Evaluation of Chloride Resistance of Early-Strength Concrete Using Blended Binder and Polycarboxylate-Based Chemical Admixture
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Experimental Plan and Mix Proportions
2.3. Experimental Methods
2.3.1. Fresh and Hardened Properties of Concrete
2.3.2. Chloride Penetration Depth and Chloride Ion Diffusion Coefficient
2.3.3. Porosity of Inner Concrete
3. Results and Discussion
3.1. Fresh and Hardened Properties of Concrete
3.2. Relation between Chloride Penetration Depth and Compressive Strength
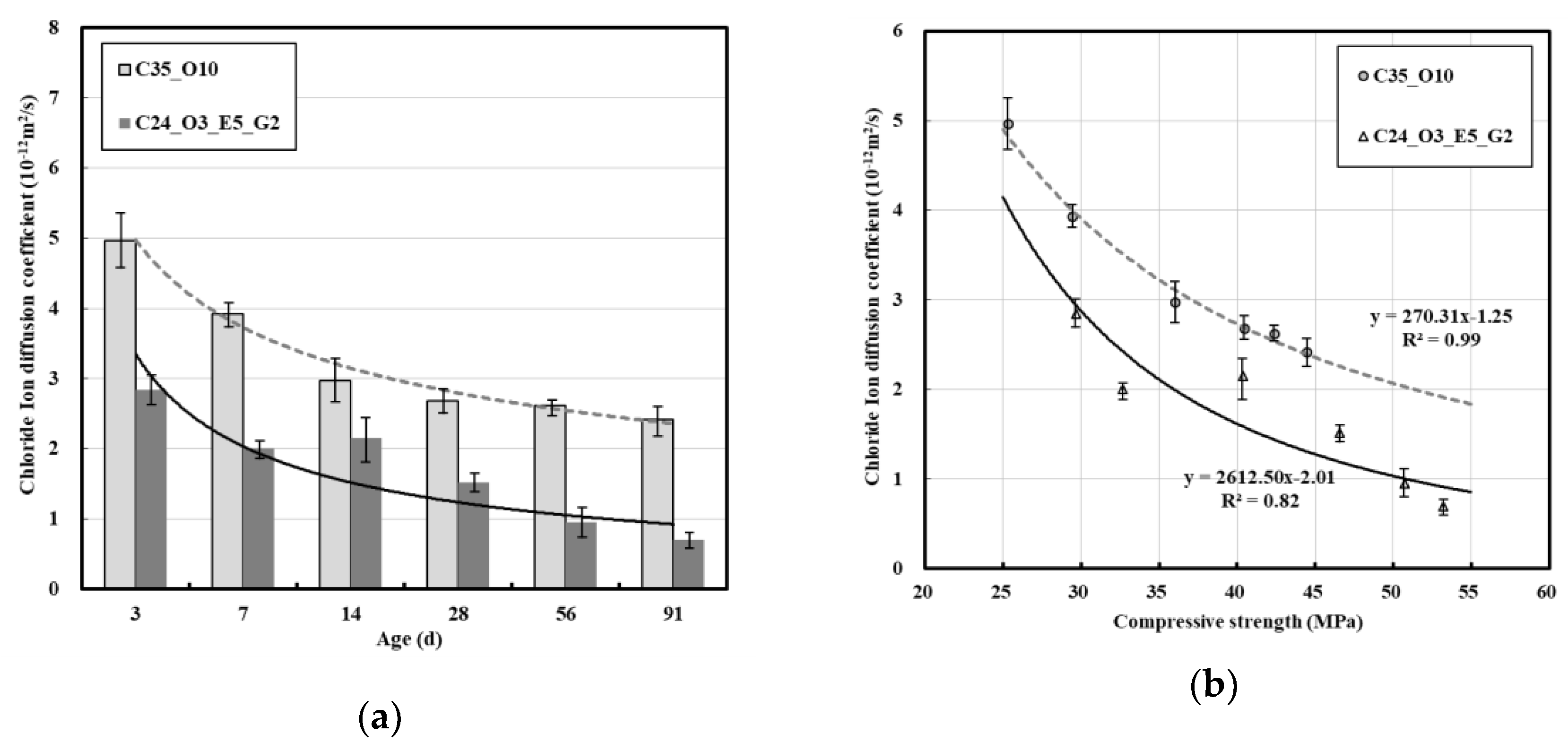
3.3. Relation between Chloride Ion Diffusion Coefficient and Compressive Strength
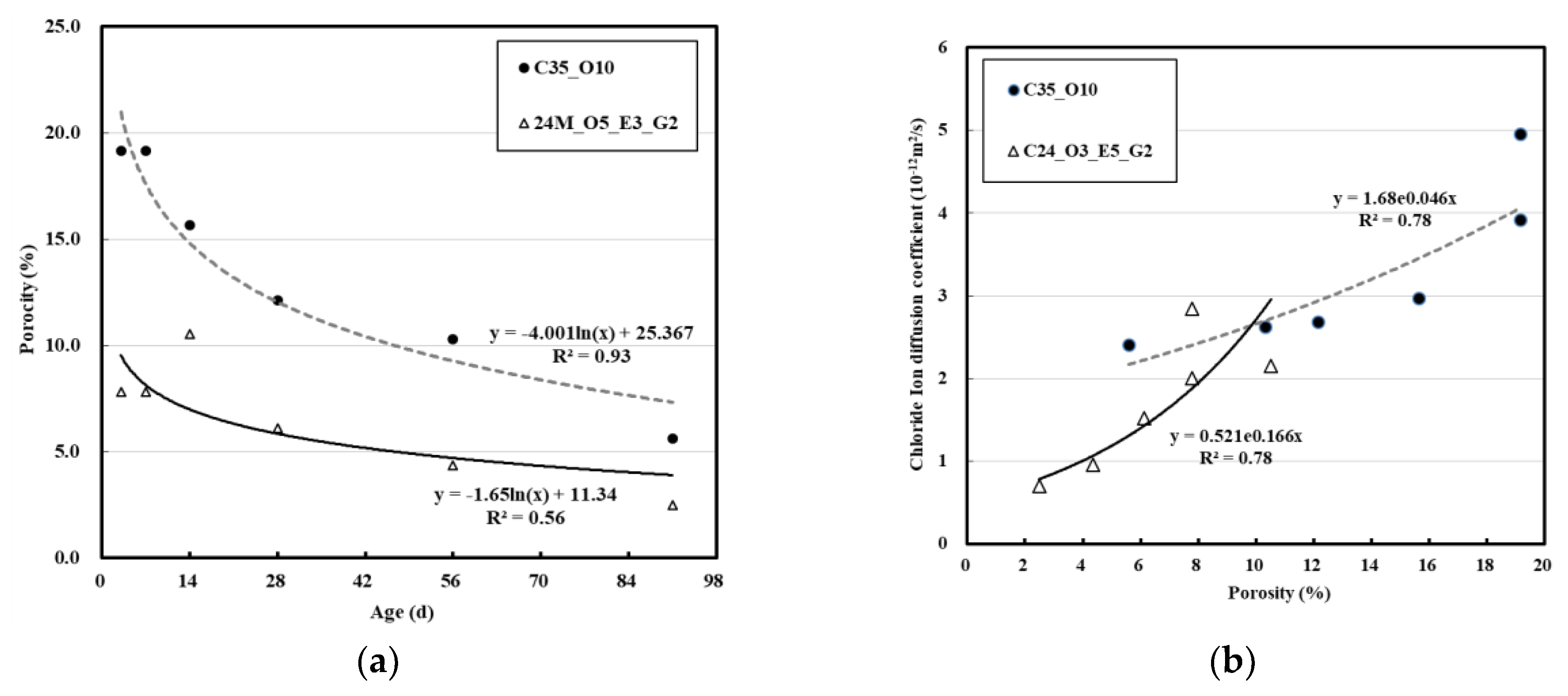
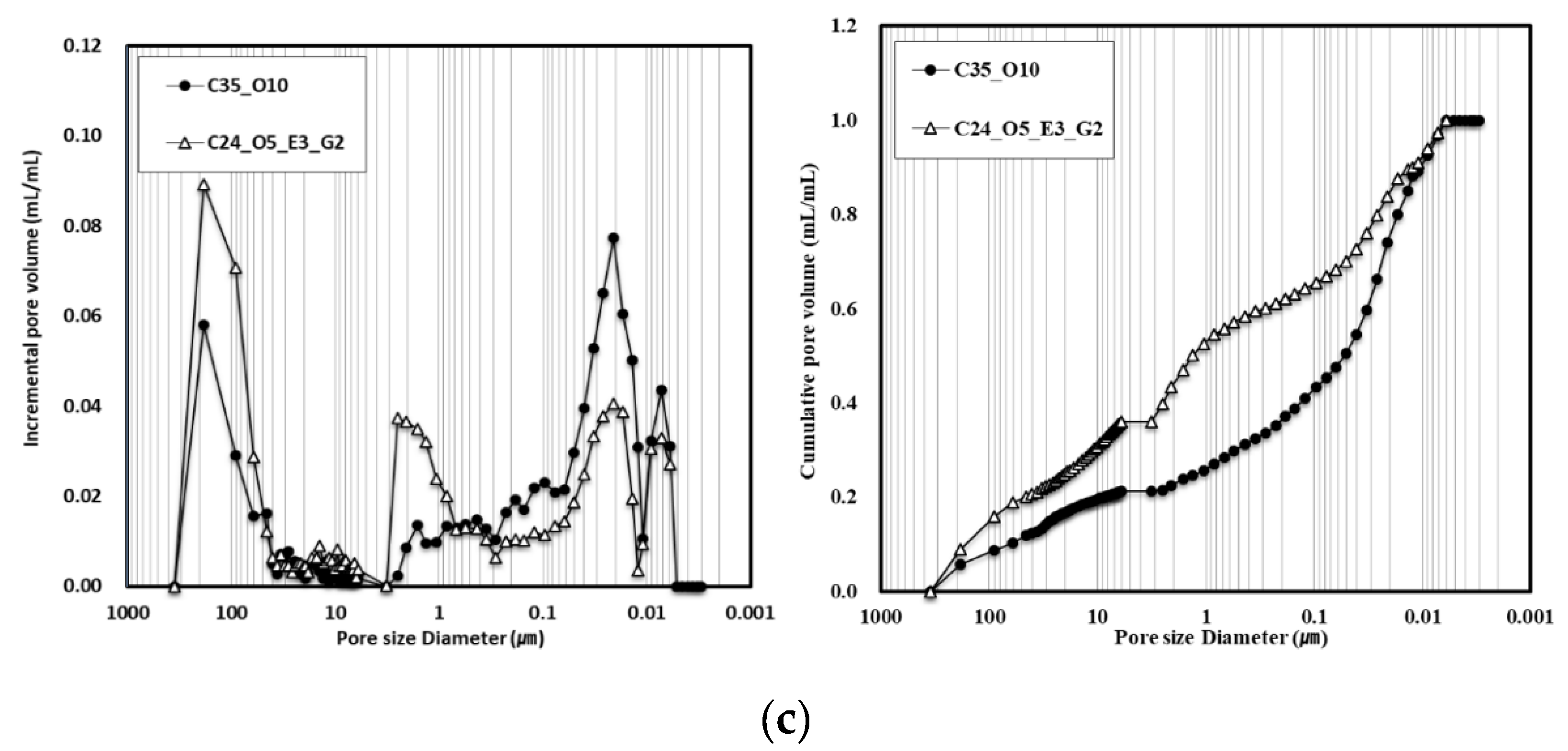
3.4. Effect of Porosity of Concrete
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Ismail, M.; Ohtsu, M. Corrosion rate of ordinary and high-performance concrete subjected to chloride attack by AC impedance spectroscopy. Constr. Build. Mater. 2006, 20, 458–469. [Google Scholar] [CrossRef]
