Polyethylenimine-Modified Magnetic Chitosan for the Uptake of Arsenic from Water
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Fe3O4 Magnetic Nanoparticles
2.3. Synthesis of CS-Modified Fe3O4 Magnetic Nanoparticles
2.4. Synthesis of Polyethylenimine-Modified Fe3O4/CS
2.5. Batch Adsorption Experiment
2.6. Regeneration and Reusability Study
3. Results and Discussion
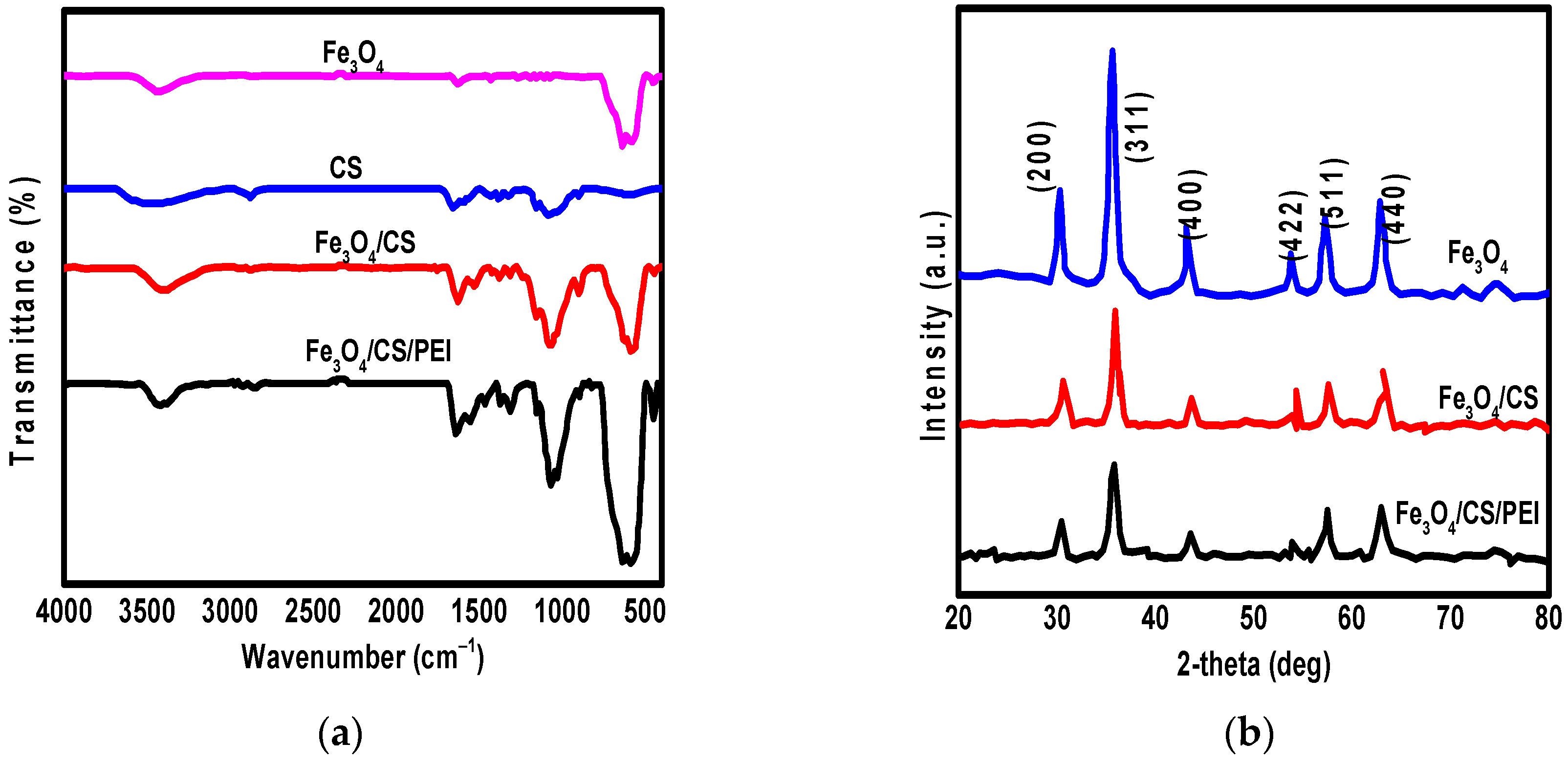
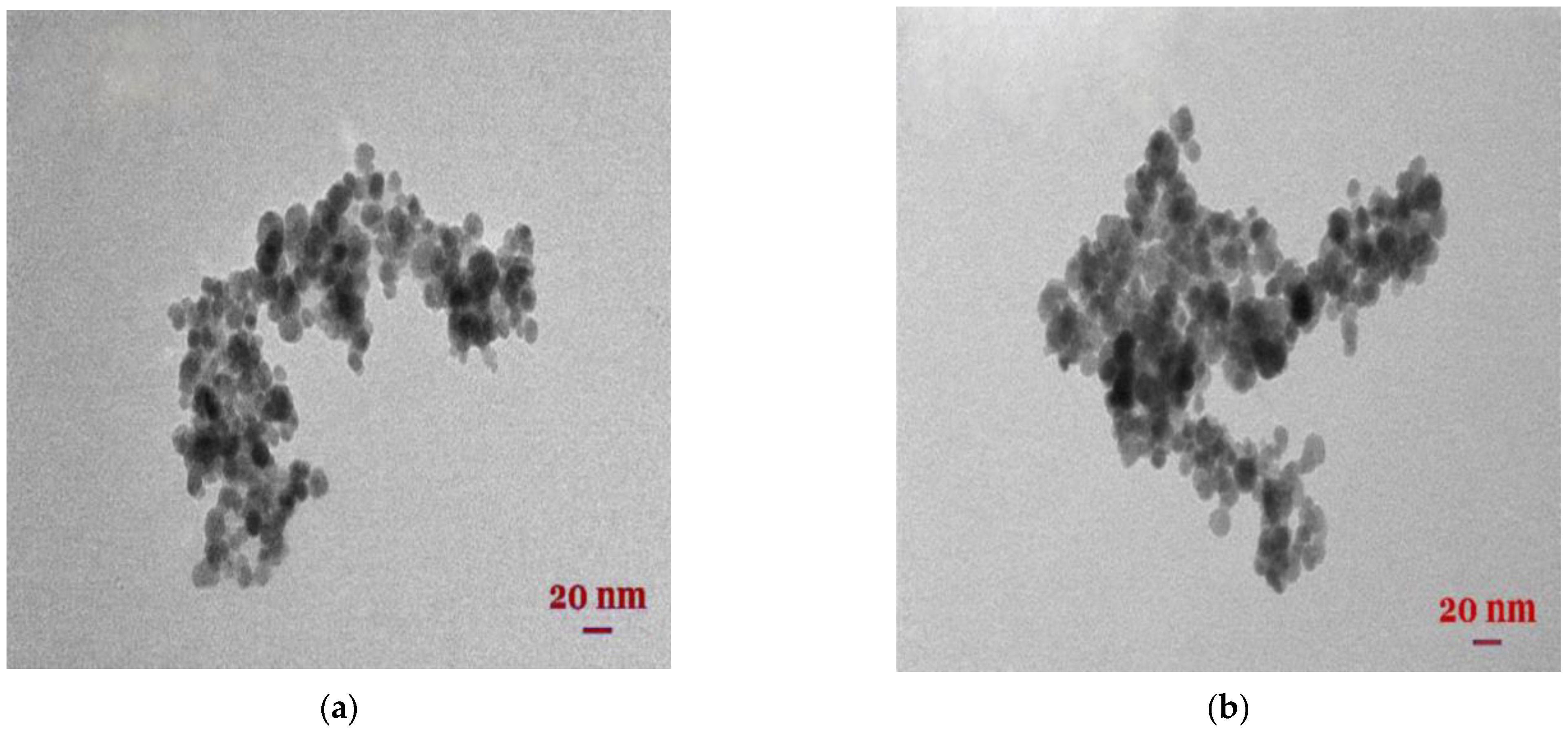
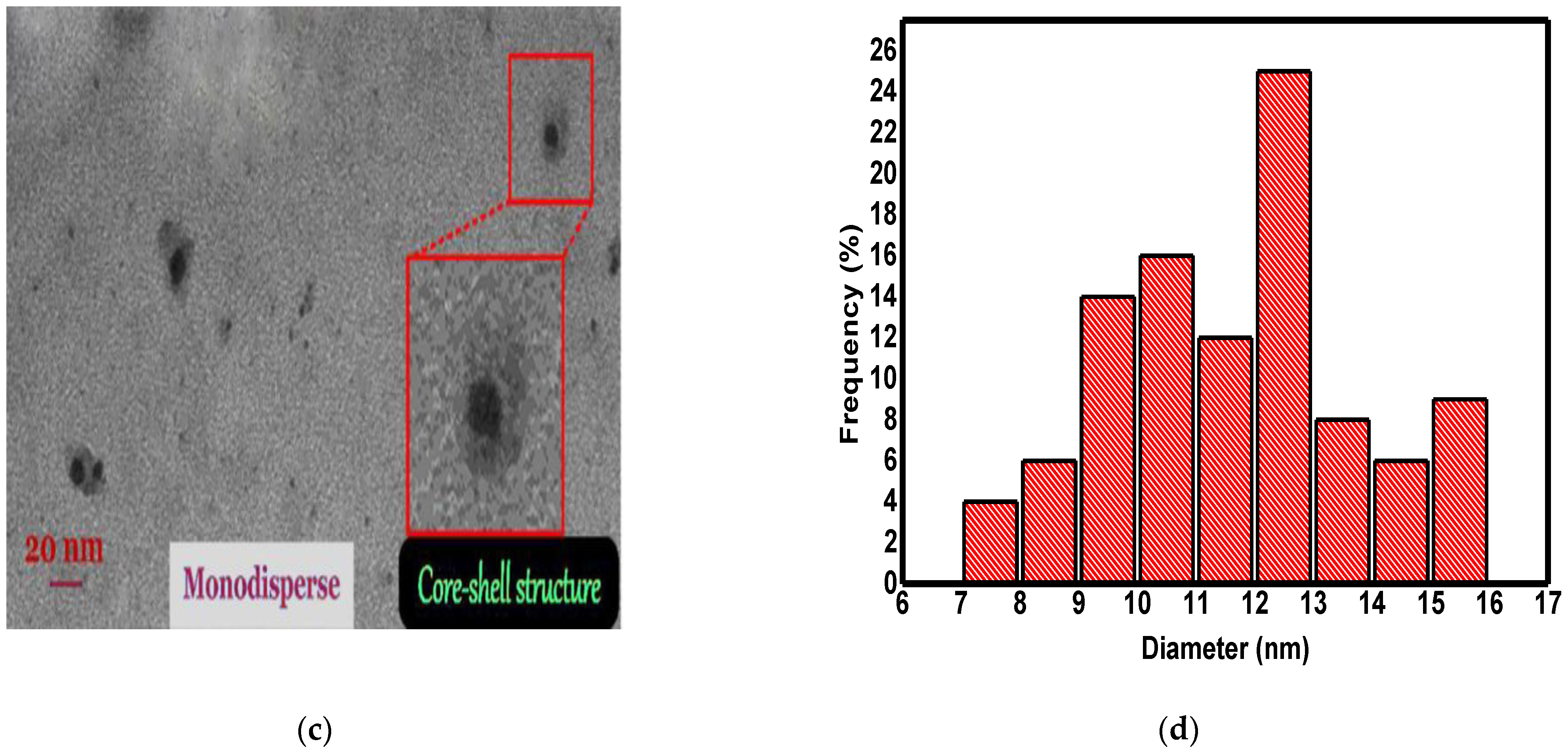
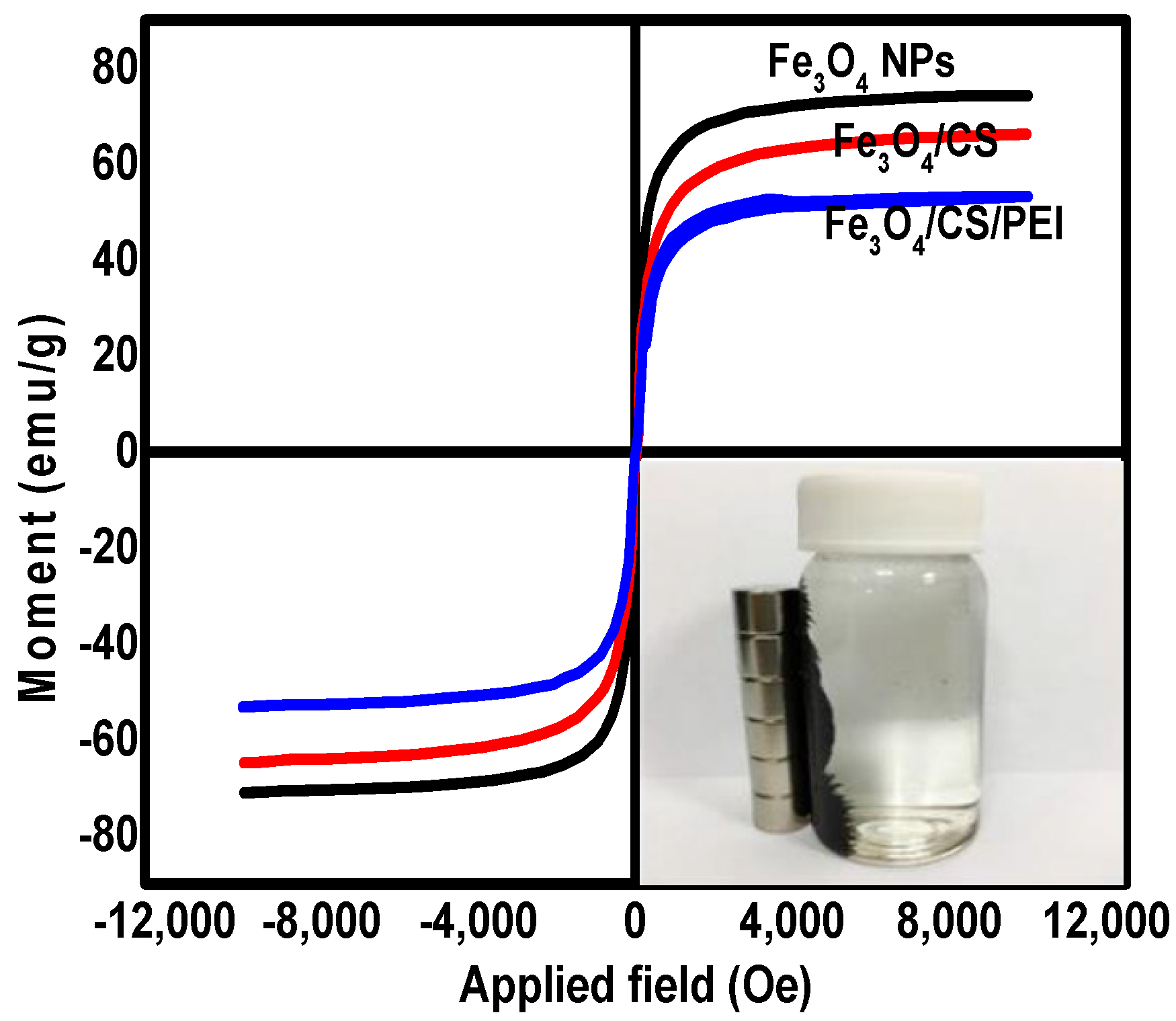
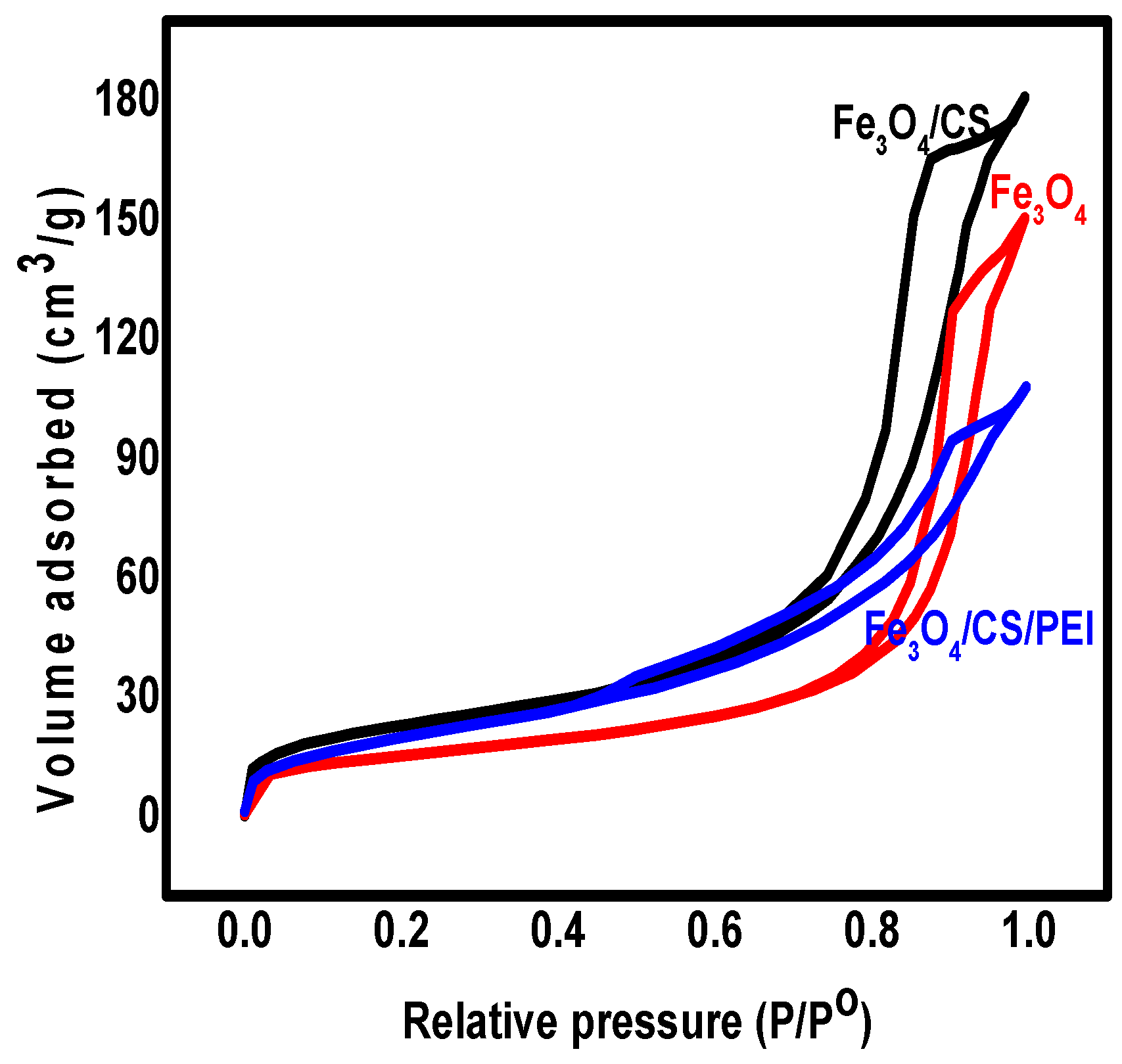
3.1. The Characterization of Synthesized Nanomaterials
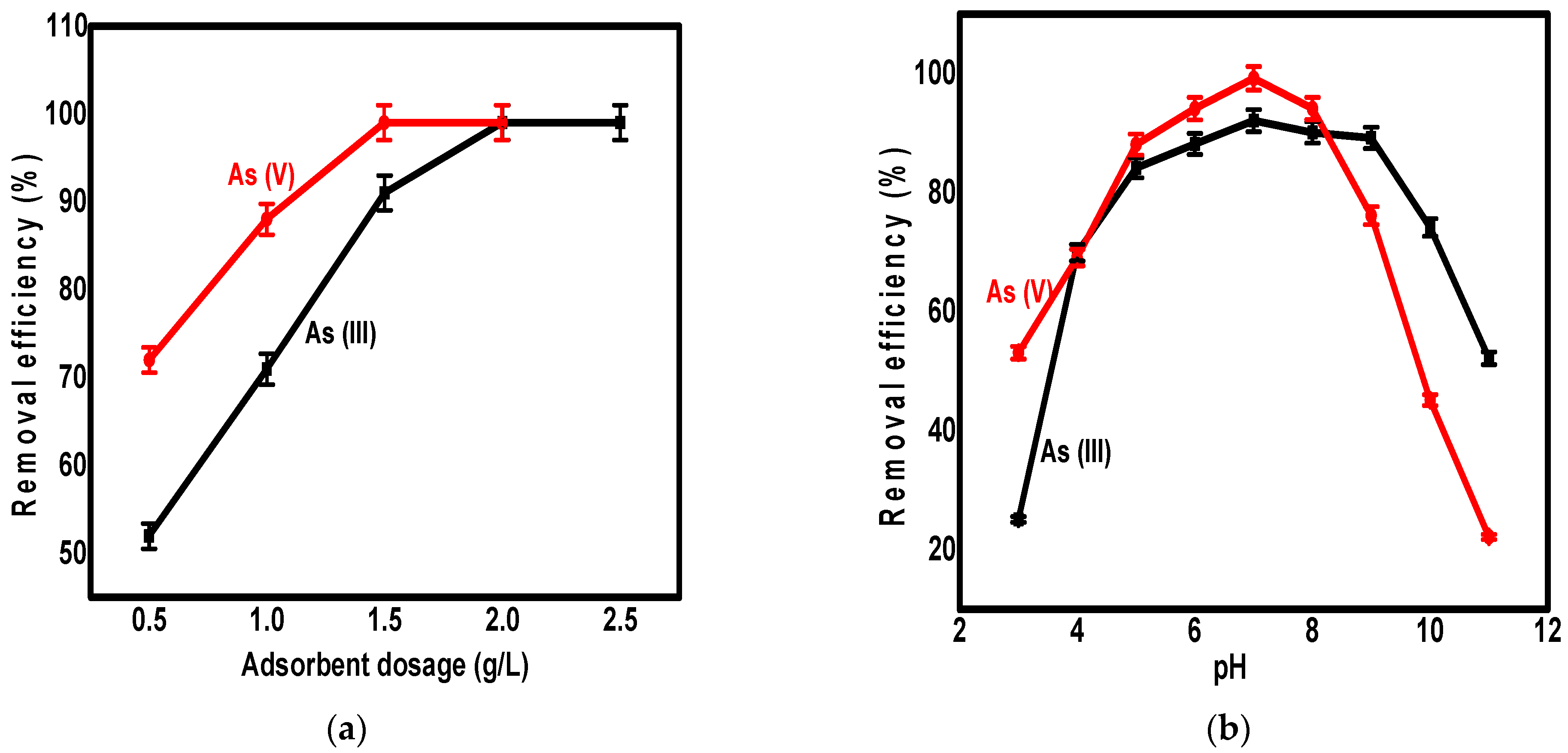
3.2. The Effect of Adsorbent Dosage
3.3. The Effect of PH Value
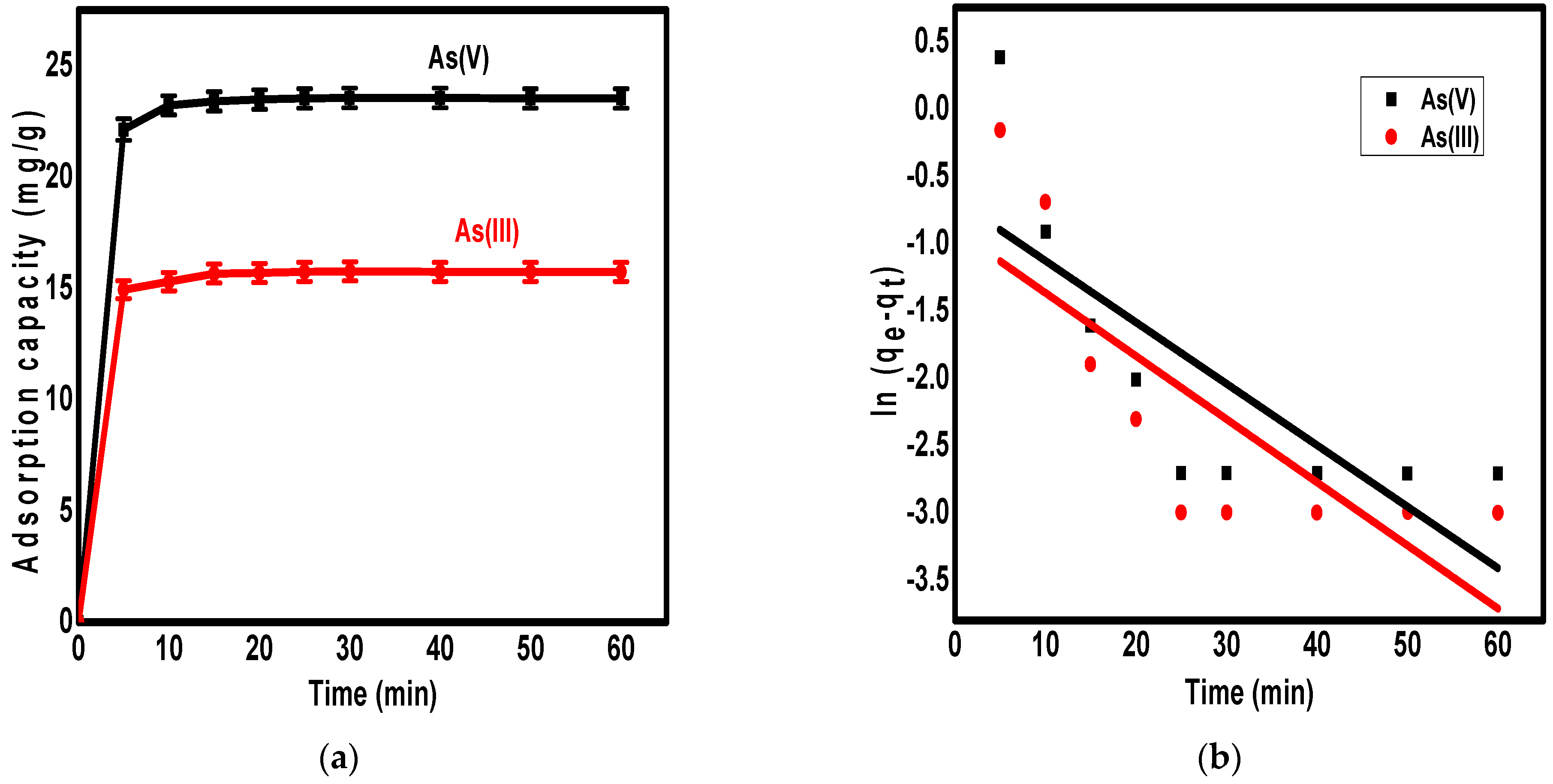
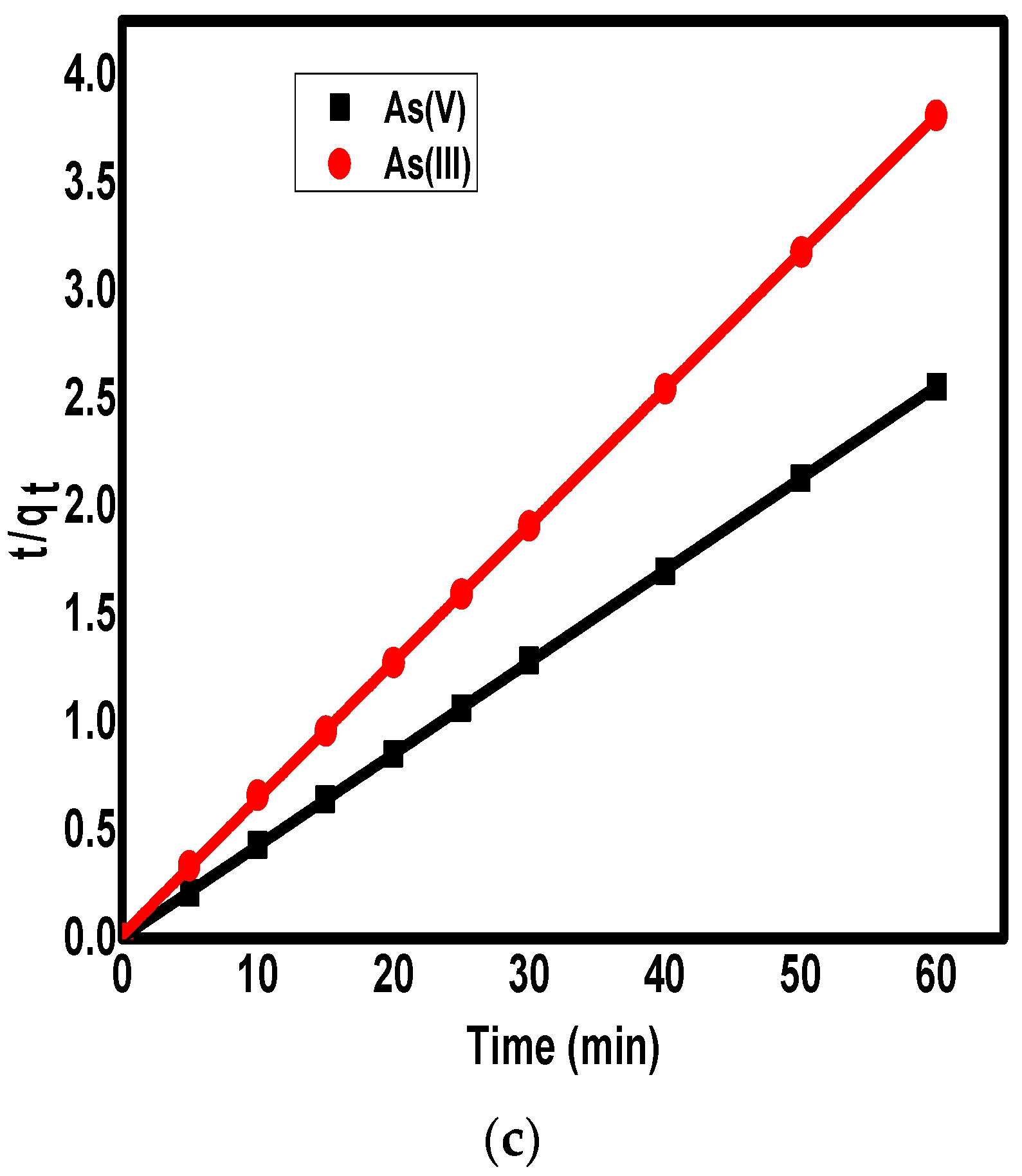
3.4. The Adsorption Kinetics
3.5. The Adsorption Isotherm
3.6. Regeneration and Reusability Study
3.7. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centeno, J.A.; Tseng, C.-H.; Van Der Voet, G.B.; Finkelman, R.B. Global Impacts Of Geogenic Arsenic: A Medical Geology Research Case. Ambio 2007, 36, 78–81. [Google Scholar] [CrossRef]
- Smith, A.H.; Goycolea, M.; Haque, R.; Biggs, M.L. Marked Increase in Bladder and Lung Cancer Mortality in a Region of Northern Chile Due to Arsenic in Drinking Water. Am. J. Epidemiol. 1998, 147, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Bakar, A.; Uda, M.; Hashim, U.; Parmin, N.; Anuar, A.; Bakar, M.; Sulaiman, M. Potential of Syntesized Silica Nanoparticles (Si-NPs) using Corn Cob for Arsenic Heavy Metal Removal. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd Joint Conference on Green Engineering Technology & Applied Computing 2020, Bangkok, Thailand, 4–5 February 2020; IOP Publishing: Bristol, UK, 2020; Volume 864, p. 012187. [Google Scholar]
- Adeloju, S.; Khan, S.; Patti, A. Arsenic Contamination of Groundwater and Its Implications for Drinking Water Quality and Human Health in Under-Developed Countries and Remote Communities—A Review. Appl. Sci. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Huong, P.T.L.; Huy, L.T.; Lan, H.; Thang, L.H.; An, T.T.; Van Quy, N.; Tuan, P.A.; Alonso, J.; Phan, M.-H.; Le, A.-T. Magnetic iron oxide-carbon nanocomposites: Impacts of carbon coating on the As(V) adsorption and inductive heating responses. J. Alloys Compd. 2018, 739, 139–148. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: Application of isotherm, kinetic and thermodynamics. Process. Saf. Environ. Prot. 2018, 119, 138–163. [Google Scholar] [CrossRef]
