Diagnosis and Monitoring of Volatile Fatty Acids Production from Raw Cheese Whey by Multiscale Time-Series Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analytical Methods
2.3. Multiscale Time Series Analysis
Multifractal Analysis
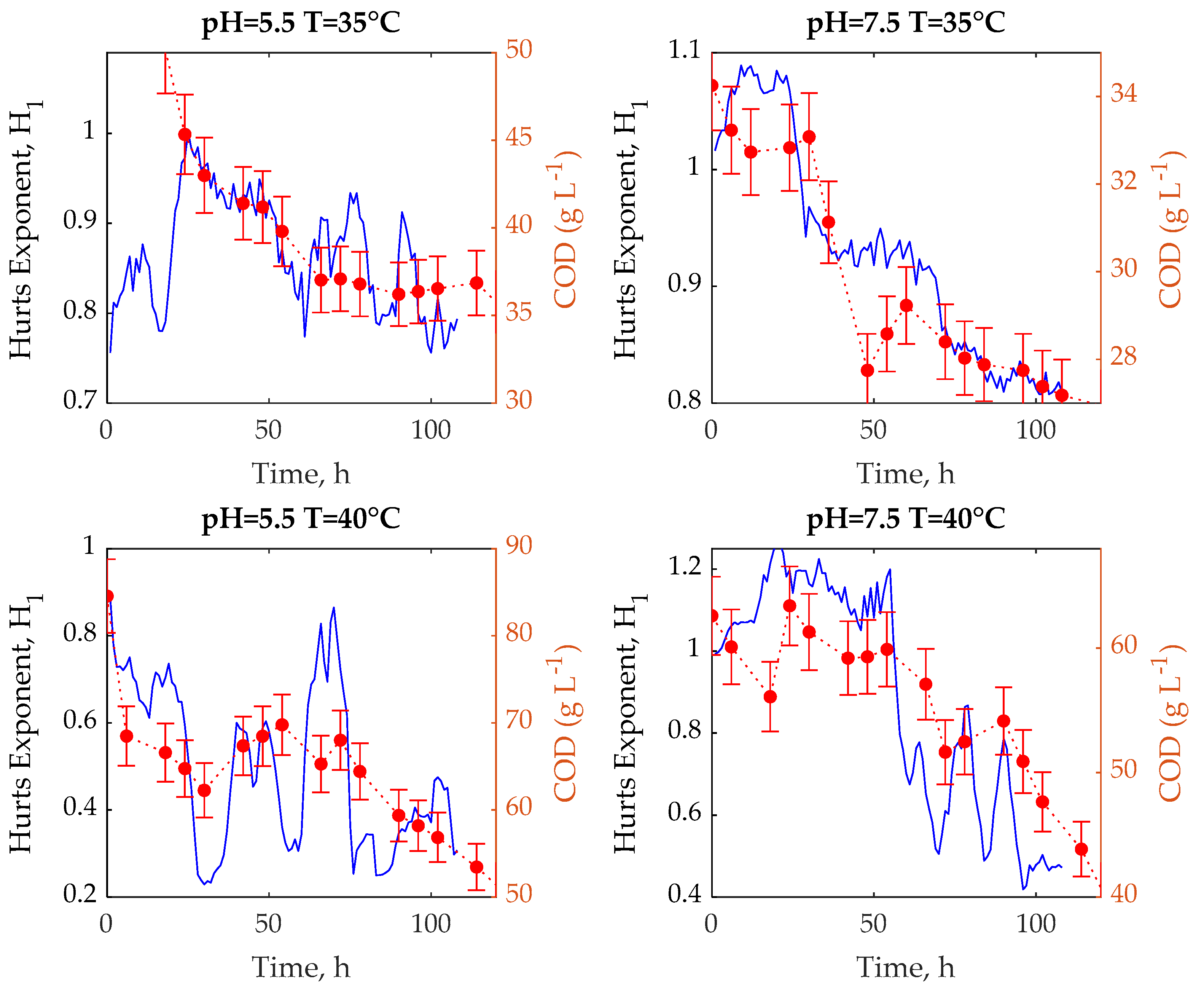
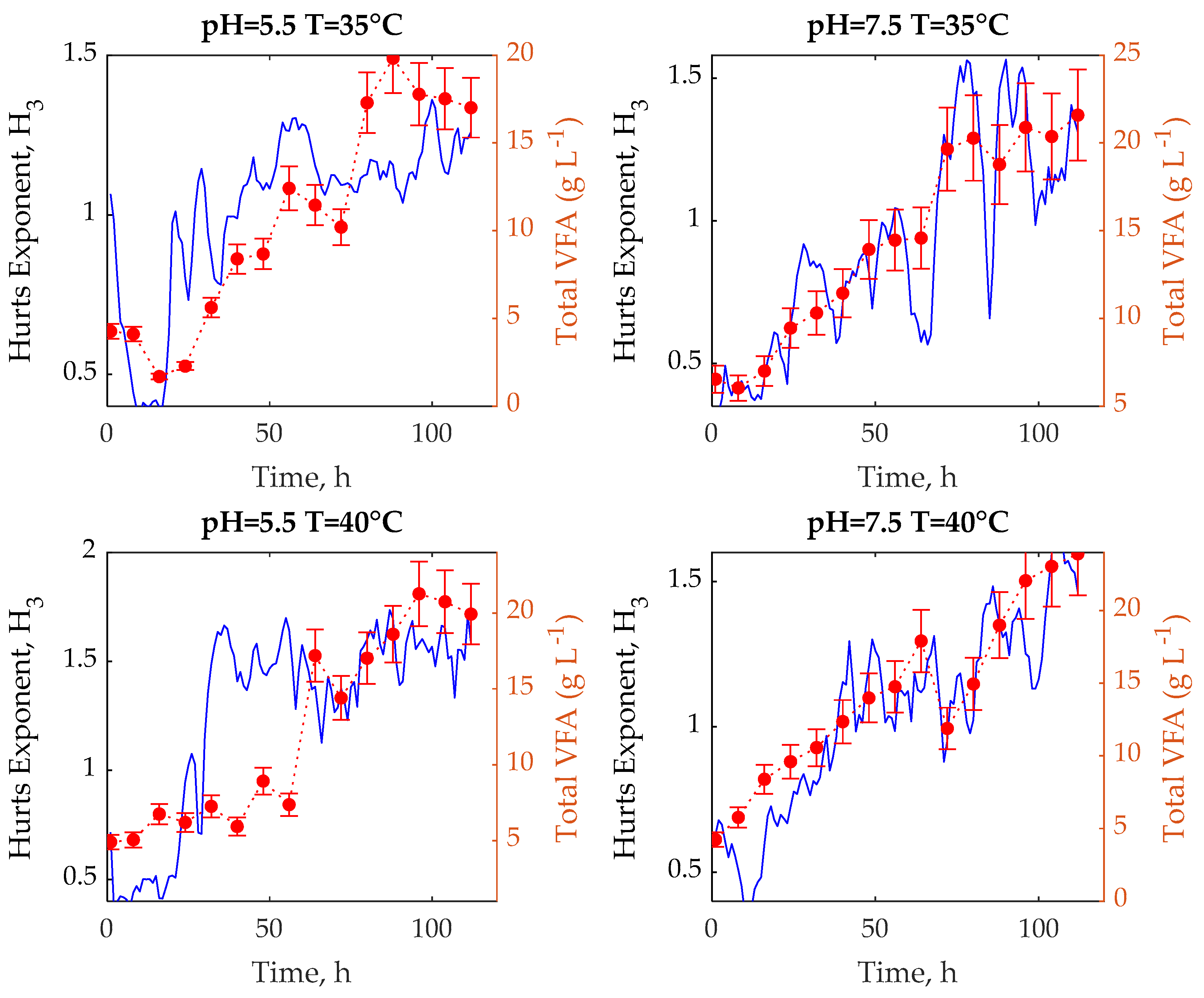
3. Results and Discussion
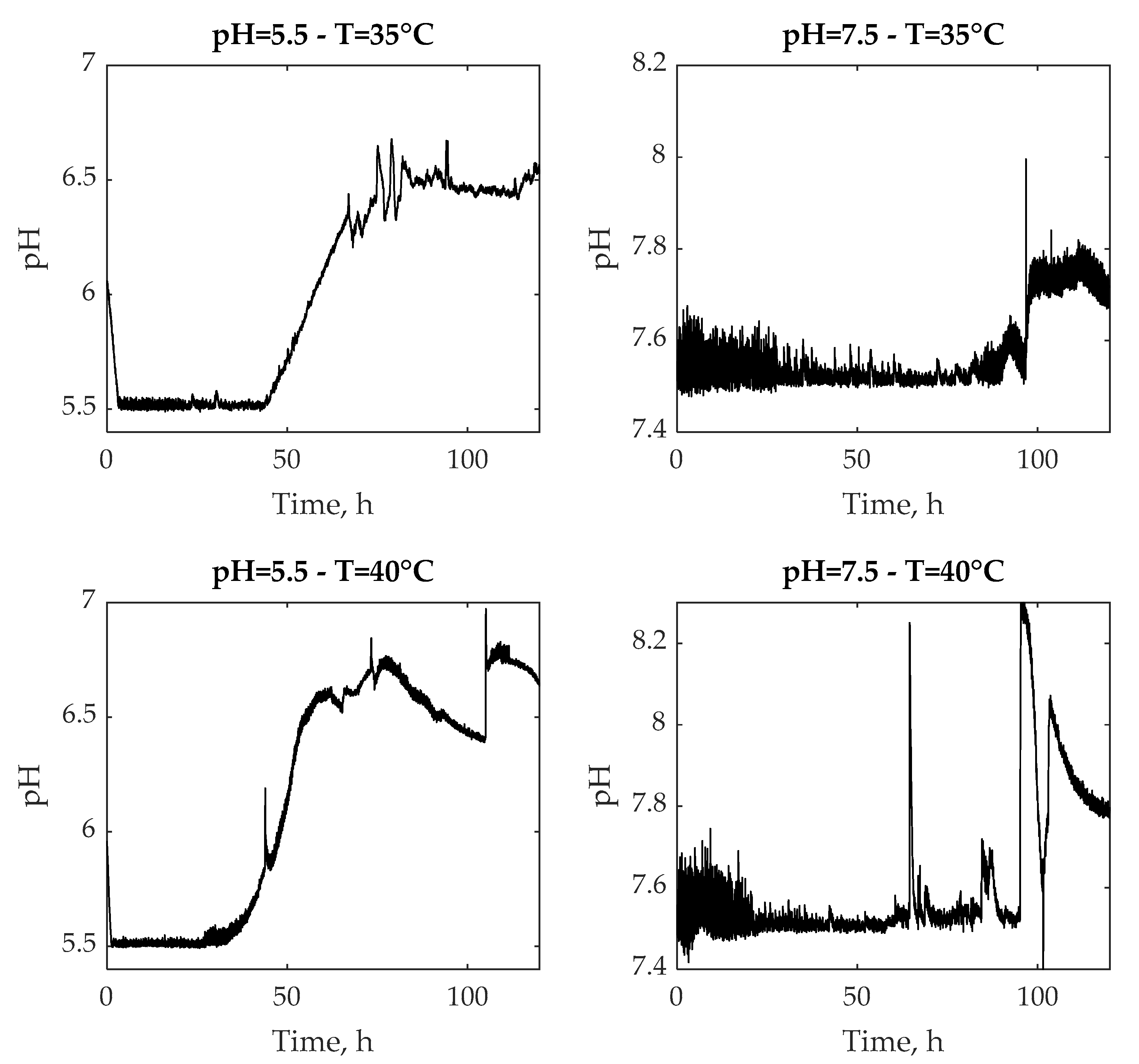
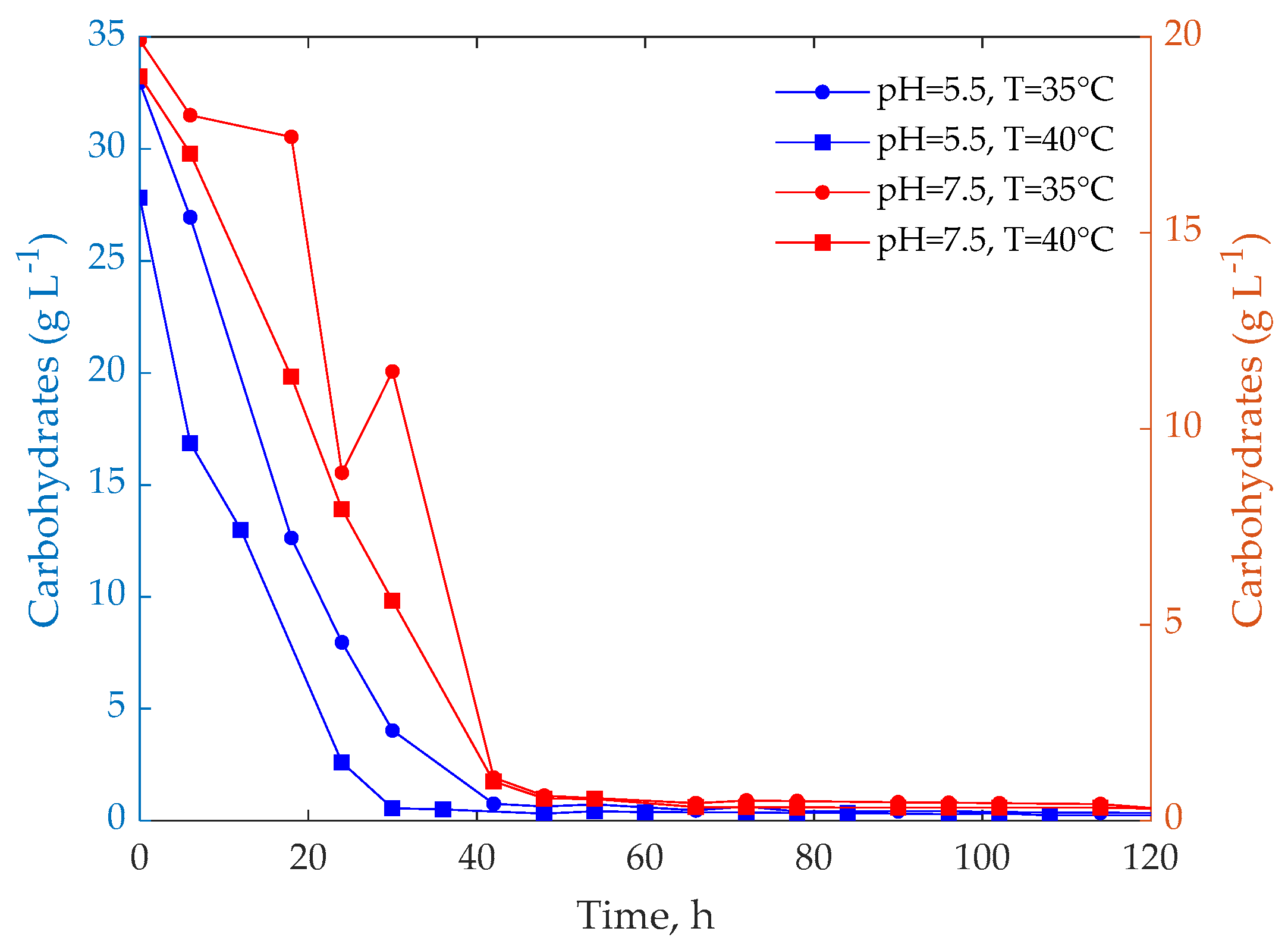
3.1. Experimental Tests
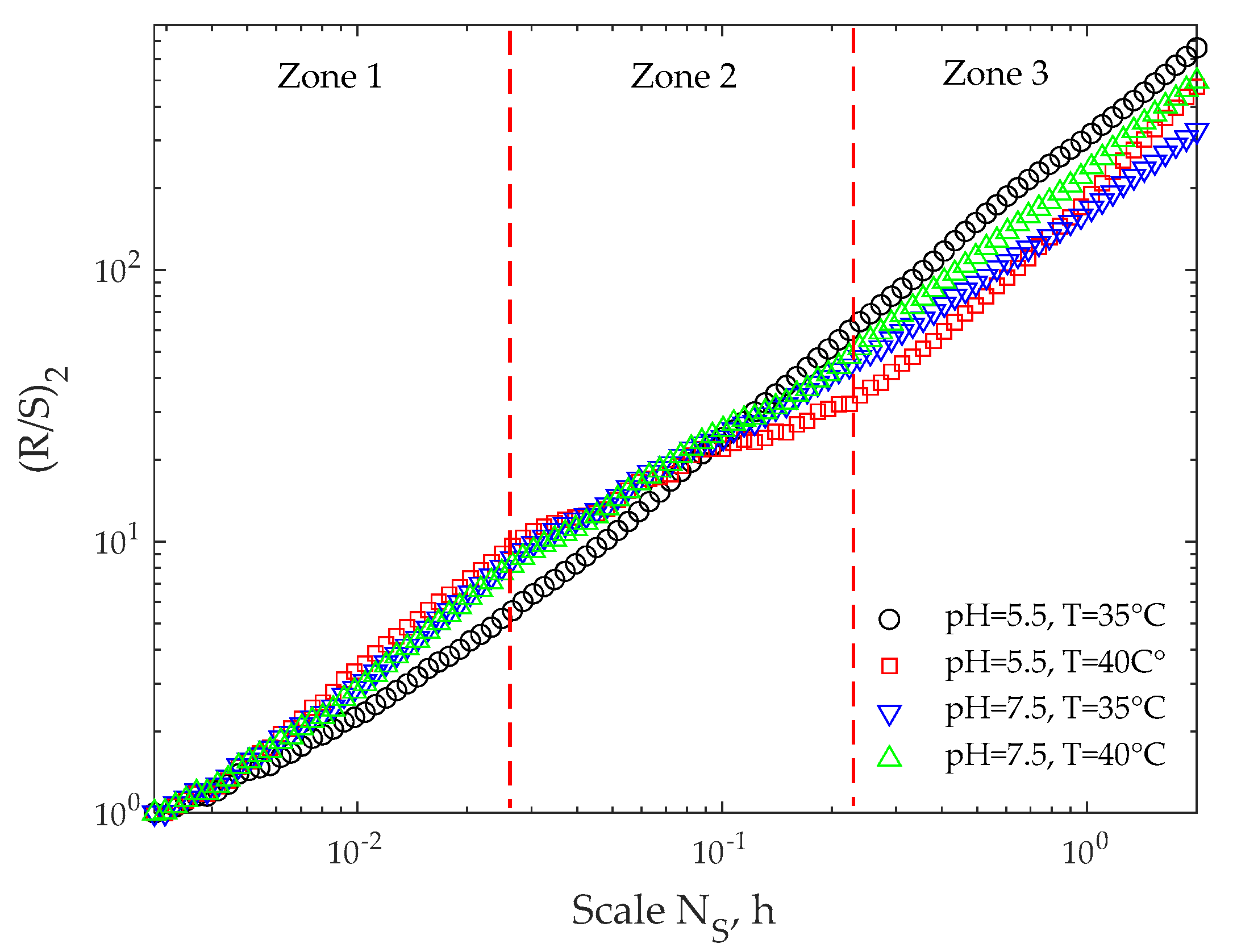
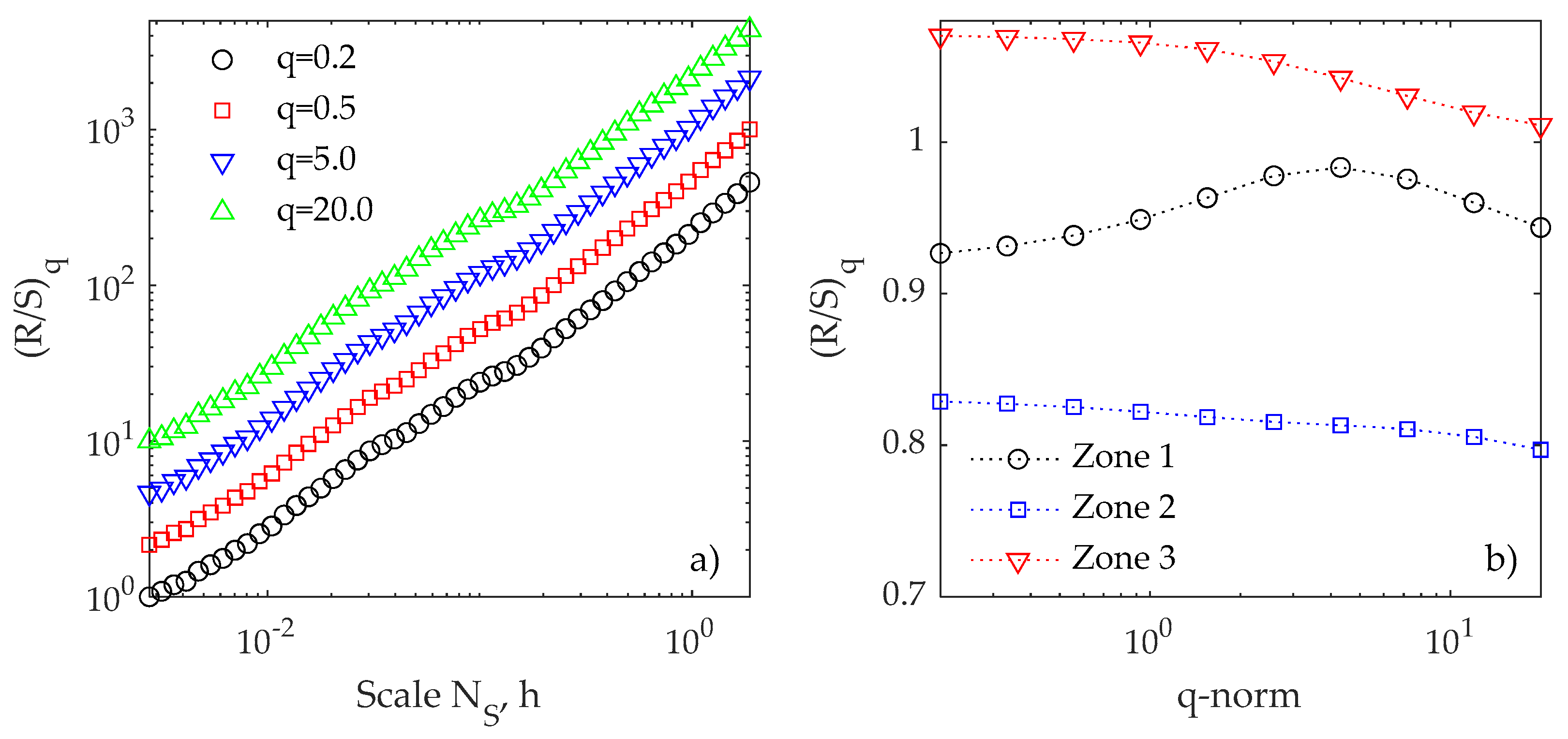
3.2. R/S Analysis
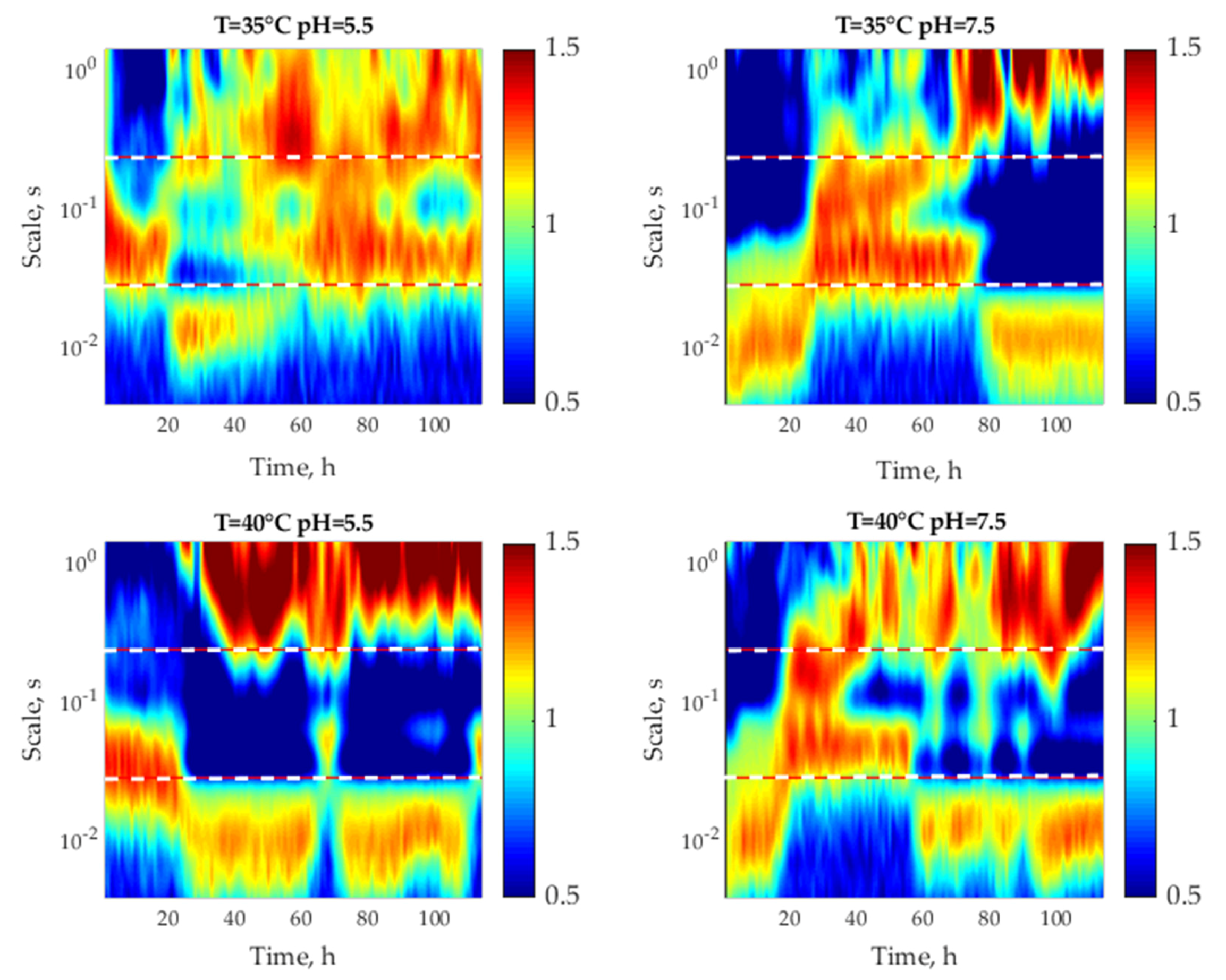
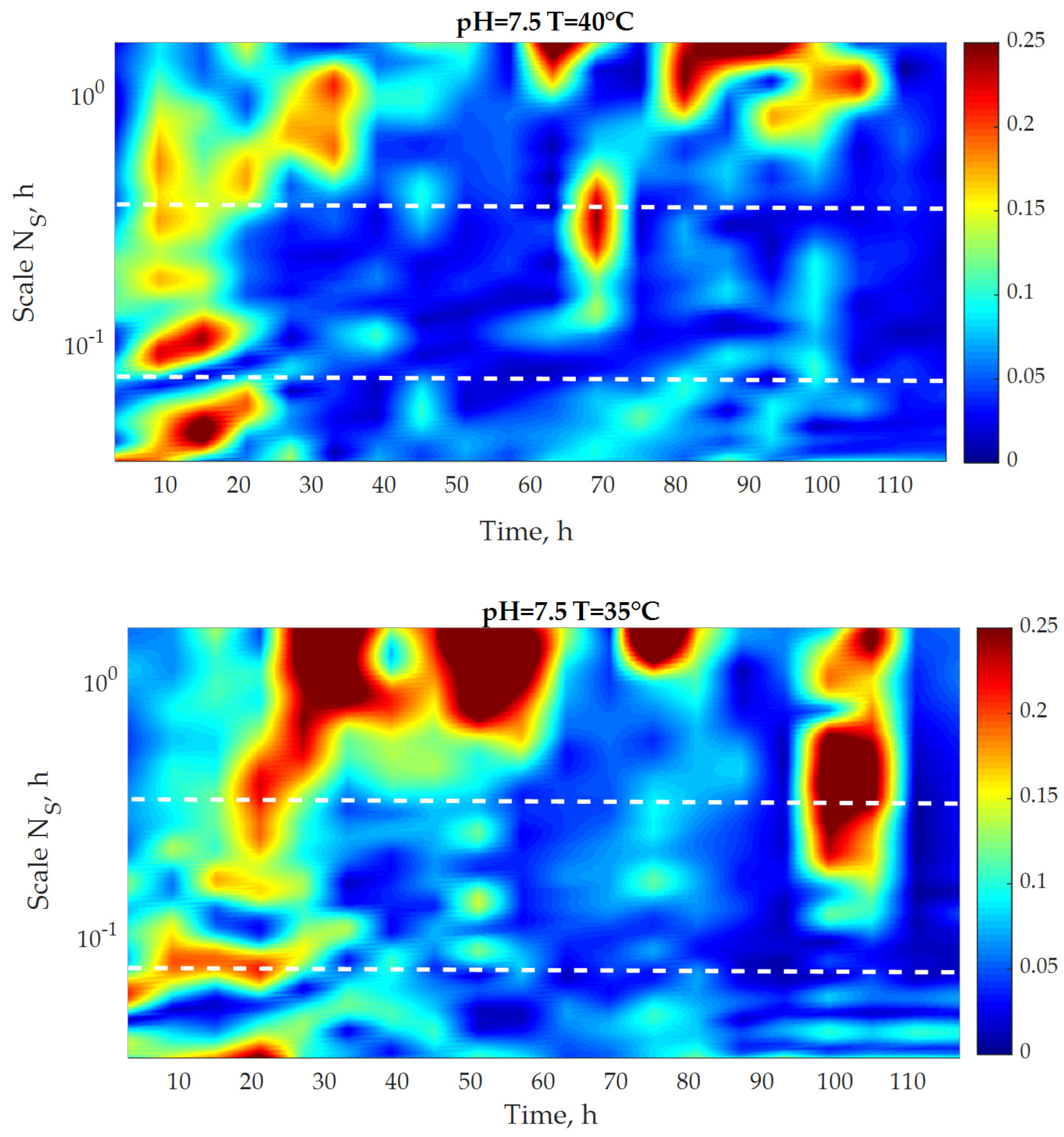
3.3. Multifractal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VFA | g L−1 | volatile fatty acids |
| R/S | - | rescaled range |
| COD | g L−1 | chemical oxygen demand |
| AD | - | Anaerobic digestion |
| FRB | - | fluidized bed reactor |
| CSTR | - | continuous stirred tank reactor |
| AnSBR | - | anaerobic sequencing batch reactor |
| OLR | gCOD L−1 d−1 | organic loading rate |
| HRT | d | hydraulic retention time |
| VSS | g L−1 | volatile suspended solids |
| TCH | gglucose L−1 | total carbohydrates |
| TS | g L−1 | total solids |
| ORP | mV | oxide reduction potential |
| IM | - | multifractal Index |
References
- Mockaitis, G.; Ratusznei, S.M.; Rodrigues, J.A.; Zaiat, M.; Foresti, E. Anaerobic whey treatment by a stirred sequencing batch reactor (ASBR): Effects of organic loading and supplemented alkalinity. J. Environ. Manag. 2006, 79, 198–206. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J. Biogas from anaerobic digestion processes: Research updates. Renew. Energy 2016, 98, 108–119. