady, M.F. Physiological and anatomical comparison between four different apple cultivars under cold-storage conditions. Afr. J. Plant Sci. 2009, 3, 133–138. [Google Scholar]
- Clarke, C.J.; McGlone, V.A.; Jordan, R.B. Detection of brownheart in ‘Braeburn’ apple by transmission NIR spectroscopy. Postharvest Biol. Technol. 2003, 28, 87–96. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Feygenberg, O.; Maurer, D.; Alkan, N. Improved cold tolerance of mango fruit with enhanced anthocyanin and flavonoid contents. Molecules 2018, 23, 1832. [Google Scholar] [CrossRef] [Green Version]
- Dobrzañski, B.; Rybczyñski, R. Colour change of apple as a result of storage, shelf-life, and bruising. Int. Agrophysics 2002, 16, 261–268. [Google Scholar]
- Telias, A.; Bradeen, J.M.; Luby, J.J.; Hoover, E.E.; Allen, A.C. Regulation of anthocyanin accumulation in apple peel. Hortic. Rev. 2011, 38, 357. [Google Scholar]
- Sethi, S.; Joshi, A.; Arora, B.; Bhowmik, A.; Sharma, R.R.; Kumar, P. Significance of FRAP, DPPH, and CUPRAC assays for antioxidant activity determination in apple fruit extracts. Eur. Food Res. Technol. 2020, 246, 591–598. [Google Scholar] [CrossRef]
- Deun, E.L.; Werf, R.; Lebail, G.; Quéré, J.M.; Guyot, S. HPLC-DAD-MS profiling of polyphenols responsible for yellow-orange color in apple juices of different French cider apple varieties. J. Agric. Food Chem. 2015, 63, 7675–7684. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical profiles of new red-fleshed apple varieties compared with old and new white-fleshed varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverrí, G.; Lopez, M.L. Fruit color development, anthocyanin content, standard quality, volatile compound emissions and consumer acceptability of several ‘Fuji’ apple strains. Sci. Hortic. 2012, 137, 138–147. [Google Scholar] [CrossRef]
