Theoretical Study on Specific Loss Power and Heating Temperature in CoFe2O4 Nanoparticles as Possible Candidate for Alternative Cancer Therapy by Superparamagnetic Hyperthemia
Abstract
1. Introduction
2. Basic Theoretical Aspects
3. Results and Discussion
3.1. Characteristic Observables of CoFe2O4 Nanoparticles and Study Method
3.2. The Maximum Specific Loss Power in the Case of Superparamagnetic Hyperthermia with CoFe2O4 Nanoparticles
- (i)
- (ii)
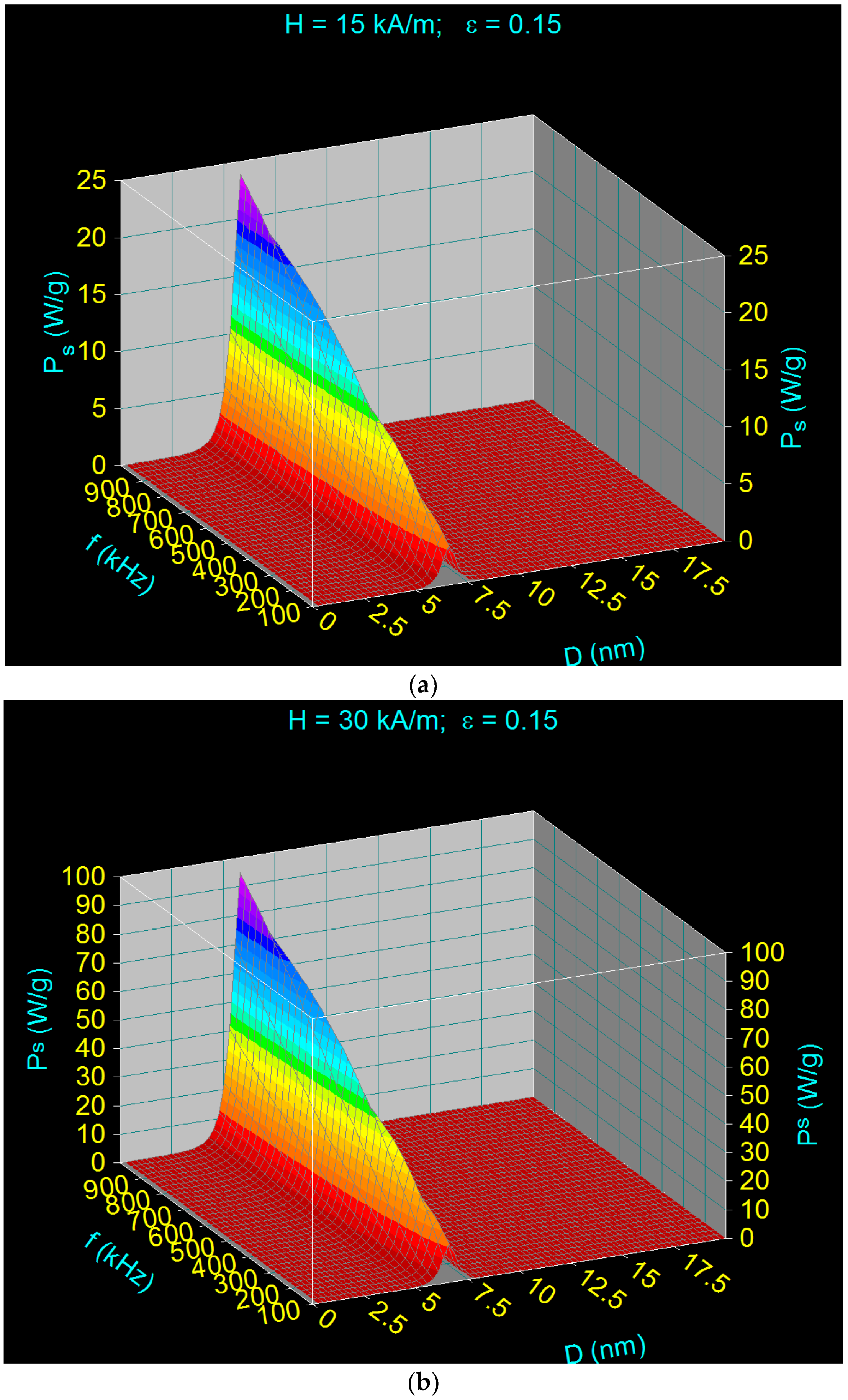
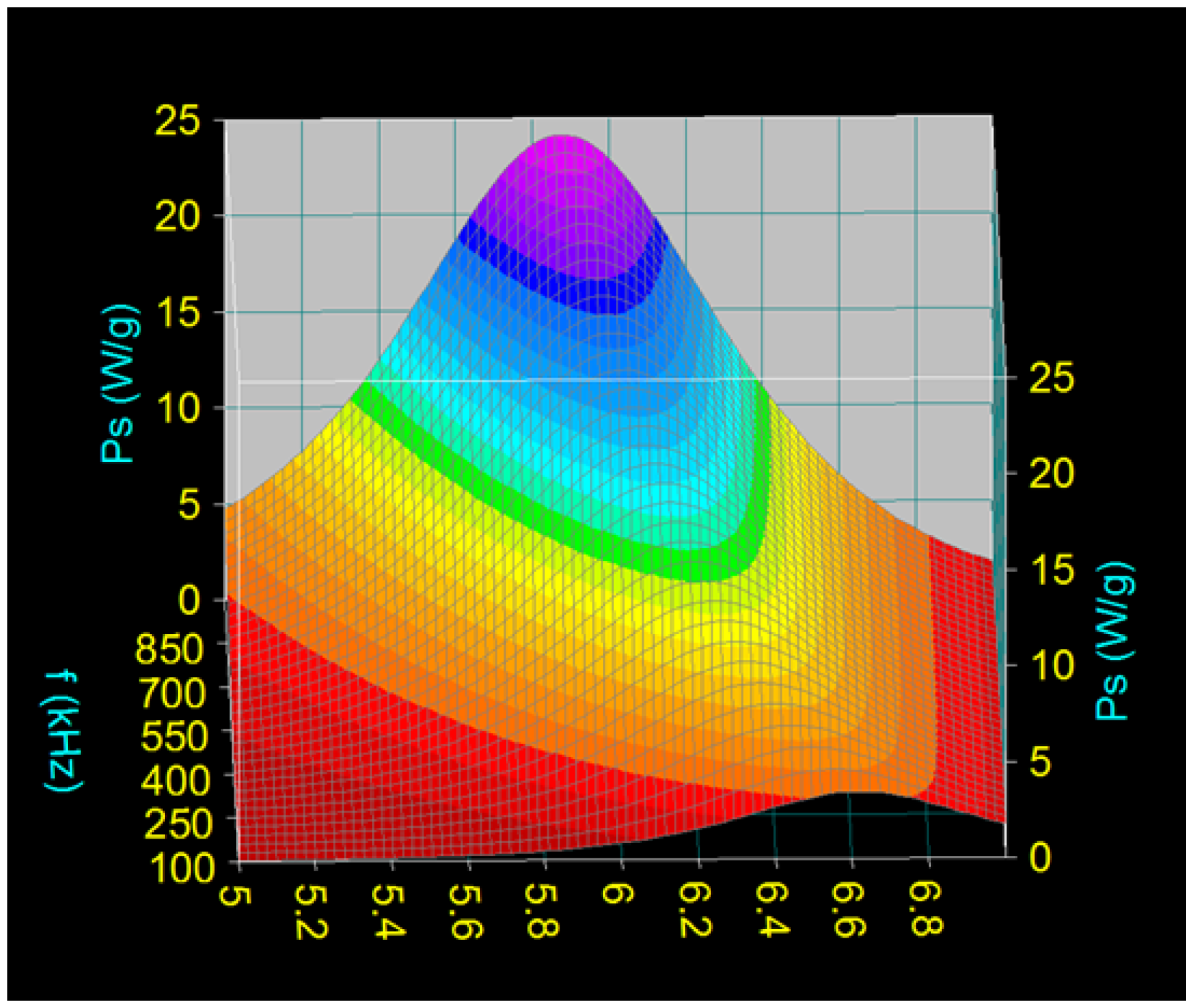
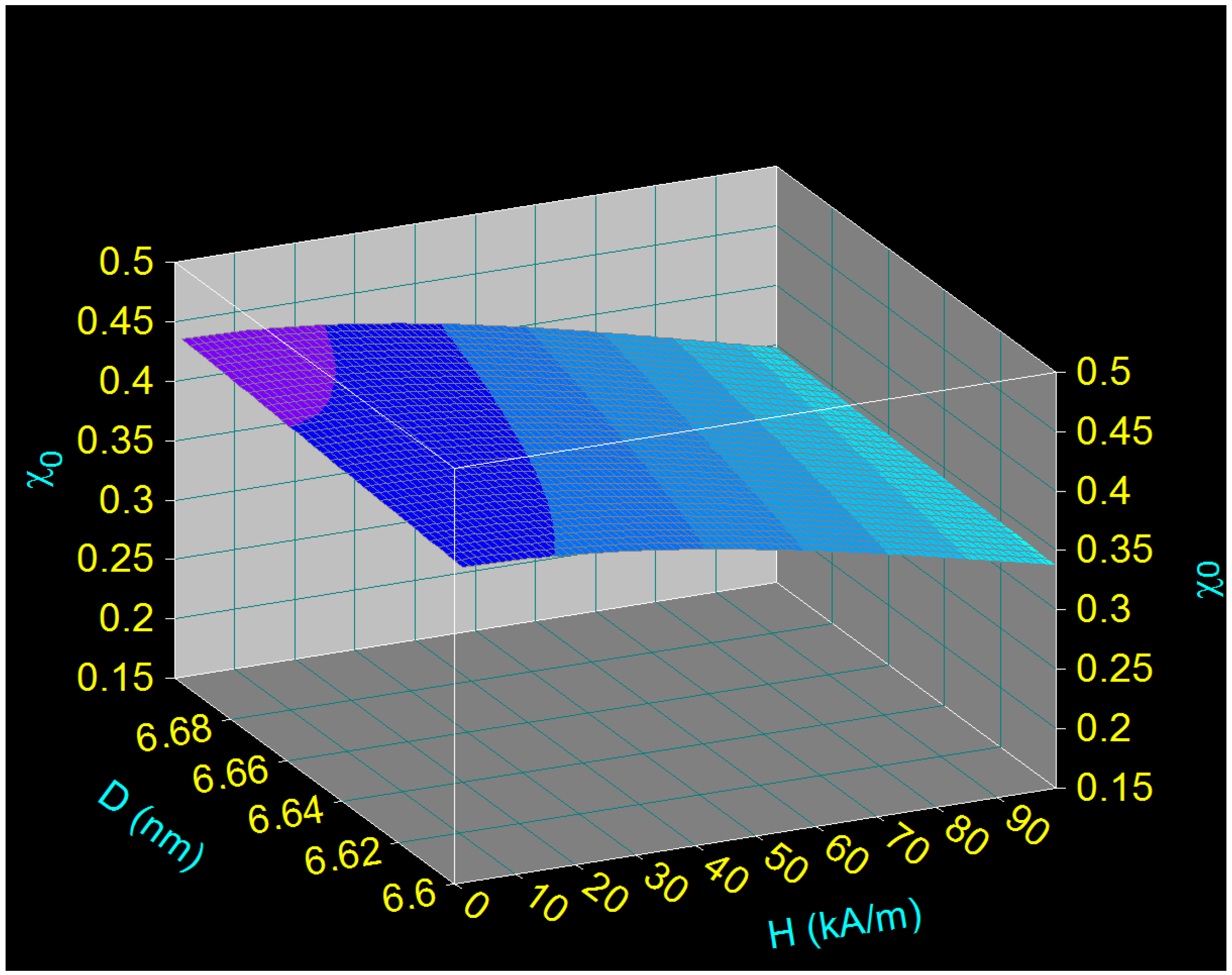
- There is a dependence of the maximum specific loss power PsM on the diameter of the magnetic nanoparticles D as a function of the frequency of the magnetic