Bioactive Compounds from Herbal Medicine Targeting Multiple Myeloma
Abstract
1. Introduction
- Proteasome inhibitors [19,20,21]. The proteasome is a multicatalytic target responsible for the degradation of the proteins. To date, there are only three proteasome inhibitors approved for clinical: ixazomib [22], bortezomib [23], and carfilzomib [24]. These drugs can stimulate osteogenesis in MM patients [25]. Proteasome modulation can also be achieved by binding lenalidomide to thalidomide and acting as E3-Ligase inhibitors [26].
- DNA damaging agents. This category includes alkylating drugs, such as melphalan, or other agents, as doxorubicin, as well as histone deacetylase inhibitors such as panobinostat [27].
- Monoclonal antibodies [31,32,33]. There are two monoclonal-based therapy approved for the treatment of MM: daratumumab (CD38 pathway) [34], and elotuzumab (targetSLAMF7 pathway) [35]. The efficacy of monoclonal antibodies is the highest, but this therapy has a high cost [6]. In 2020, the FDA approved a third monoclonal antibody for MM therapy, Sarclisa (isatuximab—irfc) in combination with pomalidomide and dexamethasone [36]. Isatuximab is a monoclonal antibody that targets the transmembrane receptor and the ectoenzyme CD38, a protein overexpressed by malignant hematological cells [37]. Isatuximab is a new MM treatment for patient’s refractory to lenalidomide and proteasome inhibitor [38].
2. Molecular Pathways Involved in Multiple Myeloma Progression
3. Flavonoids in Multiple Myeloma
3.1. Apigenin
3.2. Baicalein
3.3. Chrysoeriol
3.4. Luteolin
3.5. Scutellarin
3.6. Wogonin
3.7. Fisetin
3.8. Myricetin
3.9. Quercetin
3.10. Epigallocatechin-Gallate
3.11. Daidzin
3.12. Formononetin
3.13. Genistein
3.14. Chalcones
3.15. Butein
3.16. Cardamonin
3.17. Isobavachalcone
3.18. Isoliquiritigenin
3.19. Xanthohumol
3.20. Bavachin
3.21. Icariin
3.22. Icaritin
3.23. Icariside II
4. Plant Extracts in Multiple Myeloma
5. Conclusions and Therapeutic Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergsagel, P.L.; Kuehl, W.M. Chromosome translocations in multiple myeloma. Oncogene 2001, 20, 5611–5622. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Spaan, I.; Raymakers, R.A.; Van de Stolpe, A.; Peperzak, V. Wnt signaling in multiple myeloma: A central player in disease with therapeutic potential. J. Hematol. Oncol. 2018, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Abouzaid, S.; Bonafede, M.; Cai, Q.; Parikh, K.; Cosler, L.; Richardson, P. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia 2017, 31, 1915–1921. [Google Scholar] [CrossRef]
- Turesson, I.; Bjorkholm, M.; Blimark, C.H.; Kristinsson, S.; Velez, R.; Landgren, O. Rapidly changing myeloma epidemiology in the general population: Increased incidence, older patients, and longer survival. Eur. J. Haematol. 2018. [Google Scholar] [CrossRef]
- Malacrida, A.; Cavalloro, V.; Martino, E.; Cassetti, A.; Nicolini, G.; Rigolio, R.; Cavaletti, G.; Mannucci, B.; Vasile, F.; Giacomo, M.D.; et al. Anti-multiple myeloma potential of secondary metabolites from Hibiscus sabdariffa. Molecules 2019, 24, 2500. [Google Scholar] [CrossRef]
- Mirzaei, H.; Bagheri, H.; Ghasemi, F.; Khoi, J.M.; Pourhanifeh, M.H.; Heyden, Y.V.; Mortezapour, E.; Nikdasti, A.; Jeandet, P.; Khan, H.; et al. Anti-cancer activity of curcumin on multiple myeloma. Anticancer Agents Med. Chem. 2021, 21, 575–586. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Wang, J. Progress in the identification of gene mutations involved in multiple myeloma. Oncotargets Ther. 2019, 12, 4075–4080. [Google Scholar] [CrossRef]
- Vrábel, D.; Pour, L.; Ševčíková, S. The impact of NF-κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 2019, 34, 56–66. [Google Scholar] [CrossRef]
- Moschetta, M.; Kawano, Y.; Sacco, A.; Belotti, A.; Ribolla, R.; Chiarini, M.; Giustini, V.; Bertoli, D.; Sottini, A.; Valotti, M.; et al. Bone marrow stroma and vascular contributions to myeloma bone homing. Curr. Osteoporos. Rep. 2017, 15, 499–506. [Google Scholar] [CrossRef]
- Shupp, A.B.; Kolb, A.D.; Mukhopadhyay, D.; Bussard, K.M. Cancer metastases to bone: Concepts, mechanisms, and interactions with bone osteoblasts. Cancers 2018, 10, 182. [Google Scholar] [CrossRef]
- Mahindra, A.; Laubach, J.; Raje, N.; Munshi, N.; Richardson, P.G.; Anderson, K. Latest advances and current challenges in the treatment of multiple myeloma. Nat. Rev. Clin. Oncol. 2012, 9, 135–143. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Burwick, N.; Sharma, S. Glucocorticoids in multiple myeloma: Past, present, and future. Ann. Hematol. 2019, 98, 19–28. [Google Scholar] [CrossRef]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Annunziata, C.M.; Davis, R.E.; Demchenko, Y.; Bellamy, W.; Gabrea, A.; Zhan, F.; Lenz, G.; Hanamura, I.; Wright, G.; Xiao, W.; et al. Frequent engagement of the classical and alternative NF-kappa B pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 2007, 12, 115–130. [Google Scholar] [CrossRef]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous mutations activate the noncanonical NF-kappa B pathway in multiple myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Fan, F.; Podar, K. The role of AP-1 transcription factors in plasma cell biology and multiple myeloma pathophysiology. Cancers 2021, 13, 2326. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Rajan, A.M.; Kumar, S. New investigational drugs with single-agent activity in multiple myeloma. Blood Cancer J. 2016, 6, e451. [Google Scholar] [CrossRef]
- Moreau, P.; Richardson, P.G.; Cavo, M.; Orlowski, R.Z.; San Miguel, J.F.; Palumbo, A.; Harousseau, J.L. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012, 120, 947–959. [Google Scholar] [CrossRef]
- Shirley, M. Ixazomib: First global approval. Drugs 2016, 76, 405–411. [Google Scholar] [CrossRef]
- Guerrero-Garcia, T.A.; Mogollon, R.J.; Castillo, J.J. Bortezomib in plasmablastic lymphoma: A glimpse of hope for a hard-to-treat disease. Leuk. Res. 2017, 62, 12–16. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Terpos, E.; Kastritis, E.; Dimopoulos, M.A. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expert Opin. Pharmacother. 2017, 18, 1883–1897. [Google Scholar] [CrossRef]
- Pennisi, A.; Li, X.; Ling, W.; Khan, S.; Zangari, M.; Yaccoby, S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am. J. Hematol. 2009, 84, 6–14. [Google Scholar] [CrossRef]
- Driscoll, J. Expression of E3 ubiquitin ligases in multiple myeloma patients after treatment with the proteasome inhibitor bortezomib. Cancer Transl. Med. 2015, 1, 153. [Google Scholar] [CrossRef]
- López-Iglesias, A.A.; González-Méndez, L.; San-Segundo, L.; Herrero, A.B.; Hernández-García, S.; Martín-Sánchez, M.; Gutiérrez, N.C.; Paíno, T.; Avilés, P.; Mateos, M.V.; et al. Synergistic DNA-damaging effect in multiple myeloma with the combination of zalypsis, bortezomib and dexamethasone. Haematologica 2017, 102, 168–175. [Google Scholar] [CrossRef]
- Costa, F.; Das, R.; Bailur, J.K.; Dhodapkar, K.; Dhodapkar, M.V. Checkpoint inhibition in myeloma: Opportunities and challenges. Front. Immunol. 2018, 9, 2204. [Google Scholar] [CrossRef]
- Hoy, S.M. Pomalidomide: A review in relapsed and refractory multiple myeloma. Drugs 2017, 77, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Kocoglu, M.; Badros, A. The role of immunotherapy in multiple myeloma. Pharmaceuticals 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T. Therapeutic antibodies for multiple myeloma. Jpn. J. Clin. Oncol. 2018, 48, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.F.; Lin, L.; Xing, L.; Yu, T.; Wen, K.; Anderson, K.C.; Tai, Y.T. Monoclonal antibody: A new treatment strategy against multiple myeloma. Antibodies 2017, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Sherbenou, D.W.; Mark, T.M.; Forsberg, P. Monoclonal antibodies in multiple myeloma: A new wave of the future. Clin. Lymphoma Myeloma Leuk. 2017, 17, 545–554. [Google Scholar] [CrossRef]
- Blair, H.A. Daratumumab: A review in relapsed and/or refractory multiple myeloma. Drugs 2017, 77, 2013–2024. [Google Scholar] [CrossRef]
- Passey, C.; Sheng, J.; Mora, J.; Tendolkar, A.; Robbins, M.; Dodge, R.; Roy, A.; Bello, A.; Gupta, M. The clinical pharmacology of elotuzumab. Clin. Pharmacokinet. 2018, 57, 297–313. [Google Scholar] [CrossRef]
- US Food and Drug Administration. New Drugs Approval Report 2020. Available online: https://www.fda.gov/media/144982/download (accessed on 20 April 2021).
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.Y.; et al. Isatuximab acts through Fc-dependent, independent, and direct pathways to kill multiple myeloma cells. Front. Immunol. 2020, 11, 1771. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Gay, F.; Palumbo, A. Multiple myeloma: Management of adverse events. Med. Oncol. 2010, 27, 646–653. [Google Scholar] [CrossRef]
- Meregalli, C. An Overview of bortezomib-induced neurotoxicity. Toxics 2015, 3, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Tacchetti, P.; Terragna, C.; Galli, M.; Zamagni, E.; Petrucci, M.T.; Pezzi, A.; Montefusco, V.; Martello, M.; Tosi, P.; Baldini, L.; et al. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: Clinical and molecular analyses of a phase 3 study. Am. J. Hematol. 2014, 89, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Caivano, A.; La Rocca, F.; Laurenzana, I.; Annese, T.; Tamma, R.; Famigliari, U.; Simeon, V.; Trino, S.; De Luca, L.; Villani, O.; et al. Epha3 acts as proangiogenic factor in multiple myeloma. Oncotarget 2017, 8, 34298–34309. [Google Scholar] [CrossRef]
- La Rocca, F.; Airoldi, I.; Di Carlo, E.; Marotta, P.; Falco, G.; Simeon, V.; Laurenzana, I.; Trino, S.; De Luca, L.; Todoerti, K.; et al. EphA3 targeting reduces in vitro adhesion and invasion and in vivo growth and angiogenesis of multiple myeloma cells. Cell Oncol. 2017, 40, 483–496. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A neolignan from the Magnolia family for the prevention and treatment of cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Antiulcer properties of fruits and vegetables: A mechanism based perspective. Food Chem. Toxicol. 2017, 108, 104–119. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef]
- Karikas, G.A. Anticancer and chemopreventing natural products: Some biochemical and therapeutic aspects. J. BUON 2010, 15, 627–638. [Google Scholar]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Basu, A. Utilisation of bioactive compounds derived from waste in the food industry. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; Vuong, Q.V., Ed.; CRC Press: Boca Ratón, FL, USA, 2017; pp. 342–357. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Ballard, C.R.; Maróstica, M.R. Health benefits of flavonoids. In Bioactive Compounds: Health Benefits and Potential Applications; Segura-Campos, M.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 185–201. [Google Scholar]
- Gioxari, A.; Kogiannou, D.A.A.; Kalogeropoulos, N.; Kaliora, A.C. Phenolic compounds: Bioavailability and health effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Cambridge, MA, USA, 2016; pp. 339–345. [Google Scholar]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic phytochemicals in cancer prevention and therapy: Bioavailability versus bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Kou, X.; Han, L.; Li, X.; Xue, Z.; Zhou, F. Antioxidant and antitumor effects and immunomodulatory activities of crude and purified polyphenol extract from blueberries. Front. Chem. Sci. Eng. 2016, 10, 108–119. [Google Scholar] [CrossRef]
- Pojero, F.; Poma, P.; Spanò, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting multiple myeloma with natural polyphenols. Eur. J. Med. Chem. 2019, 180, 465–485. [Google Scholar] [CrossRef]
- Zhu, F.; Jiang, D.; Zhang, M.; Zhao, B. 2,4-Dihydroxy-3’-methoxy-4’-ethoxychalcone suppresses cell proliferation and induces apoptosis of multiple myeloma via the PI3K/akt/mTOR signaling pathway. Pharm. Biol. 2019, 57, 641–648. [Google Scholar] [CrossRef]
- Dai, Y.; Jin, S.; Li, X.; Wang, D. The involvement of Bcl-2 family proteins in AKT-regulated cell survival in cisplatin resistant epithelial ovarian cancer. Oncotarget 2017, 8, 1354–1368. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef]
- Long, F.; Wang, T.; Jia, P.; Wang, H.; Qing, Y.; Xiong, T.; He, M.; Wang, X. Anti-tumor effects of atractylenolide-I on human ovarian cancer cells. Med. Sci. Monit. 2017, 23, 571–579. [Google Scholar] [CrossRef]