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.M.; Reichling, P.; Andersen, C.; Benz, R. Online monitoring of concentration and dynamics of volatile fatty acids in anaerobic digestion processes with mid-infrared spectroscopy. Bioprocess Biosyst. Eng. 2014, 38, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Pind, P.F.; Angelidaki, I.; Ahring, B.K.; Stamatelatou, K.; Lyberatos, G. Monitoring and Control of Anaerobic Reactors. Blue Biotechnol. 2003, 82, 135–182. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Noike, T.; Endo, G.; Chang, J.-E.; Yaguchi, J.-I.; Matsumoto, J.-I. Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol. Bioeng. 1985, 27, 1482–1489. [Google Scholar] [CrossRef]
- Spanjers, H.; Van Lier, J. Instrumentation in anaerobic treatment—Research and practice. Water Sci. Technol. 2006, 53, 63–76. [Google Scholar] [CrossRef]
- Madsen, M.; Holm-Nielsen, J.B.; Esbensen, K.H. Monitoring of anaerobic digestion processes: A review perspective. Renew. Sustain. Energy Rev. 2011, 15, 3141–3155. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.; Latrille, E.; Harmand, J.; Robles, Á.; Ferrer, J.; Gaida, D.; Wolf, C.; Mairet, F.; Bernard, O.; Alcaraz-Gonzalez, V.; et al. Instrumentation and control of anaerobic digestion processes: A review and some research challenges. Rev. Environ. Sci. Bio/Technol. 2015, 14, 615–648. [Google Scholar] [CrossRef]
- Boe, K.; Batstone, D.J.; Steyer, J.-P.; Angelidaki, I. State indicators for monitoring the anaerobic digestion process. Water Res. 2010, 44, 5973–5980. [Google Scholar] [CrossRef]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I.J.A.M. Volatile fatty acids as indicators of process imbalance in anaerobic di-gestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Uellendahl, H.; Ahring, B.K. Regulation and optimization of the biogas process: Propionate as a key pa-rameter. Biomass Bioenergy 2007, 31, 820–830. [Google Scholar] [CrossRef] [Green Version]
