Effect of Photosensitization Mediated by Curcumin on Carotenoid and Aflatoxin Content in Different Maize Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Photosensitizer Solutions
2.3. Light Source
2.4. Maize Kernel Samples
2.5. In Vitro Trials-Effect of Photosensitization on A. flavus Spores
2.5.1. Study Design
2.5.2. Evaluation of Photosensitization Effect on A. flavus Spores
2.5.3. Determination of Reactive Oxygen Species (ROS) Formation
2.5.4. Morphological Changes on A. flavus Spores
2.6. In Vivo Trials-Effect of Photosensitization on Maize Kernels
2.6.1. Inoculation of Kernels
2.6.2. Photosensitization Treatment
2.7. Extraction and Quantification of Aflatoxin B1
2.8. Effect of Photosensitization on Maize Kernel Colour
2.9. Carotenoid Extraction and Quantification
2.10. Data Analysis
3. Results and Discussion
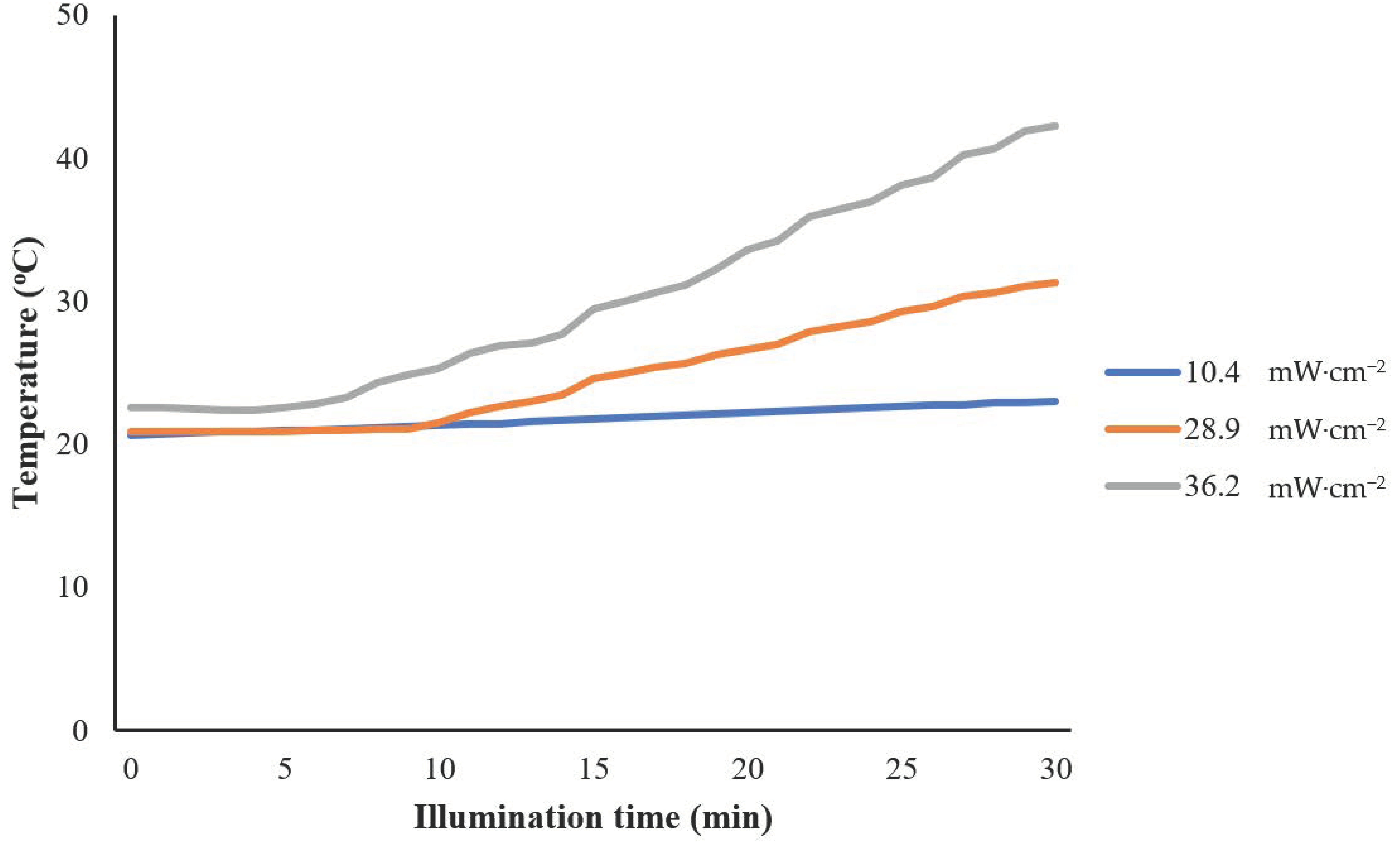
3.1. LED Light Dose and Temperature Profile
3.2. In Vitro Experiments
3.2.1. Effect of Photosensitization on A. flavus Spores
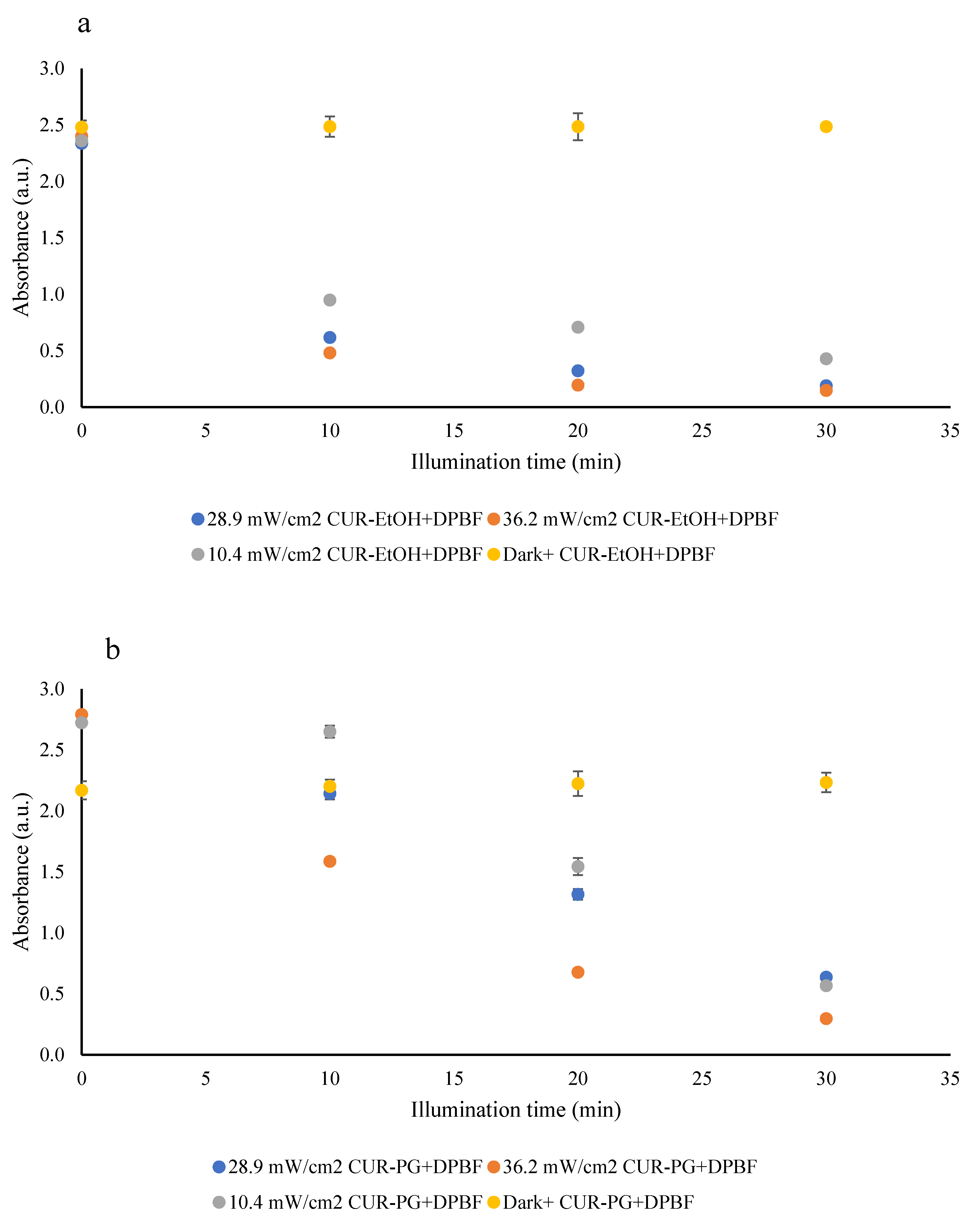
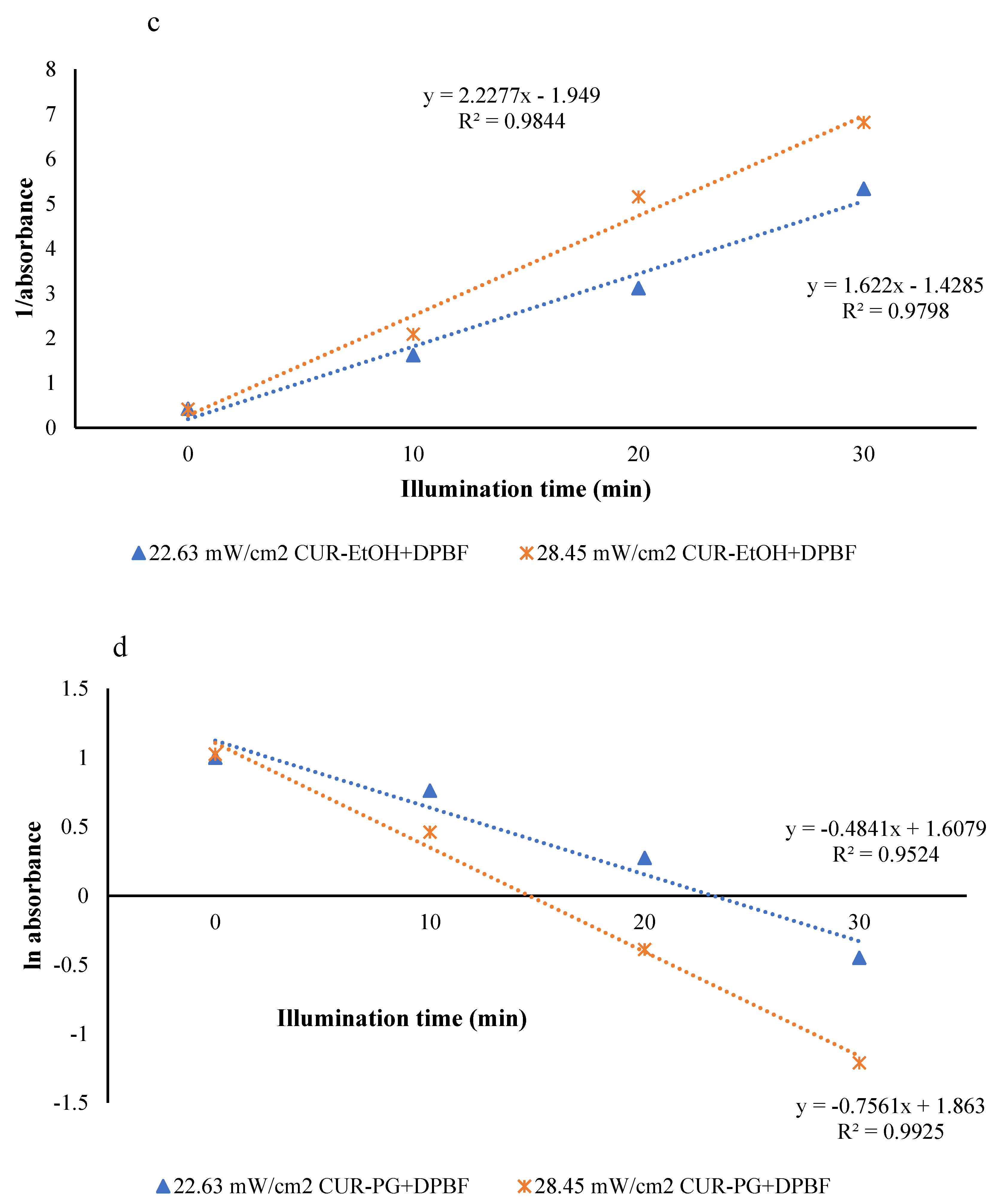
3.2.2. Reactive Oxygen Species (ROS) Generation
3.2.3. Morphological Changes of A. flavus Spores
3.3. In Vivo Experiment
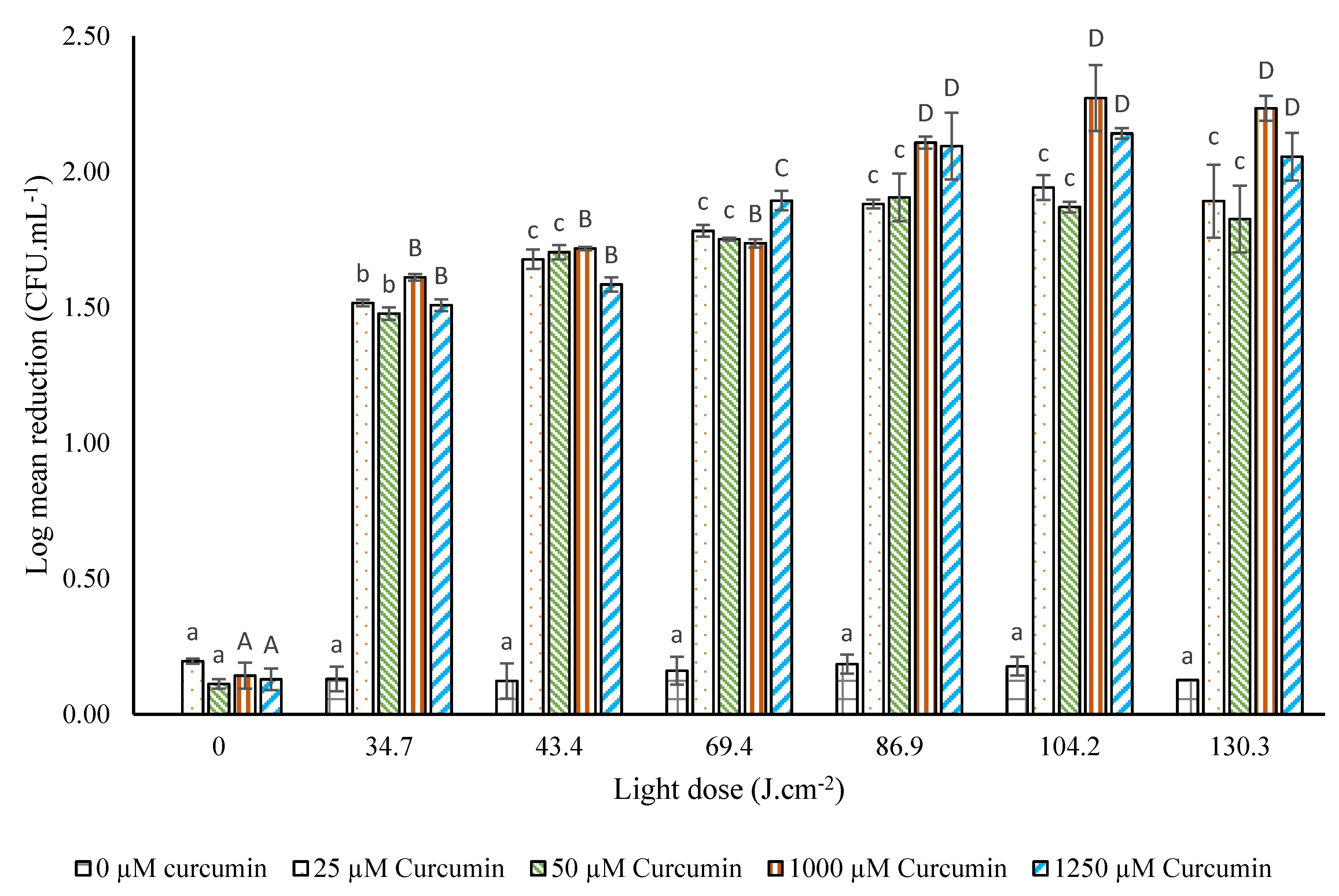
3.3.1. Photodecontamination of A. flavus on Maize
3.3.2. Effect of Photosensitization on AFB1 Production
3.3.3. Effect of Photosensitization on Maize Colour
3.4. Effect of Blue Light on Carotenoid Content in Maize Kernels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fink-Gremmels, J.; van der Merwe, D. Mycotoxins in the food chain: Contamination of foods of animal origin. In Chemical Hazards in Foods of Animal Origin; Smulders, F.J.M., Rietjens, I.M.C.M., Rose, M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; Volume 7, pp. 1190–1198. [Google Scholar]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on health. Indian J. Clin. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef]
