Correlation between Lesion Progression and Depolarization Assessed by Polarization-Sensitive Optical Coherence Tomography
Abstract
:1. Introduction
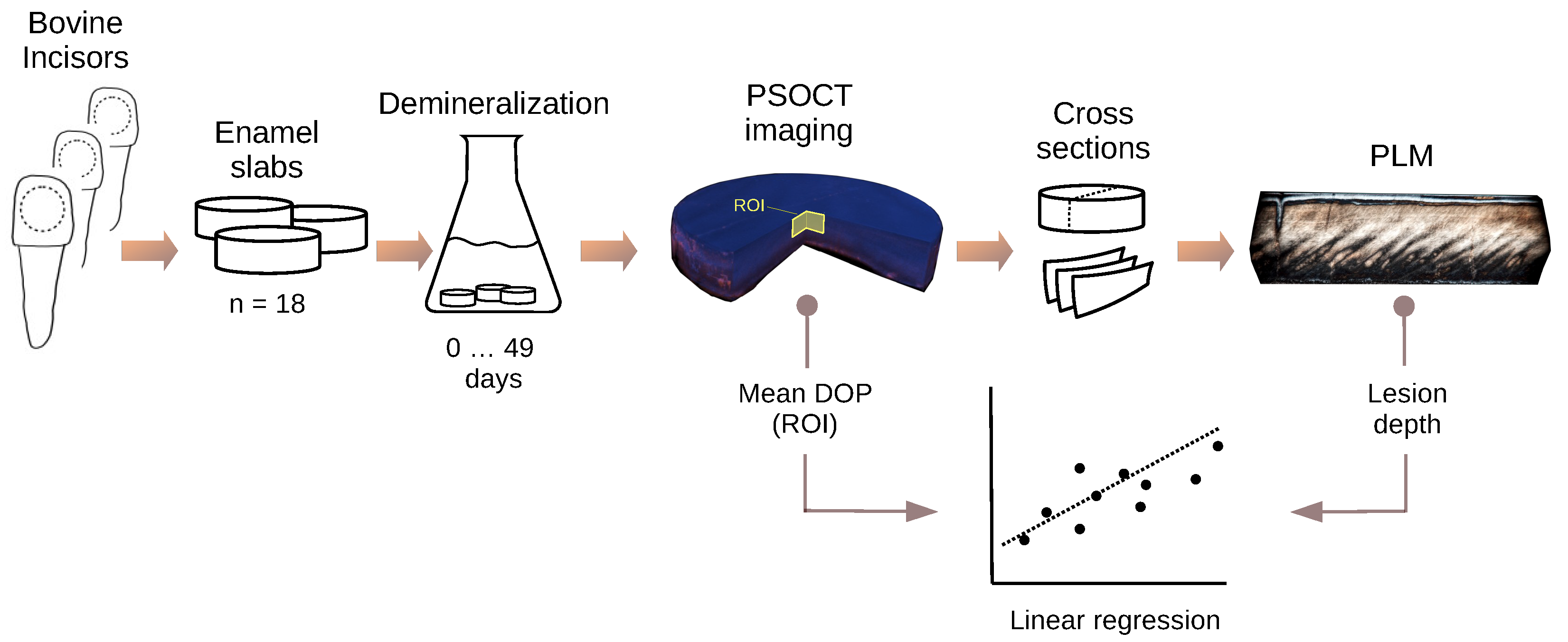
2. Materials and Methods
2.1. Specimen Preparation
2.2. Artificial Demineralization Model
2.3. Polarization-Sensitive OCT Imaging
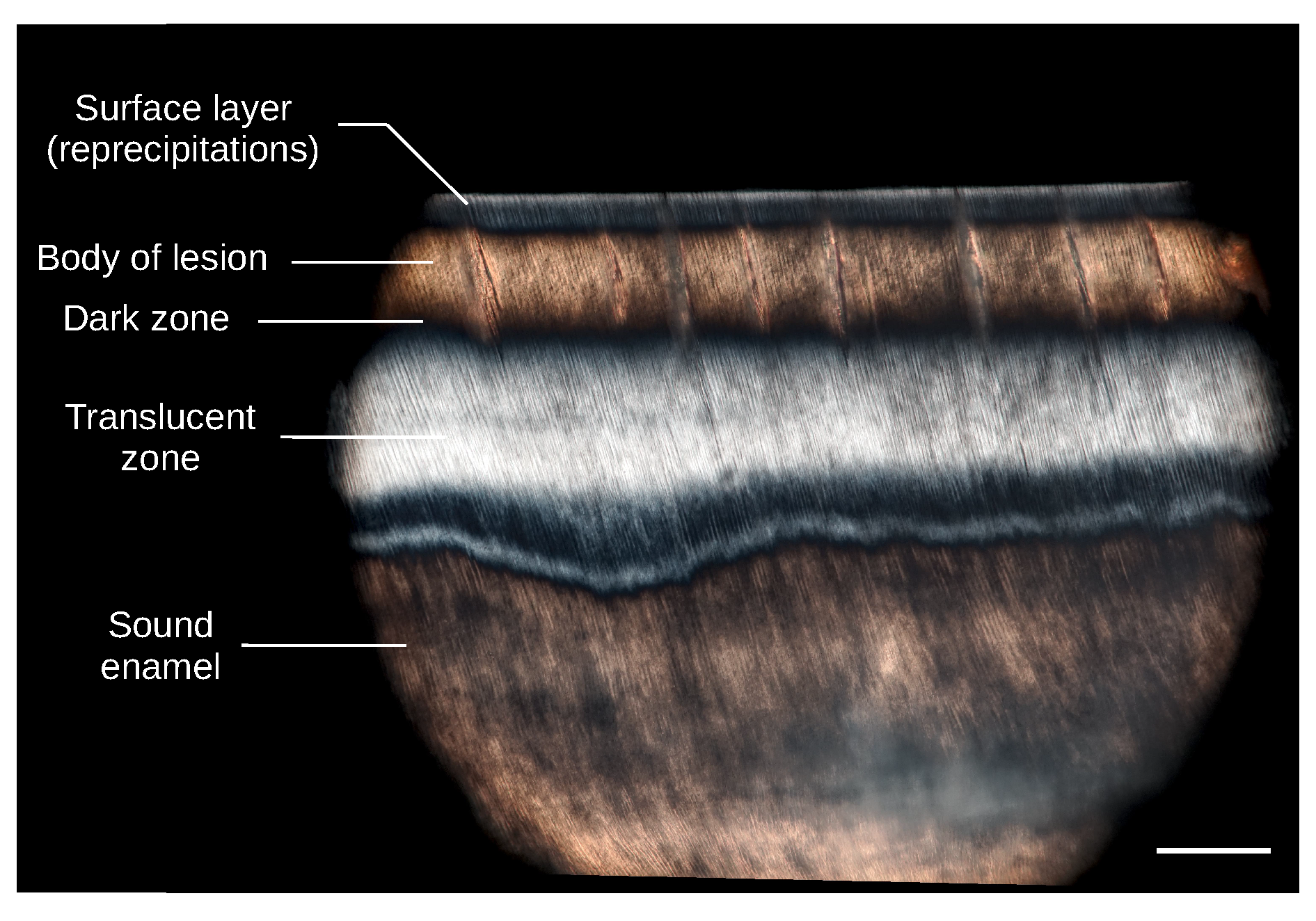
2.4. Polarized Light Microscopy of Thin Sections
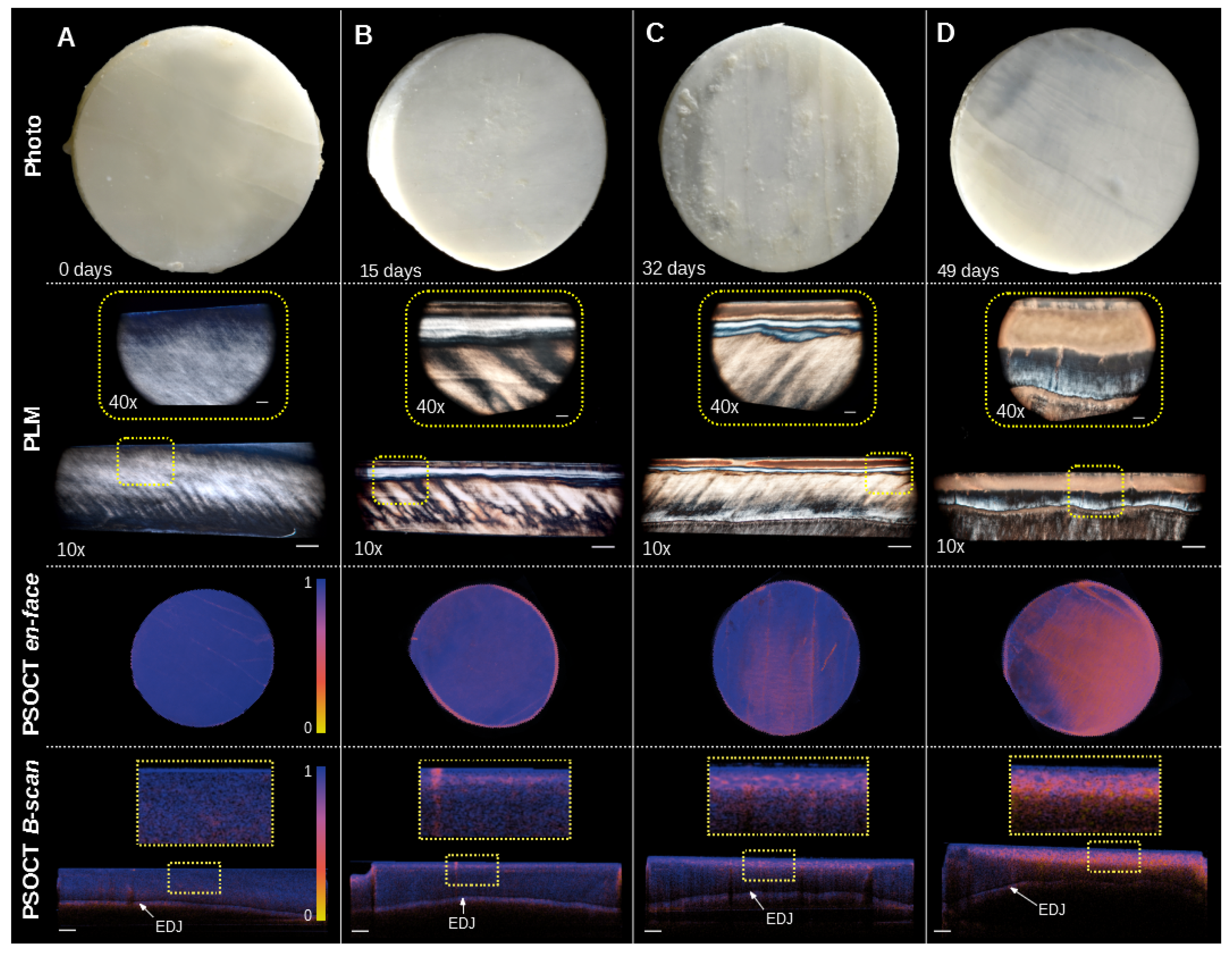
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darling, A.I. Studies of the early lesion of enamel caries with transmitted light, polarized light and radiography. Br. Dent. J. 1956, 101, 289–297. [Google Scholar]
- Darling, A.I. Studies of the early lesion of enamel caries: Its nature, mode of spread, and points of entry. Br. Dent. J. 1958, 105, 119–135. [Google Scholar]
- Baumgartner, A.; Dichtl, S.; Hitzenberger, C.K.; Sattmann, H.; Robl, B.; Moritz, A.; Fercher, A.F.; Sperr, W. Polarization-Sensitive Optical Coherence Tomography of Dental Structures. Caries Res. 2000, 34, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Everett, M.J.; Colston, B.W., Jr.; Sathyam, U.S.; Da Silva, L.B.; Fried, D.; Featherstone, J.D.B. Noninvasive Diagnosis of Early Caries with Polarization-Sensitive Optical Coherence Tomography (PS-OCT). In Proceedings of the BIOS ’99 International Biomedical Optics Symposium, San Jose, CA, USA, 23–29 January 1999; Lasers in Dentistry V. Featherstone, J.D.B., Rechmann, P., Fried, D., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; Volume 3593, p. 177. [Google Scholar] [CrossRef]
- Fried, D.; Xie, J.; Shafi, S.; Featherstone, J.D.B.; Breunig, T.M.; Le, C. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J. Biomed. Opt. 2002, 7, 618. [Google Scholar] [CrossRef]
- Jones, R.S.; Darling, C.L.; Featherstone, J.D.; Fried, D. Imaging artificial caries on the occlusal surfaces with polarization-sensitive optical coherence tomography. Caries Res. 2006, 40, 81–89. [Google Scholar] [CrossRef]