- Molina, F.; Castellano, M.; García, C.; Roca, E.; Lema, J.M. Selection of variables for on-line monitoring, diagnosis, and control of anaerobic digestion processes. Water Sci. Technol. 2009, 60, 615–622. [Google Scholar] [CrossRef]
- Gaida, D.; Wolf, C.; Meyer, C.; Stuhlsatz, A.; Lippel, J.; Bäck, T.; Bongards, M.; McLoone, S. State estimation for anaero-bic digesters using the ADM1. Water Sci. Technol. 2012, 66, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Tidriri, K.; Chatti, N.; Verron, S.; Tiplica, T. Bridging data-driven and model-based approaches for process fault diagnosis and health monitoring: A review of researches and future challenges. Annu. Rev. Control. 2016, 42, 63–81. [Google Scholar] [CrossRef]
- Montiel-Escobar, J.L.; Alcaraz-González, V.; Méndez-Acosta, H.O.; González-Álvarez, V. ADM1-Based Robust Interval Observer for Anaerobic Digestion Processes. Clean Soil Air Water 2012, 40, 933–940. [Google Scholar] [CrossRef]
- Lara-Cisneros, G.; Dochain, D. Software Sensor for Online Estimation of the VFA’s Concentration in Anaerobic Digestion Processes via a High-Order Sliding Mode Observer. Ind. Eng. Chem. Res. 2018, 57, 14173–14181. [Google Scholar] [CrossRef]
- Dewasme, L.; Sbarciog, M.; Rocha-Cózatl, E.; Haugen, F.; Wouwer, A.V. State and unknown input estimation of an an-aerobic digestion reactor with experimental validation. Control Eng. Pract. 2019, 85, 280–289. [Google Scholar] [CrossRef]