- Al-Jaal, B.; Salama, S.; Al-Qasmi, N.; Jaganjac, M. Mycotoxin contamination of food and feed in the Gulf Cooperation Council countries and its detection. Toxicon 2019, 171, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New horizons in microbiological food safety: Photodynamic Decontamination based on a curcumin derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Tan, B.; Hamzah, S.; Lin, Y.; Kong, Z.; Zhang, Y.; Zheng, B.; Zeng, S. Photodynamic inactivation of Burkholderia cepacia by curcumin in combination with EDTA. Food Res. Int. 2018, 111, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ghate, V.S.; Zhou, W.; Yuk, H.G. Perspectives and trends in the application of photodynamic inactivation for microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.; Tosati, J.; Tikekar, R.; Monteiro, A.; Nitin, N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157: H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. The effect of photosensitization mediated by curcumin on storage life of fresh date (Phoenix dactylifera L.) fruit. Food Control. 2018, 93, 305–309. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Cao, B.; Wu, J.; Wang, Y.; Xue, Y.; Xu, J.; Xue, C.; Tang, Q.J. The effect of a novel photodynamic activation method mediated by curcumin on oyster shelf life and quality. Food Res. Int. 2016, 87, 204–210. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Thrane, J.-E.; Kyle, M.; Striebel, M.; Haande, S.; Grung, M.; Rohrlack, T.; Andersen, T. Spectrophotometric analysis of pigments: A critical assessment of a high-throughput method for analysis of algal pigment mixtures by spectral deconvolution. PLoS ONE 2015, 10, e0137645. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4. 3.1–F4. 3.8. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.A. Effect of carotenoids on aflatoxin B1 synthesis by Aspergillus flavus. Phytopathology 1997, 87, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.; Norton, R.; McAlpin, C. β-Carotene inhibition of aflatoxin biosynthesis among Aspergillus flavus genotypes from Illinois corn. Mycoscience 1998, 39, 167–172. [Google Scholar] [CrossRef]
- Suwarno, W.; Hannok, P.; Palacios-Rojas, N.; Windham, G.; Crossa, J.; Pixley, K. Provitamin A carotenoids in grain reduce aflatoxin contamination of maize while combating vitamin A deficiency. Front. Plant Sci. 2019, 10, 30. [Google Scholar] [CrossRef]
- Aman, R.; Schieber, A.; Carle, R. Effects of heating and illumination on trans− cis isomerization and degradation of β-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef]
- Jung, M.; Min, D. Effects of quenching mechanisms of carotenoids on the photosensitized oxidation of soybean oil. J. Am. Oil Chem. Soc. 1991, 68, 653–658. [Google Scholar] [CrossRef]
- Dutta, D.; Chaudhuri, U.; Chakraborty, R. Structure, health benefits, antioxidant property and processing and storage of carotenoids. Afr. J. Biotechnol. 2005, 4, 1510–1520. [Google Scholar] [CrossRef]
- Oshima, S.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Inhibitory effect of β-carotene and astaxanthin on photosensitized oxidation of phospholipid bilayers. J. Nutr. Sci. Vitaminol. 1993, 39, 607–615. [Google Scholar] [CrossRef]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.J.W.; Sultanbawa, Y. Inactivation of Aspergillus flavus spores by curcumin-mediated photosensitization. Food Control 2016, 59, 708–713. [Google Scholar] [CrossRef]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.; Okoth, S.A.; Sultanbawa, Y. Curcumin-based photosensitization inactivates Aspergillus flavus and reduces aflatoxin B1 in maize kernels. Food Microbiol. 