- Azamat, J.; Khataee, A.; Sadikoglu, F. Computational study on the efficiency of MoS 2 membrane for removing arsenic from contaminated water. J. Mol. Liq. 2018, 249, 110–116. [Google Scholar] [CrossRef]
- Lizama-Allende, K.; Ayala, J.; Jaque, I.; Echeverría, P. The removal of arsenic and metals from highly acidic water in horizontal subsurface flow constructed wetlands with alternative supporting media. J. Hazard. Mater. 2021, 408, 124832. [Google Scholar] [CrossRef]
- Mahato, B.N.; Krithiga, T.; Thangam, M.M. Rapid adsorption of As(V) from aqueous solution by ZnO embedded in mesoporous aluminosilicate nanocomposite adsorbent: Parameter optimization, kinetic, and isotherms studies. Surf. Interfaces 2021, 23, 100636. [Google Scholar] [CrossRef]
- Dhar, R.; Zheng, Y.; Rubenstone, J.; van Geen, A. A rapid colorimetric method for measuring arsenic concentrations in groundwater. Anal. Chim. Acta 2004, 526, 203–209. [Google Scholar] [CrossRef]
- Schmidt, S.-A.; Gukelberger, E.; Hermann, M.; Fiedler, F.; Großmann, B.; Hoinkis, J.; Ghosh, A.; Chatterjee, D.; Bundschuh, J. Pilot study on arsenic removal from groundwater using a small-scale reverse osmosis system—Towards sustainable drinking water production. J. Hazard. Mater. 2016, 318, 671–678. [Google Scholar] [CrossRef]
- Pu, L.; Xu, Y.; Xia, Q.; Ding, J.; Wang, Y.; Shan, C.; Wu, D.; Zhang, Q.; Gao, G.; Pan, B. Ferroelectric membrane for water purification with arsenic as model pollutant. Chem. Eng. J. 2021, 403, 126426. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Fuentes, R.; Thiam, A.; Salazar, R. Arsenic and fluoride removal by electrocoagulation process: A general review. Sci. Total. Environ. 2021, 753, 142108. [Google Scholar] [CrossRef] [PubMed]
- Pallier, V.; Feuillade-Cathalifaud, G.; Serpaud, B.; Bollinger, J.-C. Effect of organic matter on arsenic removal during coagulation/flocculation treatment. J. Colloid Interface Sci. 2010, 342, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ostermeyer, P.; Bonin, L.; Folens, K.; Verbruggen, F.; García-Timermans, C.; Verbeken, K.; Rabaey, K.; Hennebel, T. Effect of speciation and composition on the kinetics and precipitation of arsenic sulfide from industrial metallurgical wastewater. J. Hazard. Mater. 2021, 409, 124418. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Shabbir, M.; Rebah, F.B. Application of Functionalized Nanomaterials as Effective Adsorbents for the Removal of Heavy Metals from Wastewater: A Review. Curr. Anal. Chem. 2020, 17, 4–22. [Google Scholar] [CrossRef]
