Abstract
Substantial evidence now suggests that a positive diversity–stability relationship exists. Yet few studies examine the facets of biodiversity that contribute to this relationship, and empirical research is predominantly conducted on grassland communities under controlled conditions. We investigate the roles of species richness, environmental condition (vegetation cover), asynchrony, and weighted population stability in driving community stability across multiple taxa. We used data from a Long-term Ecological Research project to investigate temporal stability of annual plants, beetles, reptiles, and rodents in Nizzanim Coastal Sand Dune Nature Reserve in Israel. All four taxa had a strong positive relationship between asynchrony and community stability. Only rodents showed a positive richness–stability relationship. Perennial plant cover had a significant relationship with community stability for three taxa, but the direction of the correlation varied. Asynchrony had a stronger relationship with perennial plant cover than it did with richness for both plants and beetles. We suggest that community stability is driven by asynchrony for flora as well as fauna. Stability appears to be determined by species’ interactions and their responses to the environment, and not always by diversity. This has important consequences for understanding the effects of environmental degradation on ecosystem stability and productivity, which have destabilizing consequences beyond biodiversity loss.
1. Introduction
Substantial evidence has demonstrated that diversity stabilizes ecosystem functioning over time [1,2,3,4,5,6,7]. Meanwhile, the current degradation of ecosystems and resultant losses of biodiversity reported globally could be reducing the capacity of ecosystems to maintain stable levels of function and productivity [4,8,9,10,11]. Thus, understanding the drivers of temporal stability has become critically important for conserving biodiversity and ecosystem function.
Diversity–stability relationship (DSR) theories suggest that species richness can contribute to community stability through various mechanisms, such as statistical averaging [12], overyielding [4], species asynchrony and other species interactions [3,4,13,14,15,16,17,18]. Nevertheless, species-rich communities may be more susceptible to disruption of key interactions and could, therefore, be less stable at the population level [7,19,20].
Community stability can be modelled as a function of species population stability weighted by their dominance, and the covariance (synchrony) between species [18]. The relative importance of different facets of biodiversity underlying the diversity–stability relationship remains unclear [21]. Asynchrony has played a key part in theoretical studies to predict DSR in modelled communities [4,18,21,22,23,24]. Yet only a handful studies have investigated the relationship between asynchrony and stability empirically [21,25,26,27]. The dominance of a species is also likely to affect its contribution to overall stability [26,28,29]. Weighted population stability and asynchrony should therefore both affect community stability. We investigate the balance between them in the context of DSR.
Stability cannot be understood outside the context of the environmental condition, and examining DSR in real -world ecosystems has been recognized as a key research need in ecology [30,31,32]. Yet most empirical studies of DSR focus on controlled, short-term, and small-scale experiments, under standardized environmental conditions with constant community composition, often only considering the role of species richness in driving community stability [27,33].
Empirical support for the DSR in natural terrestrial ecosystems comes overwhelmingly from plants in grasslands and trees in mixed forests, due to the relative simplicity of the communities in these habitats [8,32,34,35,36,37]. The insights gained in considering cross-taxon congruence have been highlighted in a range of studies, from the effect of spatial heterogeneity on species distributions (e.g., [38,39], to anthropogenic disturbance and fragmentation on species composition [40,41,42], and functional diversity [43]. Nevertheless, few studies examine multiple taxa in natural systems in the context of DSR [27].
Studying stability in natural systems and across multiple taxa is challenging. Data from Long-Term Ecological Research (LTER) networks (see https://www.ilter.network/ accessed on 28 June 2021) help to overcome this challenge [8,36,44,45]. We take advantage of a multi-taxa coastal dune LTER site with a natural gradient of increasing perennial vegetation to examine the mechanisms through which environmental condition and richness could affect the community stability of four taxonomic groups.
Coastal dune systems provide a convenient system for studying the impact of environmental gradients on DSR because they are highly dynamic, relatively simple in terms of species diversity and complexity, and for Mediterranean coasts, most species are well documented [46]. In addition, coastal dunes are subject to a gradient of disturbance and stress, coupled with increasing Perennial Plant Cover (PPC), and disturbance has been shown to affect plant and animal community stability [47,48,49,50].
Given that shrub encroachment is a major issue for biodiversity loss in coastal systems across the world [51,52,53], an investigation of the impact of perennial plant cover on stability is important. Dunes with greater PPC are expected to have greater species diversity and therefore, create more stable communities [54,55]. Conversely, shrub encroachment can reduce species diversity and community stability in sand dune habitats [56,57]. Alternatively, intermediate levels of plant cover could confer the highest levels of diversity [58,59], and therefore, could be the most stable.
Only two research groups have explicitly investigated stability in relation to diversity within coastal dunes [60,61,62], and both found evidence for a positive DSR for plant communities (dominated by perennial species with >10% annuals). However, the taxonomic scope in these studies was limited to plants only. Moreover, these studies did not investigate the underlying mechanisms of stability, such as asynchrony among species.
In this article, we empirically investigate the roles of species richness and environmental condition (vegetation cover), and compare asynchrony and weighted population stability in driving the community stability across multiple taxonomic groups.
2. Materials and Methods
2.1. Study Site
Biodiversity trends in Nizzanim coastal sand dune Nature reserve (NDNR) in Israel have been monitored across four major taxonomic groups as part of a Long-Term Ecological Research (LTER) project since 2004. The Nizzanim LTER is an ongoing collaborative project that monitors plant, arthropod, rodent, and reptile diversity [53,57,63,64,65,66,67], and their responses to restoration practices [57,63]. NDNR (31°42′–31°44′ N, 34°35′–34°36′ E) is the largest remaining natural coastal dune system in Israel, covering an area of 20 km2. The climate is Mediterranean, with an annual average temperature of 20 °C and annual rainfall of 450 mm falling almost exclusively between November–April.
The LTER site consists of coastal sand dunes classified into three categorical states: mobile, semi-fixed, and fixed dunes, which are separated by densely vegetated inter-dune depressions [68,69,70]. The classification of fixation states is based on perennial plant cover (PPC), sand movement, and visual indicators such as dune geomorphic structure, perennial plant distribution, dominant perennial species, and soil characteristics [68,71,72,73,74,75]. Fixation state is a stronger explanatory variable than perennial plant cover alone [53,66] likely due to these local scale factors [76]. However, PPC can be used as a continuous environmental gradient that may affect stability dynamics, and so was the preferred parameter for this study.