- Afzadi, M.A.; Tahir, I.; Nybom, H. Impact of harvesting time and fruit firmness on the tolerance to fungal storage diseases in an apple germplasm collection. Postharvest Biol. Technol. 2013, 82, 51–58. [Google Scholar] [CrossRef]
- Billy, L.; Mehinagic, E.; Royer, G.; Renard, C.M.G.C.; Arvisenet, G.; Prost, C.; Jourjon, F. Relationship between texture and pectin composition of two apple cultivars during storage. Postharvest Biol. Technol. 2008, 47, 315–324. [Google Scholar] [CrossRef]
- Gajewski, M.; Szymczak, P.; Radzanowska, J. Sensory quality of orange, purple and yellow carrots stored under controlled atmosphere. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 169–176. [Google Scholar]
- Čekanavičius, V.; Murauskas, G. Applied Regression Analysis in Social Research; Vilnius University Press: Vilnius, Lithuania, 2014; p. 124. [Google Scholar]
- Kamiloglu, O. Influence of some cultural practices on yield, fruit quality and individual anthocyanins of table grape cv. ‘Horoz Karasi’. J. Anim. Plant Sci. 2011, 21, 240–245. [Google Scholar]
- Peck, G.M.; Andrews, P.K.; Reganold, J.P.; Fellman, J.K. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hortic. Sci. 2006, 41, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Golias, J.; Mylova, P.; Nemcova, A. A comparison of apple cultivars regarding ethylene production and physico-chemical changes during cold storage. Hortic. Sci. 2008, 35, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Hoehn, E.; Gasser, F.; Guggenenbuhl, B.; Kunsch, U. Efficacy of instrumental measurements for determination of minimum requirements of firmness, soluble solids, and acidity of varieties in comparison to consumer expectations. Postharvest Biol. Technol. 2003, 27, 27–37. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, S.; Kasi, M.A.; Khair, S.M. Post harvest and cold storage losses in apple of Balochistan. Asian J. Plant Sci. 2002, 1, 65–66. [Google Scholar]
- Ilyas, M.B.; Ghazanfar, M.U.; Khan, M.A.; Khan, C.A.; Bhatti, M.A.R. Postharvest losses in apple and banana during transport and storage. Pakistan J. Agric. Sci. 2007, 44, 534. [Google Scholar]
