Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biomass
2.3. Nitrogen Content and Crude Protein Estimation
2.4. Ash Content and Mineral Composition
2.5. Fat Content and Fatty Acid Profile
2.6. Dietary Fiber
2.7. Statistics
3. Results and Discussion
3.1. Crude Protein
3.2. Ash and Mineral Profile
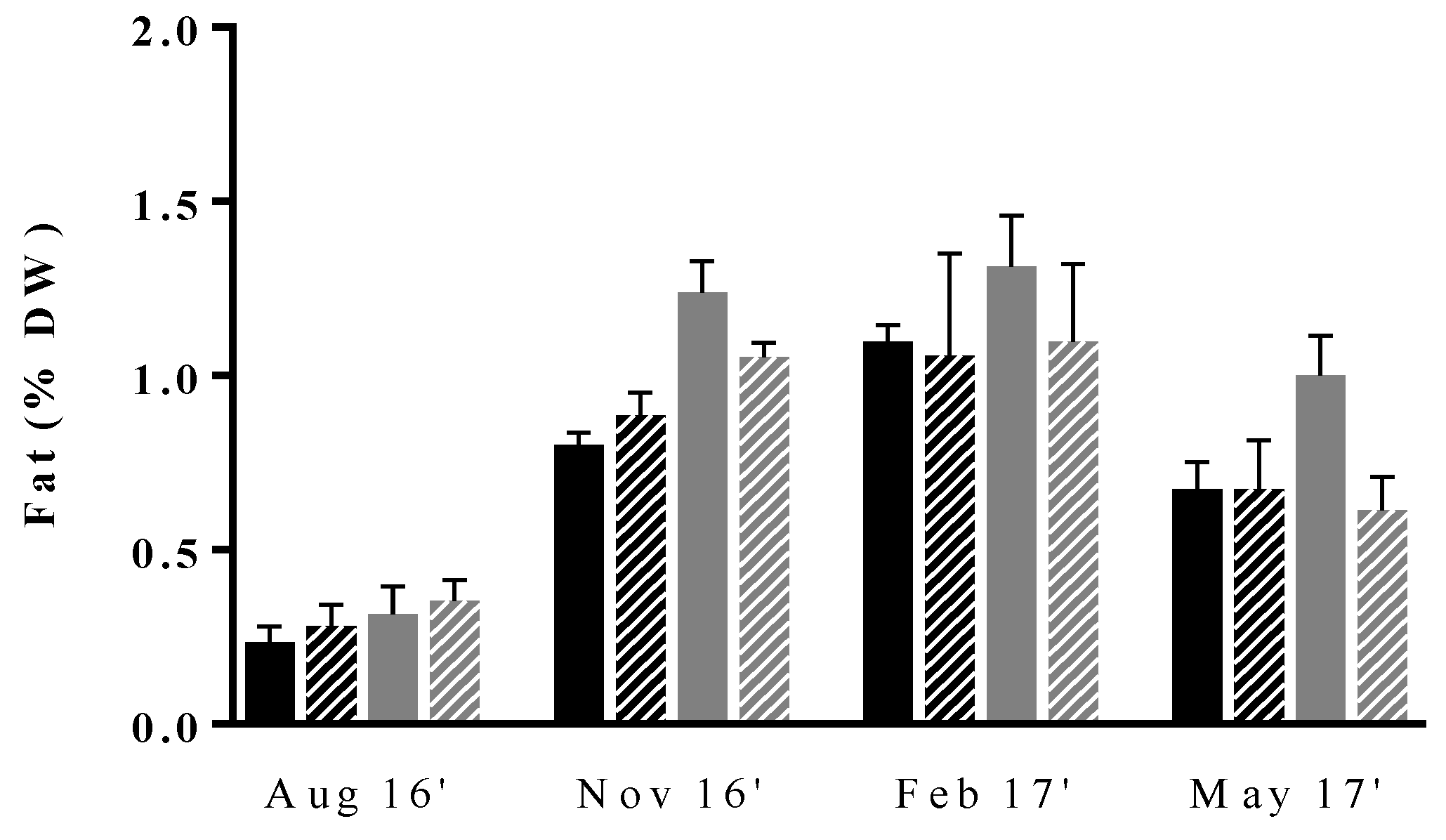
3.3. Fat and Fatty Acid Profile
3.4. Dietary Fiber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diana, J.S.; Egna, H.S.; Chopin, T.; Peterson, M.S.; Cao, L.; Pomeroy, R.; Verdegem, M.; Slack, W.T.; Bondad-Reantaso, M.G.; Cabello, F. Responsible aquaculture in 2050: Valuing local conditions and human innovations will be key to success. BioScience 2013, 63, 255–262. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action: Rome, Italy, 2020. [Google Scholar]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F.L. Is Integrated Multitrophic Aquaculture the solution to the sectors’ major challenges? A review. Rev. Aquacult. 2016, 8, 283–300. [Google Scholar] [CrossRef]
- Olesen, I.; Myhr, A.I.; Rosendal, G.K. Sustainable aquaculture: Are We getting there? Ethical perspectives on salmon farming. J. Agric. Environ. Ethics 2011, 24, 381–408. [Google Scholar] [CrossRef]
- Jansen, H.; Broch, O.; Bannister, R.; Cranford, P.; Handå, A.; Husa, V.; Jiang, Z.; Strohmeier, T.; Strand, Ø. Spatio-temporal dynamics in the dissolved nutrient waste plume from Norwegian salmon cage aquaculture. Aquacult. Environ. Interac. 2018, 10, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Pal, D.; Maiti, S.K. Seasonal variation of heavy metals in water, sediment, and highly consumed cultured fish (Labeo rohita and Labeo bata) and potential health risk assessment in aquaculture pond of the coal city, Dhanbad (India). Environ. Sci. Pollut. Res. Int. 2018, 25, 12464–12480. [Google Scholar] [CrossRef] [PubMed]
- De Casabianca, M.; Laugier, T.; Marinho-Soriano, E. Seasonal changes of nutrients in water and sediment in a Mediterranean lagoon with shellfish farming activity (Thau Lagoon, France). ICES J. Mar. Sci. 1997, 54, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Chopin, T. Aquaculture, Integrated Multi-Trophic (IMTA). In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013; pp. 184–205. [Google Scholar]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Copertino, M.d.S.; Tormena, T.; Seeliger, U. Biofiltering efficiency, uptake and assimilation rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) cultivated in shrimp aquaculture waste water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Neori, A. Essential role of seaweed cultivation in integrated multi-trophic aquaculture farms for global expansion of mariculture: An analysis. In Nineteenth International Seaweed Symposium. Developments in Applied Phycology; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 2, pp. 567–570. [Google Scholar]