2.2. Collection Methods
The Nizzanim LTER has been monitoring 10 undisturbed dunes, including three mobile, four semi-fixed, and three fixed, since 2005 (here we present 2006–2017). Each dune was sampled yearly for all taxa (dunes for plants, beetles, reptiles, and rodents) using various methods [57]: perennial plants were sampled using 100 m transects, annuals were sampled in 40 × 40 cm quadrats, beetles were sampled with dry pitfall traps, rodents were mark-recaptured using Sherman traps and their abundance was estimated using the Lincoln–Petersen Index [77], and reptiles were sampled using a combination of methods and later combined to give a rank abundance measure between 1 and 5. Full sampling methods are described in Supplementary Materials in Section S1.
There may be heterogeneity within dunes themselves; however, we did not consider intra-dune differences here and all the data within a dune were pooled to provide a single measure of stability/richness for each dune per taxa. Herewith, a “sample” refers to the monitoring of a single dune in one year for a given taxa.
2.3. Measures of Stability
The most commonly used measure of community stability is temporal variability [2,13,32,78,79,80]. A frequently used measure for temporal variability is the Coefficient of Variation (CV). This is the temporal standard deviation over the temporal mean of abundance, such that a higher CV implies lower stability [81]. We used Community variability (CVcomm) as an inverse measure of stability for each dune separately, referring to a community as the assemblage of species in a single taxonomic group found on a single dune.
CVcomm ranges from 0 to 1 and is a measure of the standard deviation (s.d.) over the mean (µ):
CVcomm = s.dcomm/µcomm
CVcomm can also be derived using Thibaut and Connolly’s [18] function:
where ϕ (or Phi) is Loreau’s synchrony [14] and CVpop_av.weighted is the CV for each species, weighted by its relative abundance and averaged across all species [18]. Synchrony (ϕ) measures covariance among species within a community and ranges from 0 (highly asynchronous) to 1 (complete synchrony) [14]. Note that a value of ϕ = 0.5 suggests that any synchrony between species is random.
Dominant species contribute to CVpop_av.weighted, and dominance has been shown to have an important role in regulating community stability [8,26,35,36,82]. Meanwhile, communities with high levels of asynchrony could have higher community stability, irrespective of the stability of individual populations. Unless species abundances are perfectly synchronous, CVcomm must be always be smaller than CVpop_av.weighted [27].
We tested the relationships between parameters using CVcomm, synchrony, and CVpop_av.weighted as the inverse measures of community stability, asynchrony, and weighted population stability, respectively, in order to maintain the calculations given in Equations (1) and (2). However, when discussing the significance of relationships, we refer to stability and asynchrony, because conceptually they are easier to understand in relation to DSR theory. Community stability is considered CVcomm−1, population stability is CVpop_av.weighted−1, and asynchrony equates to 1 − ϕ.
To exemplify the relationship between the various measures of stability, Figure 1 represents the simulated data for simple two-species communities, showing how community stability can be affected by the balance between population stability and asynchrony (modelled data is provided in Supplementary Materials Table S1). The temporal mean for overall (community) abundance was µ = 45 in all cases. Low population stability coupled with low asynchrony (when species fluctuations are synchronized) created the most unstable community (Figure 1a). The combination of high population stability coupled with low asynchrony (Figure 1b) achieved the same degree of community stability as low population stability coupled with high asynchrony (Figure 1c). High population stability together with high asynchrony produced the most stable community in terms of overall abundance (Figure 1d).
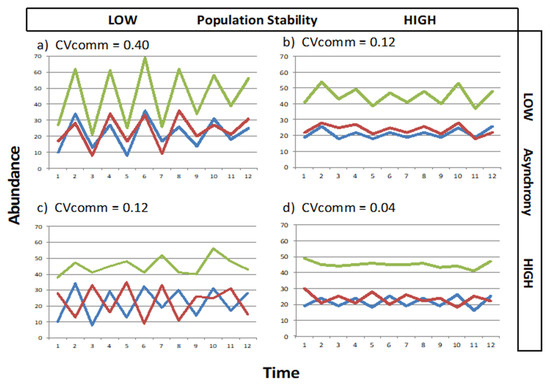
Figure 1.
Modelled abundances of two species (in red and blue) and their combined “community abundance” (in green), showing how community stability can be affected by the balance between population stability and asynchrony. (a) Low population stability and low asychrony, (b) high population stability and low asynchrony, (c) low population and stability high asynchrony, (d) high population stability and high asynchrony. CVcomm can range from 0 to 1 and is the inverse of community stability. The Temporal mean for each community (µ, pooled abundance) was 45. The Standard deviation ranged between 3.0 and 3.5 for high population stability and between 9.50 and 10.0 for low population stability. Asynchrony is measured as 1 − ϕ, with high asynchrony ranging between 0.8 and 0.9 and low asynchrony ranging between 0.1 and 0.2. Data used for these models can be found in Supplementary Materials Table S1.
2.4. Statistical Analysis
We used R version 3.4 [83] for all analyses. We ignored rare (low-abundance) species, because they can create artefacts in the analyses despite not contributing a lot to community stability. Species that were sampled less than five years across the entire data set were removed. Second, we only selected species that were present in any given dune at least five times consecutively, or where any absences were not consecutive.
Due to the fact that stability is a single measurement across many years, our large dataset was reduced to a single data point for each dune. This precluded the use of structural equation models for our data. Instead, we used a series of linear regressions of Y~X, where Y is dependent on X, using the stats package [83]. For annual plants, we found that the relationships appeared unimodal for most parameters, so we also tested Y~X2, where Y~X was not significant. Regression lines and standard error ranges for graphs were plotted with a linear model smoothing function using ggplot2 [84].. We compared the adjusted R2 of each linear regression across different independent (X) parameters for each given dependent (Y) parameter for each taxon. We did not compare the relative contribution of X parameters to Y; rather, the X parameter with the highest adjusted R2 for a given Y was regarded as the parameter that explained the most variation of Y, and was therefore considered the strongest predictor of Y.