- Veravrbeke, E.A.; Verboven, P.; Oostveldt, P.; Nicolai, B.M. Predication of moisture loss across the cuticle of apple (Malus sylvestris supsp. Mitis (Wallr.) during storage: Part 2. Model simulations and practical applications. Postharvest Biol. Technol. 2003, 30, 89–97. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Wongs-Aree, C.; Setha, S.; Komkhuntod, R.; Kondo, S.; Srilaong, V. Pre-harvest drought stress treatment improves antioxidant activity and sugar accumulation of sugar apple at harvest and during storage. Agric. Nat. Resour. 2018, 52, e146–e154. [Google Scholar] [CrossRef]
- Awad, M.A.; Jager, A.; Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Jakopič, J.; Stampar, F.; Verberič, R. The influence of exposure to light on the phenolic content of ‘Fuji’ apple. Sci. Hortic. 2009, 123, 234–239. [Google Scholar] [CrossRef]
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Lanauskas, J.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Location effects across northeastern Europe on bioactive compounds in apple fruit. J. Sci. Food Agric. 2019, 28, 93–100. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. J. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Viskelis, P.; Rubinskienė, M.; Bobinaitė, R.; Dambrauskienė, E. Bioactive compounds and antioxidant activity of small fruits in Lithuania. JFAE 2010, 8, 259–263. [Google Scholar]
- Novell, C.G.; Marin, D.P.; Amigo, J.M.; Novales, J.F.; Guerrero, J.E.; Varo, A.G. Grading and color evolution of apples using RGB and hyperspectral imaging vision cameras. J. Food Eng. 2012, 113, 281–288. [Google Scholar] [CrossRef]
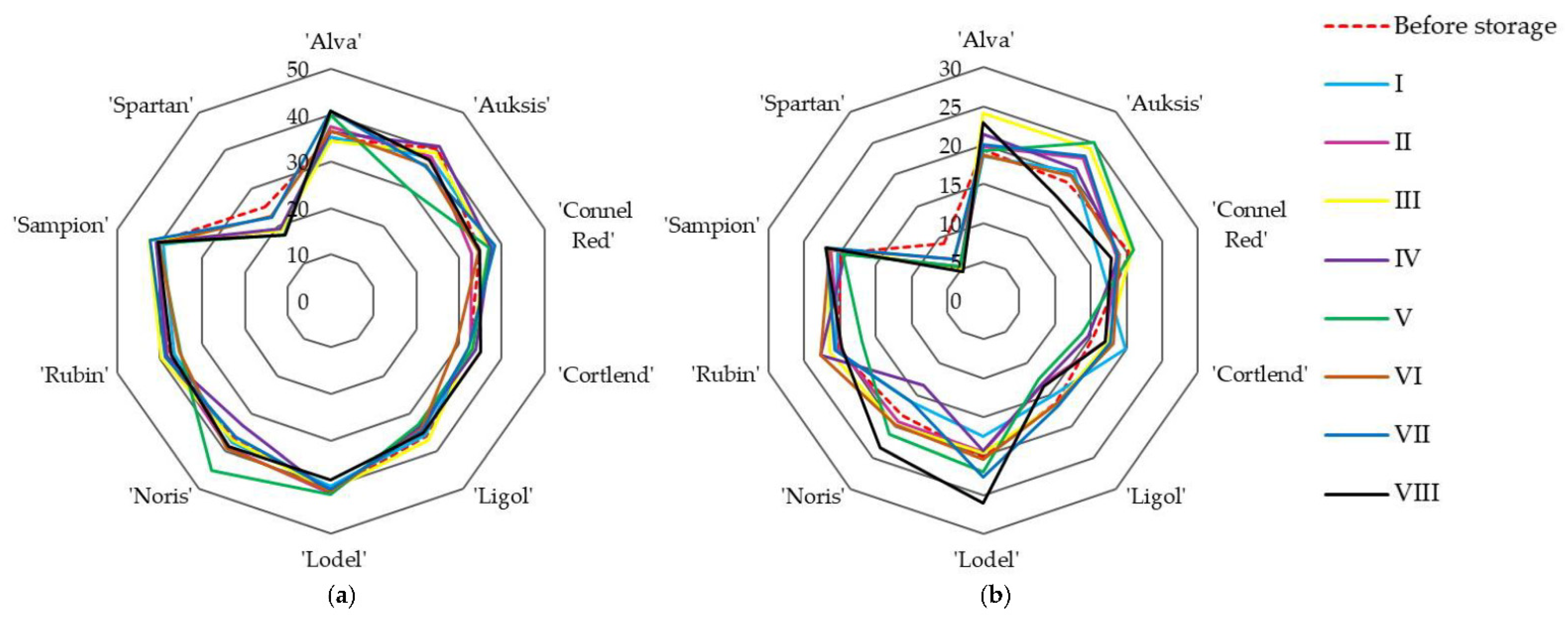
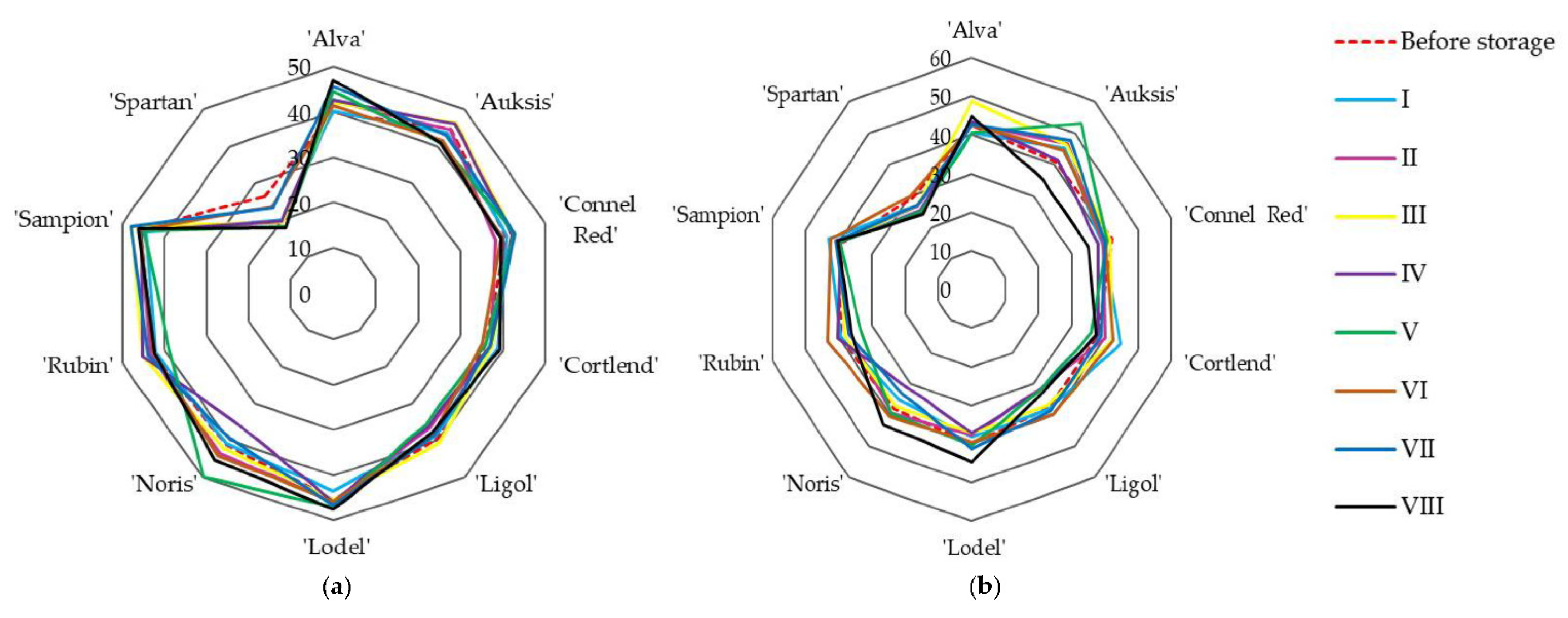
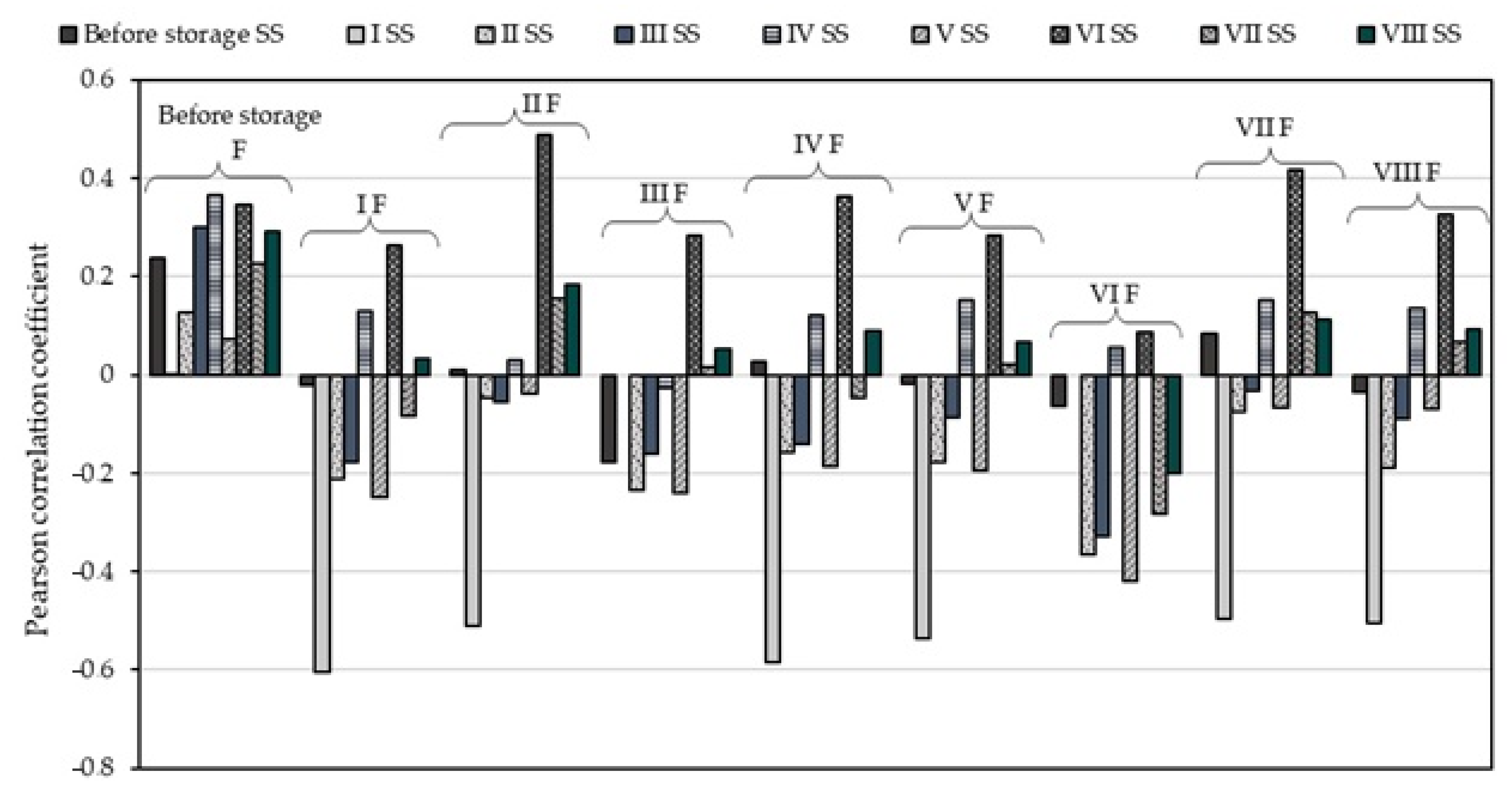
| Variant | Amount of Oxygen (O2), % | Amount of Carbon Dioxide (CO2), % | Amount of Nitrogen (N2), % | Relative Humidity, % | Temperature, °C |