- Msuya, F.E.; Neori, A. Effect of Water aeration and nutrient load level on biomass yield, N uptake and protein content of the seaweed Ulva lactuca cultured in seawater tanks. J. Appl. Phycol. 2008, 20, 1021–1031. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Sassi, J.-F. Marine algae and the global food industry. In Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; Pereira, L., Neto, J., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 300–319. [Google Scholar]
- Marinho, G.; Nunes, C.; Sousa-Pinto, I.; Pereira, R.; Rema, P.; Valente, L.M.P. The IMTA-cultivated chlorophyta Ulva spp. as a sustainable ingredient in Nile tilapia (Oreochromis Niloticus) diets. J. Appl. Phycol. 2013, 25, 1359–1367. [Google Scholar] [CrossRef]
- Shpigel, M.; Guttman, L.; Shauli, L.; Odintsov, V.; Ben-Ezra, D.; Harpaz, S. Ulva lactuca from an Integrated Multi-Trophic Aquaculture (IMTA) biofilter system as a protein supplement in gilthead seabream (Sparus Aurata) diet. Aquaculture 2017, 481, 112–118. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Coimbra, Portugal, 2011; pp. 15–49. [Google Scholar]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-promoting ingredients from four selected Azorean macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.-F.; Dion, P.; Lerat, Y.; Lahaye, M. Seasonal variability of physicochemical and rheological properties of ulvan in two Ulva species (chlorophyta) from the Brittany coast. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Serviere-Zaragoza, E.; Hurtado, M.A.; Manzano-Sarabia, M.; Mazariegos-Villarreal, A.; Reza, M.; Arjona, O.; Palacios, E. Seasonal and interannual variation of fatty acids in macrophytes from the Pacific coast of Baja California Peninsula (Mexico). J. Appl. Phycol. 2015, 27, 1297–1306. [Google Scholar] [CrossRef]
- Favero, N.; Cattalini, F.; Bertaggia, D.; Albergoni, V. Metal accumulation in a biological indicator (Ulva rigida) from the Lagoon of Venice (Italy). Arch. Environ. Contam. Toxicol. 1996, 31, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Floreto, E.A.T.; Hirata, H.; Ando, S.; Yamasaki, S. Effects of temperature, light intensity, salinity and source of nitrogen on the growth, total lipid and fatty acid composition of Ulva pertusa kjellman (chlorophyta). Bot. Mar. 1993, 36, 149–158. [Google Scholar] [CrossRef]
- Lahaye, M.; Gomez-Pinchetti, J.-L.; Del Rio, M.J.; Garcia-Reina, G. Natural decoloration, composition and increase in dietary fibre content of an edible marine algae, Ulva rigida (chlorophyta), grown under different nitrogen conditions. J. Sci. Food Agric. 1995, 68, 99–104. [Google Scholar] [CrossRef]