3. Results
In total, data points were collected in 4176 quadrats for annuals, 4140 pitfall trap-nights for beetles, and 5022 Sherman trap-nights for rodents, as well as 704 activity-transects, 352 track-transects, and 1760 pitfall trap-nights for reptiles. Overall, species richness was highest for annual plant species (n = 63), of which 41 species were included in the analysis after the exclusion of rare species. Beetles were the second most diverse group across all dunes (n = 48 morphospecies), of which 32 were included in the analysis. Twenty reptile species were recorded, of which 15 were included. Finally, a total of five rodent species were found in Nizzanim LTER, of which three species were included.
Figure 2 shows the regressions for PPC and Average species richness (Richness) against CVcomm for each taxon. Richness was not significantly correlated to community variability (CVcomm) for any taxon except rodents (Figure 2c), such that increased richness was associated with a decrease in CVcomm (an increase in community stability). Annual plants also showed a unimodal trend for PPC, with intermediate cover associated with the highest degree of community variability (Figure 2e). CVcomm for both rodents and beetles significantly increased with increasing PPC (Figure 2f,g). Reptile stability showed no correlation with either parameter (Figure 2d,h).
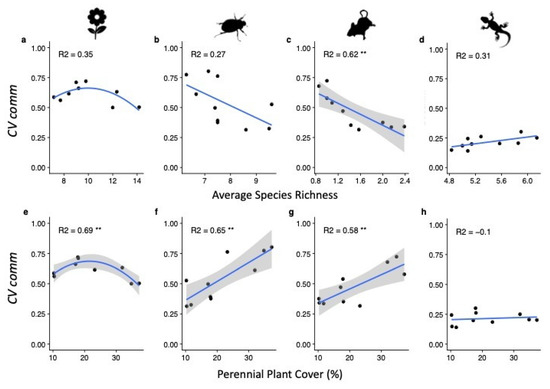
Figure 2.
Regressions of (a–d) average species richness and (e–h) of perennial plant cover (PPC) against community variability (CVcomm) for each taxon. Standard error ranges are shown for all significant regressions. Regression lines were plotted using a linear model smoothing function. NB: an increase in CVcomm equates to a reduction in community stability. Explained variation (R2) can only be compared across parameters within each taxon. ** p < 0.01.
Plots of the regressions of CVpop_av.weighted and synchrony against community variability are given in Figure 3. As can be seen above, only beetles had a positive relationship between Cvpop_av.weighted and CVcomm (Figure 3b), while all four taxa had a significant positive relationship between synchrony and CVcomm (Figure 3e–h).
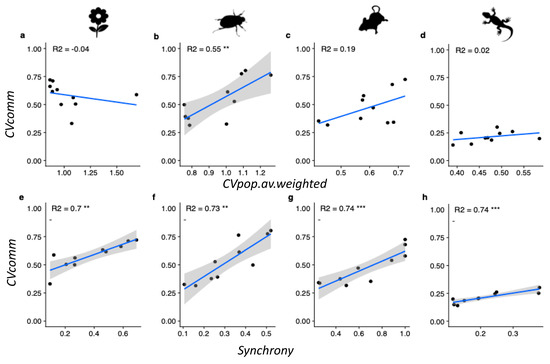
Figure 3.
Regressions of (a–d) weighted average population Coefficient of Variation (CVpop_av.weighted), and (e–h) Synchrony (ϕ), against community variability (CVcomm) for each taxon. Standard error ranges are only shown for significant regressions. Regression lines were plotted using a linear model smoothing function. Note an increase in CVcomm equates to a reduction in community stability. Explained variation (adjusted R2) can be compared across parameters within each taxon. ** p < 0.01, *** p < 0.001.
Since there were many correlations between all the different variables across four taxonomic groups, we provide a summary for each taxa in Figure 4, showing all significant correlations between parameters (including those shown in Figure 2 and Figure 3), with directionality depicted as arrows from the independent (X) to the dependent (Y) parameter Regression outputs for all taxa are reported in Supplementary Materials Table S2. As seen in Figure 4, each taxonomic group showed different strengths and directions for the relationships among parameters.
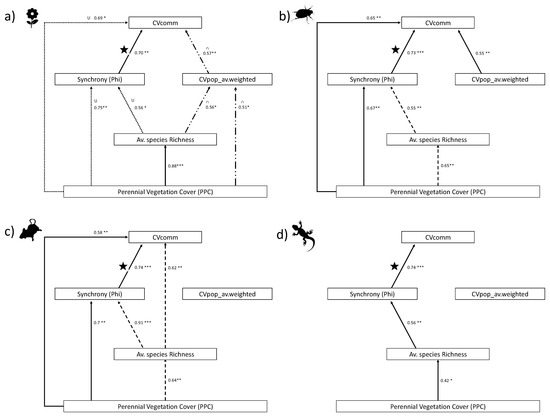
Figure 4.
Linear regressions lm(Y~X2) for (a) annual plants, and lm(Y~X) for (b) beetles (c) rodents and (d) reptiles. Arrows are drawn from independent (X) to dependent (Y) for all significant regressions (* p < 0.05, ** p < 0.01, *** p < 0.001); Adjusted R2 values are given and patterns depict directions of correlation: Solid = positive-linear, dashed= negative-linear, dotted = U shaped unimodal, double-dashed = bell-shaped unimodal. Full regression outputs are given in Supplementary Materials Table S2. ★ Depicts the strongest (highest R2) positive correlation for each taxon and is presented in full in Figure 3e–h.
Overall, no two taxa shared the same directionality in relationships across all parameters. PPC had little effect on weighted population variability (CVpop_av.weighted) except for annuals, where a unimodal bell-shaped curve was observed. For annuals, richness also presented a bell-shaped relationship with CVpop_av.weighted, and the latter had a similar bell-shaped curve with CVcomm.
Perennial plant cover (PPC) had a different relationship with synchrony depending on the taxon. A positive relationship was found for beetles and rodents, whereas a U-shaped response was present for annual plants, with intermediate cover associated with the lowest degree of synchrony. Reptiles did not show any significant relationship between synchrony and PPC; synchrony remained relatively high across all dunes, ranging between 0.7 and 0.8.
All four taxa were found to have a significant relationship between synchrony and richness, but the directionality differed between taxa; a negative relationship was found for beetles and reptiles, a positive correlation for reptiles, and a U-relationship for annuals.