|---|---|---|---|---|---|
| I | 21 | 0.03 | 78.97 | 95 ± 3 | +1.5 ± 0.5 |
| II | 5 | 1 | 94 | ||
| III | 5 | 3 | 92 | ||
| IV | 5 | 5 | 90 | ||
| V | 5 | 7 | 88 | ||
| VI | 1 | 3 | 96 | ||
| VII | 10 | 3 | 87 | ||
| VIII | 20 | 3 | 77 |
| Before Storage | I | II | III | IV | V | VI | VII | VIII | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % | TS, % | CS, % |
| ‘Alva’ | 10.09 | 2.75 | 13.55 | 3.77 | 12.63 | 3.16 | 12.87 | 3.59 | 13.02 | 3.24 | 13.02 | 3.24 | 13.04 | 2.98 | 13.17 | 3.05 | 12.78 | 2.85 |
| ‘Auksis’ | 10.31 | 3.15 | 13.44 | 3.32 | 13.42 | 3.64 | 13.00 | 3.74 | 12.75 | 3.24 | 13.58 | 3.18 | 13.45 | 2.78 | 12.79 | 2.52 | 13.35 | 2.22 |
| ‘Connel Red’ | 10.90 | 2.43 | 12.88 | 3.28 | 13.99 | 3.47 | 13.45 | 3.20 | 13.44 | 2.96 | 13.03 | 3.10 | 14.13 | 3.19 | 13.31 | 2.91 | 13.62 | 2.42 |
| ‘Cortlend’ | 9.42 | 2.22 | 13.97 | 3.72 | 14.54 | 3.60 | 14.16 | 3.22 | 13.16 | 3.29 | 13.27 | 3.76 | 12.80 | 2.34 | 13.07 | 2.40 | 13.21 | 2.41 |
| ‘Ligol’ | 9.15 | 2.07 | 12.04 | 3.79 | 12.32 | 3.74 | 11.82 | 2.75 | 12.46 | 3.54 | 12.34 | 2.96 | 13.69 | 3.44 | 13.50 | 2.28 | 13.18 | 2.91 |
| ‘Lodel’ | 10.22 | 2.68 | 14.13 | 3.73 | 14.55 | 3.33 | 14.12 | 2.99 | 12.76 | 3.16 | 13.18 | 3.00 | 13.34 | 2.40 | 13.74 | 2.54 | 12.82 | 2.09 |
| ‘Noris’ | 10.12 | 2.00 | 13.02 | 3.42 | 12.94 | 3.28 | 13.43 | 3.18 | 13.32 | 2.80 | 12.75 | 3.24 | 12.77 | 2.99 | 12.91 | 2.85 | 12.62 | 3.24 |
| ‘Rubin’ | 11.14 | 3.02 | 12.76 | 3.16 | 14.66 | 3.72 | 15.07 | 3.94 | 14.27 | 3.05 | 14.14 | 2.88 | 14.25 | 3.58 | 13.61 | 2.61 | 13.92 | 2.52 |
| ‘Sampion’ | 10.86 | 3.24 | 13.83 | 3.90 | 12.87 | 3.61 | 12.78 | 2.84 | 12.47 | 3.42 | 12.77 | 3.11 | 12.19 | 2.91 | 12.70 | 1.90 | 13.31 | 2.85 |
| ‘Spartan’ | 10.74 | 3.00 | 12.74 | 3.54 | 12.72 | 3.80 | 12.34 | 3.42 | 12.87 | 3.59 | 12.45 | 3.65 | 12.99 | 3.82 | 12.76 | 3.16 | 12.79 | 2.61 |
| Before Storage | I | II | III | IV | V | VI | VII | VIII | |