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, H.; Shen, Z.; Wang, X. Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 14782–14787. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Ikeda, H.; Hideshima, T.; Fulciniti, M.; Perrone, G.; Miura, N.; Yasui, H.; Okawa, Y.; Kiziltepe, T.; Santo, L.; Vallet, S.; et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood 2010, 116, 1460–1468. [Google Scholar] [CrossRef]
- Qu, W.; Pang, C.; Xue, Y.; Zhang, Q.; Wei, X. Dihydroartemisinin inhibits the Raf/ERK/MEK and PI3K/AKT pathways in glioma cells. Oncol. Lett. 2015, 10, 3266–3270. [Google Scholar] [CrossRef]
- Liu, T.J.; Koul, D.; LaFortune, T.; Tiao, N.; Shen, R.J.; Maira, S.M.; Garcia-Echevrria, C.; Yung, W.K. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol. Cancer Ther. 2009, 8, 2204–2210. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tian, F.; Su, M.H.; Wu, M.; Huang, Y.; Hu, L.H.; Jin, L.; Zhu, X.J. BF211, a derivative of bufalin, enhances the cytocidal effects in multiple myeloma cells by inhibiting the IL-6/JAK2/STAT3 pathway. Int. Immunopharmacol. 2018, 64, 24–32. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhu, J.; Xu, J.; Ding, K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014, 450, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.Q.; Sethi, G.; Goh, B.C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Wang, C.; Kumar, A.P.; Samy, R.P.; Lim, L.H.; Wang, L.; Goh, B.C.; et al. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol. Ther. 2016, 162, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef]
- Sikka, S.; Shanmugam, M.; Kannaiyan, R.; Surana, R.; Shin, E.M.; Kumar, A.; Sethi, G.; Ahn, K. Suppression of essential pro-inflammatory signaling pathways by natural agents for the therapy of multiple myeloma. Phytochem. Rev. 2013, 13, 79–106. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Surana, R.; Shin, E.M.; Ramachandran, L.; Sethi, G.; Kumar, A.P. Targeted Inhibition of Multiple Proinflammatory Signalling Pathways for the Prevention and Treatment of Multiple Myeloma, in Multiple Myeloma—An Overview; Gupta, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 93–128. [Google Scholar] [CrossRef]
- Bharti, A.C.; Shishodia, S.; Reuben, J.M.; Weber, D.; Alexanian, R.; Raj-Vadhan, S.; Estrov, Z.; Talpaz, M.; Aggarwal, B.B. Nuclear factor-kappa B and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 2004, 103, 3175–3184. [Google Scholar] [CrossRef]
- Quintanilla-Martinez, L.; Kremer, M.; Specht, K.; Calzada-Wack, J.; Nathrath, M.; Schaich, R.; Höfler, H.; Fend, F. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am. J. Pathol. 2003, 162, 1449–1461. [Google Scholar] [CrossRef]
- Catlett-Falcone, R.; Landowski, T.H.; Oshiro, M.M.; Turkson, J.; Levitzki, A.; Savino, R.; Ciliberto, G.; Moscinski, L.; Fernández-Luna, J.L.; Nuñez, G.; et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999, 10, 105–115. [Google Scholar] [CrossRef]
- Galm, O.; Yoshikawa, H.; Esteller, M.; Osieka, R.; Herman, J.G. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood 2003, 101, 2784–2788. [Google Scholar] [CrossRef]
- Chong, P.S.Y.; Chng, W.J.; de Mel, S. STAT3: A promising therapeutic target in multiple myeloma. Cancers 2019, 11, 731. [Google Scholar] [CrossRef]
- Savvidou, I.; Khong, T.; Cuddihy, A.; McLean, C.; Horrigan, S.; Spencer, A. β-catenin inhibitor BC2059 is efficacious as monotherapy or in combination with proteasome inhibitor bortezomib in multiple myeloma. Mol. Cancer Ther. 2017, 16, 1765–1778. [Google Scholar] [CrossRef]
- Merchant, A.A.; Matsui, W. Targeting Hedgehog—A cancer stem cell pathway. Clin. Cancer Res. 2010, 16, 3130–3140. [Google Scholar] [CrossRef]
- Sukhdeo, K.; Mani, M.; Zhang, Y.; Dutta, J.; Yasui, H.; Rooney, M.D.; Carrasco, D.E.; Zheng, M.; He, H.; Tai, Y.T.; et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc. Natl. Acad. Sci. USA 2007, 104, 7516–7521. [Google Scholar] [CrossRef]
- Behrens, J.; von Kries, J.P.; Kühl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996, 382, 638–642. [Google Scholar] [CrossRef]
- Molenaar, M.; van de Wetering, M.; Oosterwegel, M.; Peterson-Maduro, J.; Godsave, S.; Korinek, V.; Roose, J.; Destrée, O.; Clevers, H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86, 391–399. [Google Scholar] [CrossRef]
- Van Andel, H.; Kocemba, K.A.; Spaargaren, M.; Pals, S.T. Aberrant Wnt signaling in multiple myeloma: Molecular mechanisms and targeting options. Leukemia 2019, 33, 1063–1075. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Tolomelli, G.; Xu, H.; Xia, J.; Wang, H.; Zhou, W.; Zhou, Y.; Das, S.; Gu, Z.; et al. RARα2 expression confers myeloma stem cell features. Blood 2013, 122, 1437–1447. [Google Scholar] [CrossRef]
- Kramps, T.; Peter, O.; Brunner, E.; Nellen, D.; Froesch, B.; Chatterjee, S.; Murone, M.; Züllig, S.; Basler, K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 2002, 109, 47–60. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, W.; Hou, L.; Gao, Y. Scutellarin circumvents chemoresistance, promotes apoptosis, and represses tumor growth by HDAC/miR-34a-mediated down-modulation of Akt/mTOR and NF-κB-orchestrated signaling pathways in multiple myeloma. Int. J. Clin. Exp. Pathol. 2020, 13, 212–219. [Google Scholar]
- Qin, W.; Wu, H.J.; Cao, L.Q.; Li, H.J.; He, C.X.; Zhao, D.; Xing, L.; Li, P.Q.; Jin, X.; Cao, H.L. Research progress on PARP14 as a drug target. Front. Pharmacol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.H.; Zhang, J.; Yu, B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Díaz, J.G.; Carmona, A.J.; Torres, F.; Quintana, J.; Estévez, F.; Herz, W. Cytotoxic activities of flavonoid glycoside acetates from Consolida oliveriana. Planta Med. 2008, 74, 171–174. [Google Scholar] [CrossRef]
- Li, Y.L.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, Y.W.; Liu, J.W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.W. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef]
- Cui, Q.; Wen, S.; Huang, P. Targeting cancer cell mitochondria as a therapeutic approach: Recent updates. Future Med. Chem. 2017, 9, 929–949. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid-membrane interactions: Implications on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P.; Djeussi, D.E.; Zeino, M.; Kwamou, G.M.; Ngadjui, B.; Efferth, T. Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Investig. New Drugs 2014, 32, 1053–1062. [Google Scholar] [CrossRef]
- Margina, D.; Ilie, M.; Manda, G.; Neagoe, I.; Mocanu, M.; Ionescu, D.; Gradinaru, D.; Ganea, C. Quercetin and epigallocatechin gallate effects on the cell membranes biophysical properties correlate with their antioxidant potential. Gen. Physiol. Biophys. 2012, 31, 47–55. [Google Scholar] [CrossRef]
- Ingólfsson, H.I.; Thakur, P.; Herold, K.F.; Hobart, E.A.; Ramsey, N.B.; Periole, X.; de Jong, D.H.; Zwama, M.; Yilmaz, D.; Hall, K.; et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 2014, 9, 1788–1798. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yang, D.H.; Chen, Z.S. Special Issue: Natural products: Anticancer and beyond. Molecules 2018, 23, 1246. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis 1986, 7, 959–963. [Google Scholar] [CrossRef]
- Ali, F.; Naz, F.; Jyoti, S.; Siddique, Y.H. Protective effect of apigenin against N-nitrosodiethylamine (NDEA)-induced hepatotoxicity in albino rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 767, 13–20. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, S.K.; Noel, S.; Sharma, S.; Rath, S.K. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS ONE 2012, 7, e31964. [Google Scholar] [CrossRef]
- Ghițu, A.; Schwiebs, A.; Radeke, H.H.; Avram, S.; Zupko, I.; Bor, A.; Pavel, I.Z.; Dehelean, C.A.; Oprean, C.; Bojin, F.; et al. A comprehensive assessment of apigenin as an antiproliferative, proapoptotic, antiangiogenic and immunomodulatory phytocompound. Nutrients 2019, 11, 858. [Google Scholar] [CrossRef]
- Chen, V.; Staub, R.E.; Baggett, S.; Chimmani, R.; Tagliaferri, M.; Cohen, I.; Shtivelman, E. Identification and analysis of the active phytochemicals from the anti-cancer botanical extract Bezielle. PLoS ONE 2012, 7, e30107. [Google Scholar] [CrossRef]
- Lefort, E.C.; Blay, J. The dietary flavonoid apigenin enhances the activities of the anti-metastatic protein CD26 on human colon carcinoma cells. Clin. Exp. Metastasis 2011, 28, 337–349. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, J.T.; Lee, M.G.; Lee, H.W.; Ahn, S.J.; Lee, Y.J.; Lee, Y.L.; Yoo, J.; Ahn, B.C.; Ha, J.H. In vitro antiproliferative characteristics of flavonoids and diazepam on SNU-C4 colorectal adenocarcinoma cells. J. Nat. Med. 2009, 63, 124–129. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 287, 914–920. [Google Scholar] [CrossRef]
- Wu, Y.X.; Fang, X. Apigenin, chrysin, and luteolin selectively inhibit chymotrypsin-like and trypsin-like proteasome catalytic activities in tumor cells. Planta Med. 2010, 76, 128–132. [Google Scholar] [CrossRef]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Zhu, H.Y.; Zhang, X.H.; Du, Z.Y.; Xu, Y.J.; Yu, X.D. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol. Cancer 2011, 10, 104. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Yeh, C.H.; Ma, K.H.; Liu, P.S.; Kuo, J.K.; Chueh, S.H. Baicalein decreases hydrogen peroxide-induced damage to NG108-15 cells via upregulation of Nrf2. J. Cell. Physiol. 2015, 230, 1840–1851. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Zhou, E.; Chen, L.; Kou, J.; Wang, J.; Yang, Z. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int. Immunopharmacol. 2015, 28, 470–476. [Google Scholar] [CrossRef]
- Michaelis, M.; Sithisarn, P.; Cinatl, J., Jr. Effects of flavonoid-induced oxidative stress on anti-H5N1 influenza a virus activity exerted by baicalein and biochanin A. BMC Res. Notes 2014, 7, 384. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tsai, K.W.; Li, Y.Z.; Chang, Y.S.; Lai, Y.C.; Laio, Y.H.; Wu, J.D.; Liu, Y.W. Anti-bladder-tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid. Based Complement. Altern. Med. 2013, 2013, 579751. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, X.; Xing, Z.; Xing, R.; Lv, R.; Cheng, X.; Su, J.; Zhou, Z.; Xu, Z.; Nilsson, S.; et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol. Cell Biochem. 2015, 406, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Guo, C.; Yang, Y.; Li, F.; Zhang, Y.; Jiang, B.; Li, Q. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol. Med. Rep. 2015, 11, 2129–2134. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Selvamani, A.; Subramanian, R.; Pandi, A.; Thiruvengadam, D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in vivo. Toxicol. Appl. Pharmacol. 2012, 261, 10–21. [Google Scholar] [CrossRef]
- Liu, X.P.; He, L.; Zhang, Q.P.; Zeng, X.T.; Liu, S.Q. Baicalein inhibits proliferation of myeloma U266 cells by downregulating IKZF1 and IKZF3. Med. Sci. Monit. 2018, 24, 2809–2817. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, L.P.; Qin, J.; Zhang, M.; Chen, Y.; Wang, D.; Li, Z.; Tang, J.Z.; Mo, S.L. Baicalein decreases side population proportion via inhibition of ABCG2 in multiple myeloma cell line RPMI 8226 in vitro. Fitoterapia 2014, 94, 21–28. [Google Scholar] [CrossRef]
- Stacy, A.E.; Jansson, P.J.; Richardson, D.R. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol. Pharmacol. 2013, 84, 655–669. [Google Scholar] [CrossRef]
- Lin, M.G.; Liu, L.P.; Li, C.Y.; Zhang, M.; Chen, Y.; Qin, J.; Gu, Y.Y.; Li, Z.; Wu, X.L.; Mo, S.L. Scutellaria extract decreases the proportion of side population cells in a myeloma cell line by down-regulating the expression of ABCG2 protein. Asian Pac. J. Cancer Prev. 2013, 14, 7179–7186. [Google Scholar] [CrossRef]
- Zhang, R.B.; He, L.; Huang, Z.; Ma, Z.; Liu, S.Q. Synergistic effect and mechanism of baicalein in combination with lenalidomide-induced apoptosis of myeloma cells. Zhonghua Xue Ye Xue Za Zhi 2013, 34, 546–547. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, Z.; Cai, H.; Li, Q.; Rong, W.; Kawano, M. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur. J. Haematol. 2010, 84, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Otsuyama, K.I.; Ma, Z.; Abroun, S.; Amin, J.; Shamsasenjan, K.; Asaoku, H.; Kawano, M.M. PPAR beta-mediated growth suppression of baicalein and dexamethasone in human myeloma cells. Leukemia 2007, 21, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Otsuyama, K.; Liu, S.; Abroun, S.; Ishikawa, H.; Tsuyama, N.; Obata, M.; Li, F.J.; Zheng, X.; Maki, Y.; et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood 2005, 105, 3312–3318. [Google Scholar] [CrossRef]