field f (Figure 2), namely: the maximum power shifts from higher values to lower values of the nanoparticle diameter when the magnetic field frequency increases from 100 kHz to 1000 kHz.Table 2 shows some values for the diameters of CoFe2O4 nanoparticles for which the maximum loss power is obtained for the frequency range limits (100 kHz and 1000 kHz) as well as at the mid-value of those limits (500 kHz).
- (iii)
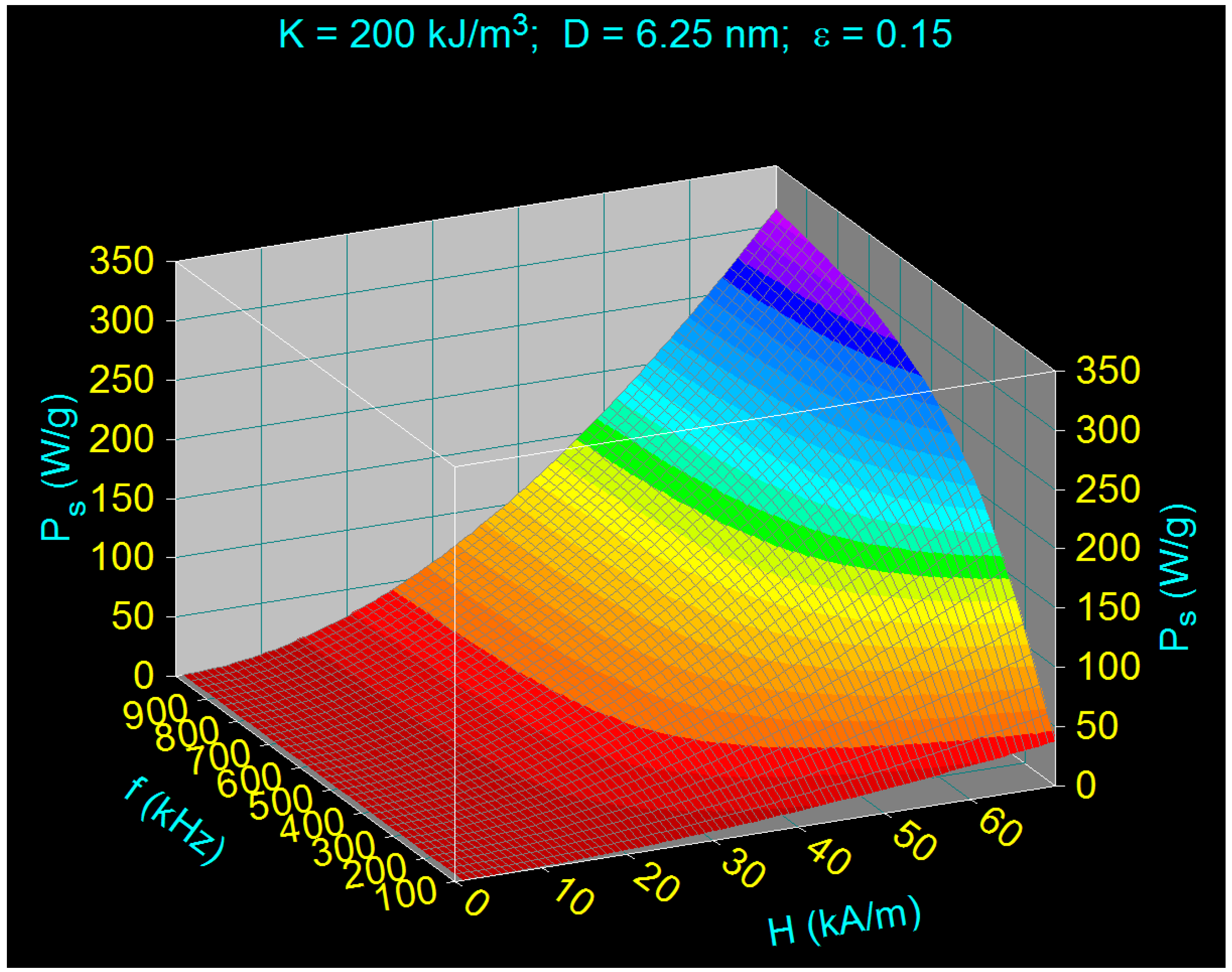
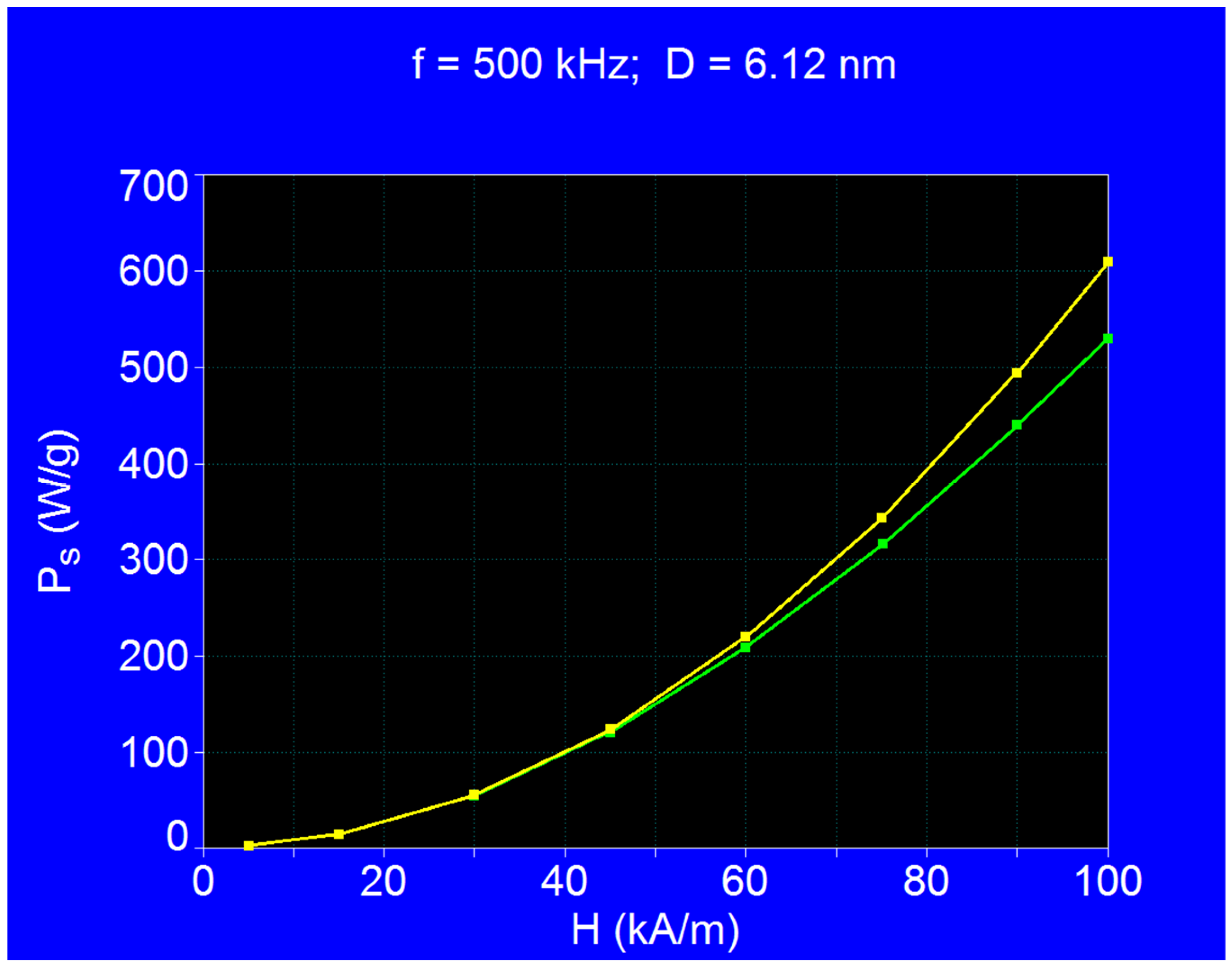
- The maximum loss power increases both with the amplitude and the frequency of magnetic field as shown in Figure 3. However, the increase is more accentuated with the increase of the magnetic field amplitude (see Figure 1a,b). However, for