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | mg/100 g | ||||||||
| ‘Alva’ | 7.20 | 6.40 | 6.40 | 6.00 | 6.40 | 6.00 | 6.00 | 6.40 | 6.40 |
| ‘Auksis’ | 7.20 | 6.40 | 6.00 | 6.00 | 6.40 | 6.00 | 5.60 | 6.00 | 6.00 |
| ‘Connel Red’ | 8.00 | 7.20 | 7.20 | 6.00 | 5.60 | 6.40 | 6.40 | 6.40 | 6.40 |
| ‘Cortlend’ | 7.20 | 6.00 | 6.00 | 5.60 | 6.00 | 6.40 | 6.00 | 6.40 | 6.00 |
| ‘Ligol’ | 7.20 | 6.40 | 6.40 | 6.40 | 6.80 | 6.40 | 6.40 | 6.00 | 6.80 |
| ‘Lodel’ | 8.00 | 6.40 | 7.20 | 6.40 | 7.20 | 6.40 | 6.40 | 6.80 | 6.40 |
| ‘Noris’ | 8.00 | 5.60 | 6.00 | 6.00 | 6.00 | 5.60 | 6.40 | 6.80 | 6.40 |
| ‘Rubin’ | 7.20 | 6.40 | 6.00 | 6.00 | 6.00 | 6.00 | 6.40 | 6.00 | 6.40 |
| ‘Sampion’ | 8.80 | 8.00 | 8.00 | 8.00 | 6.40 | 7.20 | 7.20 | 7.20 | 8.00 |
| ‘Spartan’ | 8.00 | 6.80 | 6.40 | 6.40 | 6.40 | 6.80 | 6.00 | 6.40 | 7.20 |
| Before Storage | I | II | III | IV | V | VI | VII | VIII | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % | SS, % | TA, % |
| ‘Alva’ | 13.00 | 0.50 | 15.00 | 0.37 | 14.20 | 0.37 | 14.00 | 0.37 | 14.50 | 0.34 | 14.40 | 0.41 | 14.50 | 0.37 | 14.60 | 0.34 | 14.00 | 0.37 |
| ‘Auksis’ | 13.00 | 0.50 | 14.80 | 0.40 | 15.00 | 0.42 | 14.40 | 0.42 | 14.00 | 0.37 | 14.80 | 0.40 | 14.80 | 0.37 | 14.00 | 0.34 | 14.80 | 0.33 |
| ‘Connel Red’ | 13.60 | 0.24 | 14.60 | 0.21 | 16.00 | 0.20 | 14.80 | 0.17 | 15.20 | 0.18 | 14.40 | 0.21 | 15.50 | 0.18 | 14.60 | 0.17 | 15.40 | 0.21 |
| ‘Cortlend’ | 13.50 | 0.61 | 15.40 | 0.45 | 15.80 | 0.40 | 16.00 | 0.42 | 15.80 | 0.46 | 14.60 | 0.50 | 14.60 | 0.48 | 15.00 | 0.45 | 15.00 | 0.48 |
| ‘Ligol’ | 12.40 | 0.32 | 13.50 | 0.18 | 14.00 | 0.18 | 14.30 | 0.18 | 14.00 | 0.21 | 14.20 | 0.18 | 15.00 | 0.16 | 14.80 | 0.20 | 15.00 | 0.16 |
| ‘Lodel’ | 13.20 | 0.45 | 15.60 | 0.34 | 16.80 | 0.36 | 16.00 | 0.36 | 14.40 | 0.34 | 15.20 | 0.37 | 15.00 | 0.34 | 17.20 | 0.32 | 15.20 | 0.34 |
| ‘Noris’ | 13.50 | 0.61 | 14.60 | 0.50 | 14.70 | 0.45 | 15.20 | 0.45 | 16.00 | 0.48 | 14.00 | 0.52 | 14.20 | 0.48 | 14.20 | 0.45 | 14.20 | 0.49 |