2019, 82, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J. Photochem. Photobiol. B 2017, 173, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Rout, B.; Liu, C.-H.; Wu, W.-C. Photosensitizer in lipid nanoparticle: A nano-scaled approach to antibacterial function. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- George, S.; Kishen, A. Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection. J. Biomed. Opt. 2007, 12, 034029. [Google Scholar] [CrossRef]
- Smijs, T.; Mulder, A.; Pavel, S.; Onderwater, J.; Koerten, H.; Bouwstra, J. Morphological changes of the dermatophyte Trichophyton rubrum after photodynamic treatment: A scanning electron microscopy study. Med. Mycol. 2008, 46, 315–325. [Google Scholar] [CrossRef][Green Version]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Kim, M.-J.; Bang, W.-S.; Zhou, W.; Yuk, H.-G. Effect of 460 nm light emitting diode illumination on survival of Salmonella spp. on fresh-cut pineapples at different irradiances and temperatures. J. Food Eng. 2017, 196, 130–138. [Google Scholar] [CrossRef]
- Yu, J.; Fedorova, N.D.; Montalbano, B.G.; Bhatnagar, D.; Cleveland, T.E.; Bennett, J.W.; Nierman, W.C. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-Seq. FEMS Microbiol. Lett. 2011, 322, 145–149. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef] [PubMed]
- Nsubuga, A.; Mandl, G.A.; Capobianco, J.A. Investigating the reactive oxygen species production of Rose Bengal and Merocyanine 540-loaded radioluminescent nanoparticles. Nanoscale Adv. 2021, 3, 1375–1381. [Google Scholar] [CrossRef]
- Diaz-Diestra, D.; Beltran-Huarac, J.; Bracho-Rincon, D.P.; González-Feliciano, J.A.; González, C.I.; Weiner, B.R.; Morell, G. Biocompatible ZnS: Mn quantum dots for reactive oxygen generation and detection in aqueous media. J. Nanopart. Res. 2015, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Koo, M.