the magnetic fields higher than 60–70 kA/m there is a limiting effect (saturation) of increasing the specific loss power at frequencies above 600–700 kHz.
- (iv)
- The specific loss power for CoFe2O4 nanoparticles is significantly lower than in the case of magnetite nanoparticles. However, if the power obtained under these conditions were sufficient to heat the nanoparticles to an optimum temperature of ~43 °C, and in a relatively short period of time (see Section 3.5), then the reduced power would not come as a disadvantage in the use of CoFe2O4 nanoparticles in superparamagnetic hyperthermia for tumor therapy. In addition, we’ve shown a major advantage in point (ii), regarding intracellular therapy, which will increase the effectiveness of CoFe2O4 nanoparticles in the hyperthermic destruction of tumor cells, much more efficiently from within.
3.3. The Specific Loss Power in the Linear Approximation
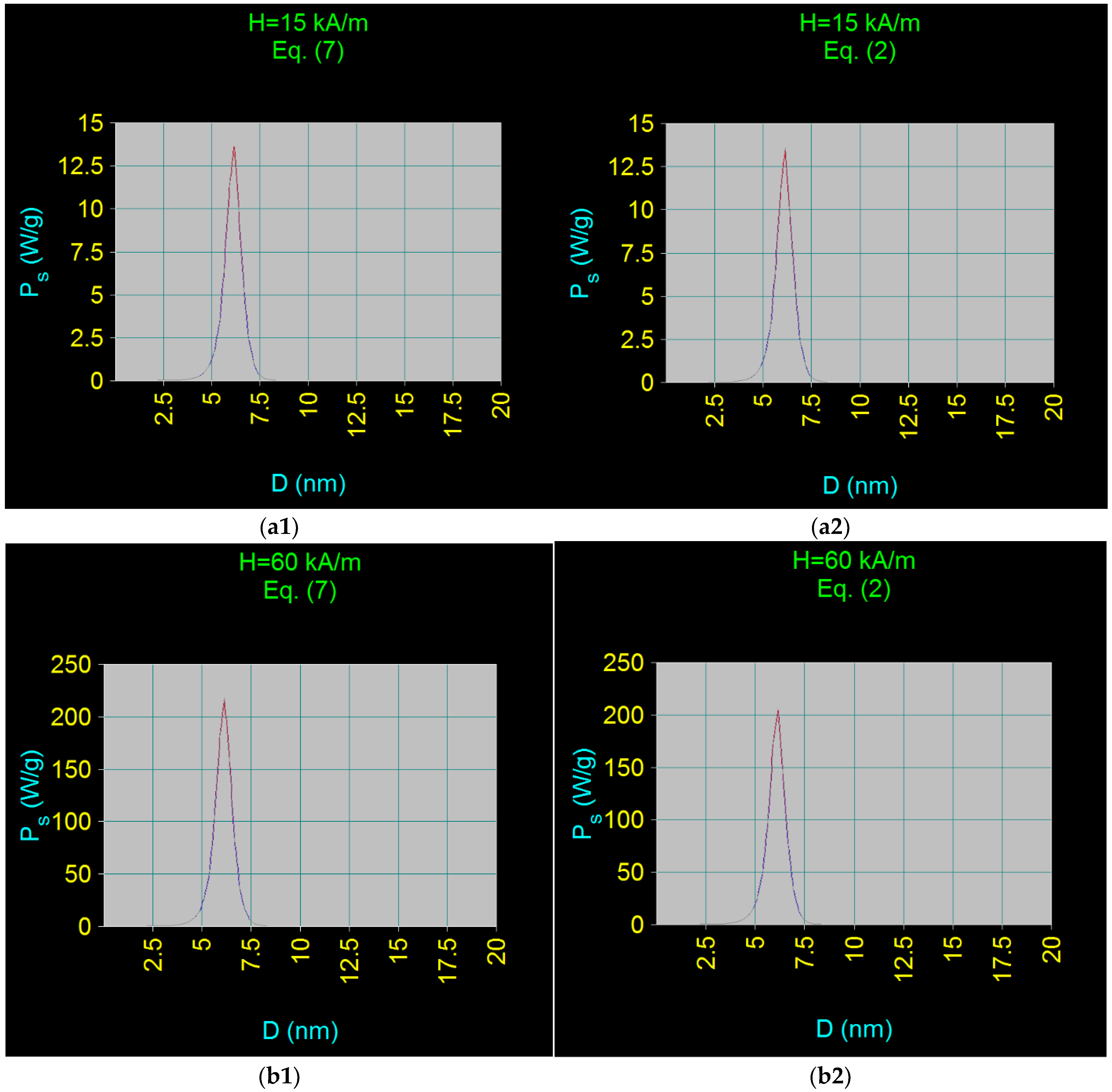
3.3.1. Maximum Specific Loss Power
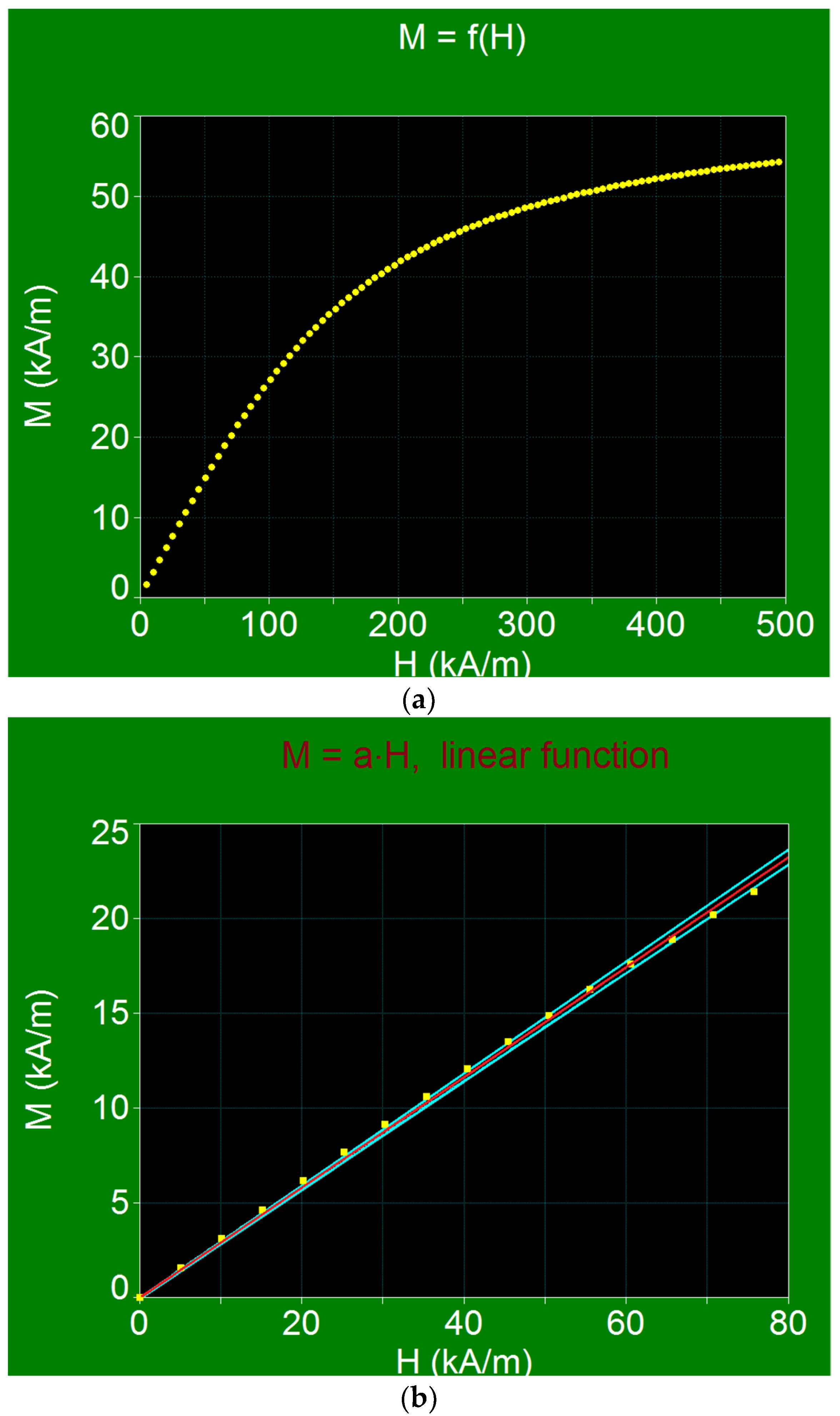
3.3.2. Magnetic Behavior of Small CoFe2O4 Nanoparticles and Linearity of Magnetization
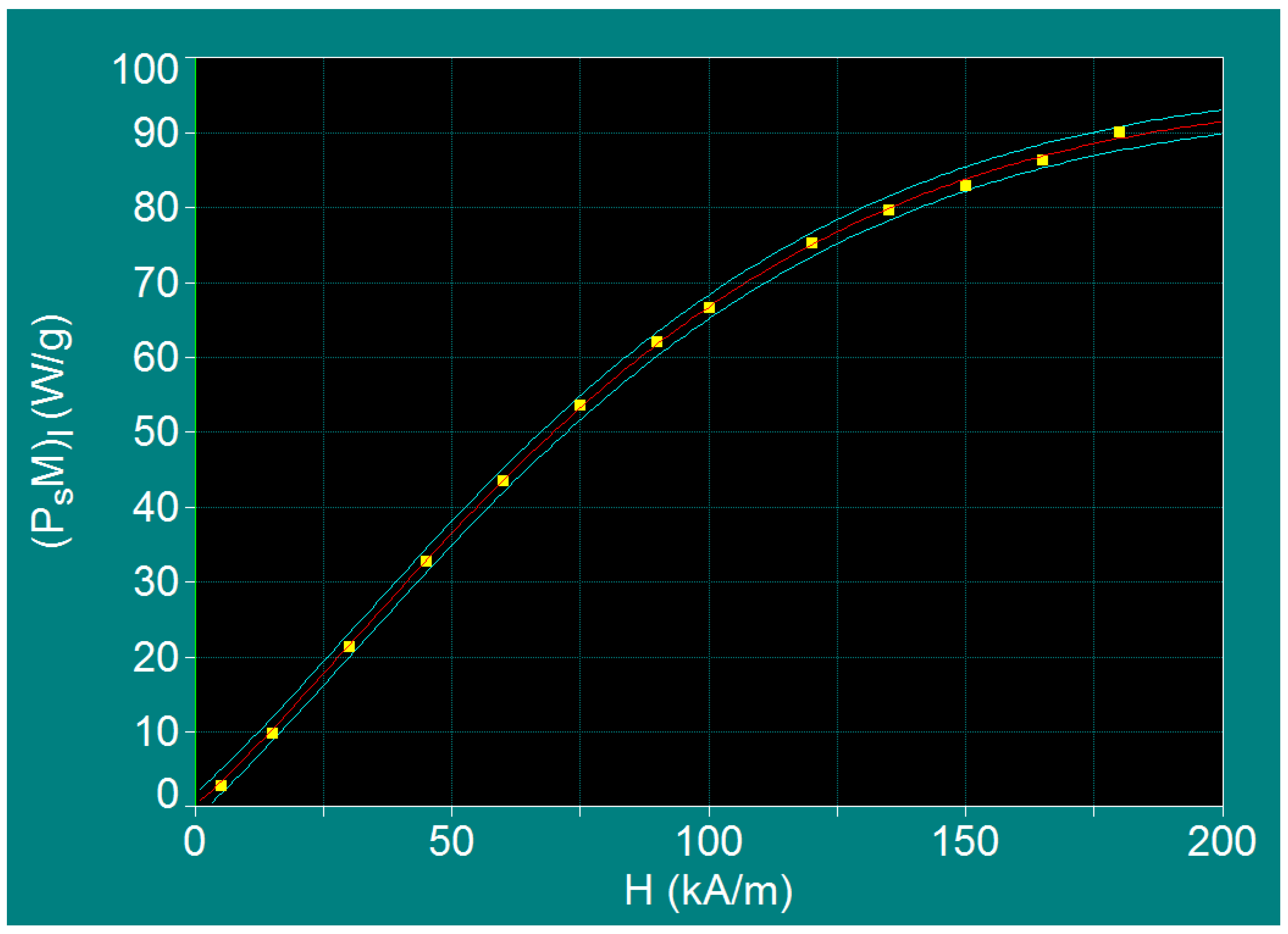
3.4. Maximum Specific Loss Power in Superparamagnetic Hyperthermia under Optimal Conditions and within Biologically Permissible Limits
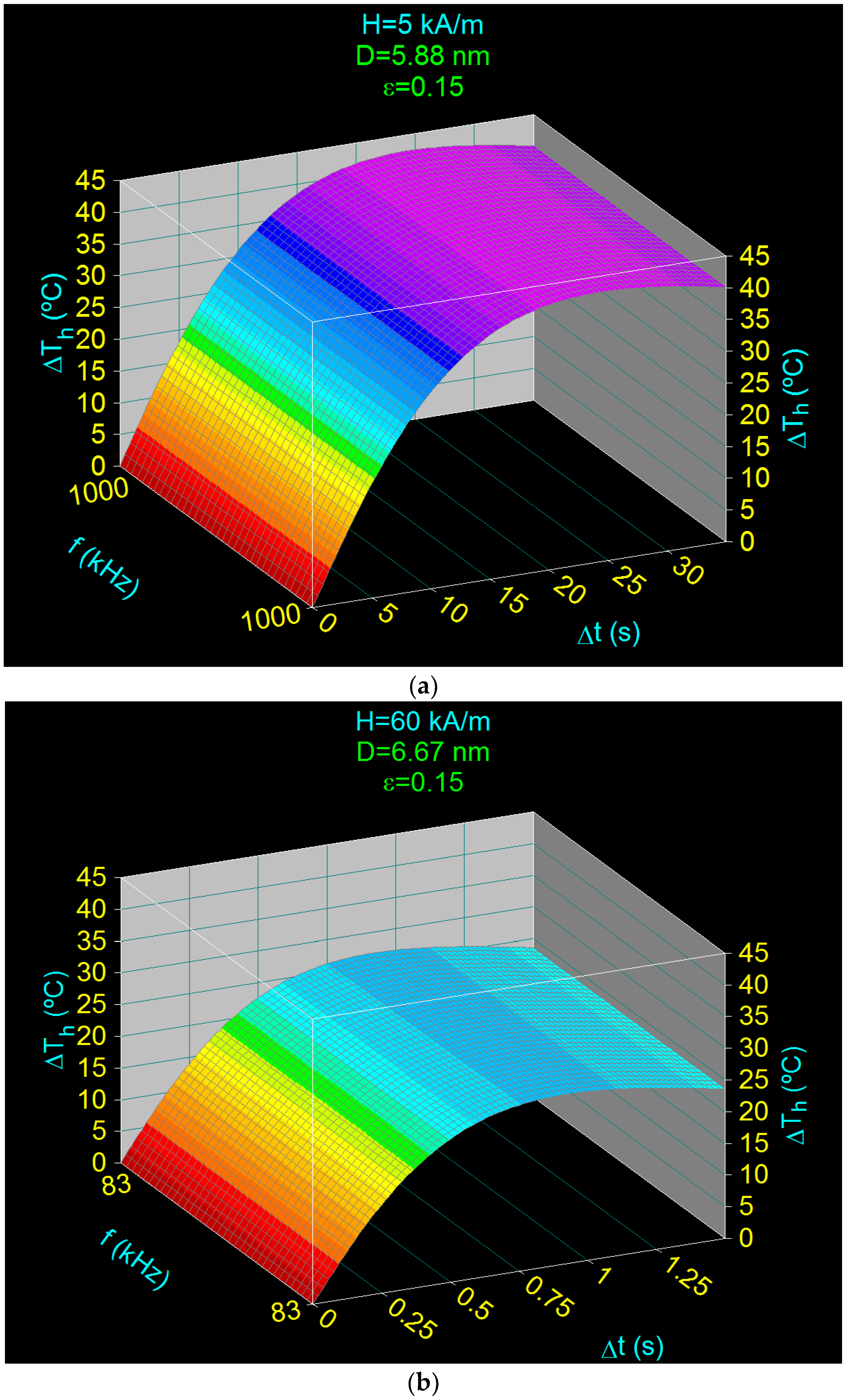
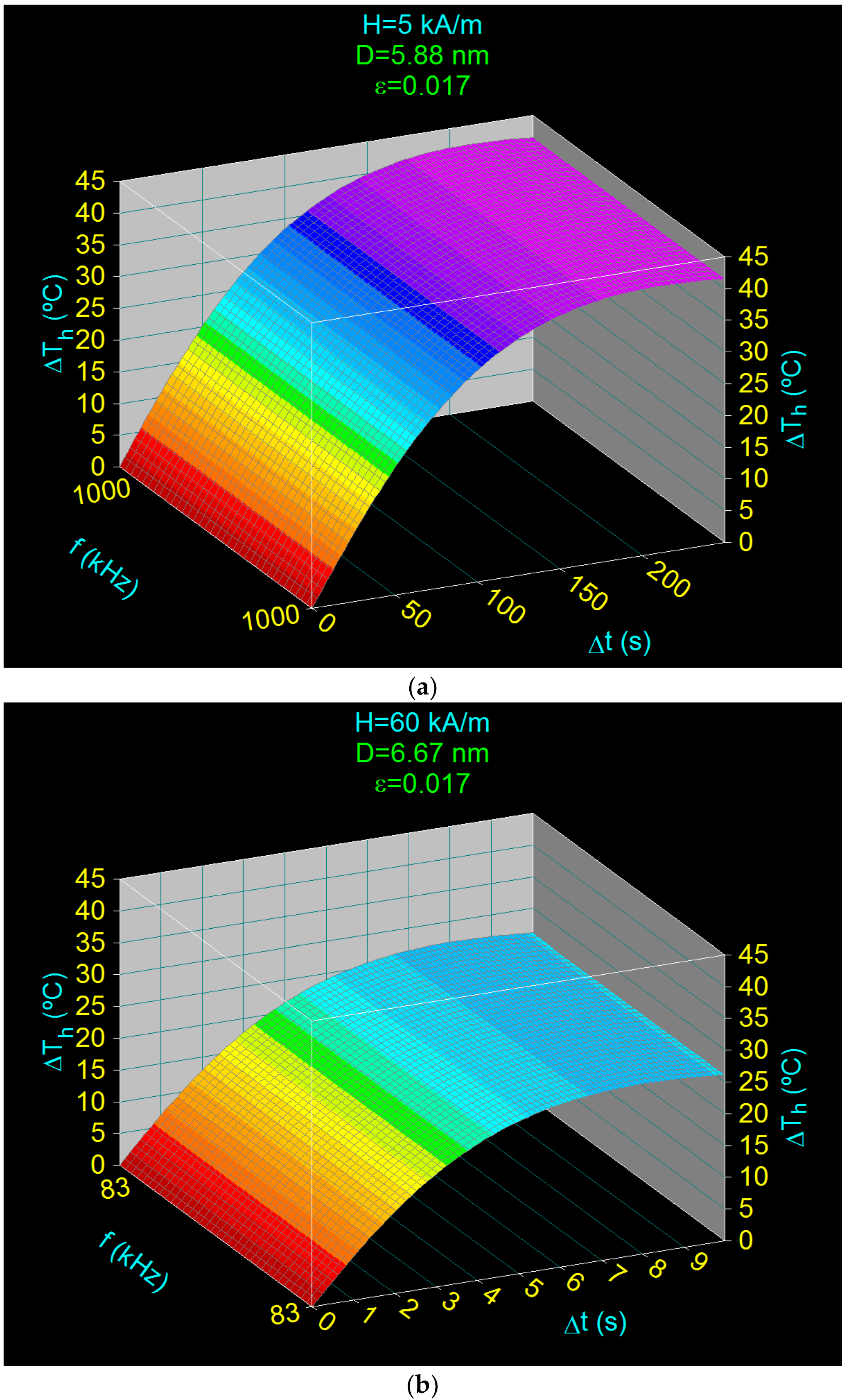
3.5. Heating Characteristics and Optimum Heating Time in the Case of Superparamagnetic Hyperthermia with CoFe2O4 Nanoparticles
4. Conclusions
- (1)
- Obtaining the heating temperature of the nanoparticles at 43 °C for the amplitudes of the magnetic field of 5–60 kA/m, the frequencies of the magnetic field of 83–1000 kHz, and the diameters of the nanoparticles in the range 5.88–6.67 nm (Section 3.4 and Section 3.5);
- (2)
- Optimal heating of the nanoparticles, by 18 °C above room temperature (25 °C), in a short period of time: 0.35–44.19 s, depending on the volume packing fractions;
- (3)
- The use of the linear approximation up to large magnetic fields, of ~60–70 kA/m, for the maximum specific loss power, which greatly simplifies the practical implementation of magnetic hyperthermia in the case of CoFe2O4 nanoparticles.