| ‘Rubin’ | 14.00 | 0.50 | 15.00 | 0.40 | 17.60 | 0.37 | 16.60 | 0.40 | 16.20 | 0.37 | 16.60 | 0.42 | 16.00 | 0.37 | 16.80 | 0.36 | 16.50 | 0.34 |
| ‘Sampion’ | 12.60 | 0.27 | 15.00 | 0.16 | 14.70 | 0.18 | 13.80 | 0.18 | 13.80 | 0.16 | 14.00 | 0.21 | 14.00 | 0.18 | 14.00 | 0.17 | 14.40 | 0.18 |
| ‘Spartan’ | 12.80 | 0.48 | 14.00 | 0.37 | 14.20 | 0.34 | 13.60 | 0.42 | 14.00 | 0.34 | 14.00 | 0.37 | 14.40 | 0.34 | 14.00 | 0.37 | 14.00 | 0.33 |
| Before Storage | I | II | III | IV | V | VI | VII | VIII | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | Peel, N cm−2 | Flesh, N cm−2 | |
| Cultivar | Mean ± SD | |||||||||||||||||
| ‘Alva’ | 278.2 ± 22.7 | 70.3 ± 6.6 | 229.2 ± 12.3 | 56.1 ± 4.5 | 257.8 ± 28.7 | 59.5 ± 8.8 | 235.7 ± 16.6 | 66.7 ± 9.1 | 247.7 ± 26.5 | 59.5 ± 6.1 | 251.7 ± 20.8 | 61.1 ± 7.7 | 239.4 ± 32.3 | 61.5 ± 5.0 | 263.0 ± 16.0 | 58.2 ± 7.5 | 251.8 ± 12.0 | 58.9 ± 8.8 |
| ‘Auksis’ | 170.0 ± 40.1 | 47.7 ± 10.2 | 128.4 ± 19. | 40.0 ± 6.8 | 179.2 ± 12.2 | 40.4 ± 6.6 | 150.6 ± 6.8 | 41.2 ± 2.9 | 155.8 ± 5.7 | 40.1 ± 3.6 | 121.9 ± 31.6 | 37.3 ± 1.9 | 162.2 ± 42.3 | 48.9 ± 11.5 | 155.5 ± 13.2 | 39.8 ± 3.5 | 128.7 ± 10.4 | 41.5 ± 9.4 |
| ‘Connel Red’ | 310.3 ± 39.8 | 82.9 ± 15.9 | 283.1 ± 19.9 | 79.6 ± 11.2 | 310.1 ± 36.9 | 72.7 ± 25.4 | 280.9 ± 39.7 | 87.1 ± 25.4 | 316.8 ± 34.1 | 78.3 ± 7.3 | 295.1 ± 54.9 | 78.7 ± 17.1 | 302.3 ± 14.7 | 70.8 ± 13.6 | 301.8 ± 14.7 | 79.3 ± 9.3 | 241.5 ± 22.6 | 68.6 ± 10.3 |
| ‘Cortlend’ | 215.0 ± 26.9 | 52.6 ± 2.2 | 174.5 ± 10.1 | 39.5 ± 2.5 | 188.2 ± 19.3 | 43.5 ± 4.8 | 190.7 ± 9.7 | 44.9 ± 4.9 | 179.1 ± 18.2 | 45.4 ± 5.9 | 174.6 ± 9.2 | 48.4 ± 7.4 | 170.6 ± 15.2 | 41.6 ± 3.9 | 182.5 ± 12.8 | 38.8 ± 0.9 | 164.0 ± 31.9 | 42.6 ± 4.9 |
| ‘Ligol’ | 307.9 ± 38.4 | 62.2 ± 5.3 | 275.2 ± 42.4 | 46.9 ± 2.7 | 303.1 ± 42.5 | 57.5 ± 8.4 | 314.2 ± 34.6 | 53.8 ± 10.9 | 295.8 ± 17.6 | 48.6 ± 2.4 | 309.5 ± 23.3 | 52.5 ± 5.2 | 275.7 ± 25.5 | 50.4 ± 4.3 | 291.6 ± 27.7 | 49.2 ± 4.4 | 275.5 ± 31.3 | 47.0 ± 5.9 |