- Lin, L.Z.; Lu, S.; Harnly, J.M. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC-DAD-ESI/MS. J. Agric. Food Chem. 2007, 55, 1321–1326. [Google Scholar] [CrossRef]
- Snijman, P.W.; Swanevelder, S.; Joubert, E.; Green, I.R.; Gelderblom, W.C. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): Some dose-response effects on mutagen activation-flavonoid interactions. Mutat. Res. 2007, 631, 111–123. [Google Scholar] [CrossRef]
- Khan, A.U.; Gilani, A.H. Selective bronchodilatory effect of Rooibos tea (Aspalathus linearis) and its flavonoid, chrysoeriol. Eur. J. Nutr. 2006, 45, 463–469. [Google Scholar] [CrossRef]
- Choi, D.Y.; Lee, J.Y.; Kim, M.R.; Woo, E.R.; Kim, Y.G.; Kang, K.W. Chrysoeriol potently inhibits the induction of nitric oxide synthase by blocking AP-1 activation. J. Biomed. Sci. 2005, 12, 949–959. [Google Scholar] [CrossRef]
- Duke, J.A.; Bogenschutz, M.J.; du Cellier, J.; Duke, P.A.K. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 612–613. [Google Scholar]
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine 2020, 68, 153173. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, Y.H.; Park, S.M.; Lee, K.E.; Lee, J.J.; Lee, B.C.; Pyo, H.B.; Song, K.S.; Park, H.D.; Yun, Y.P. Antioxidants and inhibitor of matrix metalloproteinase-1 expression from leaves of Zostera marina L. Arch. Pharm. Res. 2004, 27, 177–183. [Google Scholar] [CrossRef]
- Han, L.K.; Sumiyoshi, M.; Zheng, Y.N.; Okuda, H.; Kimura, Y. Anti-obesity action of Salix matsudana leaves (Part 2). Isolation of anti-obesity effectors from polyphenol fractions of Salix matsudana. Phytother. Res. 2003, 17, 1195–1198. [Google Scholar] [CrossRef]
- Schinella, G.R.; Giner, R.M.; Recio, M.C.; Mordujovich de Buschiazzo, P.; Ríos, J.L.; Máñez, S. Anti-inflammatory effects of South American Tanacetum vulgare. J. Pharm. Pharmacol. 1998, 50, 1069–1074. [Google Scholar] [CrossRef]
- Wei, W.; He, J.; Ruan, H.; Wang, Y. In vitro and in vivo cytotoxic effects of chrysoeriol in human lung carcinoma are facilitated through activation of autophagy, sub-G1/G0 cell cycle arrest, cell migration and invasion inhibition and modulation of MAPK/ERK signalling pathway. J. BUON 2019, 24, 936–942. [Google Scholar]
- Takemura, H.; Uchiyama, H.; Ohura, T.; Sakakibara, H.; Kuruto, R.; Amagai, T.; Shimoi, K. A methoxyflavonoid, chrysoeriol, selectively inhibits the formation of a carcinogenic estrogen metabolite in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2010, 118, 70–76. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Xiao, M.; Hong, Z.; Gong, Q.; Jiang, L.; Zhou, J. Discovery of chrysoeriol, a PI3K-AKT-mTOR pathway inhibitor with potent antitumor activity against human multiple myeloma cells in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 734–740. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Sui, J.Q.; Xie, K.P.; Xie, M.J. Inhibitory effect of luteolin on the proliferation of human breast cancer cell lines induced by epidermal growth factor. Sheng Li Xue Bao 2016, 68, 27–34. [Google Scholar]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Goyette, S.; Mafuvadze, B.; Hyder, S.M. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus 2015, 4, 444. [Google Scholar] [CrossRef]
- Sun, D.W.; Zhang, H.D.; Mao, L.; Mao, C.F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.X.; Jing, C.W.; Wang, Z.; et al. Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing Notch signaling and regulating miRNAs. Cell. Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef]
- Park, S.H.; Ham, S.; Kwon, T.H.; Kim, M.S.; Lee, D.H.; Kang, J.W.; Oh, S.R.; Yoon, D.Y. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 219–231. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Han, K.; Meng, W.; Zhang, J.J.; Zhou, Y.; Wang, Y.L.; Su, Y.; Lin, S.C.; Gan, Z.H.; Sun, Y.N.; Min, D.L. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. OncoTargets Ther. 2016, 9, 3085–3094. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Kim, D.; Divya, S.P.; et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE 2012, 7, e52279. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem. Biophys. Res. Commun. 2005, 333, 833–838. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Chiao, M.T.; Liang, Y.J.; Yang, Y.C.; Shen, C.C.; Yang, C.Y. Luteolin inhibits migration of human glioblastoma U-87 MG and T98G cells through downregulation of Cdc42 expression and PI3K/AKT activity. Mol. Biol. Rep. 2013, 40, 5315–5326. [Google Scholar] [CrossRef]
- Tjioe, K.C.; Tostes Oliveira, D.; Gavard, J. Luteolin impacts on the DNA damage pathway in oral squamous cell carcinoma. Nutr. Cancer 2016, 68, 838–847. [Google Scholar] [CrossRef]
- Yang, S.F.; Yang, W.E.; Chang, H.R.; Chu, S.C.; Hsieh, Y.S. Luteolin induces apoptosis in oral squamous cancer cells. J. Dent. Res. 2008, 87, 401–406. [Google Scholar] [CrossRef]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef]
- Ou, Y.C.; Li, J.R.; Kuan, Y.H.; Raung, S.L.; Wang, C.C.; Hung, Y.Y.; Pan, P.H.; Lu, H.C.; Chen, C.J. Luteolin sensitizes human 786-O renal cell carcinoma cells to TRAIL-induced apoptosis. Life Sci. 2014, 100, 110–117. [Google Scholar] [CrossRef]
- Wu, H.; Huang, M.; Liu, Y.; Shu, Y.; Liu, P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 2015, 14, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Li, Y.H.; Xiao, X.W.; Li, X.B. Inhibitory effects of luteolin on human gastric carcinoma xenografts in nude mice and its mechanism. Zhonghua Yi Xue Za Zhi 2013, 93, 142–146. (In Chinese) [Google Scholar] [PubMed]
- Zhang, Q.; Wan, L.; Guo, Y.; Cheng, N.; Cheng, W.; Sun, Q.; Zhu, J. Radiosensitization effect of luteolin on human gastric cancer SGC-7901 cells. J. Biol. Regul. Homeost. Agents 2009, 23, 71–78. [Google Scholar] [PubMed]
- Xu, H.; Yang, T.; Liu, X.; Tian, Y.; Chen, X.; Yuan, R.; Su, S.; Lin, X.; Du, G. Luteolin synergizes the antitumor effects of 5-fluorouracil against human hepatocellular carcinoma cells through apoptosis induction and metabolism. Life Sci. 2016, 144, 138–147. [Google Scholar] [CrossRef]
- Lee, L.T.; Huang, Y.T.; Hwang, J.J.; Lee, P.P.; Ke, F.C.; Nair, M.P.; Kanadaswam, C.; Lee, M.T. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002, 22, 1615–1627. [Google Scholar]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.F.; Wang, J.F.; Zhou, S.; Fang, F. Effects of luteolin on proliferation and programmed cell death of human multiple myeloma cell RPMI-8226. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 1425–1429. (In Chinese) [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhu, Y.; Li, X.F.; Wang, X.Q.; Tang, L.P.; Su, Z.Q.; Li, C.Y.; Zheng, G.J.; Feng, B. Scutellarin increases cisplatin-induced apoptosis and autophagy to overcome cisplatin resistance in non-small cell lung cancer via ERK/p53 and c-met/AKT signaling pathways. Front. Pharmacol. 2018, 9, 92. [Google Scholar] [CrossRef]
- Cao, P.; Liu, B.; Du, F.; Li, D.; Wang, Y.; Yan, X.; Li, X.; Li, Y. Scutellarin suppresses proliferation and promotes apoptosis in A549 lung adenocarcinoma cells via AKT/mTOR/4EBP1 and STAT3 pathways. Thorac. Cancer 2019, 10, 492–500. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Wu, J.; Qi, F.; Wang, H.; Wang, Z.; Xu, Z. Scutellarin protects cardiomyocyte ischemia-reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 2016, 157, 200–207. [Google Scholar] [CrossRef]
- Yuan, Y.; Zha, H.; Rangarajan, P.; Ling, E.A.; Wu, C. Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia. BMC Neurosci. 2014, 15, 125. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Han, J.; Zhou, M.; Ren, H.; Pan, Q.; Zheng, C.; Zheng, Q. Neuroprotective effect of Scutellarin on ischemic cerebral injury by down-regulating the expression of angiotensin-converting enzyme and AT1 receptor. PLoS ONE 2016, 11, e0146197. [Google Scholar] [CrossRef]
- Ke, Y.; Bao, T.; Wu, X.; Tang, H.; Wang, Y.; Ge, J.; Fu, B.; Meng, X.; Chen, L.; Zhang, C.; et al. Scutellarin suppresses migration and invasion of human hepatocellular carcinoma by inhibiting the STAT3/Girdin/Akt activity. Biochem. Biophys. Res. Commun. 2017, 483, 509–515. [Google Scholar] [CrossRef]
- Zhu, P.T.; Mao, M.; Liu, Z.G.; Tao, L.; Yan, B.C. Scutellarin suppresses human colorectal cancer metastasis and angiogenesis by targeting ephrinb2. Am. J. Transl. Res. 2017, 9, 5094–5104. [Google Scholar]
- Li, H.; Huang, D.; Gao, Z.; Chen, Y.; Zhang, L.; Zheng, J. Scutellarin inhibits the growth and invasion of human tongue squamous carcinoma through the inhibition of matrix metalloproteinase-2 and -9 and αvβ6 integrin. Int. J. Oncol. 2013, 42, 1674–1681. [Google Scholar] [CrossRef]
- Misso, G.; Zarone, M.R.; Lombardi, A.; Grimaldi, A.; Cossu, A.M.; Ferri, C.; Russo, M.; Vuoso, D.C.; Luce, A.; Kawasaki, H.; et al. miR-125b upregulates miR-34a and sequentially activates stress adaption and cell death mechanisms in multiple myeloma. Mol. Ther. Nucleic Acids 2019, 16, 391–406. [Google Scholar] [CrossRef]
- Baumann, S.; Fas, S.C.; Giaisi, M.; Müller, W.W.; Merling, A.; Gülow, K.; Edler, L.; Krammer, P.H.; Li-Weber, M. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood 2008, 111, 2354–2363. [Google Scholar] [CrossRef]
- Piao, H.Z.; Jin, S.A.; Chun, H.S.; Lee, J.C.; Kim, W.K. Neuroprotective effect of wogonin: Potential roles of inflammatory cytokines. Arch. Pharm. Res. 2004, 27, 930–936. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.O.; Kim, H.; Kim, S.Y.; Noh, H.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Suk, K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003, 17, 1943–1944. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, C.; Zhang, L.; Lee, Y.J. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem. Pharmacol. 2008, 75, 2020–2033. [Google Scholar] [CrossRef]
- Himeji, M.; Ohtsuki, T.; Fukazawa, H.; Tanaka, M.; Yazaki, S.; Ui, S.; Nishio, K.; Yamamoto, H.; Tasaka, K.; Mimura, A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007, 245, 269–274. [Google Scholar] [CrossRef]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Chang, Y.L.; Shen, J.J.; Wung, B.S.; Cheng, J.J.; Wang, D.L. Chinese herbal remedy wogonin inhibits monocyte chemotactic protein-1 gene expression in human endothelial cells. Mol. Pharmacol. 2001, 60, 507–513. [Google Scholar]
- Chi, Y.S.; Cheon, B.S.; Kim, H.P. Effect of wogonin, a plant flavone from Scutellaria radix, on the suppression of cyclooxygenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW 264.7 cells. Biochem. Pharmacol. 2001, 61, 1195–1203. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Kim, S.Y.; Suk, K. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci. Lett. 2001, 309, 67–71. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Yasui, K. Wogonin inhibits inducible prostaglandin E(2) production in macrophages. Eur. J. Pharmacol. 2000, 406, 477–481. [Google Scholar] [CrossRef]
- Huynh, D.L.; Kwon, T.; Zhang, J.J.; Sharma, N.; Gera, M.; Ghosh, M.; Kim, N.; Cho, S.; Lee, D.S.; Park, Y.H.; et al. Wogonin suppresses stem cell-like traits of CD133 positive osteosarcoma cell via inhibiting matrix metallopeptidase-9 expression. BMC Complement. Altern. Med. 2017, 17, 304. [Google Scholar] [CrossRef]
- Huynh, D.L.; Sharma, N.; Kumar Singh, A.; Singh Sodhi, S.; Zhang, J.J.; Mongre, R.K.; Ghosh, M.; Kim, N.; Ho, P.Y.; Kee Jeong, D. Anti-tumor activity of wogonin, an extract from Scutellaria baicalensis, through regulating different signaling pathways. Chin. J. Nat. Med. 2017, 15, 15–40. [Google Scholar] [CrossRef]
- Enomoto, R.; Koshiba, C.; Suzuki, C.; Lee, E. Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. Cancer Chemother. Pharmacol. 2011, 67, 1063–1072. [Google Scholar] [CrossRef]
- Bhaskar, A.; Gupta, R.; Vishnubhatla, S.; Kumar, L.; Sharma, A.; Sharma, M.C.; Das, P.; Thakur, S.C. Angiopoietins as biomarker of disease activity and response to therapy in multiple myeloma. Leuk. Lymphoma 2013, 54, 1473–1478. [Google Scholar] [CrossRef]