- Alsaiari, N.; Amari, A.; Katubi, K.; Alzahrani, F.; Rebah, F.; Tahoon, M. Innovative Magnetite Based Polymeric Nanocomposite for Simultaneous Removal of Methyl Orange and Hexavalent Chromium from Water. Processes 2021, 9, 576. [Google Scholar] [CrossRef]
- Katubi, K.; Alsaiari, N.; Alzahrani, F.; Siddeeg, S.M.; Tahoon, M.A. Synthesis of Manganese Ferrite/Graphene Oxide Magnetic Nanocomposite for Pollutants Removal from Water. Processes 2021, 9, 589. [Google Scholar] [CrossRef]
- Amari, A.; Elboughdiri, N.; Ghernaout, D.; Lajimi, R.H.; Alshahrani, A.M.; Tahoon, M.A.; Ben Rebah, F. Multifunctional crosslinked chitosan/nitrogen-doped graphene quantum dot for wastewater treatment. Ain Shams Eng. J. 2021. [Google Scholar] [CrossRef]
- Amari, A.; Alzahrani, F.M.; Katubi, K.M.; Alsaiari, N.S.; Tahoon, M.; Ben Rebah, F. Clay-Polymer Nanocomposites: Preparations and Utilization for Pollutants Removal. Materials 2021, 14, 1365. [Google Scholar] [CrossRef]
- Katubi, K.; Amari, A.; Harharah, H.; Eldirderi, M.; Tahoon, M.; Ben Rebah, F. Aloe vera as Promising Material for Water Treatment: A Review. Processes 2021, 9, 782. [Google Scholar] [CrossRef]
- Goswami, A.; Raul, P.; Purkait, M. Arsenic adsorption using copper (II) oxide nanoparticles. Chem. Eng. Res. Des. 2012, 90, 1387–1396. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Lin, X.; Huang, P.; Huang, Y. Synthesis of magnetic wheat straw for arsenic adsorption. J. Hazard. Mater. 2011, 193, 10–16. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.-G.; Liu, S.-B.; Liu, H.-Y.; Zeng, G.-M.; Tan, X.; Yang, C.-P.; Ding, Y.; Yan, Z.-L.; Cai, X.-X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Z.; Luo, H.; Hu, B.; Dang, Z.; Yang, C.; Li, L. Adsorption of arsenic on modified montmorillonite. Appl. Clay Sci. 2014, 97–98, 17–23. [Google Scholar] [CrossRef]