- Korzen, L.; Abelson, A.; Israel, A. Growth, Protein and carbohydrate contents in Ulva rigida and Gracilaria bursa-pastoris integrated with an offshore fish farm. J. Appl. Phycol. 2016, 28, 1835–1845. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Neto, R.; Marçal, C.; Queirós, A.; Abreu, H.; Silva, A.; Cardoso, S. Screening of Ulva rigida, Gracilaria Sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.S.P.; da Costa, E.; Melo, T.; Sulpice, R.; Cardoso, S.M.; Pitarma, B.; Pereira, R.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Seasonal plasticity of the polar lipidome of Ulva rigida cultivated in a sustainable integrated multi-trophic aquaculture. Algal Res. 2020, 49, 101958. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Pinheiro, V.F.; Marçal, C.; Abreu, H.; Lopes da Silva, J.A.; Silva, A.M.S.; Cardoso, S.M. Physicochemical Changes of Air-Dried and Salt Processed Ulva rigida over Storage Time. Molecules 2020, 24, 2955. [Google Scholar] [CrossRef] [Green Version]
- Gadberry, B.A.; Colt, J.; Maynard, D.; Boratyn, D.C.; Webb, K.; Johnson, R.B.; Saunders, G.W.; Boyer, R.H. Intensive land-based production of red and green macroalgae for human consumption in the Pacific Northwest: An evaluation of seasonal growth, yield, nutritional composition, and contaminant levels. ALGAE 2018, 33, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Robertson-Andersson, D.V.; Maneveldt, G.W.; Naidoo, K. Effects of wild and farm-grown macroalgae on the growth of juvenile South African abalone Haliotis midae linnaeus. Afr. J. Aquat. Sci. 2011, 36, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Robertson-Andersson, D.V.; Potgieter, M.; Hansen, J.; Bolton, J.J.; Troell, M.; Anderson, R.J.; Halling, C.; Probyn, T. Integrated seaweed cultivation on an abalone farm in South Africa. J. Appl. Phycol. 2008, 20, 579–595. [Google Scholar] [CrossRef]
- Rouxel, C.; Bonnabeze, E.; Daniel, A.; Jerôme, M.; Etienne, M.; Fleurence, J. Identification by SDS PAGE of green seaweeds (Ulva and Enteromorpha) used in the food industry. J. Appl. Phycol. 2001, 13, 215–219. [Google Scholar] [CrossRef]
- Taboada, C.; Millán, R.; MÃguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2009, 90, 445–449. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Duke, C.S. Biochemical strategies for growth of Gracilaria tikvahiae (Rhodophyta) in relation to light intensity and nitrogen availability. J. Phycol. 1984, 20, 488–495. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Del Río, M.J.; Ramazanov, Z.; García-Reina, G. Ulva rigida (Ulvales, Chlorophyta) tank culture as biofilters for dissolved inorganic nitrogen from fishpond effluents. Hydrobiologia 1996, 326, 61–66. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Matos, J.; Costa, S.; Rodrigues, A.; Pereira, R.; Sousa Pinto, I. Experimental integrated aquaculture of fish and red seaweeds in Northern Portugal. Aquaculture 2006, 252, 31–42. [Google Scholar] [CrossRef]
- Cohen, I.; Neori, A. Ulva lactuca biofilters for marine fishpond effluents I. Ammonia uptake kinetics and nitrogen content. Bot. Mar. 1991, 34, 475–482. [Google Scholar] [CrossRef]