- Draa, K.C.; Zemouche, A.; Alma, M.; Voos, H.; Darouach, M. A Nonlinear observer-based trajectory tracking method applied to an anaerobic digestion process. J. Process. Control. 2019, 75, 120–135. [Google Scholar] [CrossRef]
- Flores-Mejia, H.; Lara-Musule, A.; Hernández-Martínez, E.; Aguilar-López, R.; Puebla, H. Indirect Monitoring of Anaero-bic Digestion for Cheese Whey Treatment. Processes 2021, 9, 539. [Google Scholar] [CrossRef]
- Lee, J.-W.; Hong, Y.-S.T.; Suh, C.; Shin, H.-S. Online nonlinear sequential Bayesian estimation of a biological wastewater treatment process. Bioprocess Biosyst. Eng. 2011, 35, 359–369. [Google Scholar] [CrossRef]
- Jones, R.M.; MacGregor, J.F.; Murphy, K.L. State estimation in wastewater engineering: Application to an anaerobic process. Environ. Monit. Assess. 1989, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Kumar, G.; Rani, M.D.; Srinivasan, B. A novel approach to evaluate state estimation approaches for anaerobic digester units under modeling uncertainties: Application to an industrial dairy unit. J. Environ. Chem. Eng. 2017, 5, 4004–4013. [Google Scholar] [CrossRef]
- MacGregor, J.; Cinar, A. Monitoring, fault diagnosis, fault-tolerant control and optimization: Data driven methods. Comput. Chem. Eng. 2012, 47, 111–120. [Google Scholar] [CrossRef]
- Yin, S.; Ding, S.X.; Xie, X.; Luo, H. A Review on Basic Data-Driven Approaches for Industrial Process Monitoring. IEEE Trans. Ind. Electron. 2014, 61, 6418–6428. [Google Scholar] [CrossRef]
- Nair, V.V.; Dhar, H.; Kumar, S.; Thalla, A.K.; Mukherjee, S.; Wong, J.W. Artificial neural network based modeling to evaluate methane yield from biogas in a laboratory-scale anaerobic bioreactor. Bioresour. Technol. 2016, 217, 90–99. [Google Scholar] [CrossRef]