- Zhu, N.; Qiao, J.; Ye, Y.; Yan, T. Synthesis of mesoporous bismuth-impregnated aluminum oxide for arsenic removal: Adsorption mechanism study and application to a lab-scale column. J. Environ. Manag. 2018, 211, 73–82. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Gutiérrez-Martínez, J.; Rangel-Méndez, J.R. Modified activated carbon with interconnected fibrils of iron-oxyhydroxides using Mn2+ as morphology regulator, for a superior arsenic removal from water. J. Environ. Sci. 2019, 76, 403–414. [Google Scholar] [CrossRef]
- Alsaiari, N.; Katubi, K.; Alzahrani, F.; Siddeeg, S.; Tahoon, M. The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review. Micromachines 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; Al Mesfer, M.K.; Alsaiari, N.S.; Danish, M.; Alshahrani, A.M.; Tahoon, M.A.; Rebah, F.B. Electrochemical and Optical Properties of Tellurium Dioxide (TeO2) Nanoparticles. Int. J. Electrochem. Sci. 2021, 16, 210235. [Google Scholar] [CrossRef]
- Amari, A.; Alalwan, B.; Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Rebah, F.B. Biomolecules Behavior on a Surface of Boron Doped/un-doped Graphene Nanosheets. Int. J. Electrochem. Sci. 2020, 15, 11427–11436. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Alsaiari, N.S.; Tahoon, M.A.; Rebah, F.B. The application of nanomaterials as electrode modifiers for the electrochemical detection of ascorbic acid. Int. J. Electrochem. Sci. 2020, 15, 3327–3346. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Alsaiari, N.S.; Mnif, W.; Ben Rebah, F. Effective Heavy Metals Removal from Water Using Nanomaterials: A Review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Alzahrani, F.; Alsaiari, N.; Katubi, K.; Amari, A.; Ben Rebah, F.; Tahoon, M. Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers 2021, 13, 1742. [Google Scholar] [CrossRef] [PubMed]
- Simonescu, C.M.; Tătăruş, A.; Culiţă, D.C.; Stănică, N.; Ionescu, I.A.; Butoi, B.; Banici, A.-M. Comparative study of CoFe2O4 nanoparticles and CoFe2O4-chitosan composite for Congo Red and Methyl Orange removal by adsorption. Nanomaterials 2021, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Fouda, A.; Elwakeel, K.Z.; Wei, Y.; Guibal, E.; Hamad, N.A. Phosphorylation of Guar Gum/Magnetite/Chitosan Nanocomposites for Uranium (VI) Sorption and Antibacterial Applications. Molecules 2021, 26, 1920. [Google Scholar] [CrossRef] [PubMed]