Within each taxon, the adjusted R2 (adj.R2) values for each regression were used as an indication of which parameter explained the most variation for a given dependent variable. Thus, PPC explained more variation than richness for asynchrony of annuals (adj.R2 = 0.75 and adj.R2 = 0.56, respectively), and beetles (adj.R2 = 0.67 and 0.55, respectively). However, PPC explained less than richness for rodent synchrony (adj.R2 = 0.70 and 0.91, respectively) and reptile synchrony (adj.R2 = 0.01 and 0.56, respectively).
Beetles were the only taxon to show a significant positive correlation between CVpop_av.weighted and CVcomm, but even for this taxon, there was a stronger adjusted R2 for synchrony and CVcomm.
All taxa displayed different relationships and directionality among parameters, except for the relationship between synchrony and CVcomm. The key finding in our study was that despite the differences in strengths and directions of relationships between other parameters, the strongest positive relationship for all four taxa was consistently between synchrony and CVcomm (as shown by the highest adj.R2 for a positive correlation in a given taxon as depicted by the star ★ in Figure 4a–d, and see Figure 3e–h). In other words, a high degree of asynchrony was associated with the highest levels of community stability.
4. Discussion
Diversity–stability relationship studies often focus exclusively on richness and stability, without considering the environmental factors or interspecific relationships that may drive stability [21]. We investigated the roles of species richness, environmental condition (perennial vegetation cover), synchrony, and weighted population variability in driving community variability across multiple taxonomic groups. Our empirical data consistently presented synchrony as the most strongly and positively correlated parameter for community variability (CVcomm) for all taxa. In addition, synchrony was a better predictor from community variability than weighted population variability, despite both parameters being mathematically linked to community variability [18]. In stability terms (as the inverse of variability), the asynchrony–community stability relationship was always significant and positive across all taxa; a high degree asynchrony appeared to confer more stable communities no matter which taxon and despite the different directionalities and strengths in the relationships among all other parameters. In contrast, a positive diversity–stability relationship (DSR) was only supported in rodents (a negative richness–CVcomm correlation equates to a positive richness–community stability relationship).
Asynchrony has been recognized as a key driver in theoretical and modelled DSR [18,24,85]. In empirical studies, asynchrony can play a key role in stabilizing real plant communities in grasslands, but other studies had conflicting results [8,21,25,26,29,86,87]. Despite some of the founding theory of DSR being focused on fluctuations of animal populations [88,89] few studies have looked at animal stability in relation to asynchrony. Blüthgen et al. [27] considered asynchrony in relation to community stability for plants, arthropods, birds, and bats, and found (as we did) a consistent positive relationship between asynchrony and community stability for animal and plant taxa in both grassland and forest systems. As with our findings, they too suggest that asynchrony was a stronger driver of community stability than richness directly.
In terms of asynchrony and richness, several empirical studies have also reported a positive relationship in experimental [85] and naturally assembled grasslands [90], whereas Blüthgen et al. [27] reported the opposite for grassland plants. We found support for a positive asynchrony–richness relationship for beetles and rodents (i.e., a negative synchrony–richness correlation), whereas the asynchrony–richness relationship was negative for reptiles and unimodal for annuals.
Asynchrony could be expected to increase with increasing richness due to processes such as niche partitioning at the evolutionary scale or competitive exclusion at the ecological scale [14,18,91]. The mechanisms for this relationship thus remain unclear. Asynchrony can reflect either heterogeneity in species (functional) responses to environmental conditions (response diversity), or simply their demographic stochasticity [8,12,27,29,32].
The degree to which asynchrony is linked to diversity has been shown to be influenced by environmental condition in both empirical [86,90] and modelled [29] systems. Disturbance and stochastic environments are known to have direct as well as indirect effects on the DSR via the changes they create in species richness, community composition, and species traits [92,93]. Spatial heterogeneity in natural systems increases species diversity and stability of birds [94], and riverine fish [95]. In our findings, the direction of the relationship between asynchrony and PPC was dependent on the taxon, but all taxa had a positive asynchrony–community stability relationship.
Environmental conditions can also be more important than richness in predicting community stability across a range of ecosystems [33]. Eutrophication weakened the stabilizing effect of richness on a grassland community by increasing the temporal variation of productivity and decreasing species asynchrony in more diverse communities, rather than by reducing diversity per se [34]. It is perhaps not surprising, then, that the DSR was not supported in our findings for most taxa, whereas a significant effect of PPC on community stability was found for three out of four taxa (all except reptiles).
The species richness gradient of annuals in the Nizzanim dunes was similar to other sites along the coast of Israel, with the highest richness in fixed dunes, where the PPC was also highest [57]. PPC had a significant U-shaped relationship with annuals’ community stability (depicted as a bell shape with CVcomm in Figure 2e and Figure 3a), whereas the richness–stability relationship was weak. Species asynchrony was also found to have a unimodalrelationship with PPC, such that the lowest levels of asynchrony and stability were found in semi-fixed dunes. Kuiters [62] found that plant stability in coastal dunes was largely explained by diversity rather than by abiotic factors, but he mostly considered perennial plants, which are not as vulnerable to environmental conditions.
Rodents were the only taxon to show a significant positive DSR in Nizzanim, and it was a stronger predictor than PPC. It is apparent that the stabilizing effect operated through the effect of richness on asynchrony; rodent communities in Nizzanim Dunes Nature Reserve have very low overall richness and are almost exclusively dominated by two species: the Greater Egyptian gerbil, Gerbillus pyramidum, and Allenby’s gerbil, Gerbillus andersoni allenbyi, comprising 98.5% of rodent captures (with three other species presenting 1.5% of the captures). An increase from one to two species was positively associated with a significant increase in rodent community stability. These species are known to have low covariance due to spatio-temporal niche differentiation [96,97].
Reptiles did not appear to be as influenced by PPC as other taxa, both in terms of asynchrony and stability. Asynchrony was in general quite high across all sites for reptiles, which could infer a high degree of niche differentiation for this taxon irrespective of plant cover. Alternatively, there may be some dampening of the range of values for covariance due to the ranking methodology that was applied.
Dominance of species in their communities has been shown to be an important role in regulating population and community stability [8,26,35,36,82]. Surprisingly, we found no support for any effect of weighted population stability (CVpop_av.weighted−1) on community stability except for beetles. For the latter, a positive relationship between population stability and community stability was found despite the theoretical expectations of a negative relationship [7,18,98]. Nevertheless, for beetles the asynchrony–community stability relationship was stronger than population stability–community stability, consistent with other taxa.