- Kang, H.; Darling, C.L.; Fried, D. Nondestructive monitoring of the repair of enamel artificial lesions by an acidic remineralization model using polarization-sensitive optical coherence tomography. Dent. Mater. 2012, 28, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.C.; Kang, H.; Darling, C.L.; Fried, D. Automated assessment of the remineralization of artificial enamel lesions with polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2014, 5, 2950. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.H.; Chan, A.C.; Fried, W.A.; Simon, J.C.; Darling, C.L.; Fried, D. Use of 2D images of depth and integrated reflectivity to represent the severity of demineralization in cross-polarization optical coherence tomography. J. Biophotonics 2015, 8, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Golde, J.; Tetschke, F.; Walther, J.; Rosenauer, T.; Hempel, F.; Hannig, C.; Koch, E. Detection of carious lesions utilizing depolarization imaging by polarization sensitive optical coherence tomography. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [Green Version]
- Golde, J.; Tetschke, F.; Walther, J.; Rosenauer, T.; Hempel, F.; Hannig, C.; Koch, E.; Kirsten, L. Cross-sectional and en-face depolarization imaging for the assessment of dental lesions. Curr. Dir. Biomed. Eng. 2018, 4, 301–304. [Google Scholar] [CrossRef]
- Makita, S.; Hong, Y.J.; Miura, M.; Yasuno, Y. Degree of polarization uniformity with high noise immunity using polarization-sensitive optical coherence tomography. Opt. Lett. 2014, 39, 6783. [Google Scholar] [CrossRef] [PubMed]
- Golde, J.; Tetschke, F.; Vosahlo, R.; Walther, J.; Hannig, C.; Koch, E.; Kirsten, L. Assessment of Occlusal Enamel Alterations Utilizing Depolarization Imaging Based on PS-OCT. In Proceedings of the SPIE 11078, Optical Coherence Imaging Techniques and Imaging in Scattering Media III, Munich, Germany, 23–27 June 2019; p. 24. [Google Scholar] [CrossRef]
- Xuelian, H.; Qiang, G.; Biao, R.; Yuqing, L.; Xuedong, Z. Models in Caries Research. In Dental Caries: Principles and Management; Springer: Berlin/Heidelberg, Germany, 2016; pp. 157–173. [Google Scholar] [CrossRef]