- Apriceno, A.; Silvestro, I.; Girelli, A.; Francolini, I.; Pietrelli, L.; Piozzi, A. Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation. Polymers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Amari, A.; Tahoon, M.A.; Alsaiari, N.S.; Rebah, F.B. Removal of meloxicam, piroxicam and Cd+2 by Fe3O4/SiO2/glycidyl methacrylate-S-SH nanocomposite loaded with laccase. Alex. Eng. J. 2020, 59, 905–914. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Ben Rebah, F. Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida. Processes 2019, 7, 800. [Google Scholar] [CrossRef]
- Amari, A.; Alzahrani, F.; Alsaiari, N.; Katubi, K.; Rebah, F.; Tahoon, M. Magnetic Metal Organic Framework Immobilized Laccase for Wastewater Decolorization. Processes 2021, 9, 774. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Veintemillas-Verdaguer, S.; Tartaj, P.; Mazarío, E.; Morales, M.; Ovejero, J. Engineering Iron Oxide Nanocatalysts by a Microwave-Assisted Polyol Method for the Magnetically Induced Degradation of Organic Pollutants. Nanomaterials 2021, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.L.; Arnal, M.C.; De La Guardia, M. Removal of heavy metals by using adsorption on alumina or chitosan. Anal. Bioanal. Chem. 2003, 375, 820–825. [Google Scholar] [CrossRef]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Surimi wash water treatment for protein recovery: Effect of chitosan–alginate complex concentration and treatment time on protein adsorption. Bioresour. Technol. 2005, 96, 665–671. [Google Scholar] [CrossRef]
- Cheung, W.; Szeto, Y.; McKay, G. Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Kuncoro, E.P.; Roussy, J.; Guibal, E. Mercury Recovery by Polymer-Enhanced Ultrafiltration: Comparison of Chitosan and Poly(Ethylenimine) Used as Macroligand. Sep. Sci. Technol. 2005, 40, 659–684. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, S.; Wang, B.; Huang, J.; Yu, G. Removal of clofibric acid from aqueous solution by polyethylenimine-modified chitosan beads. Front. Environ. Sci. Eng. 2014, 8, 675–682. [Google Scholar] [CrossRef]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanopart. Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, P.; Luo, S.; Tu, X.; Cao, Q.; Shu, M. Synthesis of novel nanocomposite Fe3O4/ZrO2/chitosan and its application for removal of nitrate and phosphate. Appl. Surf. Sci. 2013, 284, 942–949. [Google Scholar] [CrossRef]
- Li, G.-Y.; Jiang, Y.-R.; Huang, K.-L.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Microwave assisted synthesis of poly (N-vinylimidazole) grafted chitosan as an effective adsorbent for mercury (II) removal from aqueous solution: Equilibrium, kinetic, thermodynamics and regeneration studies. J. Dispers. Sci. Technol. 2019, 41, 828–840. [Google Scholar] [CrossRef]