5. Conclusions
Our findings suggest that in sand dunes, community stability is driven by asynchrony rather than population stability. Furthermore, our results provided only equivocal evidence for a relationship between diversity and stability in coastal dune systems. Each of the four taxonomic groups in this study appeared to operate under a different mechanism in terms of the community’s response to richness and plant cover (a proxy for environmental condition), yet consistently demonstrated a positive asynchrony–stability relationship.
If this pattern is applicable to other systems, this would suggest that the emergent property of the community is the interactions among species (asynchrony), rather than the number of species present. This in turn infers a deterministic formation of community assemblages, rather than random stochasticity [99,100,101]. Hence, although individual populations may fluctuate, competition and niche differentiation allow species to fluctuate in complementary ways, maintaining certain characteristics of stability. From a generalist predator’s point of view (or an anthropocentric view of ecosystem services), resource availability remains stable as the decline of one species is compensated by an increase in another. This could also explain the contradictory theories of stability (e.g., [7,88,102,103]; diversity can beget stability [88], even if population stability is negatively correlated to diversity [102] when the community is highly asynchronous.
Understanding how biotic mechanisms confer stability in variable environments is a fundamental quest in ecology, and one that is becoming increasingly urgent due to global change [36]. If stability can be determined by collective responses of species to each other and to their environment, rather than by the richness or dominance of species present, this has important consequences for understanding the effects of environmental degradation on ecosystem function and productivity. Anthropogenic changes to our planet may be having a two-fold impact on ecosystems, by diminishing ecosystem stability in addition to the direct effects on biodiversity (species) loss.
More attention is needed to bridge our understanding of ecological theory with conserving ecosystem function [104]. Long-term studies such as those within the International LTER network are particularly well suited to a metadata analysis, which could examine DSR in natural systems and across multiple taxa. We need to better understand the drivers and effects of asynchrony among species both theoretically and with empirical data in natural systems; interspecific interactions and mechanisms that determine asynchrony and stability are far more complex in natural systems than under controlled conditions. A focus on asynchrony as a stabilizing mechanism could better inform conservation management regarding the risk of environmental degradation on ecosystem function.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11136214/s1, Section S1: Sampling methods for data collection across 5 taxonomic groups, Section S2: Supporting data, Table S1: Modelled data for demonstrating the combine effects of population stability and asynchrony on community stability as depicted in Figure 1, Table S2: Regression results for all parameters summarized in Figure 2, Figure 3 and Figure 4.
Author Contributions
Conceptualization, T.L.F.B., P.B., E.G. and A.B.; methodology, T.L.F.B., P.B., E.G. and A.B.; formal analysis, T.L.F.B.; investigation, T.L.F.B., P.B., E.G. and A.B.; resources, P.B., E.G. and A.B.; data curation, T.L.F.B.; writing—original draft preparation, T.L.F.B.; writing—review and editing, P.B., E.G. and A.B.; supervision, P.B., E.G. and A.B.; project administration, T.L.F.B.; funding acquisition, P.B., E.G. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study and the Nizzanim LTER are funded by the Israel Nature and Parks Authority, (INPA) and the International Arid Land Consortium (IALC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
For full datasets contact the corresponding author.
Acknowledgments
Special thanks to Michael Dorman, who provided substantial time and advice on the initial analyses and use of R coding. Great thanks are due to Yael Zilka, Boaz Shacham, Zehava Siegal, Adi Ramot, Meirav Perry, Ittai Renan, Gal Vine, Oz Rittner, Arnon Tsairi, and the numerous team heads and graduate and undergraduate students from Ben Gurion University for their assistance in collecting monitoring data across the years. We thank the entomologists at the Steinhardt Museum of Natural History for their help with identifying beetles, in particular Chicatunov and Laibale Friedman. Thanks also to the Shikmim Field Study Center (Society for the Protection of Nature in Israel) for their hospitality during our fieldwork sessions over the years. We also acknowledge contributions from the Ministry of Science and Technology (MOST) and the International Arid Land Consortium (IALC). Finally, we thank Yehoshua Shkedi, Yariv Malihi, and the Israel Nature & Parks Authority (INPA) rangers for their continuous support and assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity–stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef]
- Loreau, M.; de Mazancourt, C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kéfi, S.; Domínguez-García, V.; Donohue, I.; Fontaine, C.; Thébault, E.; Dakos, V. Advancing our understanding of ecological stability. Ecol. Lett. 2019, 22, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.E.; Hilborn, R.; Chasco, B.; Boatright, C.P.; Quinn, T.P.; Rogers, L.; Webster, M.S. Population diversity and the portfolio effect in an exploited species. Nature 2010, 465, 609–612. [Google Scholar] [CrossRef] [PubMed]
- May, R.M. Stability and Complexity in Model Ecosystems; Princeton University Press: Princeton, NJ, USA, 2001; ISBN 9780691088617. [Google Scholar]
- Grman, E.; Lau, J.A.; Schoolmaster, D.R.; Gross, K.L. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol. Lett. 2010, 13, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S. Ecosystem consequences of biodiversity loss: The evolution of a paradigm. Ecology 2002, 83, 1537–1552. [Google Scholar] [CrossRef]