- Neori, A.; Cohen, I.; Gordin, H. Ulva lactuca biofilters for marine fishpond effluents II. Growth rate, yield and C:N ratio. Bot. Mar. 1991, 34, 483–489. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and Assessment of Antiacetylcholinesterase potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Masaló, I.; Oca, J.; Ferrer, J.; Cremades, J.; Pintado, J.; Jiménez, P. Influence of Growing Conditions on Ulva Ohnoi Composition Cultivated in an IMTA-RAS System. Aquac Eur. 2016, 16, 6–8. [Google Scholar]
- Pinchetti, J.L.G.; Fernández, E.d.C.; Díez, P.M.; Reina, G.G. Nitrogen availability influences the biochemical composition and photosynthesis of tank-cultivated Ulva rigida (Chlorophyta). J. Appl. Phycol. 1998, 10, 383–389. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Ryther, J.H. The effects of nitrogen and seawater flow rate on the growth and biochemical composition of Gracilaria foliifera Var. Angustissima in mass outdoor cultures. Bot. Mar. 1979, 22, 529–537. [Google Scholar] [CrossRef]
- Frikha, F.; Kammoun, M.; Hammami, N.; Mchirgui, R.; Belbahri, L.; Gargouri, Y.; Miled, N.; Ben-Rebah, F. Chemical composition and some biological activities of marine algae collected in Tunisia. Cienc. Mar. 2011, 37, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Astorga-España, M.S.; Rodríguez Galdón, B.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Mineral and trace element concentrations in seaweeds from the Sub-Antarctic ecoregion of Magallanes (Chile). J. Food Compost. Anal. 2015, 39, 69–76. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Sheikh, M.A. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi J. Biol. Sci. 2015, 22, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Koliaki, C.; Katsilambros, N. Dietary sodium, potassium, and alcohol: Key players in the pathophysiology, prevention, and treatment of human hypertension. Nutr. Rev. 2013, 71, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Moreda-Piñeiro, J.; Alonso-Rodríguez, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Development of a new sample pre-treatment procedure based on pressurized liquid extraction for the determination of metals in edible seaweed. Anal. Chim. Acta 2007, 598, 95–102. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the Coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef] [Green Version]
- El Maghraby, D.M.; Fakhry, E.M. Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production. Oceanologia 2015, 57, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.M.; Phleger, C.F.; Nichols, P.D. Seasonal lipid composition in macroalgae of the Northeastern Pacific Ocean. Bot. Mar. 2002, 45, 58–65. [Google Scholar] [CrossRef]
- Floreto, E.A.T.; Teshima, S.; Ishikawa, M. Effects of nitrogen and phosphorus on the growth and fatty acid composition of Ulva pertusa Kjellman (Chlorophyta). Bot. Mar. 1996, 39, 69–74. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An analysis of the nutritional and health values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two chlorophyta collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef]