- Anirudhan, T.; Rauf, T.A.; Rejeena, S. Removal and recovery of phosphate ions from aqueous solutions by amine functionalized epichlorohydrin-grafted cellulose. Desalination 2012, 285, 277–284. [Google Scholar] [CrossRef]
- Campanile, R.; Scardapane, E.; Forente, A.; Granata, C.; Germano, R.; Di Girolamo, R.; Minopoli, A.; Velotta, R.; Della Ventura, B.; Iannotti, V. Core-Shell Magnetic Nanoparticles for Highly Sensitive Magnetoelastic Immunosensor. Nanomaterials 2020, 10, 1526. [Google Scholar] [CrossRef]
- Saykova, D.; Saikova, S.; Mikhlin, Y.; Panteleeva, M.; Ivantsov, R.; Belova, E. Synthesis and Characterization of Core–Shell Magnetic Nanoparticles NiFe2O4@ Au. Metals 2020, 10, 1075. [Google Scholar] [CrossRef]
- Niemirowicz-Laskowska, K.; Mystkowska, J.; Łysik, D.; Chmielewska, S.; Tokajuk, G.; Misztalewska-Turkowicz, I.; Wilczewska, A.Z.; Bucki, R. Antimicrobial and Physicochemical Properties of Artificial Saliva Formulations Supplemented with Core-Shell Magnetic Nanoparticles. Int. J. Mol. Sci. 2020, 21, 1979. [Google Scholar] [CrossRef]
- Rekos, K.; Kampouraki, Z.-C.; Sarafidis, C.; Samanidou, V.; Deliyanni, E. Graphene Oxide Based Magnetic Nanocomposites with Polymers as Effective Bisphenol–A Nanoadsorbents. Materials 2019, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, Z.; Zhou, A.; Chen, J.; Hu, M.; Dong, X.; Xia, Q. Adsorption of phosphate onto amine functionalized nano-sized magnetic polymer adsorbents: Mechanism and magnetic effects. RSC Adv. 2015, 5, 22080–22090. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, J.-I.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Adsorptive removal of As(V) and As(III) from water by a Zr(IV)-loaded orange waste gel. J. Hazard. Mater. 2008, 154, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.I.; Singh, P.N.; Tara, N.; Pal, S.; Chaudhry, S.A.; Sinha, I. Arsenic removal from water by starch functionalized maghemite nano-adsorbents: Thermodynamics and kinetics investigations. Colloid Interface Sci. Commun. 2020, 36, 100263. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Chen, L.; Huang, X.; Liu, J. Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem. Eng. J. 2014, 251, 25–34. [Google Scholar] [CrossRef]