- Fan, G.C. Hypoxic exosomes promote angiogenesis. Blood 2014, 124, 3669–3670. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chen, Y.; Wang, X.P.; An, T.; Tao, L.; Zhou, Y.X.; Huang, Y.J.; Chen, B.A.; Li, Z.Y.; You, Q.D.; et al. Wogonin inhibits multiple myeloma-stimulated angiogenesis via c-Myc/VHL/HIF-1α signaling axis. Oncotarget 2016, 7, 5715–5727. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yao, J.; Wang, F.; Zhou, M.; Zhou, Y.; Wang, H.; Wei, L.; Zhao, L.; Li, Z.; Lu, N.; et al. Wogonin inhibits tumor angiogenesis via degradation of HIF-1α protein. Toxicol. Appl. Pharmacol. 2013, 271, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sattler, M.; Tonon, G.; Grabher, C.; Lababidi, S.; Zimmerhackl, A.; Raab, M.S.; Vallet, S.; Zhou, Y.; Cartron, M.A.; et al. Targeting angiogenesis via a c-Myc/hypoxia-inducible factor-1alpha-dependent pathway in multiple myeloma. Cancer Res. 2009, 69, 5082–5090. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.P.; Chen, Y.; Tian, X.Y.; Qin, J.; Wang, D.; Li, Z.; Mo, S.L. Wogonin induces apoptosis in RPMI 8226, a human myeloma cell line, by downregulating phospho-Akt and overexpressing Bax. Life Sci. 2013, 92, 55–62. [Google Scholar] [CrossRef]
- Brazil, D.P.; Park, J.; Hemmings, B.A. PKB binding proteins. Getting in on the Akt. Cell 2002, 111, 293–303. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Demchuk, O.M. New perspectives for fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef]
- Jash, S.K.; Mondal, S. Bioactive flavonoid fisetin—A molecule of pharmacological interest. Signpost Open Access J. Org. Biomol. Chem. 2014, 2, 89–128. [Google Scholar]
- Harborne, J.B. Progress in the chemistry of organic natural products. Am. Chem. Soc. 1975. [Google Scholar] [CrossRef]
- Kimira, M.; Arai, Y.; Shimoi, K.; Watanabe, S. Japanese intake of flavonoids and isoflavonoids from foods. J. Epidemiol. 1998, 8, 168–175. [Google Scholar] [CrossRef]
- Jang, K.Y.; Jeong, S.J.; Kim, S.H.; Jung, J.H.; Kim, J.H.; Koh, W.; Chen, C.Y.; Kim, S.H. Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett. 2012, 319, 197–202. [Google Scholar] [CrossRef]
- Sung, B.; Pandey, M.K.; Aggarwal, B.B. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol. Pharmacol. 2007, 71, 1703–1714. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef]
- Mohtar, N.; Taylor, K.M.; Sheikh, K.; Somavarapu, S. Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin. Eur. J. Pharm. Biopharm. 2017, 113, 1–10. [Google Scholar] [CrossRef]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and α-glucosidase inhibition activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 594–602. [Google Scholar] [CrossRef]
- Sowa, M.; Ślepokura, K.; Matczak-Jon, E. Improving solubility of fisetin by cocrystallization. CrystEngComm 2014, 16, 10592–10601. [Google Scholar] [CrossRef]
- Sowa, M.; Ślepokura, K.; Matczak-Jon, E. Cocrystals of fisetin, luteolin and genistein with pyridinecarboxamide coformers: Crystal structures, analysis of intermolecular interactions, spectral and thermal characterization. CrystEngComm 2013, 15, 7696. [Google Scholar] [CrossRef]
- Sengupta, B.; Banerjee, A.; Sengupta, P.K. Investigations on the binding and antioxidant properties of the plant flavonoid fisetin in model biomembranes. FEBS Lett. 2004, 570, 77–81. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, V.D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- De Whalley, C.V.; Rankin, S.M.; Hoult, J.R.; Jessup, W.; Leake, D.S. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem. Pharmacol. 1990, 39, 1743–1750. [Google Scholar] [CrossRef]
- Lee, J.D.; Huh, J.E.; Jeon, G.; Yang, H.R.; Woo, H.S.; Choi, D.Y.; Park, D.S. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009, 9, 268–276. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Khusro, F.H.; Mustafa Adhami, V.; Suh, Y.; Mukhtar, H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer 2012, 130, 1695–1705. [Google Scholar] [CrossRef]
- Suh, Y.; Afaq, F.; Johnson, J.J.; Mukhtar, H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis 2009, 30, 300–307. [Google Scholar] [CrossRef]
- Maurya, B.K.; Trigun, S.K. Fisetin modulates antioxidant enzymes and inflammatory factors to inhibit aflatoxin-B1 induced hepatocellular carcinoma in rats. Oxid. Med. Cell. Longev. 2016, 2016, 1972793. [Google Scholar] [CrossRef]
- Ravichandran, N.; Suresh, G.; Ramesh, B.; Siva, G.V. Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem. Toxicol. 2011, 49, 1141–1147. [Google Scholar] [CrossRef]
- Pal, H.C.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFκB signaling pathways in SKH-1 hairless mice. Photochem. Photobiol. 2015, 91, 225–234. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hewage, S.R.K.M.; Ryu, Y.S.; Oh, M.C.; Kwon, T.K.; Chae, S.; Hyun, J.W. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the MAPK signaling pathway. Tumour Boil. 2016, 37, 9615–9624. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.S.; Hammarback, J.A. Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J. Biol. Chem. 1994, 269, 11492–11497. [Google Scholar] [CrossRef]
- Inkielewicz-Stepniak, I.; Radomski, M.W.; Wozniak, M. Fisetin prevents fluoride and dexamethasone-induced oxidative damage in osteoblast and hippocampal cells. Food Chem. Toxicol. 2012, 50, 583–589. [Google Scholar] [CrossRef]
- Liao, Y.C.; Shih, Y.W.; Chao, C.H.; Lee, X.Y.; Chiang, T.A. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J. Agric. Food Chem. 2009, 57, 8933–8941. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.; Ha, J.H.; Lee, M.G.; Kim, J.E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Sechi, M.; Mukhtar, H. Dietary flavonoid fisetin binds to β-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015, 367, 173–183. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Meng, X.; Ou, L.; Ip, M.M. Activation of the AMP-activated protein kinase-p38 MAP kinase pathway mediates apoptosis induced by conjugated linoleic acid in p53-mutant mouse mammary tumor cells. Cell. Signal. 2010, 22, 590–599. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.K.; Kim, B.S.; Lee, S.H.; Park, Y.S.; Park, B.K.; Kim, S.J.; Kim, J.; Choi, C.; Kim, J.S.; et al. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef]
- Zhang, W.B.; Wang, Z.; Shu, F.; Jin, Y.H.; Liu, H.Y.; Wang, Q.J.; Yang, Y. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J. Biol. Chem. 2010, 285, 40461–40471. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Zhao, M.; Wang, Y.; Cheng, X.; Wang, D.; Xu, Y.; Du, Z.; Yu, X. Celastrol targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent cytotoxicity in tumor cells. BMC Cancer 2011, 11, 170. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.J.; Kwon, T.R.; Yun, S.M.; Jung, J.H.; Kim, M.; Lee, H.J.; Lee, M.H.; Ko, S.G.; Chen, C.Y.; et al. Cryptotanshinone enhances TNF-α-induced apoptosis in chronic myeloid leukemia KBM-5 cells. Apoptosis 2011, 16, 696–707. [Google Scholar] [CrossRef]
- Afroze, N.; Pramodh, S.; Hussain, A.; Waleed, M.; Vakharia, K. A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech 2020, 10, 211. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Q.; Wu, S.; Yi, D.; Yu, Y.; Liu, S.; Li, S.; Li, Z. Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells. Mol. Med. Rep. 2016, 13, 2094–2100. [Google Scholar] [CrossRef]
- Zang, W.; Wang, T.; Wang, Y.; Li, M.; Xuan, X.; Ma, Y.; Du, Y.; Liu, K.; Dong, Z.; Zhao, G. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumour Biol. 2014, 35, 12583–12592. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, S.Y.; Tang, L.; Shen, Y.Z.; Luo, L.; Xu, C.W.; Liu, Q.; Li, D. Myricetin induces apoptosis in HepG2 cells through Akt/p70S6K/bad signaling and mitochondrial apoptotic pathway. Anticancer Agents Med. Chem. 2013, 13, 1575–1581. [Google Scholar] [CrossRef]
- Phillips, P.A.; Sangwan, V.; Borja-Cacho, D.; Dudeja, V.; Vickers, S.M.; Saluja, A.K. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011, 308, 181–188. [Google Scholar] [CrossRef]
- Akhtar, S.; Najafzadeh, M.; Isreb, M.; Newton, L.; Gopalan, R.C.; Anderson, D. Anticancer potential of myricetin bulk and nano forms in vitro in lymphocytes from myeloma patients. Arch. Toxicol. 2021, 95, 337–343. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Int. J. Med. 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.W.; Li, Y.H.; Wu, G.; Ren, J.Z.; Lu, H.B.; Li, Z.M.; Han, X.W. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Deng, X.H.; Song, H.Y.; Zhou, Y.F.; Yuan, G.Y.; Zheng, F.J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 2013, 6, 1155–1158. [Google Scholar] [CrossRef]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24-) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Interactions of quercetin with receptor tyrosine kinases associated with human lung carcinoma. Nat. Prod. Res. 2018, 32, 2928–2931. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.I.; Yun, J.H.; Kim, J.H. Quercetin 3-O-glucoside suppresses epidermal growth factor-induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumour Biol. 2015, 36, 9385–9393. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Chen, D.; Ma, Q.; Zheng, Y.; Liao, S.; Wang, Y.; Zhang, J. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol. Int. 2019, 43, 117–124. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 2017, 22, 544–557. [Google Scholar] [CrossRef]
- Huang, D.Y.; Dai, Z.R.; Li, W.M.; Wang, R.G.; Yang, S.M. Inhibition of EGF expression and NF-κB activity by treatment with quercetin leads to suppression of angiogenesis in nasopharyngeal carcinoma. Saudi J. Biol. Sci. 2018, 25, 826–831. [Google Scholar] [CrossRef]
- Polukonova, N.V.; Navolokin, N.A.; Bucharskaya, A.B.; Mudrak, D.A.; Baryshnikova, M.A.; Stepanova, E.V.; Solomko, E.S.; Polukonova, A.V.; Maslyakova, G.N. The apoptotic activity of flavonoid-containing Gratiola officinalis extract in cell cultures of human kidney cancer. Russ. Open Med. J. 2018. [Google Scholar] [CrossRef]
- Li, S.Z.; Qiao, S.F.; Zhang, J.H.; Li, K. Quercetin increase the chemosensitivity of breast cancer cells to doxorubicin via PTEN/Akt pathway. Anticancer Agents Med. Chem. 2015, 15, 1185–1189. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, H.; Hu, Z.; Huang, Y.; Yao, F.; Sun, S.; Wu, B. Quercetin inhibits proliferation and drug resistance in KB/VCR oral cancer cells and enhances its sensitivity to vincristine. Nutr. Cancer 2015, 67, 126–136. [Google Scholar] [CrossRef]
- Xavier, C.P.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef]
- Xu, Y.W.; Zou, L.F.; Li, F. Effect of quercetin on proliferation and apoptosis of multiple myeloma cells and its related mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 1234–1239. (In Chinese) [Google Scholar] [CrossRef]
- He, D.; Guo, X.; Zhang, E.; Zi, F.; Chen, J.; Chen, Q.; Lin, X.; Yang, L.; Li, Y.; Wu, W.; et al. Quercetin induces cell apoptosis of myeloma and displays a synergistic effect with dexamethasone in vitro and in vivo xenograft models. Oncotarget 2016, 7, 45489–45499. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, Z.; Huang, J.; Zhou, S.; Ye, H.; Jiang, S.; Yu, K. Quercetin suppresses the proliferation of multiple myeloma cells by down-regulating IQ motif-containing GTPase activating protein 1 expression and extracellular signal-regulated kinase activation. Leuk. Lymphoma 2014, 55, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed. Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Elbling, L.; Herbacek, I.; Weiss, R.M.; Jantschitsch, C.; Micksche, M.; Gerner, C.; Pangratz, H.; Grusch, M.; Knasmüller, S.; Berger, W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radic. Biol. Med. 2010, 49, 1444–1452. [Google Scholar] [CrossRef]
- Xu, Y.; Ho, C.T.; Amin, S.G.; Han, C.; Chung, F.L. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992, 52, 3875–3879. [Google Scholar]
- Braicu, C.; Gherman, C.D.; Irimie, A.; Berindan-Neagoe, I. Epigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J. Nanosci. Nanotechnol. 2013, 13, 632–637. [Google Scholar] [CrossRef]
- Shankar, S.; Marsh, L.; Srivastava, R.K. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol. Cell Biochem. 2013, 372, 83–94. [Google Scholar] [CrossRef]
- Mantena, S.K.; Roy, A.M.; Katiyar, S.K. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem. Photobiol. 2005, 81, 1174–1179. [Google Scholar] [CrossRef]
- Jung, Y.D.; Kim, M.S.; Shin, B.A.; Chay, K.O.; Ahn, B.W.; Liu, W.; Bucana, C.D.; Gallick, G.E.; Ellis, L.M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef]