- (4)
- Possible intracellular therapy due to the use of very small nanoparticles (5.88–6.67 nm) of CoFe2O4 that lead to obtaining the optimal maximum specific loss power (PsMo), a therapy that is much more effective in destroying tumor cells.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosensweig, R. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Néel, L. Théorie du traînage magnétique des ferromagnétiques en grains fins avec application aux terres cuites. Ann. Geophys. 1949, 5, 99–136. [Google Scholar]
- Bean, C.P.; Livingston, L.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic behavior of Mn0.6Fe0.4Fe2O4 nanoparticles in ferrofluid at low temperatures. J. Magn. Magn. Mater. 2002, 251, 304. [Google Scholar] [CrossRef]
- Perigo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 41302. [Google Scholar] [CrossRef]
- Caizer, C. Nanoparticle size effect on some magnetic properties. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer: Cham, Switzerland, 2016; pp. 475–519. [Google Scholar]
- Caizer, C. Magnetic hyperthermia-using magnetic metal/oxide nanoparticles with potential in cancer therapy. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 193–218. [Google Scholar]
- Brown, W.F., Jr. Thermal fluctuations of a single-domain particle. Phys. Rev. 1963, 130, 1677–1686. [Google Scholar] [CrossRef]
- Torres, T.E.; Lima, E., Jr.; Calatayud, M.P.; Sanz, B.; Ibarra, A.; Fernández-Pacheco, R.; Mayoral, A.; Marquina, C.; Ibarra, M.R.; Goya, G.F. The relevance of brownian relaxation as power absorption mechanism in magnetic hyperthermia. Sci. Rep. 2019, 9, 3992. [Google Scholar] [CrossRef] [PubMed]
- Caizer, C. Magnetic/Superparamagnetic hyperthermia as an effective noninvasive alternative method for therapy of ma-lignant tumors. In Nanotheranostics: Applications and Limitations; Rai, M., Jamil, B., Eds.; Springer: Cham, Switzerland, 2019; pp. 297–335. [Google Scholar]
- Caizer, C. Magnetic/Superparamagnetic hyperthermia in clinical trials for noninvasive alternative cancer therapy. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Caizer, C., Rai, M., Eds.; Wiley: Oxford, UK, 2021. (In Press)
- Caizer, C.; Rai, M. Magnetic nanoparticles in alternative tumors therapy: Biocompatibility, toxicity and safety compared with classical methods. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Caizer, C., Rai, M., Eds.; Wiley: Oxford, UK, 2021. (In Press)
- Ito, A.; Tanaka, K.; Honda, H.; Abe, S.; Yamaguchi, H.; Kobayaschi, T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J. Biosci. Bioeng. 2003, 96, 364–369. [Google Scholar] [CrossRef]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Towards breast cancer treatment by magnetic heating. J. Magn. Magn. Mater. 2005, 293, 314–319. [Google Scholar] [CrossRef]
- Tanaka, K.; Ito, A.; Kobayashi, T.; Kawamura, T.; Shimada, S.; Matsumoto, K.; Saida, T.; Honda, H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int. J. Cancer 2005, 116, 624–633. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Jordan, A.; Taymoorian, K.; Gneveckow, U.; Waldöfner, N.; Scholz, R.; Koch, M.; Lein, M.; Jung, K.; et al. Magnetic fluid hyperthermia (MFH) reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate 2005, 64, 283–292. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. NeuroOncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. NeuroOncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Gazeau, F.; Levy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef]
- Hou, C.-H.; Hou, S.-M.; Hsueh, Y.-S.; Lin, J.; Wu, H.-C.; Lin, F.-H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef]
- Alphandéry, E.; Chebbi, I.; Guyot, F.; Durand-Dubief, M. Use of bacterial magnetosomes in the magnetic hyperthermia treatment of tumours: A review. Int. J. Hyperth. 2013, 29, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I. In vivo applications of magnetic nanoparticle Hyperthermia. Int. J. Hypertherm. 2013, 29, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Bugnet, M.; Radtke, G.; Neveu, S.; Botton, G.A.; Wilhelm, C.; Abou-Hassan, A. Can magneto-plasmonic nanohybrids efficiently combine photothermia with magnetic hyperthermia? Nanoscale 2015, 7, 18872–18877. [Google Scholar] [CrossRef]
- Wang, P.; Xie, X.; Wang, J.; Shi, Y.; Shen, N.; Huang, X. Ultra-small superparamagnetic iron oxide mediated magnetic hyperthermia in treatment of neck lymph node metastasis in rabbit pyriform sinus VX2 carcinoma. Tumor Biol. 2015, 36, 8035–8040. [Google Scholar] [CrossRef]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J. Control. Release 2017, 262, 259–272. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of Magnetic Hyperthermia for Glioblastoma Multiforme Therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- The NanoTherm® Therapy, MagForce Nanomedicine, Germany. Available online: https://www.magforce.com/en/home/our_therapy/ (accessed on 28 April 2021).