-A.; Lee, M.H.; Seon, G.M.; Park, Y.J.; Jeong, H.; Kim, D.; Park, J.-C. An effective method to generate controllable levels of ROS for the enhancement of HUVEC proliferation using a chlorin e6-immobilized PET film as a photo-functional biomaterial. Regen. Biomater. 2021, 8, rbab005. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.; Bilskj, P.; Reszka, K.; Motten, A.; Sik, R.; Dahl, T. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Ahmed, H.; Mekawey, A.; Morsy, M. Cellular structural changes in two candida species caused by photodynamic therapy. Photodiagn. Photodyn. Ther. 2016, 4, 39–54. [Google Scholar]
- Sakita, K.M.; Conrado, P.C.; Faria, D.R.; Arita, G.S.; Capoci, I.R.; Rodrigues-Vendramini, F.A.; Pieralisi, N.; Cesar, G.B.; Gonçalves, R.S.; Caetano, W. Copolymeric micelles as efficient inert nanocarrier for hypericin in the photodynamic inactivation of Candida species. Future Microbiol. 2019, 14, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Sabino, C.; Núñez, S.; Miyakawa, W.; Martin, A.; Ribeiro, M. Photodynamic damage predominates on different targets depending on cell growth phase of Candida albicans. J. Photochem. Photobiol. B 2017, 177, 76–84. [Google Scholar] [CrossRef]
- Pudziuvyte, B.; Bakiene, E.; Bonnett, R.; Shatunov, P.; Magaraggia, M.; Jori, G. Alterations of Escherichia coli envelope as a consequence of photosensitization with tetrakis (N-ethylpyridinium-4-yl) porphyrin tetratosylate. Photochem. Photobiol. Sci. 2011, 10, 1046–1055. [Google Scholar] [CrossRef]
- Dong, S.; Shi, H.; Zhang, X.; Chen, X.; Cao, D.; Mao, C.; Gao, X.; Wang, L. Difunctional bacteriophage conjugated with photosensitizers for Candida albicans-targeting photodynamic inactivation. Int. J. Nanomed. 2018, 13, 2199. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mou, H.; Xue, C.; Leung, A.; Xu, C.; Tang, Q.