- Godard, M.; Décordé, K.; Ventura, E.; Soteras, G.; Baccou, J.-C.; Cristol, J.-P.; Rouanet, J.-M. Polysaccharides from the green alga Ulva rigida improve the antioxidant status and prevent fatty streak lesions in the high cholesterol fed hamster, an animal model of nutritionally-induced atherosclerosis. Food Chem. 2009, 115, 176–180. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Lahaye, M. Marine Algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Tenore, K.R. Experimental outdoor studies with Ulva fasciata Delile. I. Interaction of light and nitrogen on nutrient uptake, growth, and biochemical composition. J. Exp. Mar. Biol. Ecol. 1981, 53, 135–152. [Google Scholar] [CrossRef]


| Mineral | Condition | Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| August 2016 | September 2016 | October 2016 | November 2016 | January 2017 | February 2017 | May 2017 | June 2017 | ||
| Iron | LFr/LSd | 81.1 a | 82.1 | 81.3 a | 59.1 | 81.1 a | 96.0 a | 101.7a | 82.0 |
| HFr/LSd | 67.5 | 77.8 | 51.7 | 103.6 | 72.9 | 96.7 a | 85.0 | 130.0 a | |
| LFr/HSd | 43.5 | 51.8 a | 35.7 | 80.2 | 66.0 a | 136.7 | 86.0 a | 90.8 | |
| HFr/HSd | 67.6 | 70.3 | 51.7 | 62.3 a | 86.3 | 100.0 | 123.3 | 170.0 | |
| Manganese | LFr/LSd | 10.4 | 10.5 | 9.5 | 11.4 | 6.7 a | 3.4 a | 4.1 | 4.2 |
| HFr/LSd | 9.2 | 7.5 a | 10.3 | 9.9 a | 10.3 | 2.7 | 5.9 | 8.4 | |
| LFr/HSd | 6.4a | 6.8 | 6.5 | 7.6 a | 8.3 | 6.7 | 7.3 | 5.0 | |
| HFr/HSd | 5.9 | 4.8 a | 5.0 | 7.1 a | 9.6 | 5.5 a | 11.0 | 11.5 | |
| Copper | LFr/LSd | 2.7 | 2.7 a | 3.0 | 2.5 | 2.6 | 1.9 | 1.1 | 1.2 |
| HFr/LSd | 2.9 | 2.7 | 2.8 | 2.5 | 2.5 | 1.9 | 1.2 | 1.6 | |
| LFr/HSd | 2.1 | 2.3 | 2.7 | 2.8 | 2.6 | 2.1 | 1.1 | 1.4 | |
| HFr/HSd | 2.3 | 3.2 a | 3.1 | 2.8 | 3.0 | 2.2 | 1.6 | 2.0 | |
| Zinc | LFr/LSd | 1.6 | 1.2 | 1.6 | 1.6 | 1.5 | 1.5 | 0.8 | 0.9 |
| HFr/LSd | 1.5 | 1.5 | 1.4 | 1.5 | 1.1 a | 1.7 | 1.0 | 1.0 a | |
| LFr/HSd | 1.0 | 1.2 | 1.5 | 1.7 | 1.9 | 1.8 | 1.0 | 1.1 | |
| HFr/HSd | 1.1 | 0.8 | 1.6 | 1.6 | 1.7 | 1.9 | 1.5 | 1.7 | |
| Fatty Acid | August 2016 | November 2016 | February 2017 | May 2017 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LFr/LSd | HFr/LSd | LFr/HSd | HFr/HSd | LFr/LSd | HFr/LSd | LFr/HSd | HFr/HSd | LFr/LSd | HFr/LSd | LFr/HSd | HFr/HSd | LFr/LSd | HFr/LSd | LFr/HSd | HFr/HSd | |
| Saturated | ||||||||||||||||
| C14:0 | 14 | 12 | 9 | 9 | 467 a | 67 a | 37 | 196 | 220 | 188 a | 105 | 134 | 10 | 36 | 9 | 12 |
| C16:0 | 591 | 625 | 458 | 396 | 977 | 2547 | 1045 | 1303 | 3495 a | 2942 | 1099 | 2714 | 592 | 1115 | 968 | 779 |
| C18:0 | 39 | 26 | 18 | 17 | 37 | 45 | 23 | 36 | 67 | 57 | 56 | 59 | 18 | 22 | 17 | 23 |
| Unsaturated | ||||||||||||||||
| C16:1 (n-7) | 57 | 42 | 36 | 32 | 121 a | 118 | 85 | 169 | 184 | 140 | 92 | 109 | 42 | 79 | 62 | 47 |
| C18:1 (n-9) | 195 | 176 | 149 | 126 | 340 | 535 | 334 | 380 | 739 | 568 | 326 | 498 | 228 | 349 | 277 | 269 |
| C18:2 (n-6) | 27 | 14 | 17 | 14 | 59 | 69 | 45 | 58 | 121 | 106 | 52 | 84 | 25 | 43 | 33 | 26 |