| ‘Lodel’ | 231.4 ± 31.0 | 44.2 ± 2.9 | 174.3 ± 21.9 | 36.8 ± 8.6 | 235.6 ± 58.5 | 43.5 ± 13.8 | 210.8 ± 37.1 | 45.3 ± 6.8 | 193.8 ± 18.3 | 40.7 ± 2.2 | 192.8 ± 29.6 | 37.5 ± 2.9 | 184.9 ± 8.1 | 33.9 ± 2.7 | 222.2 ± 44.8 | 35.3 ± 10.4 | 170.2 ± 21.2 | 30.9 ± 2.8 |
| ‘Noris’ | 275.2 ± 35.7 | 59.9 ± 10.5 | 223.1 ± 21.3 | 50.7 ± 4.5 | 218.3 ± 60.4 | 59.6 ± 15.7 | 219.1 ± 33.6 | 48.3 ± 10.5 | 235.5 ± 42.3 | 56.85 ± 6.48 | 242.0 ± 43.3 | 56.4 ± 10.5 | 267.2 ± 44.7 | 61.4 ± 9.1 | 234.9 ± 49.1 | 55.1 ± 8.2 | 203.9 ± 42.8 | 46.9 ± 7.1 |
| ‘Rubin’ | 224.4 ± 31.4 | 63.7 ± 2.9 | 204.3 ± 12.6 | 58.8 ± 5.2 | 229.2 ± 33.4 | 58.1 ± 7.3 | 202.9 ± 44.5 | 56.6 ± 5.0 | 216.7 ± 27.1 | 57.9 ± 10.9 | 210.2 ± 12.5 | 57.6 ± 5.1 | 193.0 ± 18.9 | 54.2 ± 5.9 | 227.4 ± 14.8 | 58.8 ± 5.2 | 210.5 ± 37.9 | 58.9 ± 8.9 |
| ‘Sampion’ | 165.7 ± 41.3 | 37.1 ± 6.6 | 175.5 ± 7.9 | 32.8 ± 5.1 | 160.2 ± 24.5 | 31.4 ± 5.6 | 175.4 ± 18.8 | 29.4 ± 5.6 | 166.6 ± 12.0 | 28.3 ± 4.2 | 168.0 ± 28.4 | 34.9 ± 5.2 | 173.1 ± 32.8 | 31.8 ± 7.2 | 146.5 ± 25.6 | 26.6 ± 3.4 | 156.8 ± 21.5 | 32.1 ± 6.1 |
| ‘Spartan’ | 289.5 ± 51.0 | 61.6 ± 16.4 | 217.2 ± 37.8 | 29.2 ± 7.6 | 235.2 ± 43.2 | 36.3 ± 11.3 | 226.4 ± 44.9 | 38.5 ± 13.8 | 225.2 ± 34.8 | 37.3 ± 8.9 | 198.9 ± 26.6 | 36.7 ± 7.5 | 264.0 ± 51.9 | 47.8 ± 16.5 | 234.7 ± 44.8 | 31.1 ± 5.9 | 178.8 ± 25.1 | 47.7 ± 11.6 |
| I | II | III | IV | V | VI | VII | VIII | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | WL, % | QL, % | WL, % | QL, % | WL, % | QL, % | WL, % | QL, % | WL, % | QL, % | WL, % | QL, % | WL, % | QL, % | WL, % | QL, % |
| ‘Alva’ | 8.30 | 17.10 | 8.20 | 12.60 | 3.90 | 13.00 | 4.70 | 13.80 | 3.40 | 12.60 | 3.90 | 14.50 | 5.10 | 10.50 | 5.20 | 14.60 |
| ‘Auksis’ | 5.20 | 35.80 | 4.70 | 31.60 | 5.10 | 26.20 | 7.00 | 37.60 | 4.10 | 34.30 | 4.70 | 37.90 | 5.00 | 33.50 | 5.00 | 33.90 |