- Tahoon, M.; Gomaa, E.; Suleiman, M. Aqueous Micro-hydration of Na+ (H2O)n=1–7 Clusters: DFT Study. Open Chem. 2019, 17, 260–269. [Google Scholar]
- Gomaa, E.A.; Tahoon, M.A.; Negm, A. Aqueous micro-solvation of Li + ions: Thermodynamics and energetic studies of Li+-(H2O)n (n = 1–6) structures. J. Mol. Liq. 2017, 241, 595–602. [Google Scholar] [CrossRef]
- Gomaa, E.A.; Tahoon, M.A. Ion association and solvation behavior of copper sulfate in binary aqueous–methanol mixtures at different temperatures. J. Mol. Liq. 2016, 214, 19–23. [Google Scholar] [CrossRef]
- Ryu, S.-R.; Jeon, E.-K.; Yang, J.-S.; Baek, K. Adsorption of As(III) and As(V) in groundwater by Fe–Mn binary oxide-impregnated granular activated carbon (IMIGAC). J. Taiwan Inst. Chem. Eng. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.; Bundschuh, J.; et al. Exploring the arsenic removal potential of various biosorbents from water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Kumar, M.; Mukherjee, S.; Goswami, R. Sustainable removal of pernicious arsenic and cadmium by a novel composite of MnO2 impregnated alginate beads: A cost-effective approach for wastewater treatment. J. Environ. Manag. 2019, 234, 8–20. [Google Scholar] [CrossRef]
- Kim, N.; Park, M.; Yun, Y.-S.; Park, D. Removal of anionic arsenate by a PEI-coated bacterial biosorbent prepared from fermentation biowaste. Chemosphere 2019, 226, 67–74. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Enhanced Arsenic (III) adsorption from aqueous solution by magnetic pine cone biomass. Mater. Chem. Phys. 2019, 222, 20–30. [Google Scholar] [CrossRef]
- Boddu, S.; Dulla, J.B.; Alugunulla, V.N.; Khan, A.A. An assessment on removal performance of arsenic with treated Turbinaria vulgaris as an adsorbent: Characterization, optimization, isotherm, and kinetics study. Environ. Prog. Sustain. Energy 2020, 39, 13313. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal from Water by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance Evaluation of Small Sized Powdered Ferric Hydroxide as Arsenic Adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic(V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef] [PubMed]
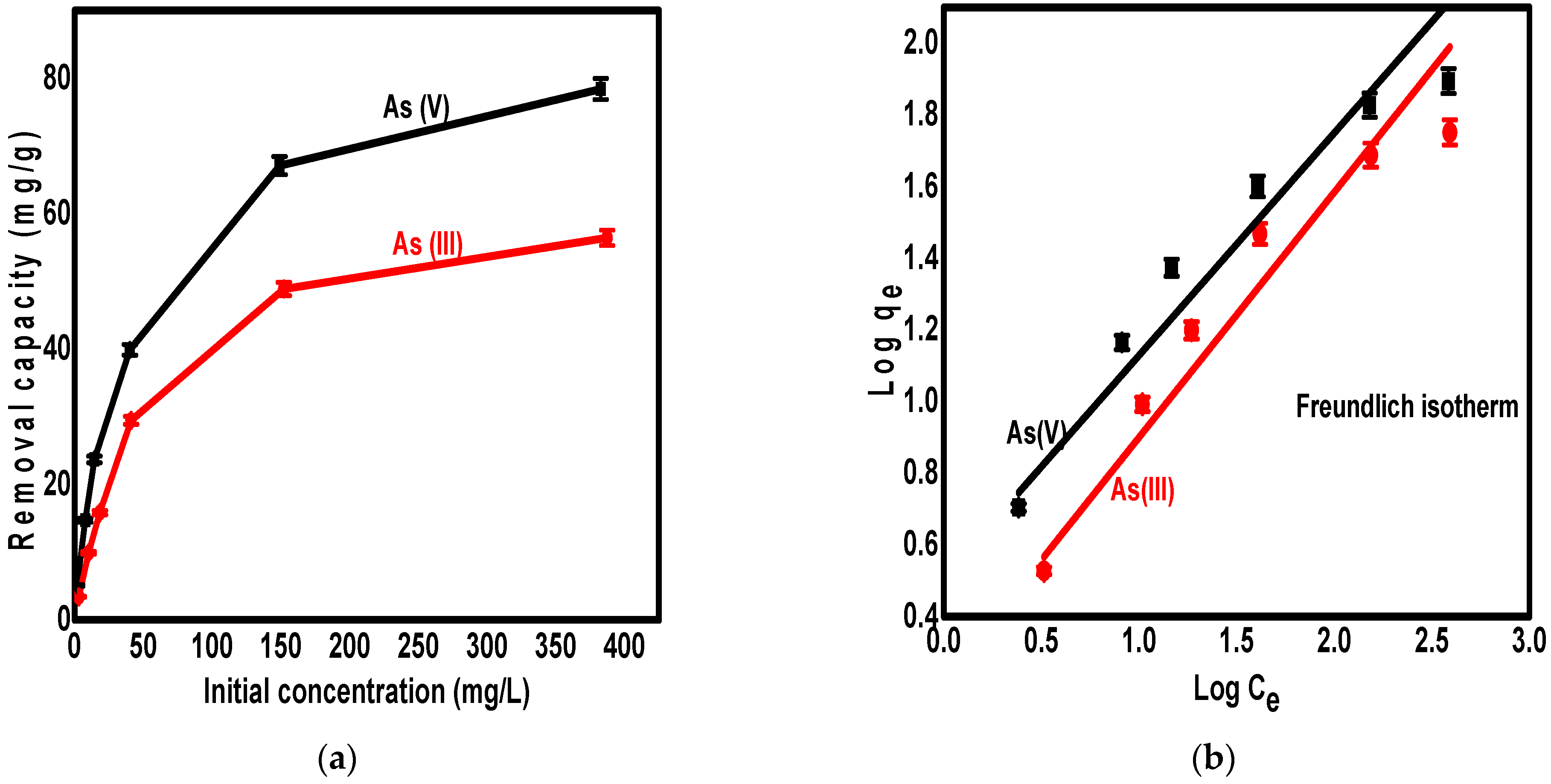
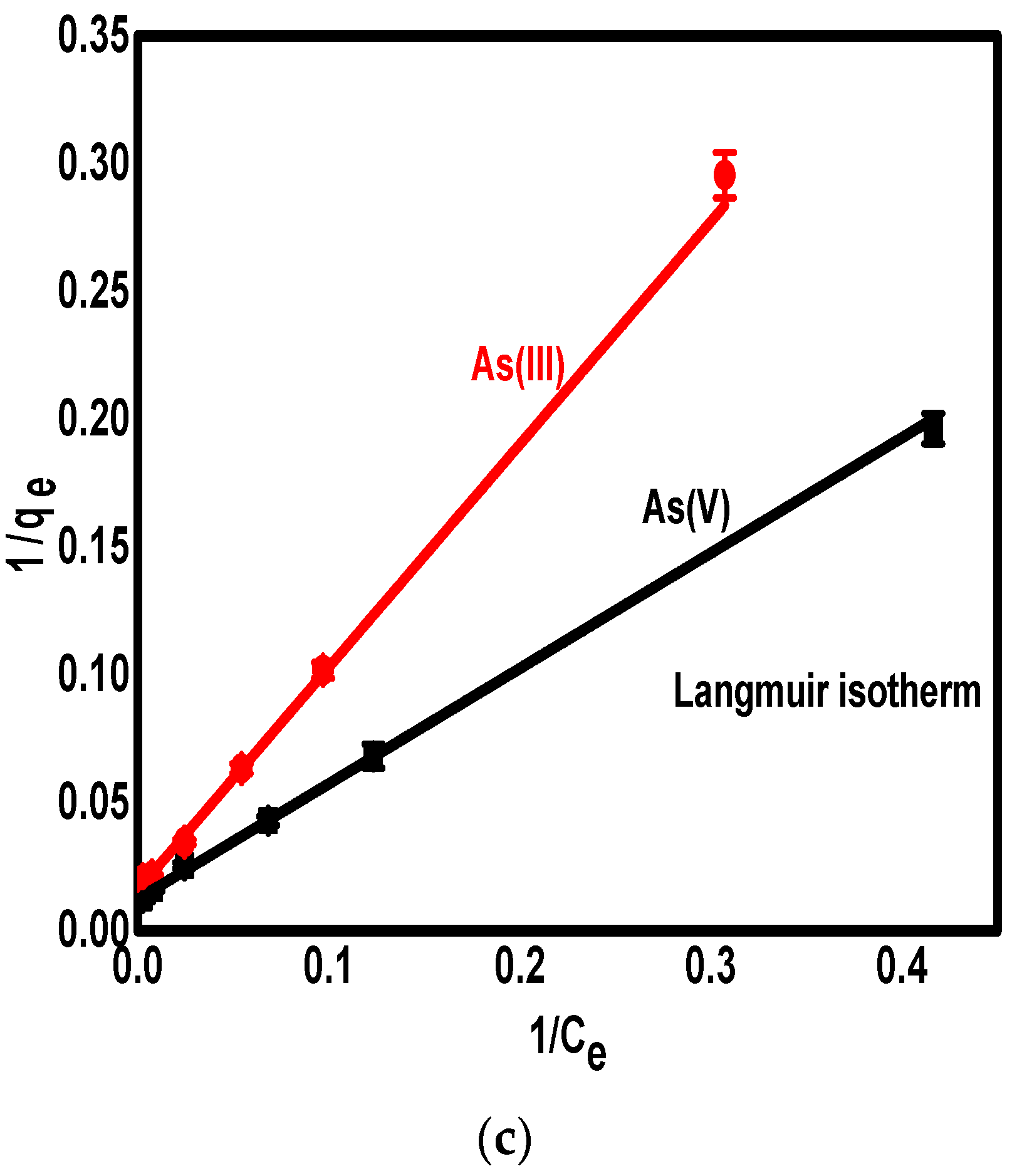
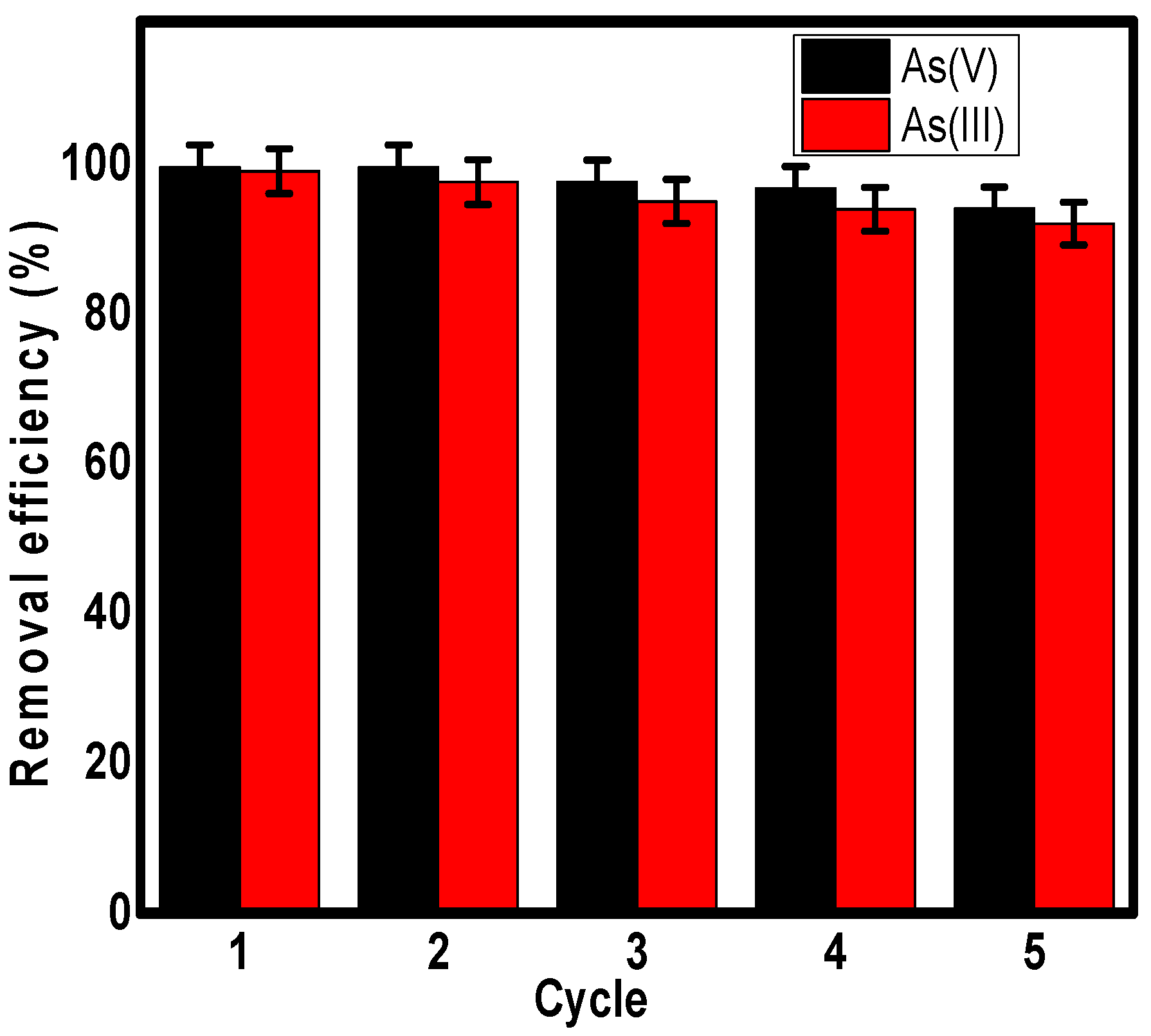
| Item | Coefficient | Pollutant | |
|---|---|---|---|
| As(III) | As(V) | ||