- Hu, M.; Long, F.; Tang, M. The thaumasite form of sulfate attack in concrete of Yongan Dam. Concrete 2006, 36, 2006–2008. [Google Scholar]
- Ma, B.; Gao, X.; Byars, E.A.; Zhou, Q. Thaumasite formation in a tunnel of Bapanxia Dam in Western China. Cem. Concr. Res. 2006, 36, 716–722. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete, 5th ed.; Trans-Atlantic, Publications, Inc.: Philadelphia, PA, USA, 2012. [Google Scholar]
- De Medeiros-Junior, R.A.; de Lima, M.G.; de Brito, P.C.; de Medeiros, M.H.F. Chloride penetration into concrete in an offshore platform-analysis of exposure conditions. Ocean. Eng. 2015, 103, 78–87. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Glass, G.K.; Stevenson, G.M.; Buenfeld, N.R. Chloride-binding isotherms from the diffusion cell test. Cem. Concr. Res. 1998, 28, 939–945. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F.; Coats, A.M.; Glasser, F.P. Chloride ingress in cement paste and mortar. Cem. Concr. Res. 1999, 29, 1497–1504. [Google Scholar] [CrossRef]
- Li, L.Y.; Page, C.L. Modelling of electrochemical chloride extraction from concrete: Influence of ionic activity coefficients. Comput. Mater. Sci. 1998, 9, 303–308. [Google Scholar] [CrossRef]
- Li, D.W.; Li, L.Y.; Wang, X.F. Chloride diffusion model in concrete in marine environment with considering binding effect. Mar. Struct. 2019, 66, 44–51. [Google Scholar] [CrossRef]
- Korea Architectural Standard Specification Reinforced Concrete Work; Architectural Institute of Korea: Seoul, Korea, 2009.
- Japanese Architectural Standard Specification Reinforced Concrete Work JASS 5; Architectural Institute of Japan: Tokyo, Japan, 2009.
- ACI 318-99: Building Code Requirements for Structural Concrete; American Concrete Institute: Farmington Hills, MI, USA, 1999.
- Thomas, M.; Bremner, T. Performance of lightweight aggregate concrete containing slag after 25 years in a harsh marine environment. Cem. Concr. Res. 2012, 42, 358–364. [Google Scholar] [CrossRef]
- Song, H.-W.; Lee, C.-H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Padaratz, I.J.; Alonso, C.; Borba, J.C., Jr. Chloride penetration into concrete structures in the marine atmosphere zone—Relationship between deposition of chlorides on the wet candle and chlorides accumulated into concrete. Cem. Concr. Compos. 2007, 29, 667–676. [Google Scholar] [CrossRef]
- CEB-FIP Model Code 1990: Design Code; Thomas Telford Publishing: London, UK, 1993.
- BS EN 13670:2009, Execution of Concrete Structures; BSI: London, UK, 2010.
- ACI 201.2R-16: Guide to Durable Concrete; American Concrete Institute: New York, NY, USA, 2016.
- EN 1992-1-1: Eurocode 2: Design of Concrete Structures—Part 1-1: General Rules and Rules for Buildings; BSI: London, UK, 2004.