- Koh, Y.W.; Choi, E.C.; Kang, S.U.; Hwang, H.S.; Lee, M.H.; Pyun, J.; Park, R.; Lee, Y.; Kim, C.H. Green tea (-)-epigallocatechin-3-gallate inhibits HGF-induced progression in oral cavity cancer through suppression of HGF/c-Met. J. Nutr. Biochem. 2011, 22, 1074–1083. [Google Scholar] [CrossRef]
- Kwak, I.H.; Shin, Y.H.; Kim, M.; Cha, H.Y.; Nam, H.J.; Lee, B.S.; Chaudhary, S.C.; Pai, K.S.; Lee, J.H. Epigallocatechin-3-gallate inhibits paracrine and autocrine hepatocyte growth factor/scatter factor-induced tumor cell migration and invasion. Exp. Mol. Med. 2011, 43, 111–120. [Google Scholar] [CrossRef]
- Kushima, Y.; Iida, K.; Nagaoka, Y.; Kawaratani, Y.; Shirahama, T.; Sakaguchi, M.; Baba, K.; Hara, Y.; Uesato, S. Inhibitory effect of (-)-epigallocatechin and (-)-epigallocatechin gallate against heregulin beta1-induced migration/invasion of the MCF-7 breast carcinoma cell line. Biol. Pharm. Bull. 2009, 32, 899–904. [Google Scholar] [CrossRef]
- Lim, Y.C.; Park, H.Y.; Hwang, H.S.; Kang, S.U.; Pyun, J.H.; Lee, M.H.; Choi, E.C.; Kim, C.H. (-)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 2008, 271, 140–152. [Google Scholar] [CrossRef]
- Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Cancer chemoprevention by phytochemicals: Nature’s healing touch. Molecules 2017, 22, 395. [Google Scholar] [CrossRef]
- Jin, G.; Yang, Y.; Liu, K.; Zhao, J.; Chen, X.; Liu, H.; Bai, R.; Li, X.; Jiang, Y.; Zhang, X.; et al. Combination curcumin and (-)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis 2017, 6, e384. [Google Scholar] [CrossRef]
- Chan, M.M.; Chen, R.; Fong, D. Targeting cancer stem cells with dietary phytochemical—Repositioned drug combinations. Cancer Lett. 2018, 433, 53–64. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Zhu, K. SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J. Exp. Clin. Cancer Res. 2018, 37, 37. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.Y. The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumour Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef]
- Mayr, C.; Wagner, A.; Neureiter, D.; Pichler, M.; Jakab, M.; Illig, R.; Berr, F.; Kiesslich, T. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement. Altern. Med. 2015, 15, 194. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Nazari Soltan Ahmad, S.; Barghi, S.; Maroufi, N.F.; Taheri, R.A. Improved anticancer effects of epigallocatechin gallate using rgd-containing nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Yuan, X.; He, Y.; Zhou, G.; Li, X.; Feng, A.; Zheng, W. Target challenging-cancer drug delivery to gastric cancer tissues with a fucose graft epigallocatechin-3-gallate-gold particles nanocomposite approach. J. Photochem. Photobiol. 2018, 183, 147–153. [Google Scholar] [CrossRef]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (-)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (-)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]
- Luo, K.W.; Lung, W.Y.; Chun, X.; Luo, X.L.; Huang, W.R. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget 2018, 9, 12261–12272. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Gu, J.J.; Qiao, K.S.; Sun, P.; Chen, P.; Li, Q. Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4557–4563. [Google Scholar] [CrossRef]
- Olotu, F.A.; Agoni, C.; Adeniji, E.; Abdullahi, M.; Soliman, M.E. Probing gallate-mediated selectivity and high-affinity binding of epigallocatechin gallate: A way-forward in the design of selective inhibitors for anti-apoptotic Bcl-2 proteins. Appl. Biochem. Biotechnol. 2019, 187, 1061–1080. [Google Scholar] [CrossRef]
- Velavan, B.; Divya, T.; Sureshkumar, A.; Sudhandiran, G. Nano-chemotherapeutic efficacy of (-)-epigallocatechin 3-gallate mediating apoptosis in A549 cells: Involvement of reactive oxygen species mediated Nrf2/Keap1signaling. Biochem. Biophys. Res. Commun. 2018, 503, 1723–1731. [Google Scholar] [CrossRef]
- Shammas, M.A.; Neri, P.; Koley, H.; Batchu, R.B.; Bertheau, R.C.; Munshi, V.; Prabhala, R.; Fulciniti, M.; Tai, Y.T.; Treon, S.P.; et al. Specific killing of multiple myeloma cells by (-)-epigallocatechin-3-gallate extracted from green tea: Biologic activity and therapeutic implications. Blood 2006, 108, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Fujimura, Y.; Hidaka, S.; Kim, Y.; Murayama, K.; Takai, M.; Huang, Y.; Yamashita, S.; Murata, M.; Miura, D.; et al. Metabolic profiling-based data-mining for an effective chemical combination to induce apoptosis of cancer cells. Sci. Rep. 2015, 5, 9474. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Hirotsu, K.; Kumazoe, M.; Goto, Y.; Sugihara, K.; Suda, T.; Tsurudome, Y.; Suzuki, T.; Yamashita, S.; Kim, Y.; et al. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 2012, 443, 525–534. [Google Scholar] [CrossRef]
- Zhou, C.G.; Hui, L.M.; Luo, J.M. Epigallocatechin gallate inhibits the proliferation and induces apoptosis of multiple myeloma cells via inactivating EZH2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2093–2098. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Gu, J.; Huang, B.; Zhao, Y.; Zheng, D.; Ding, Y.; Zeng, L. Potentiation of (-)-epigallocatechin-3-gallate-induced apoptosis by bortezomib in multiple myeloma cells. Acta Biochim. Biophys. Sin. 2009, 41, 1018–1026. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Huang, Y.; Kumazoe, M.; Lesnick, C.; Yamada, S.; Ueda, N.; Suzuki, T.; Yamashita, S.; Kim, Y.H.; Fujimura, Y.; et al. Sphingosine kinase-1 protects multiple myeloma from apoptosis driven by cancer-specific inhibition of RTKs. Mol. Cancer Ther. 2015, 14, 2303–2312. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017, 7, 6665. [Google Scholar] [CrossRef]
- James, K.D.; Kennett, M.J.; Lambert, J.D. Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2018, 111, 302–309. [Google Scholar] [CrossRef]
- Church, R.J.; Gatti, D.M.; Urban, T.J.; Long, N.; Yang, X.; Shi, Q.; Eaddy, J.S.; Mosedale, M.; Ballard, S.; Churchill, G.A.; et al. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Takeuchi, C.; Hidaka, S.; Fujimura, Y.; Tachibana, H. Epigallocatechin-3-O-gallate induces acid sphingomyelinase activation through activation of phospholipase C. Biochem. Biophys. Res. Commun. 2019, 520, 186–191. [Google Scholar] [CrossRef]
- Xiao, F.; Cui, H.; Zhong, X. Beneficial effect of daidzin in dry eye rat model through the suppression of inflammation and oxidative stress in the cornea. Saudi J. Biol. Sci. 2018, 25, 832–837. [Google Scholar] [CrossRef]
- Isoda, H.; Talorete, T.P.; Kimura, M.; Maekawa, T.; Inamori, Y.; Nakajima, N.; Seki, H. Phytoestrogens genistein and daidzin enhance the acetylcholinesterase activity of the rat pheochromocytoma cell line PC12 by binding to the estrogen receptor. Cytotechnology 2002, 40, 117–123. [Google Scholar] [CrossRef]
- Hua, F.; Li, C.H.; Chen, X.G.; Liu, X.P. Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the Raf/MEK/ERK cascade. Int. J. Mol. Med. 2018, 41, 3485–3492. [Google Scholar] [CrossRef]
- Kato, K.; Takahashi, S.; Cui, L.; Toda, T.; Suzuki, S.; Futakuchi, M.; Sugiura, S.; Shirai, T. Suppressive effects of dietary genistin and daidzin on rat prostate carcinogenesis. Jpn. J. Cancer Res. 2000, 91, 786–791. [Google Scholar] [CrossRef]
- Pyo, Y.H.; Seong, K.S. Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J. Agric. Food Chem. 2009, 57, 8617–8622. [Google Scholar] [CrossRef]
- Yusakul, G.; Sakamoto, S.; Juengwatanatrakul, T.; Putalun, W.; Tanaka, H.; Morimoto, S. Preparation and application of a monoclonal antibody against the isoflavone glycoside daidzin using a mannich reaction-derived hapten conjugate. Phytochem. Anal. 2016, 27, 81–88. [Google Scholar] [CrossRef]
- Choi, H.; Tostes, R.C.; Webb, R.C. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J. Am. Soc. Hypertens. 2011, 5, 154–160. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, J.C.; Sui, S.F.; Yang, M.S. Effect of daidzin, genistin, and glycitin on osteogenic and adipogenic differentiation of bone marrow stromal cells and adipocytic transdifferentiation of osteoblasts. Acta Pharmacol. Sin. 2005, 26, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Y.; Shi, Z.; Everaert, N.; Ren, G. Synergistic effect of bioactive anticarcinogens from soybean on anti-proliferative activity in MDA-MB-231 and MCF-7 human breast cancer cells in vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Vemuri, S.; Alsahafi, S.; Castillo, R.; Cheriyath, V. Glycone-rich soy isoflavone extracts promote estrogen receptor positive breast cancer cell growth. Nutr. Cancer 2016, 68, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Jung, S.H.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Attenuation of STAT3 signaling cascade by daidzin can enhance the apoptotic potential of bortezomib against multiple myeloma. Biomolecules 2019, 10, 23. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Ko, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Formononetin regulates multiple oncogenic signaling cascades and enhances sensitivity to bortezomib in a multiple myeloma mouse model. Biomolecules 2019, 9, 262. [Google Scholar] [CrossRef]
- Sun, D.; Lu, Y.; Zhang, S.J.; Wang, K.G.; Sun, Z. Research on the effect of formononetin on photodynamic therapy in K562 cells. Gen. Physiol. Biophys. 2017, 36, 423–430. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Li, Z.; Yan, H.; Qin, J.; Li, T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct. 2017, 8, 1061–1066. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Mo, Z.; Huang, W.; Yan, H.; Ling, Z.; Ye, Y. Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell. Physiol. Biochem. 2014, 34, 1351–1358. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Ai, X.; Cheng, B.; Lu, S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8453–8461. [Google Scholar]
- Wang, H.; Zhang, D.; Ge, M.; Li, Z.; Jiang, J.; Li, Y. Formononetin inhibits enterovirus 71 replication by regulating COX-2/PGE2 expression. Virol. J. 2015, 12, 35. [Google Scholar] [CrossRef]
- Ma, Z.; Ji, W.; Fu, Q.; Ma, S. Formononetin inhibited the inflammation of LPS-induced acute lung injury in mice associated with induction of PPAR gamma expression. Inflammation 2013, 36, 1560–1566. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, S.B.; Wang, Y.C.; Li, X.Q.; Zheng, L.H.; Zhao, M.G. Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phytother. Res. 2013, 27, 1770–1775. [Google Scholar] [CrossRef]
- Huh, J.E.; Nam, D.W.; Baek, Y.H.; Kang, J.W.; Park, D.S.; Choi, D.Y.; Lee, J.D. Formononetin accelerates wound repair by the regulation of early growth response factor-1 transcription factor through the phosphorylation of the ERK and p38 MAPK pathways. Int. Immunopharmacol. 2011, 11, 46–54. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef]
- Fan, F.; Bashari, M.H.; Morelli, E.; Tonon, G.; Malvestiti, S.; Vallet, S.; Jarahian, M.; Seckinger, A.; Hose, D.; Bakiri, L.; et al. The AP-1 transcription factor JunB is essential for multiple myeloma cell proliferation and drug resistance in the bone marrow microenvironment. Leukemia 2017, 31, 1570–1581. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Kino, T.; Kawamura, A.; Isoyama, S.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; Matsuda, H.; et al. Mangiferin enhances the sensitivity of human multiple myeloma cells to anticancer drugs through suppression of the nuclear factor κB pathway. Int. J. Oncol. 2016, 48, 2704–2712. [Google Scholar] [CrossRef]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Jagadeesh, S.; Kyo, S.; Banerjee, P.P. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006, 66, 2107–2115. [Google Scholar] [CrossRef]
- Davis, J.N.; Kucuk, O.; Sarkar, F.H. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr. Cancer 1999, 35, 167–174. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.S.; Song, Y.S. Genistein as a potential anticancer agent against ovarian cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef]
- Ouyang, G.; Yao, L.; Ruan, K.; Song, G.; Mao, Y.; Bao, S. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol. Int. 2009, 33, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, T.; Lee, M.S. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007, 80, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wan, C.; Luo, Q.; Huang, Z.; Luo, Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int. J. Mol. Sci. 2014, 15, 3432–3443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.Z.; Du, G.J.; Qi, L.W.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef]