- Tufaner, F.; Demirci, Y. Prediction of biogas production rate from anaerobic hybrid reactor by artificial neural network and nonlinear regressions models. Clean Technol. Environ. Policy 2020, 22, 713–724. [Google Scholar] [CrossRef]
- Newhart, K.B.; Holloway, R.W.; Hering, A.S.; Cath, T.Y. Data-driven performance analyses of wastewater treatment plants: A review. Water Res. 2019, 157, 498–513. [Google Scholar] [CrossRef]
- Kazemi, P.; Bengoa, C.; Steyer, J.-P.; Giralt, J. Data-driven techniques for fault detection in anaerobic digestion process. Process. Saf. Environ. Prot. 2021, 146, 905–915. [Google Scholar] [CrossRef]
- Asadi, M.; Guo, H.; McPhedran, K. Biogas production estimation using data-driven approaches for cold region municipal wastewater anaerobic digestion. J. Environ. Manag. 2020, 253, 109708. [Google Scholar] [CrossRef]
- Qin, S.J. Survey on data-driven industrial process monitoring and diagnosis. Annu. Rev. Control. 2012, 36, 220–234. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, M.W.; Park, J.M. Multi-scale extension of PLS algorithm for advanced on-line process monitoring. Chemom. Intell. Lab. Syst. 2009, 98, 201–212. [Google Scholar] [CrossRef]
- Méndez-Acosta, H.O.; Hernandez-Martinez, E.; Jáuregui-Jáuregui, J.A.; Alvarez-Ramirez, J.; Puebla, H. Monitoring anaerobic sequential batch reactors via fractal analysis of pH time series. Biotechnol. Bioeng. 2013, 110, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Martinez, E.; Puebla, H.; Mendez-Acosta, H.; Alvarez-Ramirez, J. Fractality in pH time series of continuous anaerobic bioreactors for tequila vinasses treatment. Chem. Eng. Sci. 2014, 109, 17–25. [Google Scholar] [CrossRef]