-J. Photodynamic effect of curcumin on Vibrio parahaemolyticus. Photodiagn. Photodyn. Ther. 2016, 15, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Buchovec, I.; Paskeviciute, E. Inactivation of food pathogen Bacillus cereus by photosensitization in vitro and on the surface of packaging material. J. Appl. Microbiol. 2009, 107, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Tortik, N.; Spaeth, A.; Plaetzer, K. Photodynamic decontamination of foodstuff from Staphylococcus aureus based on novel formulations of curcumin. Photochem. Photobiol. Sci. 2014, 13, 1402–1409. [Google Scholar] [CrossRef]
- Luksiene, Z.; Paskeviciute, E. Novel approach to the microbial decontamination of strawberries: Chlorophyllin-based photosensitization. J. Appl. Microbiol. 2011, 110, 1274–1283. [Google Scholar] [CrossRef]
- Alananbeh, K.; BouQellah, N.; Al Harbi, M.; Ouf, S. The Efficacy of Photosensitizers on Mycelium Growth, Mycotoxin and Enzyme Activity of Alternaria spp. Jordan J. Biol. Sci. 2018, 11, 43–50. [Google Scholar]
- Shlar, I.; Droby, S.; Choudhary, R.; Rodov, V. The mode of antimicrobial action of curcumin depends on the delivery system: Monolithic nanoparticles vs. supramolecular inclusion complex. RSC Adv. 2017, 7, 42559–42569. [Google Scholar] [CrossRef]
- Gitika, A.; Mishra, R.; Panda, S.K.; Mishra, C.; Sahoo, P.R. Evaluation of antifungal activity of Curcumin against Aspergillus flavus. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2323–2329. [Google Scholar] [CrossRef]
- Pasrija, R.; Kundu, D. Interaction of curcumin with azoles and polyenes against aspergillus infections. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 271–279. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Josewin, S.; Ghate, V.; Kim, M.-J.; Yuk, H.-G. Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon. Food Control 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- da Silva, M.; Paese, K.; Guterres, S.; Pohlmann, A.; Rutz, J.; Cantillano, R.; Nora, L.; de Oliveira Rios, A. Thermal and ultraviolet–visible light stability kinetics of co-nanoencapsulated carotenoids. Food Bioprod. Process. 2017, 105, 86–94. [Google Scholar] [CrossRef]