- Kikumori, T.; Kobayashi, T.; Sawaki, M.; Imai, T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res. Treat. 2009, 113, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Caizer, C. Computational study on superparamagnetic hyperthermia with biocompatible SPIONs to destroy the cancer cells. J. Phys. Conf. Ser. 2014, 521, 12015. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.; Ling, Y.; Liu, J.; Cai, X.; Zhou, X.; Tang, X.; Liang, B.; Chen, Y.; Chen, H.; et al. Injectable and thermally contractible hydroxypropyl methyl cellulose/Fe3O4 for magnetic hyperthermia ablation of tumors. Biomaterials 2017, 128, 84–93. [Google Scholar] [CrossRef]
- Yan, H.; Shang, W.; Sun, X.; Zhao, L.; Wang, J.; Xiong, Z.; Yuan, J.; Zhang, R.; Huang, Q.; Wang, K.; et al. “All-in-One” Nanoparticles for Trimodality Imaging-Guided Intracellular Photo-magnetic Hyperthermia Therapy under Intravenous Administration. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Bhati, P.; Chakrabarty, A.; Maity, D. Systematic investigations on heating effects of carboxyl-amine functionalized superparamagnetic iron oxide nanoparticles (SPIONs) based ferrofluids for in vitro cancer hyperthermia therapy. J. Mol. Liq. 2018, 256, 224–237. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef]
- Xu, H.; Pan, Y. Experimental evaluation on the heating eficiency of magnetoferritin nanoparticles in an alternating magnetic field. Nanomaterials 2019, 9, 1457. [Google Scholar] [CrossRef]
- Zhao, S.; Lee, S. Biomaterial-modified magnetic nanoparticles γ-Fe2O3, Fe3O4 used to improve the efficiency of hyperthermia of tumors in HepG2 model. Appl. Sci. 2021, 11, 2017. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Sambasivam, S.; Saj, A.; Alaabed, S.; Issa, B.; Al-Omari, I.A.; Obaidat, I.M. Role of magnetite nanoparticles size and concentration on hyperthermia under various field frequencies and stren. Molecules 2021, 26, 796. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Samanta, G.; Sarma, H.D.; Mishra, K.P.; Bellare, J.; Banerjee, R.; Bahadur, D. Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 12–22. [Google Scholar] [CrossRef]
- Prasad, N.K.; Rathinasamy, K.; Panda, D.; Bahadur, D. Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of γ-MnxFe2–xO3 synthesized by a single step process. J. Mat. Chem. 2007, 17, 5042–5051. [Google Scholar] [CrossRef]
- Hejase, H.; Hayek, S.S.; Qadri, S.; Haik, Y. MnZnFe nanoparticles for self-controlled magnetic hyperthermia. J. Magn. Magn. Mater. 2012, 324, 3620–3628. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Ren, J.; Leng, J.; Lin, C.; Shi, D. Enhanced Magnetic Fluid Hyperthermia by Micellar Magnetic Nanoclusters Composed of MnxZn1–xFe2O4 Nanoparticles for Induced Tumor Cell Apoptosis. ACS Appl. Mater. Interfaces 2014, 6, 16867–16879. [Google Scholar] [CrossRef] [PubMed]
- Saldívar-Ramírez, M.M.G.; Sánchez-Torres, C.G.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Almanza, J.; Larson, A.; Reséndiz-Hernández, P.J.; Acuña-Gutiérrez, I.O. Study on the efficiency of nanosized magnetite and mixed ferrites in magnetic hyperthermia. J. Mater. Sci. Mater. Electron. 2014, 25, 2229–2236. [Google Scholar] [CrossRef]
- Almaki, J.H.; Nasiri, R.; Idris, A.; Majid, F.A.A.; Salouti, M.; Wong, T.S.; Dabagh, S.; Marvibaigi, M.; Amini, N. Synthesis, characterization and in vitro evaluation of exquisite targeting SPIONs–PEG–HER in HER2+ human breast cancer cells. Nanotechnology 2016, 27, 105601. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Ng, C.T.; Chandrasekharan, P.; Yang, H.T.; Zhao, L.Y.; Peng, E.; Lv, Y.B.; Xiao, W.; Fang, J.; Yi, J.B.; et al. Synthesis of ferromagnetic Fe0.6Mn0.4O nanofl owers as a new class of magnetic theranostic platform for in vivo T1-T2 dual-mode magnetic resonance imaging and magnetic hyperthermia therapy. Adv. Healthc. Mater. 2016, 5, 2092–2104. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef] [PubMed]
- Martín-Saavedra, F.; Ruíz-Hernández, E.; Boré, A.; Arcos, D.; Vallet-Regí, M.; Vilaboa, N. Magnetic mesoporous silica spheres for hyperthermia therapy. Acta Biomater. 2010, 6, 4522–4531. [Google Scholar] [CrossRef]
- Meneses-Brassea, B.P.; Borrego, E.A.; Blazer, D.S.; Sanad, M.F.; Pourmiri, S.; Gutierrez, D.A.; Varela-Ramirez, A.; Hadjipanayis, G.C.; El-Gendy, A.A. Ni-Cu Nanoparticles and Their Feasibility for Magnetic Hyperthermia. Nanomaterials 2020, 10, 1988. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F.; Delgado, Á.; Iglesias, G. Modulation of the Magnetic Hyperthermia Response Using Different Superparamagnetic Iron Oxide Nanoparticle Morphologies. Nanomaterials 2021, 11, 627. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020. [Google Scholar] [CrossRef]
- Purushotham, S.; Ramanujan, R.V. Modeling the performance of magnetic nanoparticles in multimodal cancer therapy. J. Appl. Phys. 2010, 107, 114701. [Google Scholar] [CrossRef]
- Caizer, C. Optimization Study on Specific Loss Power in Superparamagnetic Hyperthermia with Magnetite Nanoparticles for High Efficiency in Alternative Cancer Therapy. Nanomaterials 2020, 11, 40. [Google Scholar] [CrossRef]
- Smit, J.; Wijin, H.P.J. Les Ferites; Bibliotheque Technique Philips: Paris, France, 1961. [Google Scholar]
- Valenzuela, R. Magnetic Ceramics; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Fortin, J.-P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, e2003712. [Google Scholar] [CrossRef] [PubMed]
- Kharat, P.B.; Somvanshi, S.B.; Khirade, P.P.; Jadhav, K.M. Induction Heating Analysis of Surface-Functionalized Nanoscale CoFe2O4 for Magnetic Fluid Hyperthermia toward Noninvasive Cancer Treatment. ACS Omega 2020, 5, 23378–23384. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Buske, N.; Landers, J.; Gräfe, C.; Wende, H.; Clement, J.H. Biocompatible Magnetic Fluids of Co-Doped Iron Oxide Nanoparticles with Tunable Magnetic Properties. Nanomaterials 2020, 10, 1019. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.; Ahmed, N.; Manzoor, S.; Hamayun, M.; Naseer, N.; Tenório, M.; Lebaz, N.; Elaissari, A. Aminodextran coated CoFe2O4 nanoparticles for combined magnetic resonance imaging and hyperthermia. Nanomaterials 2020, 10, 2182. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Ángeles-Pascual, A.; González, M.M.; Antaño-López, R.; Vera, A.; Leija, L.; Reguera, E.; Arriaga, L.G.; Hernández, J.M.H.; et al. Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecuses 2020, 25, 4428. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Kim, N.P.; Attanayake, S.; Phan, M.-H.; Srikanth, H. Role of magnetic anisotropy on the hyperthermia efficiency in spherical Fe3-xCoxO4 (x = 0 − 1) nanoparticles. Appl. Sci. 2021, 11, 930. [Google Scholar] [CrossRef]