- Dai, W.; Wang, F.; He, L.; Lin, C.; Wu, S.; Chen, P.; Zhang, Y.; Shen, M.; Wu, D.; Wang, C.; et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol. Carcinog. 2015, 54, 301–311. [Google Scholar] [CrossRef]
- George, J.; Banik, N.L.; Ray, S.K. Genistein induces receptor and mitochondrial pathways and increases apoptosis during BCL-2 knockdown in human malignant neuroblastoma SK-N-DZ cells. J. Neurosci. Res. 2010, 88, 877–886. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Zhu, B. Genistein inhibits the proliferation of human multiple myeloma cells through suppression of nuclear factor-κB and upregulation of microRNA-29b. Mol. Med. Rep. 2016, 13, 1627–1632. [Google Scholar] [CrossRef]
- He, H.; Chen, L.; Zhai, M.; Chen, J.Z. Genistein down-regulates the constitutive activation of nuclear factor-kappaB in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Phytother. Res. 2009, 23, 868–873. [Google Scholar] [CrossRef]
- Kłósek, M.; Mertas, A.; Król, W.; Jaworska, D.; Szymszal, J.; Szliszka, E. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in prostate cancer cells after treatment with xanthohumol-a natural compound present in Humulus lupulus L. Int. J. Mol. Sci. 2016, 17, 837. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P. Isobavachalcone: An overview. Chin. J. Integr. Med. 2012, 18, 543–547. [Google Scholar] [CrossRef]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Ashour, H.F.; Abou-Zeid, L.A.; El-Sayed, M.A.; Selim, K.B. 1,2,3-Triazole-Chalcone hybrids: Synthesis, in vitro cytotoxic activity and mechanistic investigation of apoptosis induction in multiple myeloma RPMI-8226. Eur. J. Med. Chem. 2020, 189, 112062. [Google Scholar] [CrossRef]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2014, 15, 87–120. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, J.; Xiao, X.; Cao, A.; Zhou, F. Synthesis, biological evaluation and molecular docking studies of a new series of chalcones containing naphthalene moiety as anticancer agents. Bioorg. Chem. 2018, 76, 249–257. [Google Scholar] [CrossRef]
- Ušjak, D.; Ivković, B.; Božić, D.D.; Bošković, L.; Milenković, M. Antimicrobial activity of novel chalcones and modulation of virulence factors in hospital strains of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Pathog. 2019, 131, 186–196. [Google Scholar] [CrossRef]
- Castaño, L.F.; Cuartas, V.; Bernal, A.; Insuasty, A.; Guzman, J.; Vidal, O.; Rubio, V.; Puerto, G.; Lukáč, P.; Vimberg, V.; et al. New chalcone-sulfonamide hybrids exhibiting anticancer and antituberculosis activity. Eur. J. Med. Chem. 2019, 176, 50–60. [Google Scholar] [CrossRef]
- Rashid, H.U.; Xu, Y.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg. Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef]
- Polo, E.; Ibarra-Arellano, N.; Prent-Peñaloza, L.; Morales-Bayuelo, A.; Henao, J.; Galdámez, A.; Gutiérrez, M. Ultrasound-assisted synthesis of novel chalcone, heterochalcone and bis-chalcone derivatives and the evaluation of their antioxidant properties and as acetylcholinesterase inhibitors. Bioorg. Chem. 2019, 90, 103034. [Google Scholar] [CrossRef]
- Mathew, B.; Adeniyi, A.; Joy, M.; Mathew, G.E.; Singh-Pillay, A.; Sudarsanakumar, C.; Soliman, M.; Suresh, J. Anti-oxidant behavior of functionalized chalcone-a combined quantum chemical and crystallographic structural investigation. J. Mol. Struct. 2017, 1146, 301–308. [Google Scholar] [CrossRef]
- Shin, J.; Jang, M.G.; Park, J.C.; Koo, Y.D.; Lee, J.Y.; Park, K.S.; Chung, S.S.; Park, K. Antidiabetic effects of trihydroxychalcone derivatives via activation of AMP-activated protein kinase. J. Ind. Eng. Chem. 2018, 60, 177–184. [Google Scholar] [CrossRef]
- Kumar, H.; Devaraji, V.; Joshi, R.; Jadhao, M.; Ahirkar, P.; Prasath, R.; Bhavana, P.; Ghosh, S.K. Antihypertensive activity of a quinoline appended chalcone derivative and its site specific binding interaction with a relevant target carrier protein. RSC Adv. 2015, 5, 65496–65513. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Das, M.; Manna, K. Chalcone scaffold in anticancer armamentarium: A molecular insight. J. Toxicol. 2016, 2016, 7651047. [Google Scholar] [CrossRef]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef]
- Kang, H.M.; Kim, J.H.; Lee, M.Y.; Son, K.H.; Yang, D.C.; Baek, N.I.; Kwon, B.M. Relationship between flavonoid structure and inhibition of farnesyl protein transferase. Nat. Prod. Res. 2004, 18, 349–356. [Google Scholar] [CrossRef]
- Liu, S.C.; Chen, C.; Chung, C.H.; Wang, P.C.; Wu, N.L.; Cheng, J.K.; Lai, Y.W.; Sun, H.L.; Peng, C.Y.; Tang, C.H.; et al. Inhibitory effects of butein on cancer metastasis and bioenergetic modulation. J. Agric. Food Chem. 2014, 62, 9109–9117. [Google Scholar] [CrossRef]
- Pandey, M.K.; Sung, B.; Ahn, K.S.; Aggarwal, B.B. Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol. Pharmacol. 2009, 75, 525–533. [Google Scholar] [CrossRef]
- Bowman, T.; Garcia, R.; Turkson, J.; Jove, R. STATs in oncogenesis. Oncogene 2000, 19, 2474–2488. [Google Scholar] [CrossRef]
- Cavo, M. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia 2006, 20, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020, 250, 117591. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An overview on cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Kikuchi, H.; Koyano, T.; Kowithayakorn, T.; Sakai, T.; Ishibashi, M. Death receptor 5 promoter-enhancing compounds isolated from Catimbium speciosum and their enhancement effect on TRAIL-induced apoptosis. Bioorg. Med. Chem. 2009, 17, 6748–6754. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of a Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef]
- Bajgai, S.P.; Prachyawarakorn, V.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Hybrid flavan-chalcones, aromatase and lipoxygenase inhibitors, from Desmos cochinchinensis. Phytochemistry 2011, 72, 2062–2067. [Google Scholar] [CrossRef]
- Lee, M.Y.; Seo, C.S.; Lee, J.A.; Shin, I.S.; Kim, S.J.; Ha, H.; Shin, H.K. Alpinia katsumadai H(AYATA) seed extract inhibit LPS-induced inflammation by induction of heme oxygenase-1 in RAW264.7 cells. Inflammation 2012, 35, 746–757. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.S.; Giang, P.M.; Jin, X.; Lee, S.; Son, P.T.; Lee, D.; Hong, Y.S.; Lee, K.; Lee, J.J. Blockade of nuclear factor-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J. Pharmacol. Exp. Ther. 2006, 316, 271–278. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, C.Y.; Lu, F.R.; Shu, X.R.; Yang, D.; Chen, L.; She, X.M.; Gregg, N.M.; Guo, T.; Hu, Y. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-κB pathway in vitro. Leuk. Res. 2012, 36, 514–520. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kawabata, K.; Sawada, K.; Ueda, M.; Fukuda, I.; Kawasaki, K.; Murakami, A.; Ashida, H. Cardamonin stimulates glucose uptake through translocation of glucose transporter-4 in L6 myotubes. Phytother. Res. 2011, 25, 1218–1224. [Google Scholar] [CrossRef]
- Liao, Q.; Shi, D.H.; Zheng, W.; Xu, X.J.; Yu, Y.H. Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur. J. Pharmacol. 2010, 641, 179–186. [Google Scholar] [CrossRef]
- Hou, G.; Yuan, X.; Li, Y.; Hou, G.; Liu, X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Investig. New Drugs 2020, 38, 329–339. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Wu, X.; Yang, M.; Wang, W.; Wang, L.; Tang, D.; Wang, D. Cardamonin exerts anti-gastric cancer activity via inhibiting LncRNA-PVT1-STAT3 axis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Yang, C.; Zhang, H.; Li, Y.; Wu, B.; Huang, J.; Zhou, X.; Huang, B.; Yang, K.; et al. Retraction Note: Cardamonin induces ROS-mediated G2/M phase arrest and apoptosis through inhibition of NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis. 2019, 10, 289. [Google Scholar] [CrossRef]
- Chen, H.; Shi, D.; Niu, P.; Zhu, Y.; Zhou, J. Anti-inflammatory effects of cardamonin in ovarian cancer cells are mediated via mTOR suppression. Planta Med. 2018, 84, 1183–1190. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jeengar, M.K.; Thummuri, D.; Koval, A.; Katanaev, V.L.; Marepally, S.; Naidu, V.G.M. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Biofactors 2017, 43, 152–169. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, R.; Li, Q.; Jie, X.; Hong, J.; Zong, Y.; Dong, X.; Zhang, S.; Li, Z.; Wu, G. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anticancer Drugs 2019, 30, 241–250. [Google Scholar] [CrossRef]
- Park, S.; Gwak, J.; Han, S.J.; Oh, S. Cardamonin suppresses the proliferation of colon cancer cells by promoting β-catenin degradation. Biol. Pharm. Bull. 2013, 36, 1040–1044. [Google Scholar] [CrossRef]
- Zhang, J.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Rangappa, K.S.; Sethi, G.; et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef]
- James, S.; Aparna, J.S.; Paul, A.M.; Lankadasari, M.B.; Mohammed, S.; Binu, V.S.; Santhoshkumar, T.R.; Reshmi, G.; Harikumar, K.B. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci. Rep. 2017, 7, 13945. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, K.S.; Choi, K.C.; Ko, H. Cardamonin induces autophagy and an antiproliferative effect through JNK activation in human colorectal carcinoma HCT116 cells. Bioorg. Med. Chem. Lett. 2015, 25, 2559–2564. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.C.; Shih, Y.L.; Chou, J.S.; Chen, K.W.; Chen, Y.L.; Lee, M.H.; Peng, S.F.; Leu, S.J.; Chung, J.G. Cardamonin induces cell cycle arrest, apoptosis and alters apoptosis associated gene expression in WEHI-3 mouse leukemia cells. Am. J. Chin. Med. 2019, 47, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.X.; Nayak, U.R.; Dev, S. Some new flavonoids from Psoralea Corylifolia. Tetrahedron Lett. 1968, 20, 2401–2406. [Google Scholar] [CrossRef]
- Yan, C.; Wu, Y.; Weng, Z.; Gao, Q.; Yang, G.; Chen, Z.; Cai, B.; Li, W. Development of an HPLC method for absolute quantification and QAMS of flavonoids components in Psoralea corylifolia L. J. Anal. Methods Chem. 2015, 2015, 792637. [Google Scholar] [CrossRef]
- ElSohly, H.N.; Joshi, A.S.; Nimrod, A.C.; Walker, L.A.; Clark, A.M. Antifungal chalcones from Maclura tinctoria. Planta Med. 2001, 67, 87–89. [Google Scholar] [CrossRef]
- Jantan, I.; Mohd Yasin, Y.H.; Jamil, S.; Sirat, H.; Basar, N. Effect of prenylated flavonoids and chalcones isolated from Artocarpus species on platelet aggregation in human whole blood. J. Nat. Med. 2010, 64, 365–369. [Google Scholar] [CrossRef]
- Tsai, W.J.; Hsin, W.C.; Chen, C.C. Antiplatelet flavonoids from seeds of Psoralea corylifolia. J. Nat. Prod. 1996, 59, 671–672. [Google Scholar] [CrossRef]
- Jing, H.; Zhou, X.; Dong, X.; Cao, J.; Zhu, H.; Lou, J.; Hu, Y.; He, Q.; Yang, B. Abrogation of Akt signaling by Isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett. 2010, 294, 167–177. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2009, 11, 1–13. [Google Scholar] [CrossRef]
- Ohno, O.; Watabe, T.; Nakamura, K.; Kawagoshi, M.; Uotsu, N.; Chiba, T.; Yamada, M.; Yamaguchi, K.; Yamada, K.; Miyamoto, K.; et al. Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 1504–1506. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, C.M.; Liu, C.X.; Wei, W.; Sun, Y.; Yan, H.; Wu, Y.L. Autophagy inhibition enhances isobavachalcone-induced cell death in multiple myeloma cells. Int. J. Mol. Med. 2012, 30, 939–944. [Google Scholar] [CrossRef]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef]
- Wang, K.L.; Yu, Y.C.; Hsia, S.M. Perspectives on the role of isoliquiritigenin in cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Wang, K.L.; Hsia, S.M.; Chan, C.J.; Chang, F.Y.; Huang, C.Y.; Bau, D.T.; Wang, P.S. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin. Ther. Targets 2013, 17, 337–349. [Google Scholar] [CrossRef]
- Jin, H.; Lee, S.H.; Lee, S.H. Isoliquiritigenin-mediated p62/SQSTM1 induction regulates apoptotic potential through attenuation of caspase-8 activation in colorectal cancer cells. Eur. J. Pharmacol. 2018, 841, 90–97. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, T.C.; Shieh, T.M.; Wu, C.H.; Lin, L.C.; Hsia, S.M. Isoliquiritigenin induces autophagy and inhibits ovarian cancer cell growth. Int. J. Mol. Sci. 2017, 18, 2025. [Google Scholar] [CrossRef]