- Limbo, S.; Torri, L.; Piergiovanni, L. Light-induced changes in an aqueous β-carotene system stored under halogen and fluorescent lamps, affected by two oxygen partial pressures. J. Agric. Food Chem. 2007, 55, 5238–5245. [Google Scholar] [CrossRef]
- Kim, M.-J.; Bang, W.S.; Yuk, H.-G. 405 ± 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Pesek, C.; Warthesen, J. Photodegradation of carotenoids in a vegetable juice system. J. Food Sci. 1987, 52, 744–746. [Google Scholar] [CrossRef]


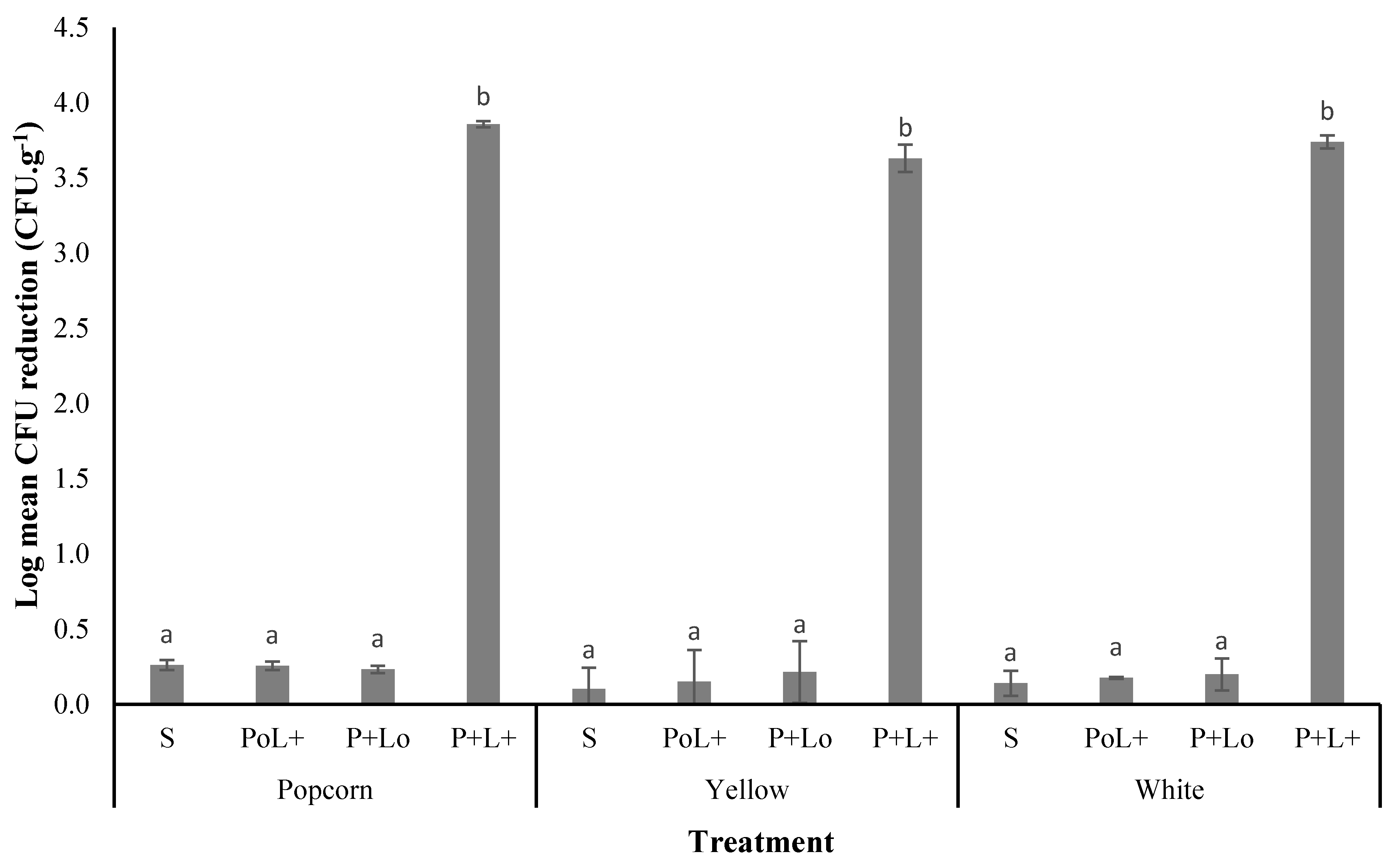
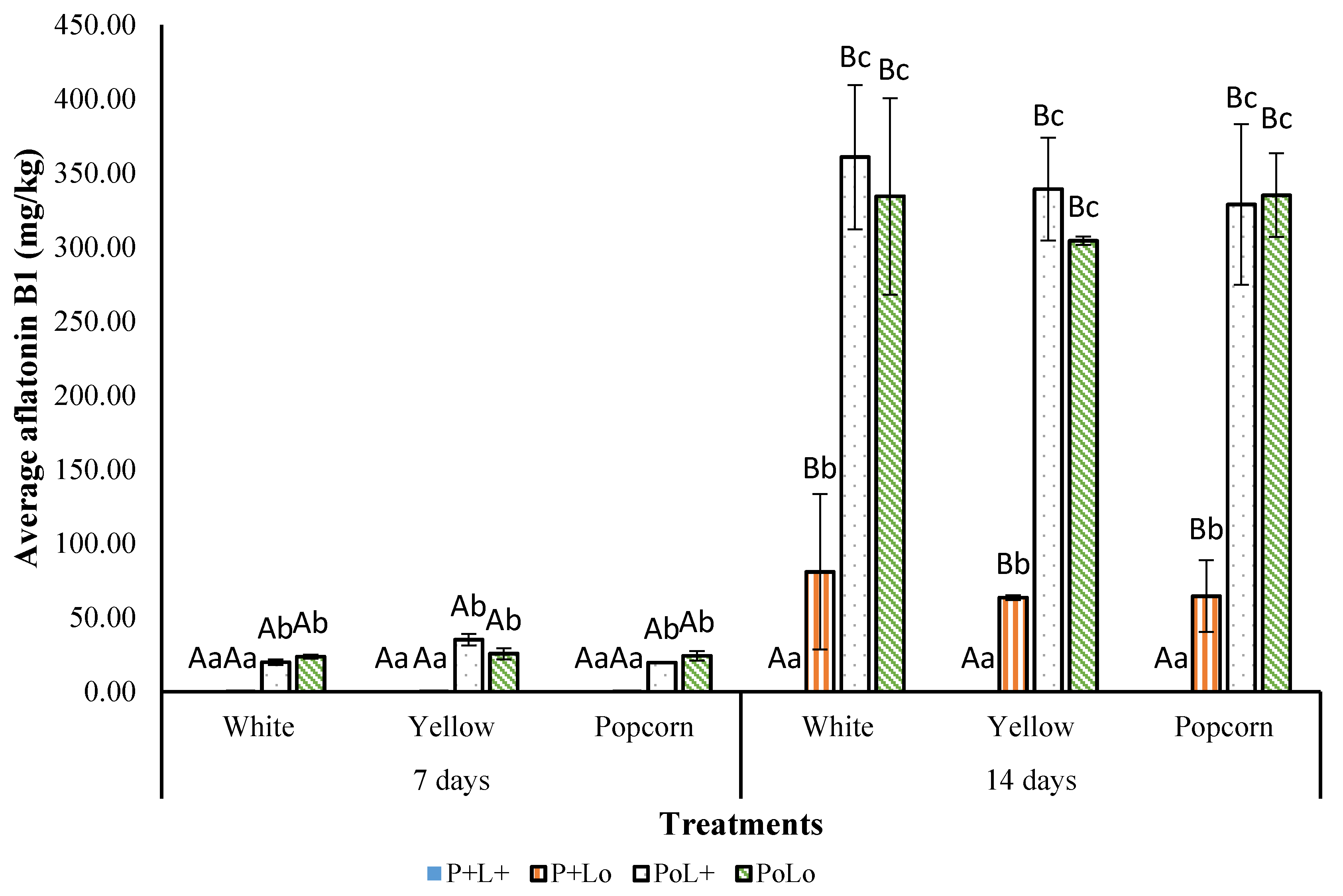
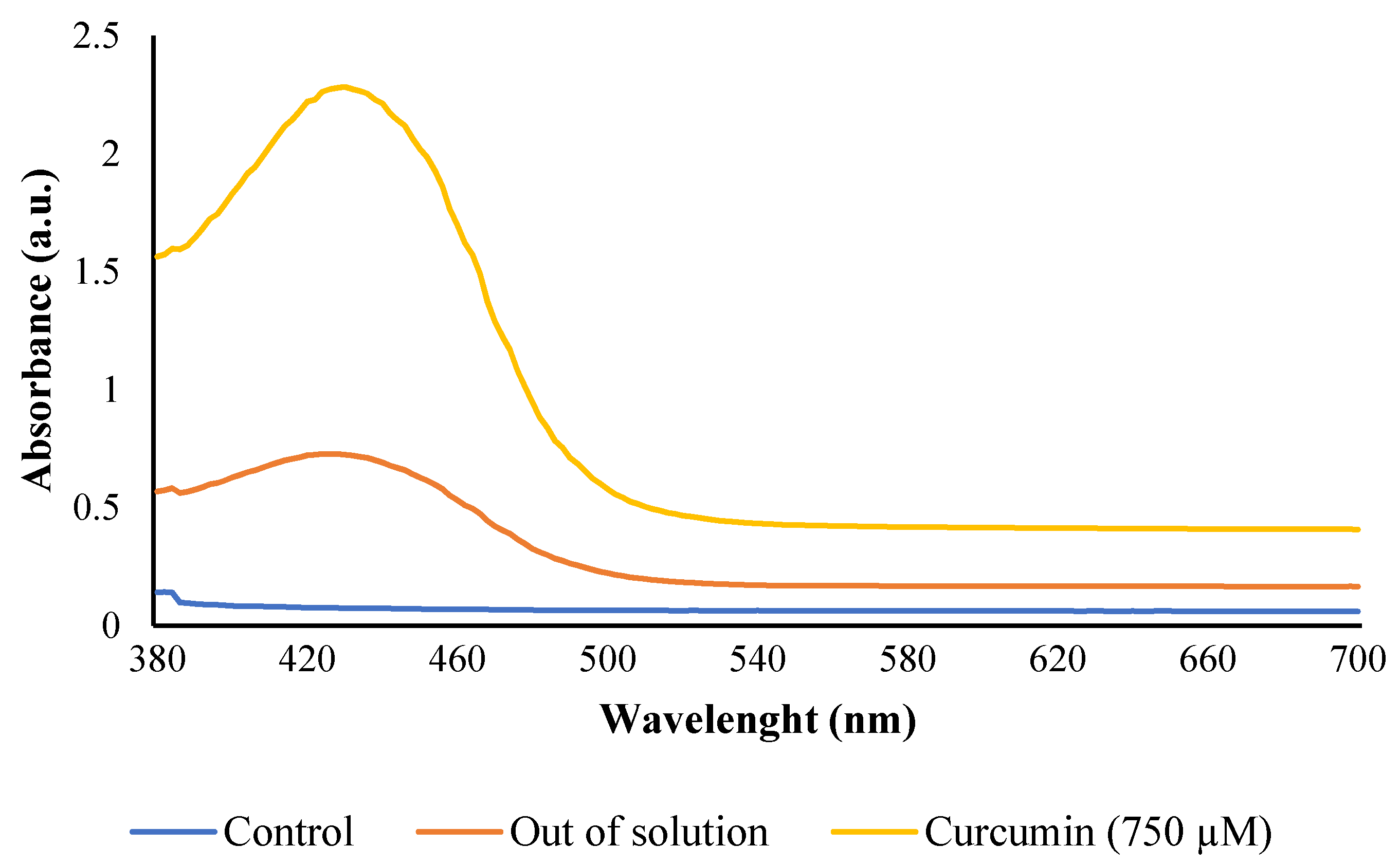
| Treatment | L* | a* | b* | C | ∆E |
|---|---|---|---|---|---|
| PoLo white | 81.9 ± 0.5 a | −0.7 ± 0.3 a | 29.4 ± 0.9 b | 29.4 ± 0.9 b | - |
| P+Lo white | 81.7 ± 0.8 a | −6.5 ± 0.4 b | 50.6 ± 1.8 a | 51.0 ± 1.7 a | 21.9 a |
| P+L+ white | 79.8 ± 0.5 a | 0.1 ± 0.4 a | 26.8 ± 0.7 b | 26.8 ± 0.7 b | 3.5 b |
| PoLo yellow | 74.8 ± 0.5 a | 6.8 ± 0.7 ab | 39.8 ± 2.5 b | 40.3 ± 2.5 b | - |
| P+Lo yellow | 77.6 ± 1.3 a | 4.4 ± 0.9 b | 54.1 ± 1.9 a | 54.2 ± 1.9 a | 14.7 a |
| P+L+ yellow | 77.2 ± 0.5 a | 7.5 ± 0.6 a | 37.6 ± 2.4 b | 38.3 ± 2.3 b | 3.3 b |
| PoLo popcorn | 74.1 ± 0.6 a | 9.8 ± 0.9 a | 28.5 ± 1.3 a | 30.2 ± 1.4 b | - |
| P+Lo popcorn | 77.4 ± 0.9 a | 5.8 ± 1.0 b | 34.9 ± 1.8 a | 35.3 ± 1.7 a | 8.7 a |
| P+L+ popcorn | 76.0 ± 0.7 a | 8.7 ± 0.7 a | 25.4 ± 1.9 a | 26.9 ± 1.8 b | 3.8 b |
| Treatment | Individual Carotenoids | Carotenoid Content (µg·g−1 DW) | ||
|---|---|---|---|---|
| White | Yellow | Popcorn | ||
| Control | Lutein | 0.22 ± 0.02 a | 5.69 ± 0.19 b | 5.67 ± 0.07 b |