- De Rooij, J.F.; Arends, J.; Kolar, Z. Diffusion of monofluorophosphate in whole bovine Enamel at pH 7. Caries Res. 1981, 15, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.; Mellberg, J.R. Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res. 1981, 15, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; Al-Ahmad, A.; Follo, M.; Spitzmüller, B.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Visualization of initial bacterial colonization on dentine and enamel in situ. J. Microbiol. Methods 2010, 81, 166–174. [Google Scholar] [CrossRef]
- Deimling, D.; Breschi, L.; Hoth-Hannig, W.; Ruggeri, A.; Hannig, C.; Nekrashevych, Y.; Prati, C.; Hannig, M. Electron microscopic detection of salivary α-amylase in the pellicle formed in situ. Eur. J. Oral Sci. 2004, 112, 503–509. [Google Scholar] [CrossRef]
- Deimling, D.; Hannig, C.; Hoth-Hannig, W.; Schmitz, P.; Schulte-Mönting, J.; Hannig, M. Non-destructive visualisation of protective proteins in the in situ pellicle. Clin. Oral Investig. 2007, 11, 211–216. [Google Scholar] [CrossRef]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Lo, E.C.M.; Chu, C.H. Effects of fluoride on two chemical models of enamel demineralization. Materials 2017, 10, 1245. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, P.; Zobrist, K.; Attin, T.; Wegehaupt, F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J. Appl. Oral Sci. 2016, 24, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Schwendicke, F.; Eggers, K.; Meyer-Lueckel, H.; Dörfer, C.; Kovalev, A.; Gorb, S.; Paris, S. In vitro induction of residual caries lesions in dentin: Comparative mineral loss and nano-hardness analysis. Caries Res. 2015, 49, 259–265. [Google Scholar] [CrossRef]
- Maia, A.M.A.; de Freitas, A.Z.; de Campello, S.L.; Gomes, A.S.L.; Karlsson, L. Evaluation of dental enamel caries assessment using Quantitative Light Induced Fluorescence and Optical Coherence Tomography. J. Biophotonics 2016, 9, 596–602. [Google Scholar] [CrossRef]