- Sánchez-García, D.; Hernández-García, H.; Mendez-Acosta, H.O.; Hernández-Aguirre, A.; Puebla, H.; Hernández-Martínez, E. Fractal Analysis of pH Time-Series of an Anaerobic Digester for Cheese Whey Treatment. Int. J. Chem. React. Eng. 2018. [Google Scholar] [CrossRef]
- Jirka, A.M.; Carter, M.J. Micro semiautomated analysis of surface and waste waters for chemical oxygen demand. Anal. Chem. 1975, 47, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B. Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Denver, CO, USA, 1995. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hurst, H.E. Long-Term Storage Capacity of Reservoirs. Trans. Am. Soc. Civ. Eng. 1951, 116, 770–799. [Google Scholar] [CrossRef]
- Kantelhardt, J.W.; Zschiegner, S.A.; Koscielny-Bunde, E.; Havlin, S.; Bunde, A.; Stanley, H. Multifractal detrended fluctuation analysis of nonstationary time series. Phys. A Stat. Mech. Its Appl. 2002, 316, 87–114. [Google Scholar] [CrossRef] [Green Version]
- Mandelbrot, B.B.; Wallis, J.R. Computer Experiments with Fractional Gaussian Noises: Part 2, Rescaled Ranges and Spectra. Water Resour. Res. 1969, 5, 242–259. [Google Scholar] [CrossRef]
- Barabási, A.-L.; Vicsek, T. Multifractality of self-affine fractals. Phys. Rev. A 1991, 44, 2730–2733. [Google Scholar] [CrossRef]
- Katsuragi, H.; Honjo, H. Multiaffinity and entropy spectrum of self-affine fractal profiles. Phys. Rev. E 1999, 59, 254–262. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Pan, L.; Wang, B.; Lou, C. Comparing Monofractal and Multifractal Analysis of Corrosion Damage Evolution in Reinforcing Bars. PLoS ONE 2012, 7, e29956. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Ortiz, W.; Andrade-Gómez, C.; Hernandez-Martinez, E.; Puebla, H. Multifractal Hurst analysis for identification of corrosion type in AISI 304 stainless steel. Int. J. Electrochem. Sci. 2015, 10, 1054–1064. [Google Scholar]