- Çakır, Ö.; Aköz, F. Effect of curing conditions on the mortars with and without GGBFS. Constr. Build. Mater. 2008, 22, 308–314. [Google Scholar] [CrossRef]
- Sojobi, A.O. Evaluation of the performance of eco-friendly lightweight interlocking concrete paving units incorporating sawdust wastes and laterite. Cogent Eng. 2016, 3, 1255168. [Google Scholar] [CrossRef]
- Rashad, A.M.; Sadek, D.M. An investigation on Portland cement replaced by high-volume GGBS pastes modified with micro-sized metakaolin subjected to elevated temperatures. Int. J. Sustain. Built Environ. 2017, 6, 91–101. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Uysal, M.; Sumer, M. Performance of self-compacting concrete containing different mineral admixtures. Constr. Build. Mater. 2011, 25, 4112–4120. [Google Scholar] [CrossRef]
- Mohan, A.; Mini, K.M. Strength and durability studies of SCC incorporating silica fume and ultra fine GGBS. Constr. Build. Mater. 2018, 171, 919–928. [Google Scholar] [CrossRef]
- Kavitha, S.; Kala, T.F. Evaluation of strength behavior of self-compacting concrete using alccofine and GGBS as partial replacement of cement. Indian J. Sci. Technol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Juradin, S.; Vlajić, D. Influence of cement type and mineral additions, silica fume and metakaolin, on the properties of fresh and hardened self-compacting concrete. In Mechanical and Materials Engineering of Modern Structure and Component Design; Springer International Publishing: Cham, Switzerland, 2015; Volume 70, pp. 251–267. [Google Scholar]
- Huang, C.H.; Wu, C.H.; Lin, S.K.; Yen, T. Effect of slag particle size on fracture toughness of concrete. Appl. Sci. 2019, 9, 805. [Google Scholar] [CrossRef]
- Ganesh, P.; Murthy, A.R. Tensile behaviour and durability aspects of sustainable ultra-high performance concrete incorporated with GGBS as cementitious material. Constr. Build. Mater. 2019, 197, 667–680. [Google Scholar] [CrossRef]