- Domínguez-García, V.; Dakos, V.; Kéfi, S. Unveiling dimensions of stability in complex ecological networks. Proc. Natl. Acad. Sci. USA 2019, 116, 25714–25720. [Google Scholar] [CrossRef]
- Thebault, E. Uncertain predictions of species responses to perturbations lead to underestimate changes at ecosystem level in diverse systems. Peer Community Ecol. 2020, 1, 100063. [Google Scholar] [CrossRef]
- Doak, D.F.; Bigger, D.; Harding, E.K.; Marvier, M.A.; O’Malley, R.E.; Thomson, D. The statistical inevitability of stability-diversity relationship in community ecology. Am. Nat. 1998, 151, 264–276. [Google Scholar] [CrossRef]
- Lehman, C.L.; Tilman, D. Biodiversity, stability, and productivity in competitive communities. Am. Nat. 2000, 156, 534–552. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; de Mazancourt, C. Species Synchrony and Its Drivers: Neutral and Nonneutral Community Dynamics in Fluctuating Environments. Am. Nat. 2008, 172, E48–E66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Hautier, Y.; Saner, P.; Wacker, L.; Bagchi, R.; Joshi, J.; Scherer-Lorenzen, M.; Spehn, E.M.; Bazeley-White, E.; Weilenmann, M.; et al. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 2010, 91, 2213–2220. [Google Scholar] [CrossRef]
- Lhomme, J.-P.; Winkel, T. Diversity–stability relationships in community ecology: Re-examination of the Portfolio effect. Theor. Popul. Biol. 2002, 279, 271–279. [Google Scholar] [CrossRef]
- Thibaut, L.M.; Connolly, S.R. Understanding diversity-stability relationships: Towards a unified model of portfolio effects. Ecol. Lett. 2013, 16, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Mrowicki, R.J.; O’Connor, N.E.; Donohue, I. Temporal variability of a single population can determine the vulnerability of communities to perturbations. J. Ecol. 2016, 104, 887–897. [Google Scholar] [CrossRef] [Green Version]
- White, L.; O’Connor, N.E.; Yang, Q.; Emmerson, M.C.; Donohue, I. Individual species provide multifaceted contributions to the stability of ecosystems. Nat. Ecol. Evol. 2020, 4, 1594–1601. [Google Scholar] [CrossRef]
- Craven, D.; Eisenhauer, N.; Pearse, W.D.; Hautier, Y.; Isbell, F.; Roscher, C.; Bahn, M.; Beierkuhnlein, C.; Bönisch, G.; Buchmann, N.; et al. Multiple facets of biodiversity drive the diversity–stability relationship. Nat. Ecol. Evol. 2018, 2, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Loreau, M. Ecosystem stability in space: Alpha, beta and gamma variability. Ecol. Lett. 2014, 17, 891–901. [Google Scholar] [CrossRef]
- Gouhier, T.C.; Guichard, F. Synchrony: Quantifying variability in space and time. Methods Ecol. Evol. 2014, 5, 524–533. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; de Mazancourt, C.; Scherer-Lorenzen, M.; Bugmann, H. Temporal stability in forest productivity increases with tree diversity due to asynchrony in species dynamics. Ecol. Lett. 2014, 17, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Lu, X.; Hirota, M.; Bai, Y. Species asynchrony and response diversity determine multifunctional stability of natural grasslands. J. Ecol. 2019, 107, 1862–1875. [Google Scholar] [CrossRef]
- Sasaki, T.; Lauenroth, W.K. Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 2011, 166, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Simons, N.K.; Jung, K.; Prati, D.; Renner, S.C.; Boch, S.; Fischer, M.; Hölzel, N.; Klaus, V.H.; Kleinebecker, T.; et al. Land use imperils plant and animal community stability through changes in asynchrony rather than diversity. Nat. Commun. 2016, 7, 10697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestre, F.T.; Castillo-Monroy, A.P.; Bowker, M.A.; Ochoa-Hueso, R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J. Ecol. 2012, 100, 317–330. [Google Scholar] [CrossRef]
- Tredennick, A.T.; de Mazancourt, C.; Loreau, M.; Adler, P.B. Environmental responses, not species interactions, determine synchrony of dominant species in semiarid grasslands. Ecology 2017, 98, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Worm, B.; Duffy, J.E. Biodiversity, productivity and stability in real food webs. Trends Ecol. Evol. 2003, 18, 628–632. [Google Scholar] [CrossRef]
- Hillebrand, H.; Langenheder, S.; Lebret, K.; Lindström, E.; Östman, Ö.; Striebel, M. Decomposing multiple dimensions of stability in global change experiments. Ecol. Lett. 2018, 21, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Ives, A.R.; Carpenter, S.R. Stability and diversity of ecosystems. Science 2007, 317, 58–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, U.; Hillebrand, H. Biodiversity and ecosystem functioning in dynamic landscapes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hautier, Y.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Bakker, J.D.; et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 2014, 508, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ren, H.; Li, M.-H.; van Ruijven, J.; Han, X.; Wan, S.; Li, H.; Yu, Q.; Jiang, Y.; Jiang, L. Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J. Ecol. 2015, 103, 1308–1316. [Google Scholar] [CrossRef]
- Hallett, L.M.; Hsu, J.S.; Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gherardi, L.A.; Gross, K.L.; Hobbs, R.J.; Turnbull, L.; et al. Biotic mechanisms of community stability shift along a precipitation gradient. Ecology 2014, 95, 1693–1700. [Google Scholar] [CrossRef] [Green Version]
- Seabloom, E.W. Compensation and the stability of restored grassland communities. Ecol. Appl. 2007, 17, 1876–1885. [Google Scholar] [CrossRef]
- Duan, M.; Liu, Y.; Yu, Z.; Baudry, J.; Li, L.; Wang, C.; Axmacher, J.C. Disentangling effects of abiotic factors and biotic interactions on cross-taxon congruence in species turnover patterns of plants, moths and beetles. Sci. Rep. 2016, 6, 23511. [Google Scholar] [CrossRef] [Green Version]
- Gossner, M.M.; Getzin, S.; Lange, M.; Pašalić, E.; Türke, M.; Wiegand, K.; Weisser, W.W. The importance of heterogeneity revisited from a multiscale and multitaxa approach. Biol. Conserv. 2013, 166, 212–220. [Google Scholar] [CrossRef]