- Perna, V.; Castelló, E.; Wenzel, J.; Zampol, C.; Fontes-Lima, D.; Borzacconi, L.; Varesche, M.; Zaiat, M.; Etchebehere, C. Hydrogen production in an upflow anaerobic packed bed reactor used to treatcheese whey. Int. J. Hydrogen Energy 2013, 38, 54–62. [Google Scholar] [CrossRef]
- Calero, R.R.; Lagoa-Costa, B.; Fernandez-Feal, M.M.D.C.; Kennes, C.; Veiga, M.C. Volatile fatty acids produc-tion from cheese whey: Influence of pH, solid retention time and organic loading rate. J. Chem. Technol. Biotechnol. Technol. 2018, 93, 1742–1747. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (AGVs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Shin, S.G.; O’Flaherty, V.; Hwang, S. Quantitative and qualitative transitions of methanogen community structure during the batch anaerobic digestion of cheese-processing wastewater. Appl. Microbiol. Biotechnol. 2010, 87, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemo-stat retention time and pH on AGV production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]

| Parameters | Whey | Inoculum |
|---|---|---|
| COD (g L−1) | 74.24 ± 1.35 | 32.52 ± 4.27 |
| Carbohydrates (CH) (gglucose L−1) | 35.04 ± 1.56 | 0.94 ± 0.07 |
| Total Solids (g L−1) | 51.21 ± 1.364 | 49.87 ± 0.95 |
| Volatile Solids (g L−1) | 36.68 ± 4.68 | 22.46 ± 4.30 |
| Volatile Sedimented solids (g L−1) | 1.20 ± 0.24 | 2.15 ± 0.41 |
| VFA (gCOD L−1) | 0.57 ± 0.08 | 28.56 ± 1.90 |
| Protein (mg L−1) | 27.32 ± 2.17 | 7.22 ± 1.09 |
| pH | 4.72 ± 0.04 | 6.85 ± 0.08 |
| Zone/Test | T = 35 °C pH = 5.5 | T = 35 °C pH = 7.5 | T = 40 °C pH = 5.5 | T = 40 °C pH = 7.5 |
|---|---|---|---|---|