| Pseudo 1st order | R2 | 0.603 | 0.630 |
| k1 | −7.90 × 10−4 | −7.70 × 10−4 | |
| qe (mg/g) | 5.1 | 6.1 | |
| Pseudo 2nd order | R2 | 0.999 | 0.999 |
| K2 | 0.43 | 0.35 | |
| qe (mg/g) | 15.90 | 23.70 | |
| Freundlich’s isotherm | R2 | 0.938 | 0.943 |
| 1/n | 0.5563 | 0.5200 | |
| KF | 2.46 | 4.54 | |
| Langmuir’s isotherm | R2 | 0.999 | 0.999 |
| RL | 0.5649 | 0.4249 | |
| KL (L/mg) | 0.0158 | 0.0394 | |
| qmax (mg/g) | 77.61 | 86.50 | |
| Adsorbent | pH Value | Removal Capacity (mg/g) | Ref. | |
|---|---|---|---|---|
| As(V) | As(III) | |||
| Fe3O4/CS/PEI | 6.70 | 86.50 | 77.61 | This study |
| Fe–Mn binary oxide-impregnated granular activated carbon | 4.0 | 16.0 | 18.0 | [63] |
| Egg shell | 4.10 | 8.0 | 12.0 | [64] |
| Tea waste | 7.0 | 5.0 | 7.30 | [64] |
| MnO2-impregnated alginate beads | 6.50 | 6.50 | - | [65] |
| PEI-coated bacterial biosorbent | 4.0 | 63.0 | - | [66] |
| Magnetic pinecone biomass | 8.0 | - | 18.0 | [67] |
| Turbinaria vulgaris sp. | 4.0 | 26.0 | - | [68] |
| Iron oxide/nanoporous carbon | 8.0 | - | 6.69 | [69] |
| MIL-53(Al)-GO nanocomposites | 9.0 | - | 65.0 | [70] |
| Dust ferric hydroxide | 7.9 | 6.90 | 3.50 | [71] |
| Micro-sized tetravalent manganese feroxyhyte | 8.0 | 15.4 | - | [72] |
| Micro-sized granular ferric hydroxide | 8.0 | 22.4 | - | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaiari, N.S.; Alzahrani, F.M.; Katubi, K.M.; Amari, A.; Rebah, F.B.; Tahoon, M.A. Polyethylenimine-Modified Magnetic Chitosan for the Uptake of Arsenic from Water. Appl. Sci. 2021, 11, 5630. https://doi.org/10.3390/app11125630
Alsaiari NS, Alzahrani FM, Katubi KM, Amari A, Rebah FB, Tahoon MA. Polyethylenimine-Modified Magnetic Chitosan for the Uptake of Arsenic from Water. Applied Sciences. 2021; 11(12):5630. https://doi.org/10.3390/app11125630
Chicago/Turabian StyleAlsaiari, Norah Salem, Fatimah Mohammed Alzahrani, Khadijah Mohammedsaleh Katubi, Abdelfattah Amari, Faouzi Ben Rebah, and Mohamed A. Tahoon. 2021. "Polyethylenimine-Modified Magnetic Chitosan for the Uptake of Arsenic from Water" Applied Sciences 11, no. 12: 5630. https://doi.org/10.3390/app11125630
APA StyleAlsaiari, N. S., Alzahrani, F. M., Katubi, K. M., Amari, A., Rebah, F. B., & Tahoon, M. A. (2021). Polyethylenimine-Modified Magnetic Chitosan for the Uptake of Arsenic from Water. Applied Sciences, 11(12), 5630. https://doi.org/10.3390/app11125630