- Lucht, N.; Friedrich, R.P.; Draack, S.; Alexiou, C.; Viereck, T.; Ludwig, F.; Hankiewicz, B. Biophysical Characterization of (Silica-coated) Cobalt Ferrite Nanoparticles for Hyperthermia Treatment. Nanomaterials 2019, 9, 1713. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Gomes, J.D.A.; Sousa, M.H.; Bakuzis, A.F. Magnetic hyperthermia investigation of cobalt ferrite nanoparticles: Comparison between experiment, linear response theory, and dynamic hysteresis simulations. J. Appl. Phys. 2012, 111, 123902. [Google Scholar] [CrossRef]
- Mohapatra, J.; Xing, M.; Liu, J.P. Magnetic and hyperthermia properties of CoxFe3-xO4 nanoparticles synthesized via cation exchange. AIP Adv. 2018, 8, 56725. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Carvalho, S.M.; Outon, L.E.F.; Ardisson, J.D.; Krambrock, K.; Mansur, H.S. Tunable magnetothermal properties of cobalt-doped magnetite–carboxymethylcellulose ferrofluids: Smart nanoplatforms for potential magnetic hyperthermia applications in cancer therapy. Nanoscale Adv. 2021, 3, 1029–1046. [Google Scholar] [CrossRef]
- Habib, A.H.; Ondeck, C.L.; Chaudhary, P.; Bockstaller, M.R.; McHenry, M.E. Evaluation of iron-cobalt/ferrite core-shell nanoparticles for cancer thermotherapy. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Langevin, P. Magnétisme et théorie des électrons. Ann. Chem. Phys. 1905, 5, 70–127. [Google Scholar]
- Jacobs, I.S.; Bean, C.P. Fine particles, thin films and exchange anisotropy. In Magnetism; Rado, G.T., Suhl, H., Eds.; Academic Press: Cambridge, MA, USA, 1963; Volume 3, pp. 271–350. [Google Scholar]
- Back, C.; Weller, D.; Heidmann, J.; Mauri, D.; Guarisco, D.; Garwin, E.L.; Siegmann, H.C. Magnetization Reversal in Ultrashort Magnetic Field Pulses. Phys. Rev. Lett. 1998, 81, 3251–3254. [Google Scholar] [CrossRef]
- Coey, J.M.D. Noncollinear spin srrangement in ultrafine ferrimagnetic crystallites. Phys. Rev. Lett. 1971, 27, 1140. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J., Jr.; Foner, S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 1996, 77, 394. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A.E.; Kodama, R.H.; Makhlouf, S.A.; Parker, F.T.; Spada, F.E.; McNiff, E.J., Jr.; Foner, S. Anomalous properties of magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 196–197, 591. [Google Scholar] [CrossRef]
- Tobia, D.; De Biasi, E.; Granada, M.; Troiani, H.E.; Zampieri, G.; Winkler, E.; Zysler, R.D. Evolution of the magnetic anisotropy with particle size in antiferromagnetic Cr2O3 nanoparticles. J. Appl. Phys. 2010, 108. [Google Scholar] [CrossRef]
- Lehlooh, A.-F.; Mahmood, S.H.; Williams, J.M. On the particle size dependence of the magnetic anisotropy energy constant. Phys. B Condens. Matter. 2002, 321, 159–162. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Néel, L. Anisotropie magnétique superficielle et surstructures d’orientation. J. Phys. 1954, 15, 225–239. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic properties of the novel nanocomposite (Zn0.15Ni0.85Fe2O4)0.15/(SiO2)0.85 at room temperature. J. Magn. Magn. Mater. 2008, 320, 1056–1062. [Google Scholar] [CrossRef]
- Caizer, C.; Savii, C.; Popovici, M. Magnetic behaviour of iron oxide nanoparticles dispersed in a silica matrix. Mater. Sci. Eng. B 2003, 97, 129–134. [Google Scholar] [CrossRef]
- Hrianca, I.; Caizer, C.; Schlett, Z. Dynamic magnetic behavior of Fe3O4 colloidal nanoparticles. J. Appl. Phys. 2002, 92, 2125–2132. [Google Scholar] [CrossRef]
- Kodama, R. Magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Kneller, E.; Seeger, A.; Kronmüller, H. Ferromagnetismus; Springer: Berlin/Heidelberg, Germany, 1962. [Google Scholar]
- Caizer, C.; Tura, V. Magnetic relaxation/stability of Co ferrite nanoparticles embedded in amorphous silica particles. J. Magn. Magn. Mater. 2006, 301, 513–520. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
| Ms (×103 A m−1) | K (×103 J m−3) | ρ (×103 kg⋅m−3) | c (Jkg−1 K−1) | ε | H (×103 A m−1) | f (×103 Hz) | D (×10−9 m) |
|---|---|---|---|---|---|---|---|
| 425 | 200 | 5.29 | 700 | 0.01–0.15 | 5–180 | 50–1000 | 1–20 |
| PsM (W/g) | f (kHz) | DM (nm) |
|---|---|---|
| 3.46 | 100 | 6.62 |
| 13.64 | 500 | 6.12 |
| 24.13 | 1000 | 5.88 |
| No. | H (kA/m) | fl (kHz) | Do (nm) | (PsM)l (W/g) |
|---|---|---|---|---|
| 1 | 5 | 1000 | 5.88 | 2.69 (+0.48) |
| 2 | 15 | 334 | 6.25 | 9.73 (+0.43) |
| 3 | 30 | 167 | 6.46 | 21.26 (+0.15) |
| 4 | 45 | 111 | 6.58 | 32.70 (+0.02) |
| 5 | 60 | 83 | 6.67 | 43.36 (+0.07) |
| 6 | 75 | 67 | 6.72 | 53.43 (−0.30) |
| 7 | 90 | 56 | 6.77 | 62.04 (−0.40) |
| 8 | 100 | 50 | 6.80 | 66.46 (+0.14) |
| 9 | 120 | 42 | 6.84 | 75.19 (−0.31) |
| 10 | 135 | 37 | 6.88 | 79.50 (+0.27) |
| 11 | 150 | 33 | 6.91 | 82.87 (+0.80) |
| 12 | 165 | 30 | 6.93 | 86.27 (+0.47) |
| 13 | 180 | 28 | 6.95 | 89.99 (−0.89) |
| H (kA/m) | fl (kHz) | Do (nm) | ΔThm (°C) | Δtm (s) | * ΔTho (°C) | ** Δto (s) |
|---|---|---|---|---|---|---|
| 5 | 1000 | 5.88 | 42.43 | 23.33 | 18 | 5.01 |
| 15 | 334 | 6.25 | 33.35 | 5.18 | 18 | 1.45 |
| 30 | 167 | 6.46 | 29.29 | 2.09 | 18 | 0.69 |
| 45 | 111 | 6.58 | 27.48 | 1.27 | 18 | 0.46 |
| 60 | 83 | 6.67 | 26.74 | 0.92 | 18 | 0.35 |
| H (kA/m) | fl (kHz) | Do (nm) | ΔThm (°C) | Δtm (s) | * ΔTho (°C) | ** Δto (s) |
|---|---|---|---|---|---|---|
| 5 | 1000 | 5.88 | 41.99 | 212.07 | 18 | 44.19 |
| 15 | 334 | 6.25 | 33.06 | 46.57 | 18 | 12.80 |
| 30 | 167 | 6.46 | 28.81 | 18.15 | 18 | 6.14 |
| 45 | 111 | 6.58 | 27.28 | 11.15 | 18 | 4.08 |
| 60 | 83 | 6.67 | 26.72 | 8.08 | 18 | 3.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caizer, C. Theoretical Study on Specific Loss Power and Heating Temperature in CoFe2O4 Nanoparticles as Possible Candidate for Alternative Cancer Therapy by Superparamagnetic Hyperthemia. Appl. Sci. 2021, 11, 5505. https://doi.org/10.3390/app11125505
Caizer C. Theoretical Study on Specific Loss Power and Heating Temperature in CoFe2O4 Nanoparticles as Possible Candidate for Alternative Cancer Therapy by Superparamagnetic Hyperthemia. Applied Sciences. 2021; 11(12):5505. https://doi.org/10.3390/app11125505
Chicago/Turabian StyleCaizer, Costica. 2021. "Theoretical Study on Specific Loss Power and Heating Temperature in CoFe2O4 Nanoparticles as Possible Candidate for Alternative Cancer Therapy by Superparamagnetic Hyperthemia" Applied Sciences 11, no. 12: 5505. https://doi.org/10.3390/app11125505
APA StyleCaizer, C. (2021). Theoretical Study on Specific Loss Power and Heating Temperature in CoFe2O4 Nanoparticles as Possible Candidate for Alternative Cancer Therapy by Superparamagnetic Hyperthemia. Applied Sciences, 11(12), 5505. https://doi.org/10.3390/app11125505