| Zone 1 | 0.85819 | 0.99983 | 1.01371 | 0.9804 |
| Zone 2 | 1.12514 | 0.73615 | 0.51536 | 0.79128 |
| Zone 3 | 1.05433 | 0.89266 | 1.28973 | 1.05855 |
| Zone/Test | T = 35 °C pH = 5.5 | T = 35 °C pH = 7.5 | T = 40 °C pH = 5.5 | T = 40 °C pH = 7.5 |
|---|---|---|---|---|
| Zone 1 | 0.0725 | 0.0919 | 0.0469 | 0.1107 |
| Zone 2 | 0.028 | 0.1929 | 0.0679 | 0.1414 |
| Zone 3 | 0.0115 | 0.1691 | 0.1756 | 0.1039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Musule, A.; Alvarez-Sanchez, E.; Trejo-Aguilar, G.; Acosta-Dominguez, L.; Puebla, H.; Hernandez-Martinez, E. Diagnosis and Monitoring of Volatile Fatty Acids Production from Raw Cheese Whey by Multiscale Time-Series Analysis. Appl. Sci. 2021, 11, 5803. https://doi.org/10.3390/app11135803
Lara-Musule A, Alvarez-Sanchez E, Trejo-Aguilar G, Acosta-Dominguez L, Puebla H, Hernandez-Martinez E. Diagnosis and Monitoring of Volatile Fatty Acids Production from Raw Cheese Whey by Multiscale Time-Series Analysis. Applied Sciences. 2021; 11(13):5803. https://doi.org/10.3390/app11135803
Chicago/Turabian StyleLara-Musule, Antonio, Ervin Alvarez-Sanchez, Gloria Trejo-Aguilar, Laura Acosta-Dominguez, Hector Puebla, and Eliseo Hernandez-Martinez. 2021. "Diagnosis and Monitoring of Volatile Fatty Acids Production from Raw Cheese Whey by Multiscale Time-Series Analysis" Applied Sciences 11, no. 13: 5803. https://doi.org/10.3390/app11135803
APA StyleLara-Musule, A., Alvarez-Sanchez, E., Trejo-Aguilar, G., Acosta-Dominguez, L., Puebla, H., & Hernandez-Martinez, E. (2021). Diagnosis and Monitoring of Volatile Fatty Acids Production from Raw Cheese Whey by Multiscale Time-Series Analysis. Applied Sciences, 11(13), 5803. https://doi.org/10.3390/app11135803