| ‘Connel Red’ | 6.70 | 16.60 | 4.90 | 16.10 | 4.40 | 18.70 | 4.20 | 19.40 | 4.40 | 28.10 | 3.60 | 31.30 | 8.00 | 35.30 | 5.60 | 39.30 |
| ‘Cortlend’ | 7.20 | 18.70 | 8.00 | 12.70 | 3.20 | 14.80 | 3.00 | 13.30 | 1.90 | 3.50 | 2.50 | 16.70 | 6.10 | 17.60 | 8.20 | 12.20 |
| ‘Ligol’ | 5.40 | 19.90 | 5.10 | 16.70 | 2.40 | 3.00 | 2.70 | 22.70 | 1.60 | 5.00 | 1.40 | 10.90 | 3.20 | 11.00 | 4.80 | 11.50 |
| ‘Lodel’ | 6.40 | 12.30 | 6.30 | 11.80 | 5.30 | 16.10 | 7.50 | 9.30 | 5.00 | 9.30 | 3.60 | 13.90 | 5.40 | 15.30 | 6.60 | 15.00 |
| ‘Noris’ | 5.50 | 25.30 | 5.30 | 8.50 | 3.00 | 13.90 | 4.20 | 16.70 | 2.30 | 16.10 | 2.20 | 15.70 | 4.80 | 15.10 | 5.20 | 16.90 |
| ‘Rubin’ | 6.00 | 16.20 | 6.50 | 10.50 | 2.90 | 18.40 | 4.90 | 18.50 | 2.50 | 9.70 | 2.00 | 18.20 | 7.90 | 19.30 | 6.30 | 25.70 |
| ‘Sampion’ | 4.20 | 13.00 | 7.50 | 7.60 | 3.40 | 10.40 | 3.90 | 8.70 | 2.20 | 8.60 | 2.60 | 8.50 | 4.50 | 8.10 | 4.90 | 8.60 |
| ‘Spartan’ | 8.00 | 8.70 | 5.60 | 3.90 | 4.20 | 8.30 | 4.40 | 4.70 | 3.50 | 11.00 | 3.70 | 7.20 | 4.90 | 7.30 | 5.10 | 6.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkeviciute, A.; Viskelis, J.; Viskelis, P.; Liaudanskas, M.; Janulis, V. Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions. Appl. Sci. 2021, 11, 6215. https://doi.org/10.3390/app11136215
Butkeviciute A, Viskelis J, Viskelis P, Liaudanskas M, Janulis V. Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions. Applied Sciences. 2021; 11(13):6215. https://doi.org/10.3390/app11136215
Chicago/Turabian StyleButkeviciute, Aurita, Jonas Viskelis, Pranas Viskelis, Mindaugas Liaudanskas, and Valdimaras Janulis. 2021. "Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions" Applied Sciences 11, no. 13: 6215. https://doi.org/10.3390/app11136215
APA StyleButkeviciute, A., Viskelis, J., Viskelis, P., Liaudanskas, M., & Janulis, V. (2021). Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions. Applied Sciences, 11(13), 6215. https://doi.org/10.3390/app11136215