- Rooney, R.C.; Azeria, E.T. The strength of cross-taxon congruence in species composition varies with the size of regional species pools and the intensity of human disturbance. J. Biogeogr. 2015, 42, 439–451. [Google Scholar] [CrossRef]
- Yong, D.L.; Barton, P.S.; Okada, S.; Crane, M.; Lindenmayer, D.B. Birds as surrogates for mammals and reptiles: Are patterns of cross-taxonomic associations stable over time in a human-modified landscape? Ecol. Indic. 2016, 69, 152–164. [Google Scholar] [CrossRef]
- Maes, D.; Bonte, D. Using distribution patterns of five threatened invertebrates in a highly fragmented dune landscape to develop a multispecies conservation approach. Biol. Conserv. 2006, 133, 490–499. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef]
- Vihervaara, P.; D’Amato, D.; Forsius, M.; Angelstam, P.; Baessler, C.; Balvanera, P.; Boldgiv, B.; Bourgeron, P.; Dick, J.; Kanka, R.; et al. Using long-term ecosystem service and biodiversity data to study the impacts and adaptation options in response to climate change: Insights from the global ILTER sites network. Curr. Opin. Environ. Sustain. 2013, 5, 53–66. [Google Scholar] [CrossRef]
- Turner, M.G.; Collins, S.L.; Lugo, A.L.; Magnuson, J.J.; Rupp, T.S.; Swanson, F.J. Disturbance dynamics and ecological response: The contribution of Long-Term Ecological Research. Bioscience 2003, 53, 46. [Google Scholar] [CrossRef] [Green Version]
- Carboni, M.; Zelený, D.; Acosta, A.T.R. Measuring ecological specialization along a natural stress gradient using a set of complementary niche breadth indices. J. Veg. Sci. 2016, 27, 892–903. [Google Scholar] [CrossRef]
- Bonte, D.; Baert, L.; Maelfait, J.-P. Spider assemblage structure and stability in a heterogeneous coastal dune system (Belgium). J. Arachnol. 2002, 30, 331–343. [Google Scholar] [CrossRef]
- Ruocco, M.; Bertoni, D.; Sarti, G.; Ciccarelli, D. Mediterranean coastal dune systems: Which abiotic factors have the most influence on plant communities? Estuar. Coast. Shelf Sci. 2014, 149, 213–222. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Bacaro, G.; Chiarucci, A. Coastline dune vegetation dynamics: Evidence of no stability. Folia Geobot. 2012, 47, 263–275. [Google Scholar] [CrossRef]
- Van Der Wurff, A.W.G.; Kools, S.A.E.; Boivin, M.E.Y.; Van Den Brink, P.J.; Van Megen, H.H.M.; Riksen, J.A.G.; Doroszuk, A.; Kammenga, J.E. Type of disturbance and ecological history determine structural stability. Ecol. Appl. 2007, 17, 190–202. [Google Scholar] [CrossRef]
- Isermann, M. Expansion of Rosa rugosa and Hippophae rhamnoides in coastal grey dunes: Effects at different spatial scales. Flora 2008, 203, 273–280. [Google Scholar] [CrossRef]
- Pye, K.; Blott, S.J.; Howe, M.A. Coastal dune stabilization in Wales and requirements for rejuvenation. J. Coast. Conserv. 2014, 18, 27–54. [Google Scholar] [CrossRef]
- Bird, T.L.F.; Dorman, M.; Ramot, A.; Bouskila, A.; Bar Kutiel, P.; Groner, E. Shrub encroachment effects on habitat heterogeneity and beetle diversity in a Mediterranean coastal dune system. Land Degrad. Dev. 2017, 28, 2553–2562. [Google Scholar] [CrossRef]
- Zuo, X.; Zhao, X.; Wang, S.; Li, Y.; Lian, J.; Zhou, X. Influence of dune stabilization on relationship between plant diversity and productivity in Horqin Sand Land, Northern China. Environ. Earth Sci. 2012, 67, 1547–1556. [Google Scholar] [CrossRef]
- Rosenzweig, M.L.; Abramsky, Z. How are diversity and productivity related? In Species Diversity in Ecological Communities: Historical and Geographical Perspectives; Ricklefs, R., Schluter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 52–65. [Google Scholar]
- Báez, S.; Collins, S.L. Shrub Invasion Decreases Diversity and Alters Community Stability in Northern Chihuahuan Desert Plant Communities. PLoS ONE 2008, 3, e2332. [Google Scholar] [CrossRef]
- Bird, T.L.F.; Bouskila, A.; Groner, E.; Bar Kutiel, P.; Kutiel, P.B. Can Vegetation Removal Successfully Restore Coastal Dune Biodiversity? Appl. Sci. 2020, 10, 2310. [Google Scholar] [CrossRef] [Green Version]
- Huston, M.A. Disturbance, productivity, and species diversity: Empiricism vs. logic in ecological theory. Ecology 2014, 95, 2382–2396. [Google Scholar] [CrossRef]
- Ferreira, S.M.; van Aarde, R.J. Maintaining diversity through intermediate disturbances: Evidence from rodents colonizing rehabilitating coastal dunes. Afr. J. Ecol. 2000, 38, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Kuiters, A.T.; Kramer, K.; Van der Hagen, H.G.J.M.; Schaminée, J.H.J. Plant diversity, species turnover and shifts in functional traits in coastal dune vegetation: Results from permanent plots over a 52-year period. J. Veg. Sci. 2009, 20, 1053–1063. [Google Scholar] [CrossRef]
- Isermann, M. Patterns in species diversity during succession of coastal dunes. J. Coast. Res. 2011, 274, 661–671. [Google Scholar]
- Kuiters, A.T. Diversity-stability relationships in plant communities of contrasting habitats. J. Veg. Sci. 2013, 24, 453–462. [Google Scholar] [CrossRef]
- Bar, P. Restoration of coastal sand dunes for conservation of biodiversity: The Israeli experience. In Restoration of Coastal Dunes; Springer Series on Environmental Management; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–185. [Google Scholar]
- Shacham, B. Dune Management and Reptiles—Implications for Habitat Reconstruction and Conservation Strategies. Ph.D. Thesis, Ben-Gurion University of the Negev, Be’er Sheva, Israel, 2010. [Google Scholar]
- Shacham, B.; Bouskila, A. Vegetation removal as a management tool in Nizzanim dunes (Israel): Preliminary assessment of effects on reptile and mammal populations. In Proceedings of the International Conference on Management and Restoration of Coastal Dunes (ICCD), Santander, Spain, 3–5 October 2007; pp. 144–145. [Google Scholar]
- Perry, M. Perennial Plants Impact on Annual Plant Diversity in Sand Dunes at Different Spatial Scales. Master’s Thesis, Ben Gurion University of the Negev, Be’er Sheva, Israel, 2008. [Google Scholar]
- Ramot, A. Effect of Plant Cover on Arthropod Community in Nizzanim Coastal Dunes. Master’s Thesis, Ben Gurion University of the Negev, Be’er Sheva, Israel, 2007. [Google Scholar]