- Oliveira, M.R.C.; Oliveira, P.H.C.; Oliveira, L.H.C.; Sfalcin, R.A.; Prates, R.A.; Navarro, R.S.; Cesar, P.F.; Deana, A.M.; Chavantes, M.C.; Bussadori, S.K.; et al. Influence of Ultrapulsed CO2 Laser, before Application of Different Types of Fluoride, on the Increase of Microhardness of Enamel in Vitro. Biomed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buskes, J.A.; Christoffersen, J.; Arends, J. Lesion formation and lesion remineralization in enamel under constant composition conditions: A new technique with applications. Caries Res. 1985, 19, 490–496. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography—A review [Invited]. Biomed. Opt. Express 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Yao, X.S.; Yao, H.; Liang, Y.; Liu, T.; Li, Y.; Wang, G.; Lan, S. Measurement of the refractive index of human teeth by optical coherence tomography. J. Biomed. Opt. 2009, 14, 034010. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Sadr, A.; Campillo-Funollet, M.; Nakashima, S.; Shimada, Y.; Tagami, J.; Sumi, Y. Effect of hydration on assessment of early enamel lesion using swept-source optical coherence tomography. J. Biophotonics 2013, 6, 171–177. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Silverstone, L.M.; Hicks, M.J.; Featherstone, M.J. Dynamic factors affecting lesion initiation and progression in human dental enamel. II. Surface morphology of sound enamel and carieslike lesions of enamel. Quintessence Int. 1988, 19. [Google Scholar]
- Robinson, C.; Shore, R.C.; Brookes, S.J.; Strafford, S.; Wood, S.R.; Kirkham, J. The chemistry of enamel caries. Crit. Rev. Oral Biol. Med. 2000, 11, 481–495. [Google Scholar] [CrossRef]
- Turani, Z.; Fatemizadeh, E.; Blumetti, T.; Daveluy, S.; Moraes, A.F.; Chen, W.; Mehregan, D.; Andersen, P.E.; Nasiriavanaki, M. Optical radiomic signatures derived from optical coherence tomography images improve identification of melanoma. Cancer Res. 2019, 79, 2021–2030. [Google Scholar] [CrossRef] [Green Version]