| Zeaxanthin | 1.54 ± 0.01 a | 7.20 ± 0.14 b | 24.04 ± 0.26 c | |
| β-cryptoxanthin | n.d. | n.d. | 1.86 ± 0.07 b | |
| β-carotene | n.d. | n.d. | 1.84 ± 0.09 b | |
| Total | 1.74 ± 0.00 Aa | 12.90 ± 0.32 Ab | 33.44 ± 0.48 Ac | |
| Illumination | Lutein | 0.17 ± 0.01 a | 6.07 ± 0.48 b | 5.76 ± 0.24 b |
| Zeaxanthin | 1.45 ± 0.02 a | 7.29 ± 0.42 b | 24.67 ± 0.70 c | |
| β-cryptoxanthin | n.d. | n.d. | 2.29 ± 0.07 a | |
| β-carotene | n.d. | n.d. | 3.30 ± 0.076 b | |
| Total | 1.62 ± 0.01 Aa | 13.36 ± 0.91 Ab | 36.01 ± 1.05 Ac | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguenha, R.; Damyeh, M.S.; Phan, A.D.T.; Hong, H.T.; Chaliha, M.; O’Hare, T.J.; Netzel, M.E.; Sultanbawa, Y. Effect of Photosensitization Mediated by Curcumin on Carotenoid and Aflatoxin Content in Different Maize Varieties. Appl. Sci. 2021, 11, 5902. https://doi.org/10.3390/app11135902
Nguenha R, Damyeh MS, Phan ADT, Hong HT, Chaliha M, O’Hare TJ, Netzel ME, Sultanbawa Y. Effect of Photosensitization Mediated by Curcumin on Carotenoid and Aflatoxin Content in Different Maize Varieties. Applied Sciences. 2021; 11(13):5902. https://doi.org/10.3390/app11135902
Chicago/Turabian StyleNguenha, Rafael, Maral Seidi Damyeh, Anh D. T. Phan, Hung T. Hong, Mridusmita Chaliha, Tim J. O’Hare, Michael E. Netzel, and Yasmina Sultanbawa. 2021. "Effect of Photosensitization Mediated by Curcumin on Carotenoid and Aflatoxin Content in Different Maize Varieties" Applied Sciences 11, no. 13: 5902. https://doi.org/10.3390/app11135902
APA StyleNguenha, R., Damyeh, M. S., Phan, A. D. T., Hong, H. T., Chaliha, M., O’Hare, T. J., Netzel, M. E., & Sultanbawa, Y. (2021). Effect of Photosensitization Mediated by Curcumin on Carotenoid and Aflatoxin Content in Different Maize Varieties. Applied Sciences, 11(13), 5902. https://doi.org/10.3390/app11135902