- Levin, N.; Kidron, G.J.; Ben-Dor, E. A field quantification of coastal dune perennial plants as indicators of surface stability, erosion or deposition. Sedimentology 2008, 55, 751–772. [Google Scholar] [CrossRef]
- Kutiel, P.; Cohen, O.; Shoshany, M.; Shub, M. Vegetation establishment on the southern Israeli coastal sand dunes between the years 1965 and 1999. Landsc. Urban Plan. 2004, 67, 141–156. [Google Scholar] [CrossRef]
- Tsoar, H.; Blumberg, D.G. Formation of parabolic dunes from barchan and transverse dunes along Israel’s Mediterranean coast. Earth Surf. Process. Landf. 2002, 27, 1147–1161. [Google Scholar] [CrossRef]
- Kutiel, P.; Danin, A.; Orshan, G. Vegetation of the sandy soils near Caesarea, Israel. I. Plant communities, environment and succession. Isr. J. Bot. 1979, 28, 20–35. [Google Scholar]
- Kutiel, P. Annual vegetation of the coastal sand dunes of the northern Sharon, Israel. Isr. J. Plant Sci. 1998, 46, 287–298. [Google Scholar] [CrossRef]
- Kutiel, P. Conservation and management of the Mediterranean coastal sand dunes in Israel. J. Coast. Conserv. 2001, 7, 183–192. [Google Scholar] [CrossRef]
- Tsoar, H. Sand dunes mobility and stability in relation to climate. Phys. A Stat. Mech. Its Appl. 2005, 357, 50–56. [Google Scholar] [CrossRef]
- Rubinstein, Y.; Groner, E.; Yizhaq, H.; Svoray, T.; Bar (Kutiel), P. An eco-spatial index for evaluating stabilization state of sand dunes. Aeolian Res. 2013, 9, 75–87. [Google Scholar] [CrossRef]
- Fenu, G.; Carboni, M.; Acosta, A.T.R.; Bacchetta, G. Environmental factors influencing coastal vegetation pattern: New insights from the Mediterranean Basin. Folia Geobot. 2013, 48, 493–508. [Google Scholar] [CrossRef]
- Brittain, S.; Böhning, D. Estimators in capture–recapture studies with two sources. AStA Adv. Stat. Anal. 2009, 93, 23–47. [Google Scholar] [CrossRef]
- Carpenter, S.; Walker, B.; Anderies, J.M.; Abel, N. From metaphor to measurement: Resilience of what to what? Ecosystems 2001, 4, 765–781. [Google Scholar] [CrossRef]
- Cottingham, K.L.; Brown, B.L.; Lennon, J.T. Biodiversity may regulate the temporal variability of ecological systems. Ecol. Lett. 2001, 4, 72–85. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 1995, 77, 350–363. [Google Scholar] [CrossRef]
- Lepš, J. Variability in population and community biomass in a grassland community affected by environmental productivity and diversity. Oikos 2004, 107, 64–71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 10 October 2018).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- de Mazancourt, C.; Isbell, F.; Larocque, A.; Berendse, F.; De Luca, E.; Grace, J.B.; Haegeman, B.; Wayne Polley, H.; Roscher, C.; Schmid, B.; et al. Predicting ecosystem stability from community composition and biodiversity. Ecol. Lett. 2013, 16, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, K.R.; Tredennick, A.T.; Koerner, S.E.; Grman, E.; Hallett, L.M.; Avolio, M.L.; La Pierre, K.J.; Houseman, G.R.; Isbell, F.; Johnson, D.S.; et al. Asynchrony among local communities stabilises ecosystem function of metacommunities. Ecol. Lett. 2017, 20, 1534–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Jiang, L.; Li, L.; Li, A.; Wu, M.; Wan, S. Diversity-dependent stability under mowing and nutrient addition: Evidence from a 7-year grassland experiment. Ecol. Lett. 2012, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Plants and Animals; Methuen: London, UK, 1958. [Google Scholar]
- Hautier, Y.; Tilman, D.; Isbell, F.; Seabloom, E.W.; Borer, E.T.; Reich, P.B. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 2015, 348, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Isbell, F.I.; Polley, H.W.; Wilsey, B.J. Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecol. Lett. 2009, 12, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.L. Disturbance frequency and community stability in native tallgrass prairie. Am. Nat. 2000, 155, 311–325. [Google Scholar] [CrossRef]
- Donohue, I.; Hillebrand, H.; Montoya, J.M.; Petchey, O.L.; Pimm, S.L.; Fowler, M.S.; Healy, K.; Jackson, A.L.; Lurgi, M.; McClean, D.; et al. Navigating the complexity of ecological stability. Ecol. Lett. 2016, 19, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Hovick, T.J.; Elmore, R.D.; Fuhlendorf, S.D.; Engle, D.M.; Hamilton, R.G. Spatial heterogeneity increases diversity and stability in grassland bird communities. Ecol. Appl. 2015, 25, 662–672. [Google Scholar] [CrossRef]
- Oberdorff, T.; Hugueny, B.; Vigneron, T. Is assemblage variability related to environmental variability? An answer for riverine fish. Oikos 2001, 93, 419–428. [Google Scholar] [CrossRef]
- Ziv, Y.; Smallwood, J.A. Gerbils and Heteromyids—Interspecific Competition and the Spatio-Temporal Niche. In Activity Patterns in Small Mammals; Springer: Berlin/Heidelberg, Germany, 2000; pp. 159–176. [Google Scholar]
- Kotler, B.P.; Brown, J.S.; Subach, A. Mechanisms of Species Coexistence of Optimal Foragers: Temporal Partitioning by Two Species of Sand Dune Gerbils. Oikos 1993, 67, 548. [Google Scholar] [CrossRef]
- Campbell, V.; Murphy, G.; Romanuk, T.N. Experimental design and the outcome and interpretation of diversity-stability relations. Oikos 2011, 120, 399–408. [Google Scholar] [CrossRef]
- Anderson, K.J. Temporal patterns in rates of community change during succession. Am. Nat. 2007, 169, 780–793. [Google Scholar] [CrossRef]
- Connor, E.F.; Simberloff, D. The Assembly of Species Communities: Chance or Competition? Ecology 1979, 60, 1132. [Google Scholar] [CrossRef]
- Cornell, H.V.; Lawton, J.H. Species Interactions, Local and Regional Processes, and Limits to the Richness of Ecological Communities: A Theoretical Perspective. J. Anim. Ecol. 1992, 61, 1. [Google Scholar] [CrossRef] [Green Version]
- May, R.M. Will a large complex system be stable? Nature 1972, 238, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Harvey, E.; Gounand, I.; Ward, C.L.; Altermatt, F. Bridging ecology and conservation: From ecological networks to ecosystem function. J. Appl. Ecol. 2017, 54, 371–379. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).