- Cao, Z.X.; Wen, Y.; He, J.L.; Huang, S.Z.; Gao, F.; Guo, C.J.; Liu, Q.Q.; Zheng, S.W.; Gong, D.Y.; Li, Y.Z.; et al. Isoliquiritigenin, an orally available natural FLT3 inhibitor from licorice, exhibits selective anti-acute myeloid leukemia efficacy in vitro and in vivo. Mol. Pharmacol. 2019, 96, 589–599. [Google Scholar] [CrossRef]
- Xiang, S.; Chen, H.; Luo, X.; An, B.; Wu, W.; Cao, S.; Ruan, S.; Wang, Z.; Weng, L.; Zhu, H.; et al. Isoliquiritigenin suppresses human melanoma growth by targeting miR-301b/LRIG1 signaling. J. Exp. Clin. Cancer Res. 2018, 37, 184. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Jiang, Y.; Zhou, Y.; Wang, Y.; Yao, Y.; Yi, C.; Gou, L.; Yang, J. Isoliquiritigenin inhibits the growth of multiple myeloma via blocking IL-6 signaling. J. Mol. Med. 2012, 90, 1311–1319. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Delle Monache, G. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 717–739. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Jiang, C.H.; Sun, T.L.; Xiang, D.X.; Wei, S.S.; Li, W.Q. Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Roehrer, S.; Stork, V.; Ludwig, C.; Minceva, M.; Behr, J. Analyzing bioactive effects of the minor hop compound xanthohumol C on human breast cancer cells using quantitative proteomics. PLoS ONE 2019, 14, e0213469. [Google Scholar] [CrossRef]
- Logan, I.E.; Miranda, C.L.; Lowry, M.B.; Maier, C.S.; Stevens, J.F.; Gombart, A.F. Antiproliferative and cytotoxic activity of xanthohumol and its non-estrogenic derivatives in colon and hepatocellular carcinoma cell lines. Int. J. Mol. Sci. 2019, 20, 1203. [Google Scholar] [CrossRef]
- Yong, W.K.; Malek, S.N.A. Xanthohumol induces growth inhibition and apoptosis in ca ski human cervical cancer cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 921306. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Zdzisińska, B.; Dmoszyńska-Graniczka, M.; Jeleniewicz, W.; Kurzepa, J.; Gagoś, M.; Stepulak, A. Xanthohumol inhibits the extracellular signal regulated kinase (ERK) signalling pathway and suppresses cell growth of lung adenocarcinoma cells. Toxicology 2016, 357–358, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Wang, C.; Sun, C.; Chen, X.; Huo, X.; Zhang, Y.; Li, G.; Xu, B.; Zhang, J.; Xie, J.; et al. Xanthohumol induces paraptosis of leukemia cells through p38 mitogen activated protein kinase signaling pathway. Oncotarget 2017, 8, 31297–31304. [Google Scholar] [CrossRef] [PubMed]
- Sławińska-Brych, A.; Zdzisińska, B.; Czerwonka, A.; Mizerska-Kowalska, M.; Dmoszyńska-Graniczka, M.; Stepulak, A.; Gagoś, M. Xanthohumol exhibits anti-myeloma activity in vitro through inhibition of cell proliferation, induction of apoptosis via the ERK and JNK-dependent mechanism, and suppression of sIL-6R and VEGF production. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129408. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Rho, M.C.; Lee, S.W.; Choi, J.N.; Kim, K.; Song, G.Y.; Kim, Y.K. Bavachin and isobavachalcone, acyl-coenzyme A: Cholesterol acyltransferase inhibitors from Psoralea corylifolia. Arch. Pharm. Res. 2008, 31, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Wang, S.C.; Suzuki, K.; Fang, S.H.; Chen, C.S.; Cheng, W.C.; Su, C.C.; Yeh, H.C.; Tu, H.P.; Liu, P.L.; et al. Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine 2019, 59, 152785. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Tomonari, Y.; Kawashima, K.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Bavachin induces the apoptosis of multiple myeloma cell lines by inhibiting the activation of nuclear factor kappa B and signal transducer and activator of transcription 3. Biomed. Pharmacother. 2018, 100, 486–494. [Google Scholar] [CrossRef]
- Lee, H.; Li, H.; Noh, M.; Ryu, J.H. Bavachin from Psoralea corylifolia improves insulin-dependent glucose uptake through insulin signaling and AMPK activation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2016, 17, 527. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, J.; Jiang, Y.; Gong, L.; Yang, B. Synthesis of prenylated flavonols and their potents as estrogen receptor modulator. Sci. Rep. 2017, 7, 12445. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Zhu, M.; Ou, Y.; Zhang, J.; Luo, H.; Luo, R.; Wu, J.; Mao, M.; Liu, X.; et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur. J. Pharmacol. 2010, 635, 40–48. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Wang, L.; Ji, S.; Zhang, J.; Zhang, C. Icariin synergizes with arsenic trioxide to suppress human hepatocellular carcinoma. Cell Biochem. Biophys. 2014, 68, 427–436. [Google Scholar] [CrossRef]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y.; et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef]
- Zhang, D.C.; Liu, J.L.; Ding, Y.B.; Xia, J.G.; Chen, G.Y. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-κB. Acta Pharmacol. Sin. 2013, 34, 301–308. [Google Scholar] [CrossRef]
- Ma, H.R.; Wang, J.; Chen, Y.F.; Chen, H.; Wang, W.S.; Aisa, H.A. Icariin and icaritin stimulate the proliferation of SKBr3 cells through the GPER1-mediated modulation of the EGFR-MAPK signaling pathway. Int. J. Mol. Med. 2014, 33, 1627–1634. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef]
- Gu, Z.F.; Zhang, Z.T.; Wang, J.Y.; Xu, B.B. Icariin exerts inhibitory effects on the growth and metastasis of KYSE70 human esophageal carcinoma cells via PI3K/AKT and STAT3 pathways. Environ. Toxicol. Pharmacol. 2017, 54, 7–13. [Google Scholar] [CrossRef]
- Fan, C.; Yang, Y.; Liu, Y.; Jiang, S.; Di, S.; Hu, W.; Ma, Z.; Li, T.; Zhu, Y.; Xin, Z.; et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016, 6, 21145. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.Y.; Sethi, G.; Ahn, K.S. Anti-myeloma effects of icariin are mediated through the attenuation of JAK/STAT3-dependent signaling cascade. Front. Pharmacol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Yang, X.J.; Xi, Y.M.; Li, Z.J. Icaritin: A novel natural candidate for hematological malignancies therapy. Biomed. Res. Int. 2019, 2019, 4860268. [Google Scholar] [CrossRef]
- Yin, L.; Qi, X.W.; Liu, X.Z.; Yang, Z.Y.; Cai, R.L.; Cui, H.J.; Chen, L.; Yu, S.C. Icaritin enhances the efficacy of cetuximab against triple-negative breast cancer cells. Oncol. Lett. 2020, 19, 3950–3958. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, N.; Dong, J.; Wang, X.; Liu, L.; Huang, J. Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 318–327. [Google Scholar] [CrossRef]
- Han, H.; Xu, B.; Hou, P.; Jiang, C.; Liu, L.; Tang, M.; Yang, X.; Zhang, Y.; Liu, Y. Icaritin sensitizes human glioblastoma cells to TRAIL-induced apoptosis. Cell Biochem. Biophys. 2015, 72, 533–542. [Google Scholar] [CrossRef]
- Tiong, C.T.; Chen, C.; Zhang, S.J.; Li, J.; Soshilov, A.; Denison, M.S.; Lee, L.S.; Tam, V.H.; Wong, S.P.; Xu, H.E.; et al. A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ERα protein. Carcinogenesis 2012, 33, 1089–1097. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Meng, J.; Wang, Z.Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur. J. Pharmacol. 2011, 658, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Chen, M.B.; Liu, Y.Y.; Wu, M.H.; Li, W.T.; Wei, M.X.; Liu, C.Y.; Qin, S.K. Identification of sphingosine kinase 1 (SphK1) as a primary target of icaritin in hepatocellular carcinoma cells. Oncotarget 2017, 8, 22800–22810. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, W.; Wei, B.; Wen, H.; Mao, S.; Xu, H.; Hu, M.; Yang, T.; Jiang, H. Antitumoral action of icaritin in LNCaP prostate cancer cells by regulating PEA3/HER2/AR signaling. Anticancer Drugs 2016, 27, 944–952. [Google Scholar] [CrossRef]
- Sun, L.; Peng, Q.; Qu, L.; Gong, L.; Si, J. Anticancer agent icaritin induces apoptosis through caspase-dependent pathways in human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 11, 3094–3100. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, W.W.; Li, B.; Chen, H.J.; Zhu, W.S.; Yang, G.X.; Chen, M.J.; He, G.Y. Anticancer effect of icaritin on human lung cancer cells through inducing S phase cell cycle arrest and apoptosis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 497–503. [Google Scholar] [CrossRef]
- Jin, L.; Miao, J.; Liu, Y.; Li, X.; Jie, Y.; Niu, Q.; Han, X. Icaritin induces mitochondrial apoptosis by up-regulating miR-124 in human oral squamous cell carcinoma cells. Biomed. Pharmacother. 2017, 85, 287–295. [Google Scholar] [CrossRef]
- Yang, J.G.; Lu, R.; Ye, X.J.; Zhang, J.; Tan, Y.Q.; Zhou, G. Icaritin reduces oral squamous cell carcinoma progression via the inhibition of STAT3 signaling. Int. J. Mol. Sci. 2017, 18, 132. [Google Scholar] [CrossRef]
- Han, S.; Gou, Y.; Jin, D.; Ma, J.; Chen, M.; Dong, X. Effects of Icaritin on the physiological activities of esophageal cancer stem cells. Biochem. Biophys. Res. Commun. 2018, 504, 792–796. [Google Scholar] [CrossRef]
- Gao, L.; Chen, M.; Ouyang, Y.; Li, R.; Zhang, X.; Gao, X.; Lin, S.; Wang, X. Icaritin induces ovarian cancer cell apoptosis through activation of p53 and inhibition of Akt/mTOR pathway. Life Sci. 2018, 202, 188–194. [Google Scholar] [CrossRef]
- Le, A.; Wang, Z.H.; Dai, X.Y.; Xiao, T.H.; Zhuo, R.; Zhang, B.; Xiao, Z.; Fan, X. Icaritin inhibits decidualization of endometrial stromal cells. Exp. Ther. Med. 2017, 14, 5949–5955. [Google Scholar] [CrossRef]
- Tong, J.S.; Zhang, Q.H.; Huang, X.; Fu, X.Q.; Qi, S.T.; Wang, Y.P.; Hou, Y.; Sheng, J.; Sun, Q.Y. Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS ONE 2011, 6, e16781. [Google Scholar] [CrossRef]
- Zhou, C.; Gu, J.; Zhang, G.; Dong, D.; Yang, Q.; Chen, M.B.; Xu, D. AMPK-autophagy inhibition sensitizes icaritin-induced anti-colorectal cancer cell activity. Oncotarget 2017, 8, 14736–14747. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, X.; Yang, J.; Teng, G.; Zhang, L.; Zhou, C. MiR-124 inhibits cell proliferation, migration and invasion by directly targeting SOX9 in lung adenocarcinoma. Oncol. Rep. 2016, 35, 3115–3121. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Liu, Y.; Li, P.; Jiang, G.; Gao, X.; Zhang, Y.; Jiang, C.; Zhu, W.; Han, H.; et al. Activation of PPARγ mediates icaritin-induced cell cycle arrest and apoptosis in glioblastoma multiforme. Biomed. Pharmacother. 2018, 100, 358–366. [Google Scholar] [CrossRef]
- Wang, Z.D.; Wang, R.Z.; Xia, Y.Z.; Kong, L.Y.; Yang, L. Reversal of multidrug resistance by icaritin in doxorubicin-resistant human osteosarcoma cells. Chin. J. Nat. Med. 2018, 16, 20–28. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, C.; Han, H.; Liu, H.; Tang, M.; Liu, L.; Ji, W.; Lu, X.; Yang, X.; Zhang, Y.; et al. Icaritin inhibits the invasion and epithelial-to-mesenchymal transition of glioblastoma cells by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1296–1307. [Google Scholar] [CrossRef]
- Zhu, J.F.; Li, Z.J.; Zhang, G.S.; Meng, K.; Kuang, W.Y.; Li, J.; Zhou, X.F.; Li, R.J.; Peng, H.L.; Dai, C.W.; et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS ONE 2011, 6, e23720. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Li, Z.; Peng, H.; Luo, Y.; Deng, M.; Li, R.; Dai, C.; Xu, Y.; Liu, S.; et al. Icaritin suppresses multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget 2015, 6, 10460–10472. [Google Scholar] [CrossRef]
- Wu, T.; Wang, S.; Wu, J.; Lin, Z.; Sui, X.; Xu, X.; Shimizu, N.; Chen, B.; Wang, X. Icaritin induces lytic cytotoxicity in extranodal NK/T-cell lymphoma. J. Exp. Clin. Cancer Res. 2015, 34, 17. [Google Scholar] [CrossRef]
- Li, Z.J.; Yao, C.; Liu, S.F.; Chen, L.; Xi, Y.M.; Zhang, W.; Zhang, G.S. Cytotoxic effect of icaritin and its mechanisms in inducing apoptosis in human burkitt lymphoma cell line. Biomed. Res. Int. 2014, 2014, 391512. [Google Scholar] [CrossRef]
- Li, Q.; Huai, L.; Zhang, C.; Wang, C.; Jia, Y.; Chen, Y.; Yu, P.; Wang, H.; Rao, Q.; Wang, M.; et al. Icaritin induces AML cell apoptosis via the MAPK/ERK and PI3K/AKT signal pathways. Int. J. Hematol. 2013, 97, 617–623. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, Z.J.; Chen, X.; Huang, X.R.; Fang, Z.Q.; Zhang, J.G.; Fang, J. Icaritin reverses multidrug resistance of multiple myeloma cell line KM3/BTZ. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 1690–1695. (In Chinese) [Google Scholar] [CrossRef]