- Meurman, J.H.; Frank, R.M. Scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Res. 1991, 25, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, W.; Hsu, S.C.Y.; Huang, Z. Optical diagnosis and characterization of dental caries with polarization-resolved hyperspectral stimulated Raman scattering microscopy. Biomed. Opt. Express 2016, 7, 1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippok, N.; Braaf, B.; Villiger, M.; Oh, W.Y.; Vakoc, B.J.; Bouma, B.E. Quantitative depolarization measurements for fiber-based polarization-sensitive optical frequency domain imaging of the retinal pigment epithelium. J. Biophotonics 2019, 12, e201800156. [Google Scholar] [CrossRef] [PubMed]
- Tetschke, F.; Kirsten, L.; Golde, J.; Walther, J.; Galli, R.; Koch, E.; Hannig, C. Application of optical and spectroscopic technologies for the characterization of carious lesions in vitro. Biomed. Tech. 2018, 63, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J. Detection and diagnosis of the early caries lesion. BMC Oral Health 2015, 15. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetschke, F.; Golde, J.; Rosenauer, T.; Basche, S.; Walther, J.; Kirsten, L.; Koch, E.; Hannig, C. Correlation between Lesion Progression and Depolarization Assessed by Polarization-Sensitive Optical Coherence Tomography. Appl. Sci. 2020, 10, 2971. https://doi.org/10.3390/app10082971
Tetschke F, Golde J, Rosenauer T, Basche S, Walther J, Kirsten L, Koch E, Hannig C. Correlation between Lesion Progression and Depolarization Assessed by Polarization-Sensitive Optical Coherence Tomography. Applied Sciences. 2020; 10(8):2971. https://doi.org/10.3390/app10082971
Chicago/Turabian StyleTetschke, Florian, Jonas Golde, Tobias Rosenauer, Sabine Basche, Julia Walther, Lars Kirsten, Edmund Koch, and Christian Hannig. 2020. "Correlation between Lesion Progression and Depolarization Assessed by Polarization-Sensitive Optical Coherence Tomography" Applied Sciences 10, no. 8: 2971. https://doi.org/10.3390/app10082971
APA StyleTetschke, F., Golde, J., Rosenauer, T., Basche, S., Walther, J., Kirsten, L., Koch, E., & Hannig, C. (2020). Correlation between Lesion Progression and Depolarization Assessed by Polarization-Sensitive Optical Coherence Tomography. Applied Sciences, 10(8), 2971. https://doi.org/10.3390/app10082971