| C18:3 (n-3) | 152 | 83 | 81 | 57 | 201 | 267 | 161 | 205 | 430 | 358 | 171 | 268 | 61 | 122 | 74 | 82 |
| C20:5 (n-3) | 10 | 3 | 4 | 4 | 105 a | 17 a | 11 | 23 a | 115 | 92 | 118 | 60 | 5 | 15 | 8 | 9 |
| ∑ SFA | 644 | 663 | 486 | 423 | 1480 | 2660 | 1106 | 1535 | 3781 | 3186 | 1260 | 2908 | 620 | 1173 | 994 | 813 |
| ∑ MUFA | 252 | 218 | 185 | 158 | 461 | 653 | 419 | 548 | 923 | 708 | 417 | 608 | 270 | 428 | 339 | 316 |
| ∑ PUFA | 189 | 100 | 103 | 75 | 365 | 353 | 217 | 286 | 666 | 556 | 341 | 411 | 91 | 180 | 115 | 117 |
| Ω6/Ω3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| AI | 1.5 | 2.1 | 1.7 | 1.9 | 3.4 | 2.8 | 1.9 | 2.5 | 2.8 | 2.9 | 2.0 | 3.2 | 1.7 | 2.1 | 2.2 | 1.9 |
| TI | 1.0 | 1.7 | 1.3 | 1.5 | 1.3 | 2.2 | 1.5 | 1.5 | 1.7 | 1.8 | 1.1 | 2.2 | 1.8 | 1.8 | 2.3 | 1.8 |
| h/H | 0.6 | 0.4 | 0.5 | 0.5 | 0.5 | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.6 | 0.3 | 0.5 | 0.5 | 0.4 | 0.5 |
| Dietary Fiber | Condition | Time | |||
|---|---|---|---|---|---|
| August 2016 | November 2016 | February 2017 | May 2017 | ||
| Insoluble | LFr/LSd | 21.0 ± 0.4 | 21.4 ± 2.0 | 19.0 ± 2.1 | 26.0 ± 0.4 |
| HFr/LSd | 21.8 ± 1.3 | 19.3 ± 3.1 | 22.2 ± 0.4 | 26.2 ± 1.2 | |
| LFr/HSd | 21.0 ± 1.3 | 20.1 ± 1.0 | 22.4 ± 1.6 | 26.3 ± 0.5 | |
| HFr/HSd | 22.0 ± 0.8 | 19.9 ± 1.2 | 22.8 ± 0.4 | 29.2 ± 1.6 | |
| Soluble | LFr/LSd | 25.9 ± 1.1 | 22.1 ± 1.1 | 22.8 ± 1.2 | 30.2 ± 1.2 |
| HFr/LSd | 24.3 ± 1.9 | 22.2 ± 1.7 | 20.8 ± 0.6 | 28.2 ± 0.5 | |
| LFr/HSd | 23.9 ± 1.3 | 21.1 ± 1.8 | 22.0 ± 0.6 | 30.6 ± 1.3 | |
| HFr/HSd | 24.1 ± 1.8 | 21.0 ± 0.6 | 21.4 ± 0.3 | 29.0 ± 1.0 | |
| Total | LFr/LSd | 46.9 ± 1.3 | 43.5 ± 2.4 | 41.8 ± 1.2 | 57.1 ± 0.4 |
| HFr/LSd | 43.4 ± 0.0 | 41.5 ± 1.7 | 43.4 ± 0.5 | 54.4 ± 1.2 | |
| LFr/HSd | 45.8 ± 0.5 | 41.2 ± 0.8 | 39.0 ± 1.4 | 56.9 ± 1.1 | |
| HFr/HSd | 47.2 ± 0.7 | 40.9 ± 1.3 | 44.0 ± 0.2 | 57.2 ± 1.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queirós, A.S.; Circuncisão, A.R.; Pereira, E.; Válega, M.; Abreu, M.H.; Silva, A.M.S.; Cardoso, S.M. Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors. Appl. Sci. 2021, 11, 6137. https://doi.org/10.3390/app11136137
Queirós AS, Circuncisão AR, Pereira E, Válega M, Abreu MH, Silva AMS, Cardoso SM. Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors. Applied Sciences. 2021; 11(13):6137. https://doi.org/10.3390/app11136137
Chicago/Turabian StyleQueirós, Ana S., Ana R. Circuncisão, Eduarda Pereira, Mónica Válega, Maria H. Abreu, Artur M. S. Silva, and Susana M. Cardoso. 2021. "Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors" Applied Sciences 11, no. 13: 6137. https://doi.org/10.3390/app11136137
APA StyleQueirós, A. S., Circuncisão, A. R., Pereira, E., Válega, M., Abreu, M. H., Silva, A. M. S., & Cardoso, S. M. (2021). Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors. Applied Sciences, 11(13), 6137. https://doi.org/10.3390/app11136137