- Xia, Q.; Xu, D.; Huang, Z.; Liu, J.; Wang, X.; Wang, X.; Liu, S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 2010, 81, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, H.J.; Ahn, K.S.; Kim, S.H.; Nam, D.; Kim, D.K.; Choi, D.Y.; Ahn, K.S.; Lu, J.; Kim, S.H. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009, 280, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Eun, J.S.; Kim, D.K.; Li, R.H.; Shin, T.Y.; Park, H.; Cho, N.P.; Soh, Y. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1alpha in human osteosarcoma cells. Eur. J. Pharmacol. 2008, 579, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ahn, K.S.; Jeong, S.J.; Kwon, T.R.; Jung, J.H.; Yun, S.M.; Han, I.; Lee, S.G.; Kim, D.K.; Kang, M.; et al. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur. J. Pharmacol. 2011, 654, 10–16. [Google Scholar] [CrossRef]
- Lamorte, D.; Faraone, I.; Laurenzana, I.; Milella, L.; Trino, S.; De Luca, L.; Del Vecchio, L.; Armentano, M.F.; Sinisgalli, C.; Chiummiento, L.; et al. Future in the past: Azorella glabra Wedd. as a source of new natural compounds with antiproliferative and cytotoxic activity on multiple myeloma cells. Int. J. Mol. Sci. 2018, 19, 3348. [Google Scholar] [CrossRef]
- Kiraz, Y.; Neergheen-Bhujun, V.S.; Rummun, N.; Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour Biol. 2016, 37, 1803–1815. [Google Scholar] [CrossRef]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef]
- Cho, J.; Rho, O.; Junco, J.; Carbajal, S.; Siegel, D.; Slaga, T.J.; DiGiovanni, J. Effect of combined treatment with ursolic acid and resveratrol on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev. Res. 2015, 8, 817–825. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Wong, Y.S. Synergistic apoptosis-inducing effects on A375 human melanoma cells of natural borneol and curcumin. PLoS ONE 2014, 9, e101277. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Yang, C.C.; Shyur, L.F. Phytomedicine-modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol. Res. 2016, 114, 128–143. [Google Scholar] [CrossRef]
- Efferth, T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 2017, 139, 56–70. [Google Scholar] [CrossRef]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Frost, B.M.; Lönnerholm, G.; Koopmans, P.; Abrahamsson, J.; Behrendtz, M.; Castor, A.; Forestier, E.; Uges, D.R.A.; De Graaf, S.S.N. Vincristine in childhood leukaemia: No pharmacokinetic rationale for dose reduction in adolescents. Acta Paediatr. 2003, 92, 551–557. [Google Scholar] [CrossRef]
- Okouneva, T.; Hill, B.T.; Wilson, L.; Jordan, M.A. The effects of vinflunine, vinorelbine, and vinblastine on centromere dynamics. Mol. Cancer Ther. 2003, 2, 427–436. [Google Scholar]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer. 2002, 2, 143–148. [Google Scholar] [CrossRef]
- İşeri, Ö.D.; Yurtcu, E.; Sahin, F.I.; Haberal, M. Corchorus olitorius (jute) extract induced cytotoxicity and genotoxicity on human multiple myeloma cells (ARH-77). Pharm. Biol. 2013, 51, 766–770. [Google Scholar] [CrossRef]
- Gao, R.; Miao, X.; Sun, C.; Su, S.; Zhu, Y.; Qian, D.; Ouyang, Z.; Duan, J. Frankincense and myrrh and their bioactive compounds ameliorate the multiple myeloma through regulation of metabolome profiling and JAK/STAT signaling pathway based on U266 cells. BMC Complement. Med. Ther. 2020, 20, 96. [Google Scholar] [CrossRef]
- Adham, A.N.; Naqishbandi, A.M.; Efferth, T. Cytotoxicity and apoptosis induction by Fumaria officinalis extracts in leukemia and multiple myeloma cell lines. J. Ethnopharmacol. 2021, 266, 113458. [Google Scholar] [CrossRef]
- Malacrida, A.; Maggioni, D.; Cassetti, A.; Nicolini, G.; Cavaletti, G.; Miloso, M. Antitumoral effect of Hibiscus sabdariffa on human squamous cell carcinoma and multiple myeloma cells. Nutr. Cancer 2016, 68, 1161–1170. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Lin, J.; Zhuang, Q.; Xu, W.; Hong, Z.; Sferra, T.J. Patrinia scabiosaefolia extract suppresses proliferation and promotes apoptosis by inhibiting the STAT3 pathway in human multiple myeloma cells. Mol. Med. Rep. 2011, 4, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Song, H.S.; Park, H.; Kim, B. Activation of ER stress-dependent miR-216b has a critical role in Salviamiltiorrhiza ethanol-extract-induced apoptosis in U266 and U937 cells. Int. J. Mol. Sci. 2018, 19, 1240. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, S.; Kang, Z.; Lin, Q. Serenoa repens induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of STAT 3 signaling. Oncol. Rep. 2009, 22, 377–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, P.S.; Prasad, M.N. Strychnos nux-vomica root extract induces apoptosis in the human multiple myeloma cell line-U266B1. Cell Biochem. Biophys. 2013, 66, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Ramanadham, M.; Prasad, M.N. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line—RPMI 8226. Food Chem. Toxicol. 2009, 47, 283–288. [Google Scholar] [CrossRef]
- Adham, A.N.; Hegazy, M.E.F.; Naqishbandi, A.M.; Efferth, T. Induction of apoptosis, autophagy and ferroptosis by Thymus vulgaris and Arctium lappa extract in leukemia and multiple myeloma cell lines. Molecules 2020, 25, 5016. [Google Scholar] [CrossRef]
- Kovacs, E. Investigation of the proliferation, apoptosis/necrosis, and cell cycle phases in several human multiple myeloma cell lines. Comparison of Viscum album QuFrF extract with vincristine in an in vitro model. Sci. World J. 2010, 10, 311–320. [Google Scholar] [CrossRef]
- Hou, J.; Qian, J.; Li, Z.; Gong, A.; Zhong, S.; Qiao, L.; Qian, S.; Zhang, Y.; Dou, R.; Li, R.; et al. Bioactive compounds from Abelmoschus manihot L. alleviate the progression of multiple myeloma in mouse model and improve bone marrow microenvironment. OncoTargets Ther. 2020, 13, 959–973. [Google Scholar] [CrossRef]
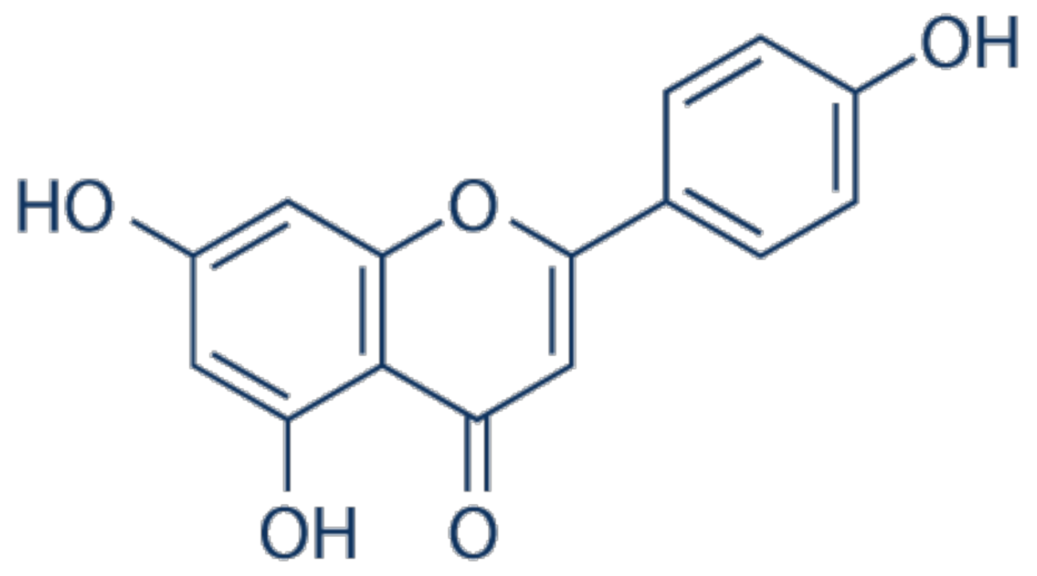
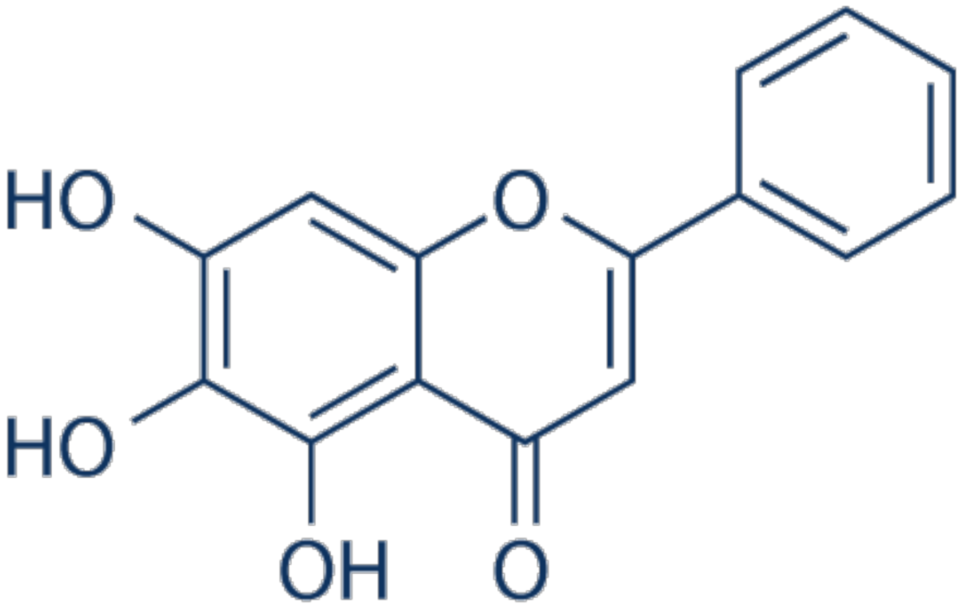
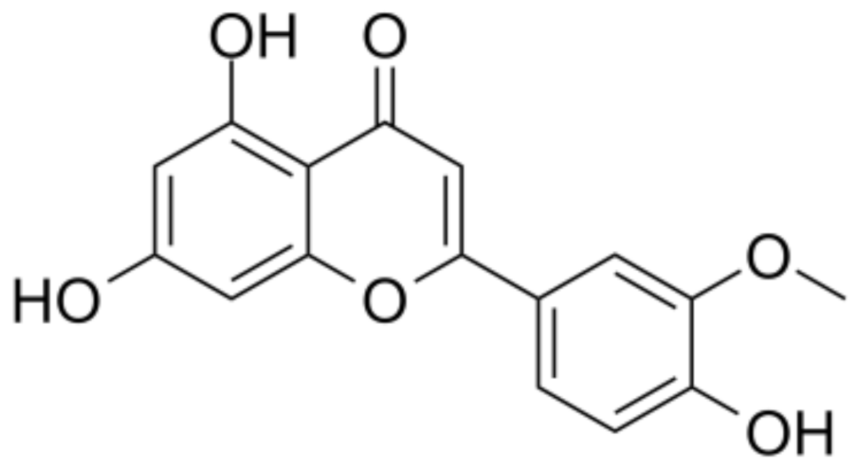
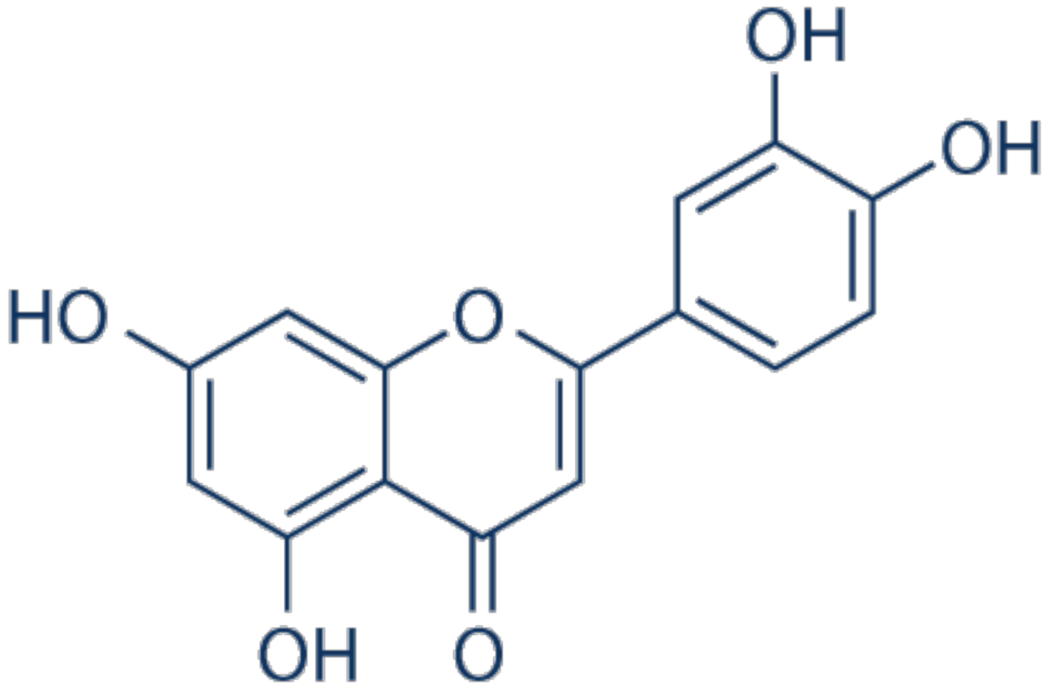

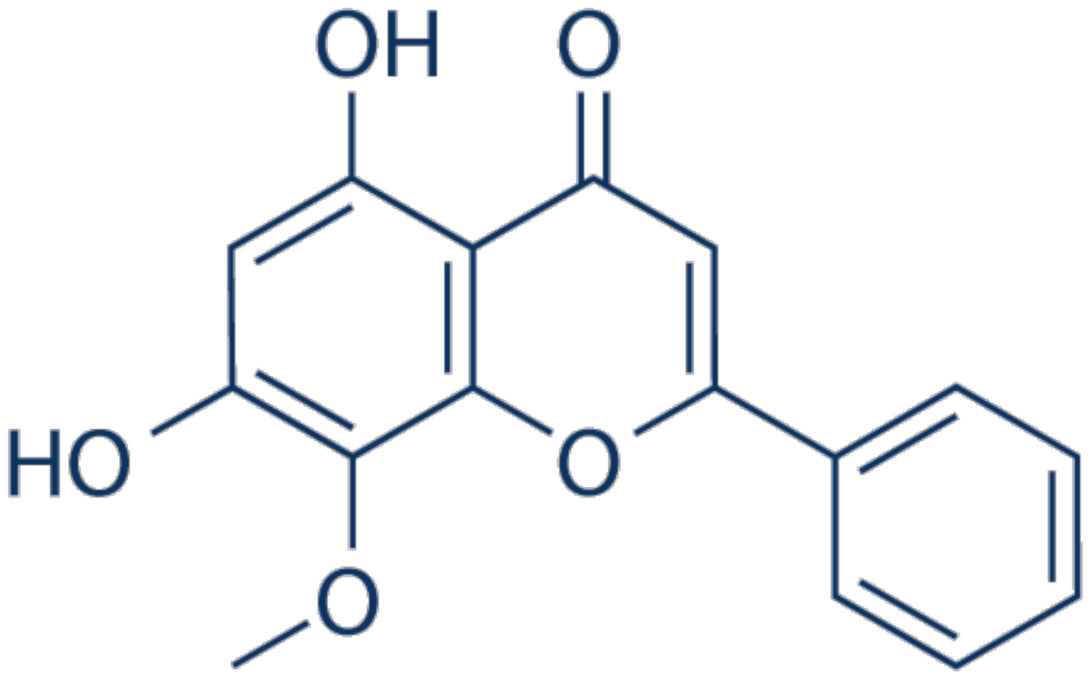


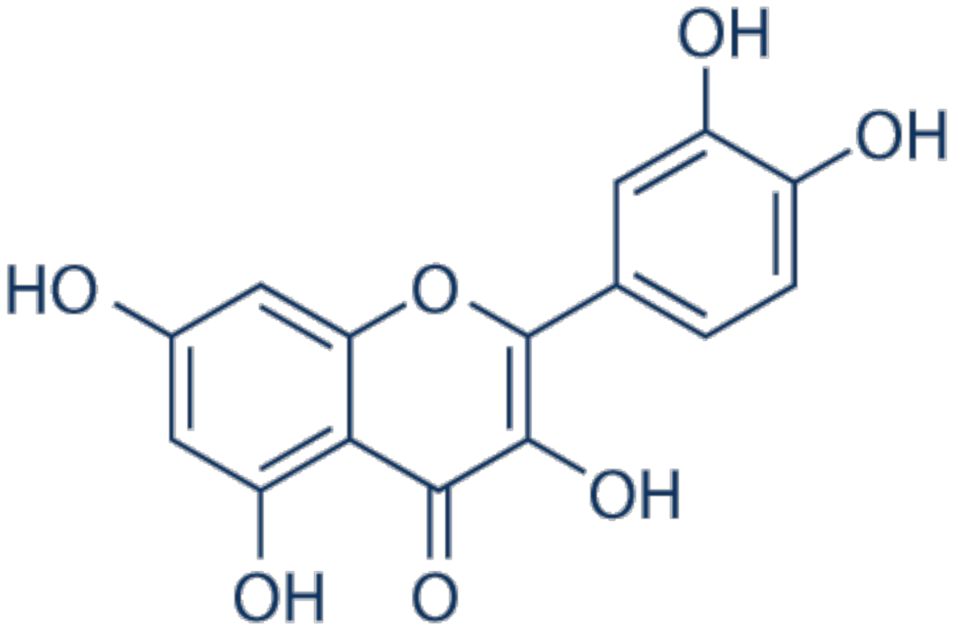
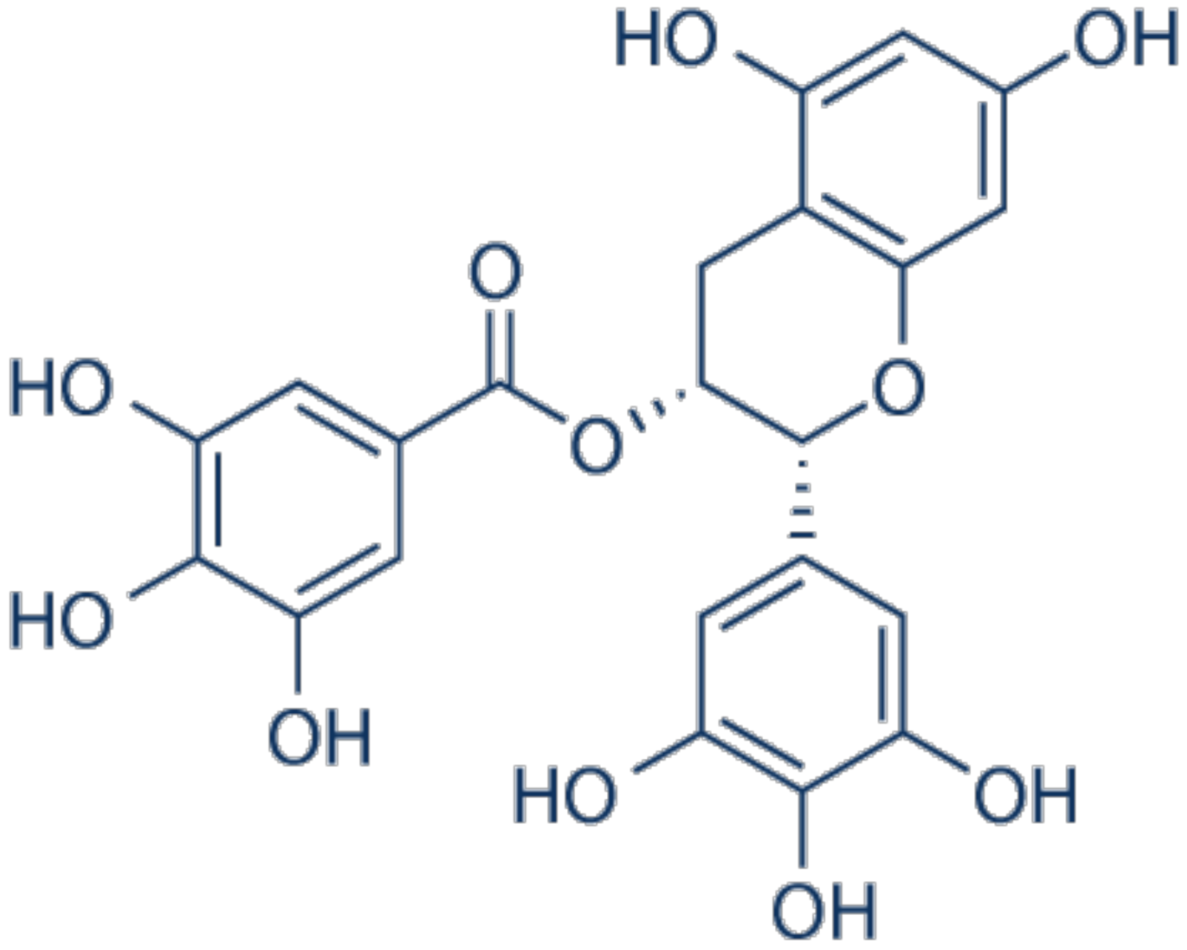
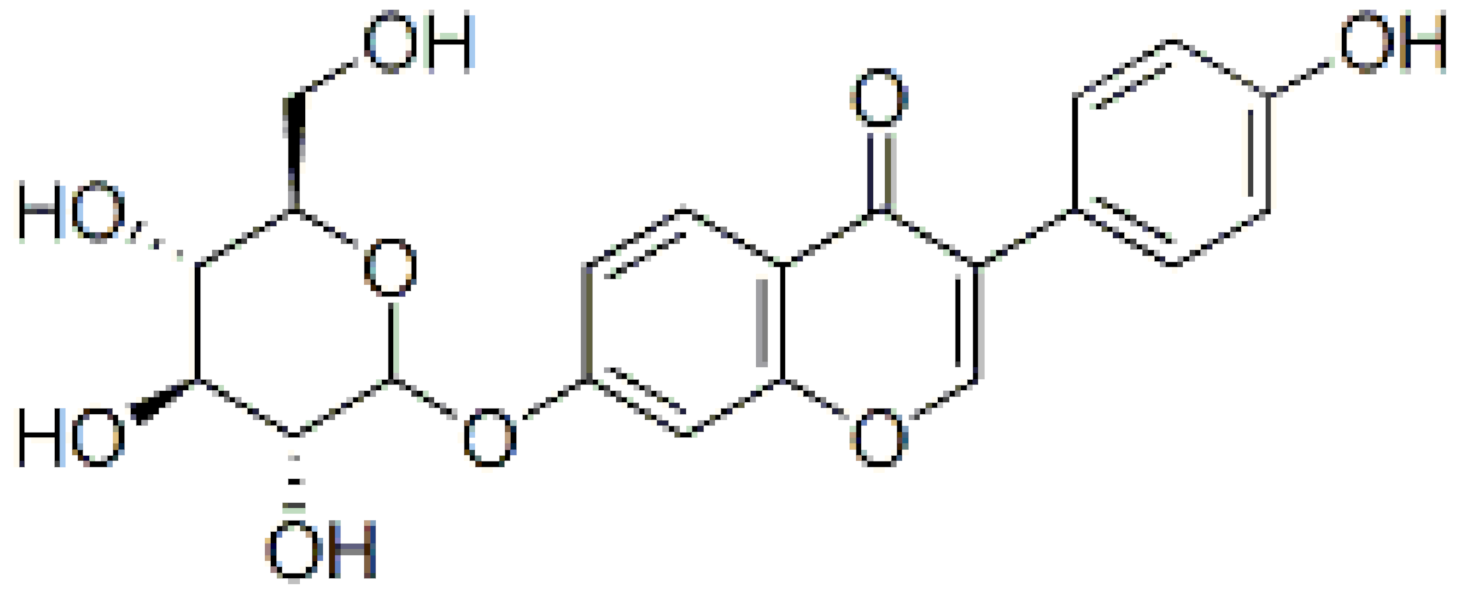
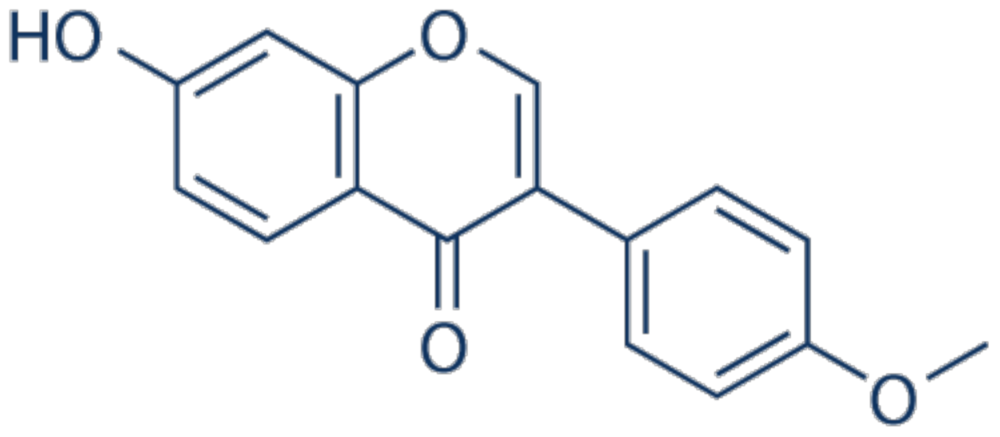

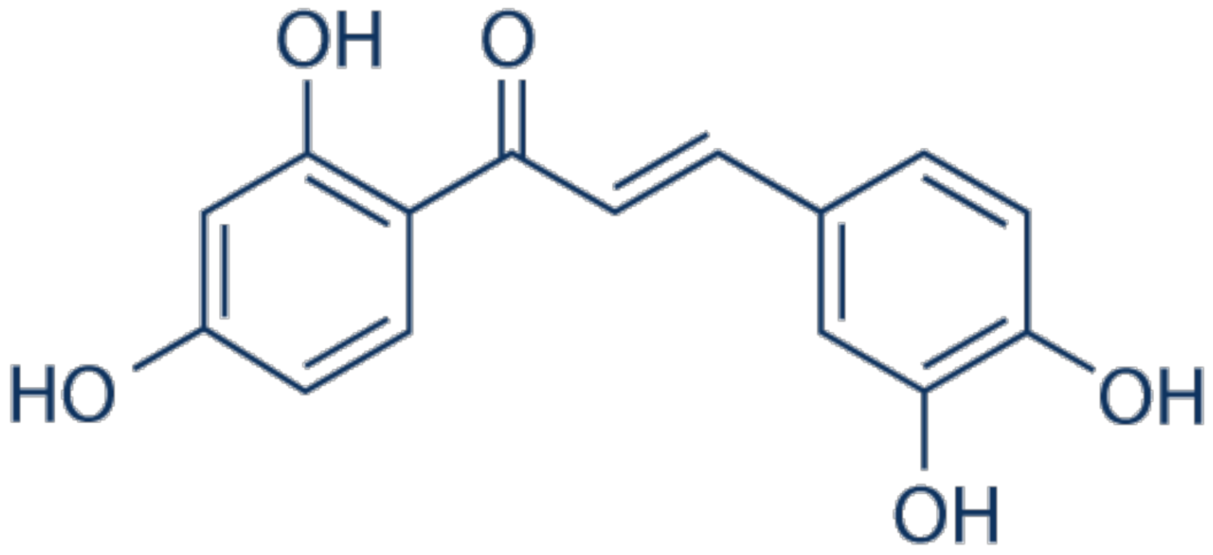
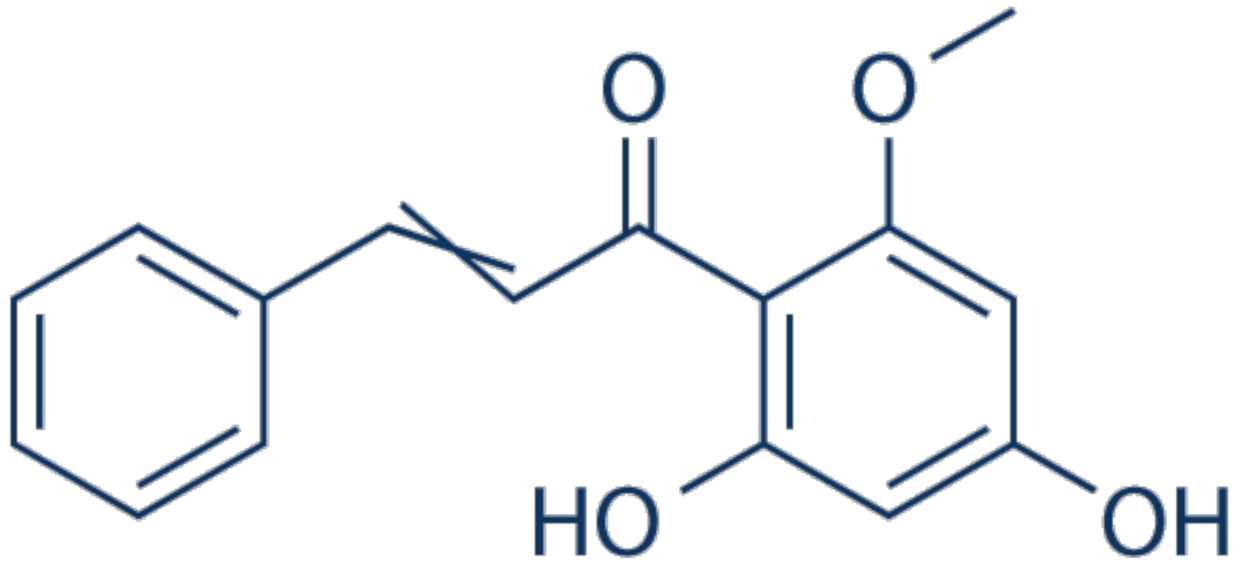

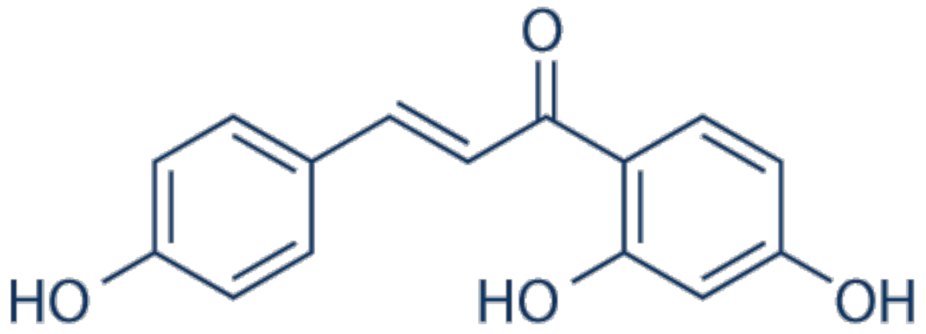


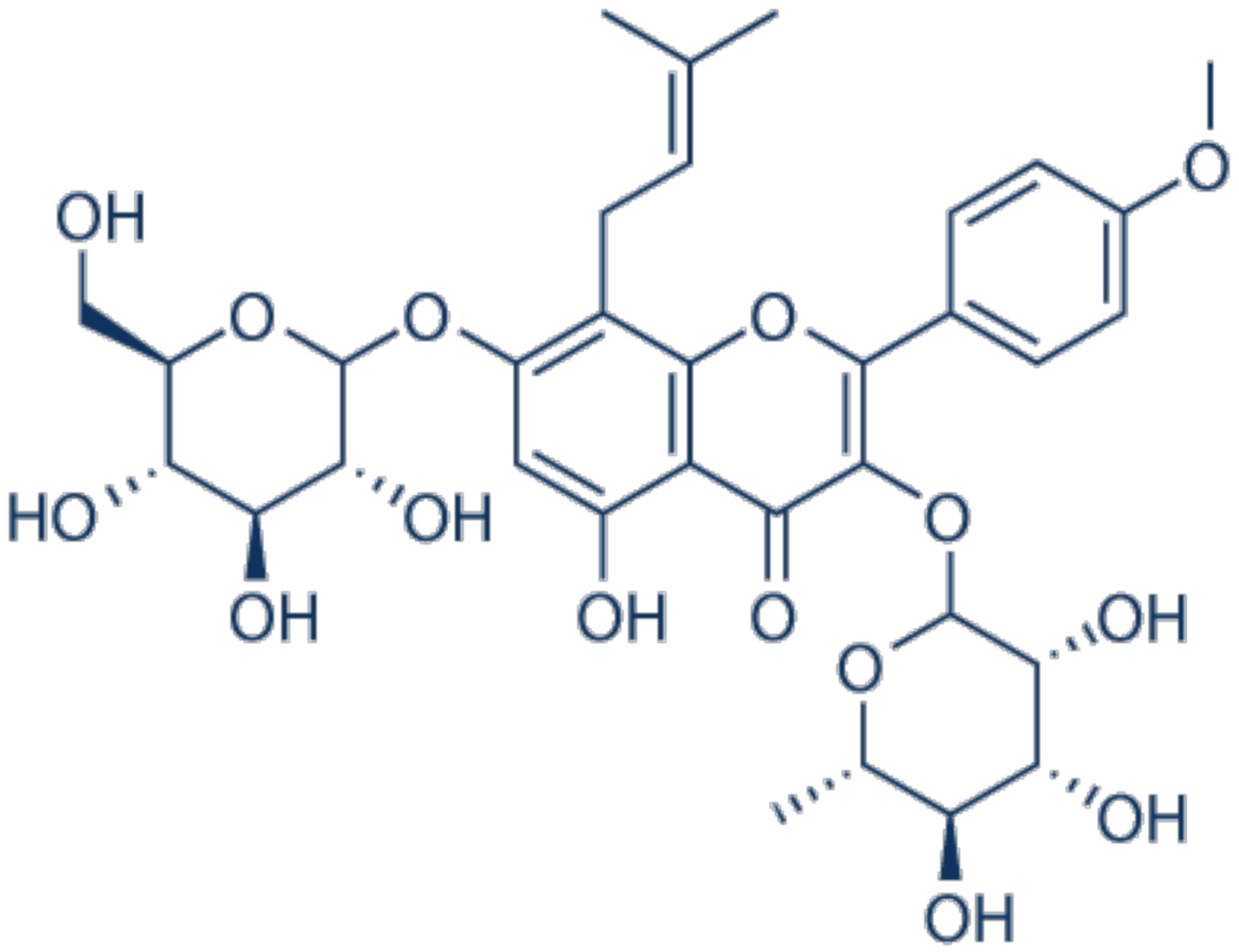
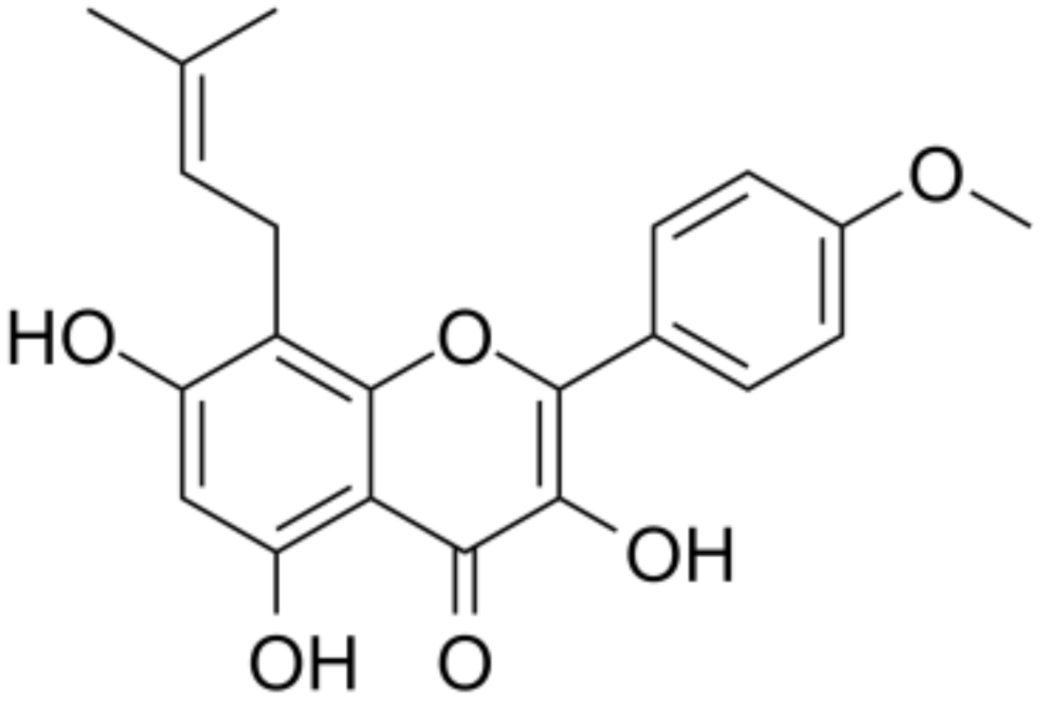
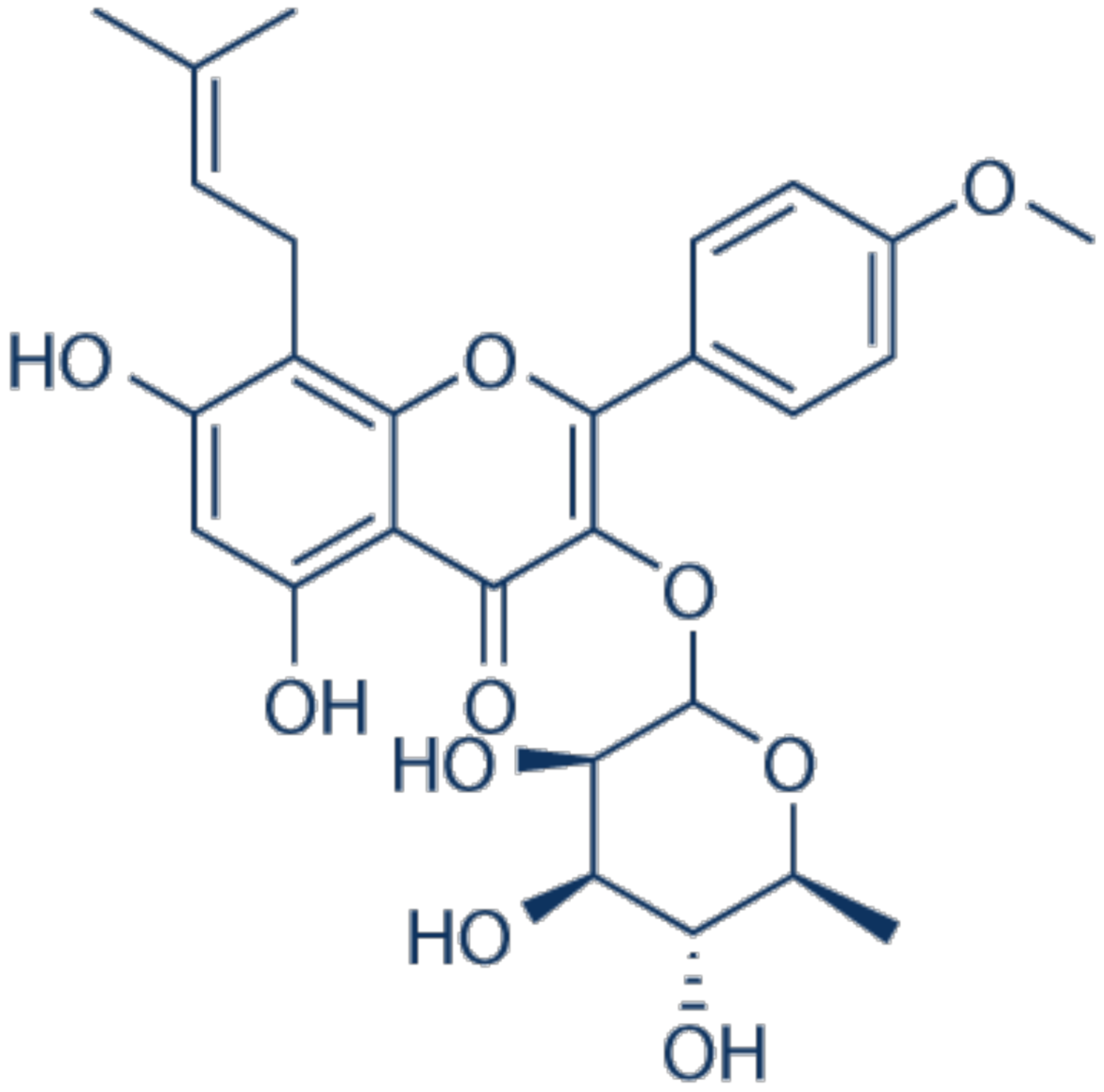
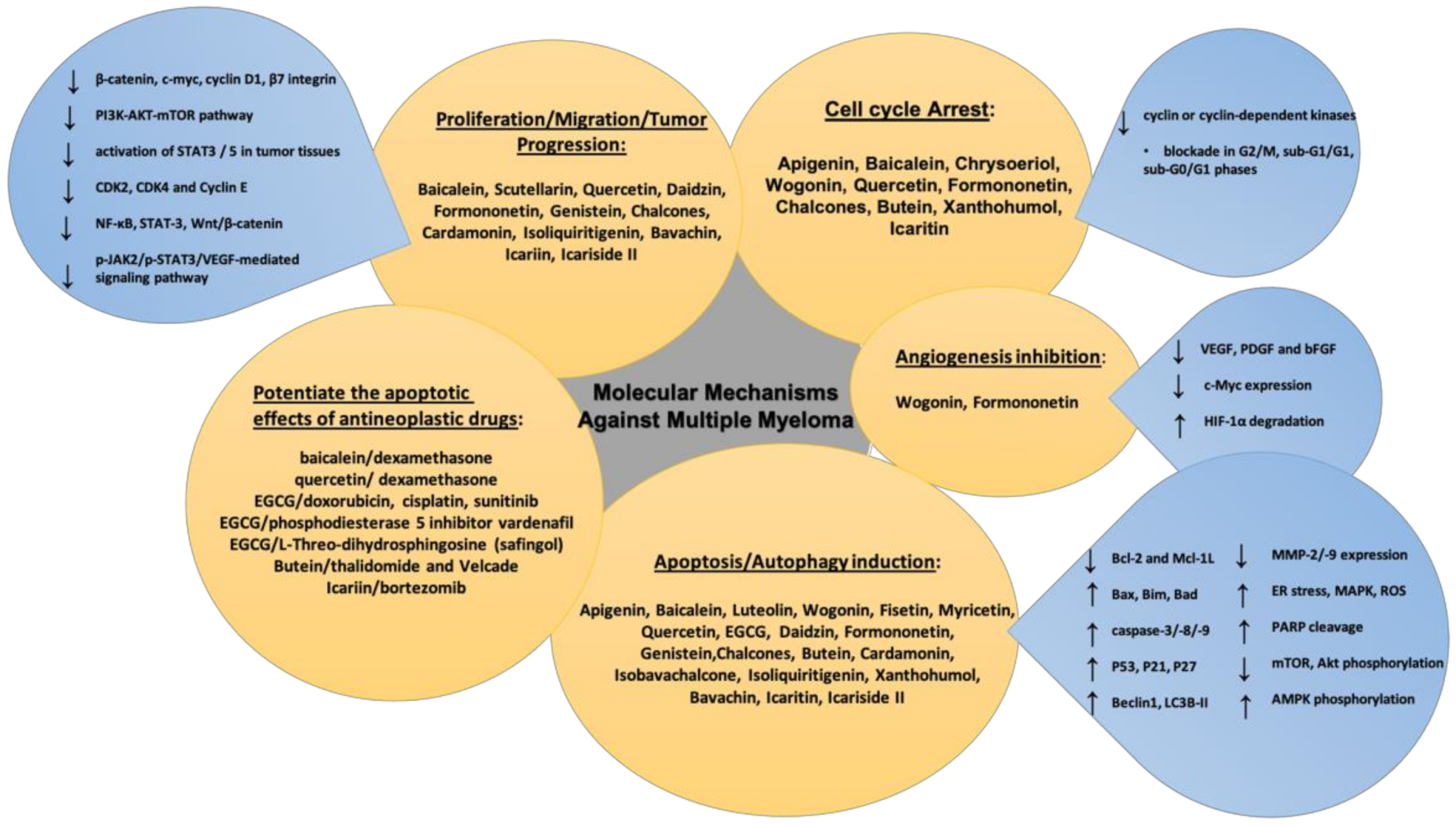
| Class of Flavonoids | Bioactive Compounds |
|---|---|
| Flavones | Apigenin, Baicalein, Crysoeriol, Luteolin, Scutellarin, Wogonin |
| Flavonols | Fisetin, Myricetin, Quercetin |
| Flavanols | Epigallocatehin-3-gallate |
| Isoflavonones | Daidzin, Genistein |
| Chalcones | Butein, Cardamonin, Isobavachalcone, Isoliquiritigenin, Xanthohumol |
| Prenylated flavonoids | Bavachin, Icariin, Icaritin, Icariside II |
| Herbal Extracts | In Vitro/In Vivo | Cancer Cell Line and Animal Model | Bioactive Effect | References |
|---|---|---|---|---|
| Azorella cglabra | In vitro | SKMM1, RPMI-8226, and MM1S cells PBMCs | ↑ antioxidant activity ↓ cell viability, induced apoptosis, and cell cycle arrest | [461] |
| Corchorus olitorius leaf extracts (LE) and seed extracts (SE) | In vitro | ARH-77 cells | - cytotoxic effects on cells (LE and SE) -SE had higher cytotoxicity on cells - genotoxicity effects in dose-dependent manner (LE and SE) | [473] |
| Frankincense and myrrh extracts | In vitro | U266 cells | - inhibited cell proliferation - ameliorated the secretion of cytokines ↓ expression of JAK/STAT signaling pathway -3-O-acetyl-α-boswellic acid, 11-keto-boswellic acid and 3-acetyl-11 keto-boswellic acid had the most significant anti-multiple myeloma activities | [474] |
| Fumaria officinalis extracts | In vitro | MOLP-8, NCI-H929, KMS-12BM, RPMI-8226, KMS-11, AMO-I, L-363, OPM-2, JJN-3 cells | - CF induced vialbility of NCI-H929 cell line - EF induced cytotoxicity on OPM-2 cells - CF and EF induced apoptosis in NCI-H929 cells by loss of MMP, generation of ROS - EF induced autophagic cell death, while CF stimulated iron-dependent cell death | [475] |
| Hibiscus sabdariffa extracts | In vitro | RPMI-8226 cells | - antiproliferative effects (Hib-ester and Hib-carbaldehyde compounds) ↓ cell migration and invasion, events involved in the process of metastasis | [6] |
| Hibiscus sabdariffa extract (HSE) | In vitro | RPMI-8226 cells | ↓ cell growth, motility and invasiveness by ERK1/2 modulation ↑ cytostatic effects dependent on p38 activation | [476] |
| Patrinia scabiosaefolia ethanol extract (EEEEPS) | In vitro | U266 cells | - inhibited the activation of STAT3 - inhibited the proliferation - induced apoptosis ↓ the expression of Bcl-2 and cyclin D1 | [477] |
| Punica granatum leaves, flowers and stem extracts | In vitro | U266 cells | - inhibited proliferation - apoptotic effect - caused cell cycle arrest in G2/M and S phases | [462] |
| Salvia miltiorrhiza (SM) extract | In vitro | U266 and U937 cells | - suppressed the growth of U266 and U937 cells ↑ ROS generation and cytotoxic effect was dependent on ROS - induced ER stress mediated apoptosis ↑ expression of miR-216b and ↓ its target, c-Jun, in U266 and U937 Cells | [478] |
| Scutellaria baicalensis extract | In vitro | RPMI-8226 cells | - Scutellaria extract riched in baicalin, wogonoside, baicalein and wogonin - inhibited the proliferation ↓ the expression level of ABCG2 protein | [135] |
| Serenoa repens | In vitro | U266, RPMI 8226 multiple myeloma cells | - induced growth arrest - induced apoptosis ↑ the expression of cleaved-PARP or p27 protein ↓ basal level of phosphorylated form of STAT 3 ↓ IL-6 induced level of phosphorylated form of STAT 3 and ERK - inhibition of STAT 3 signaling | [479] |
| Strychnos nux-vomica root extract | In vitro | U266B1 cells | - anti-proliferative effect - accumulation of the sub-G0/G1 cell population with consequent decline in other phases of cell cycle ↓ IL-6 and CD-138 | [480] |
| Strychnos nux-vomica root extract | In vitro | RPMI 8226 | - anti-proliferative activity -the SN-treated cells exhibited significant features associated with apoptosis: cell shrinkage, condensed chromatin, nuclear fragmentation and membrane blebbing ↑ the accumulation of cells at sub-G0/G1 phase - induced disruption of mitochondrial membrane potential and subsequent leakage of mitochondrial cytochrome c | [481] |
| Thymus vulgaris and Arctium lappa extracts | In vitro | MOLP-8, KMS-11, NCI-H929, RPMI-8226, KMS-12BM, JJN-3, L-363, AMO-I, and OPM-2 cells | - cytotoxicity in CCRF-CEM and CEM/ADR 5000 cell lines - TCF induced apoptosis in NCI-H929 cells with a higher ratio, compared to ACF - ACF demonstrated more potent autophagy activity than TCF - TCF and ACF induced cell cycle arrest and ferroptosis | [482] |
| Viscum album QuFrF (VAQuFrF) extract | In vitro | Molp-8, LP-1, OPM-2, Colo-677, RPMI-8226, and KMS-12-BM cells | - VAQuFrF + vincristine inhibited cell proliferation, arrested the cell cycle phases, and increased the number of apoptotic/necrotic cells. - VAQuFrF inhibited the proliferation of the cells more effectively than vincristine in Molp-8, LP-1, and RPMI-8226 cells at a dose of 10 μg/105 cells - VAQuFrF affected the tumour cells mainly via cytostatic effect | [483] |
| Bioactive compounds from Abelmoschus manihot L. | In vitro In vivo | ARP1 and H929 human MM cell lines and murine MM cell line, 5TMM3VT C57BL/KaLwRij mice | - HKC improved survival rate of MM-prone mice - promoted osteoblastogenesis and suppressed osteoclastogenesis in murine cell lines - HK-11 inhibited MM cells proliferation and ↓ β-catenin signaling | [484] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotoraci, C.; Ciceu, A.; Sasu, A.; Miutescu, E.; Hermenean, A. Bioactive Compounds from Herbal Medicine Targeting Multiple Myeloma. Appl. Sci. 2021, 11, 4451. https://doi.org/10.3390/app11104451
Cotoraci C, Ciceu A, Sasu A, Miutescu E, Hermenean A. Bioactive Compounds from Herbal Medicine Targeting Multiple Myeloma. Applied Sciences. 2021; 11(10):4451. https://doi.org/10.3390/app11104451
Chicago/Turabian StyleCotoraci, Coralia, Alina Ciceu, Alciona Sasu, Eftimie Miutescu, and Anca Hermenean. 2021. "Bioactive Compounds from Herbal Medicine Targeting Multiple Myeloma" Applied Sciences 11, no. 10: 4451. https://doi.org/10.3390/app11104451
APA StyleCotoraci, C., Ciceu, A., Sasu, A., Miutescu, E., & Hermenean, A. (2021). Bioactive Compounds from Herbal Medicine Targeting Multiple Myeloma. Applied Sciences, 11(10), 4451. https://doi.org/10.3390/app11104451