- Reddy Suda, V.B.; Srininvasa Rao, P. Experimental investigation on optimum usage of micro silica and GGBS for the strength characteristics of concrete. Mater. Today Proc. 2020, in press. [Google Scholar]
- Bost, P.; Regnier, M.; Horgnies, M. Comparison of the accelerating effect of various additions on the early hydration of Portland cement. Constr. Build. Mater. 2016, 113, 290–296. [Google Scholar] [CrossRef]
- Kurihara, R.; Maruyama, I. Effects of heating and drying on the strength and stiffness of high-early-strength Portland cement pastes. Cem. Concr. Compos. 2020, 106, 103455. [Google Scholar] [CrossRef]
- Wen, X.-D.; Feng, L.; Hu, D.-Y.; Wang, K.; Zhang, Z. Effect of side-chain length in polycarboxylic superplasticizer on the early-age performance of cement-based materials. Constr. Build. Mater. 2019, 211, 26–32. [Google Scholar] [CrossRef]
- Li, P.; Ma, Z.; Li, X.; Lu, X.; Hou, P.; Du, P. Effect of gypsum on hydration and hardening properties of alite modified calcium sulfoaluminate cement. Materials 2019, 12, 3131. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Wang, J.; Guan, X. Effects of aluminum sulfate and quicklime/fluorgypsum ratio on the properties of calcium sulfoaluminate (CSA) cement-based double liquid grouting materials. Materials 2019, 12, 1222. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T. Influences of chemical composition and fineness on the development of concrete strength by curing conditions. Materials 2019, 12, 4061. [Google Scholar] [CrossRef]
- Pradeep Kumar, M.; Mini, K.M.; Rangarajan, M. Ultrafine GGBS and calcium nitrate as concrete admixtures for improved mechanical properties and corrosion resistance. Constr. Build. Mater. 2018, 18210, 249–257. [Google Scholar] [CrossRef]
- Luo, R.; Cai, Y.; Wang, C.; Huang, X. Study of chloride binding and diffusion in GGBS concrete. Cem. Concr. Res. 2003, 33, 1–7. [Google Scholar] [CrossRef]
- Sakai, Y. Relationship between pore structure and chloride diffusion in cementitious materials. Constr. Build. Mater. 2019, 229, 116868. [Google Scholar] [CrossRef]
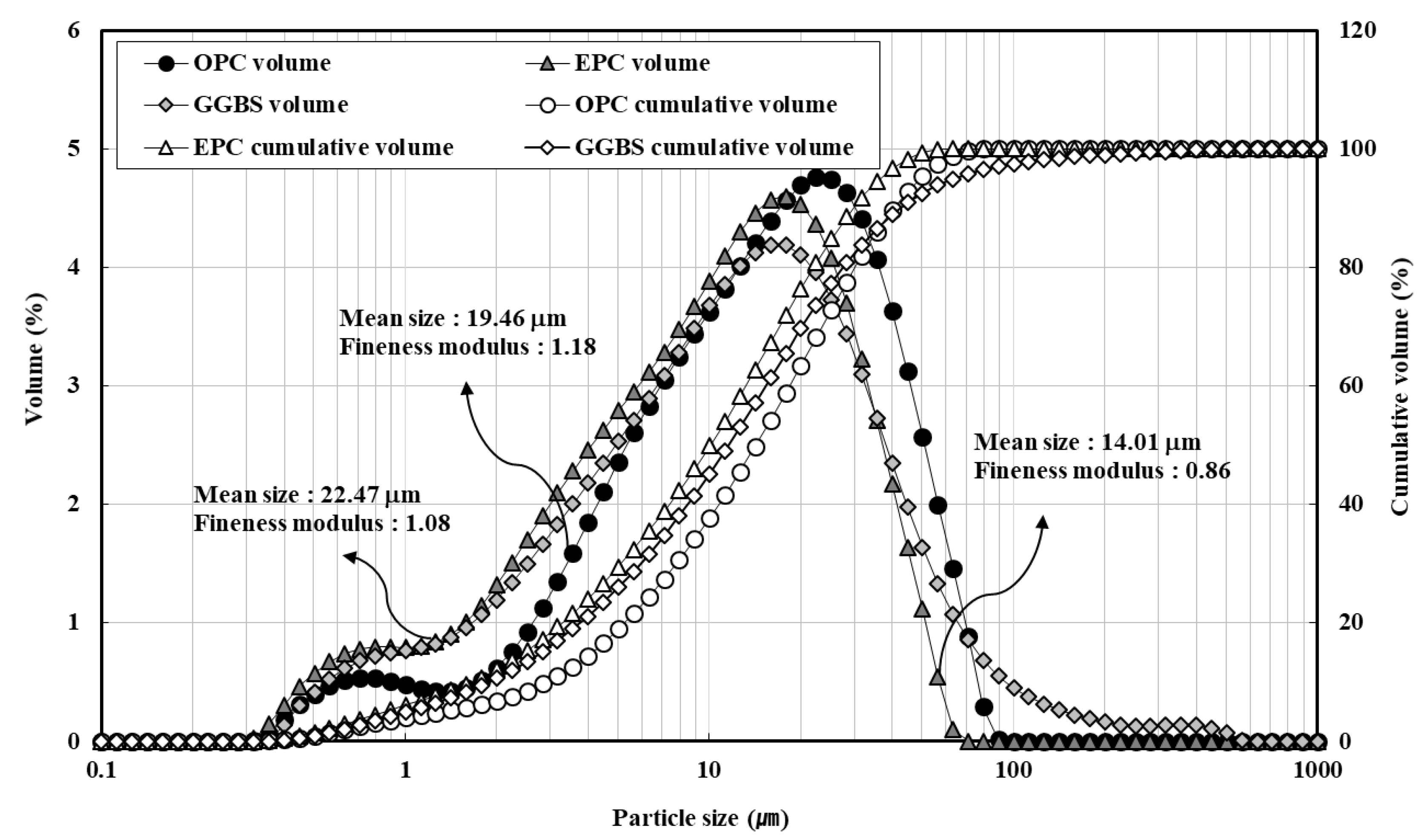
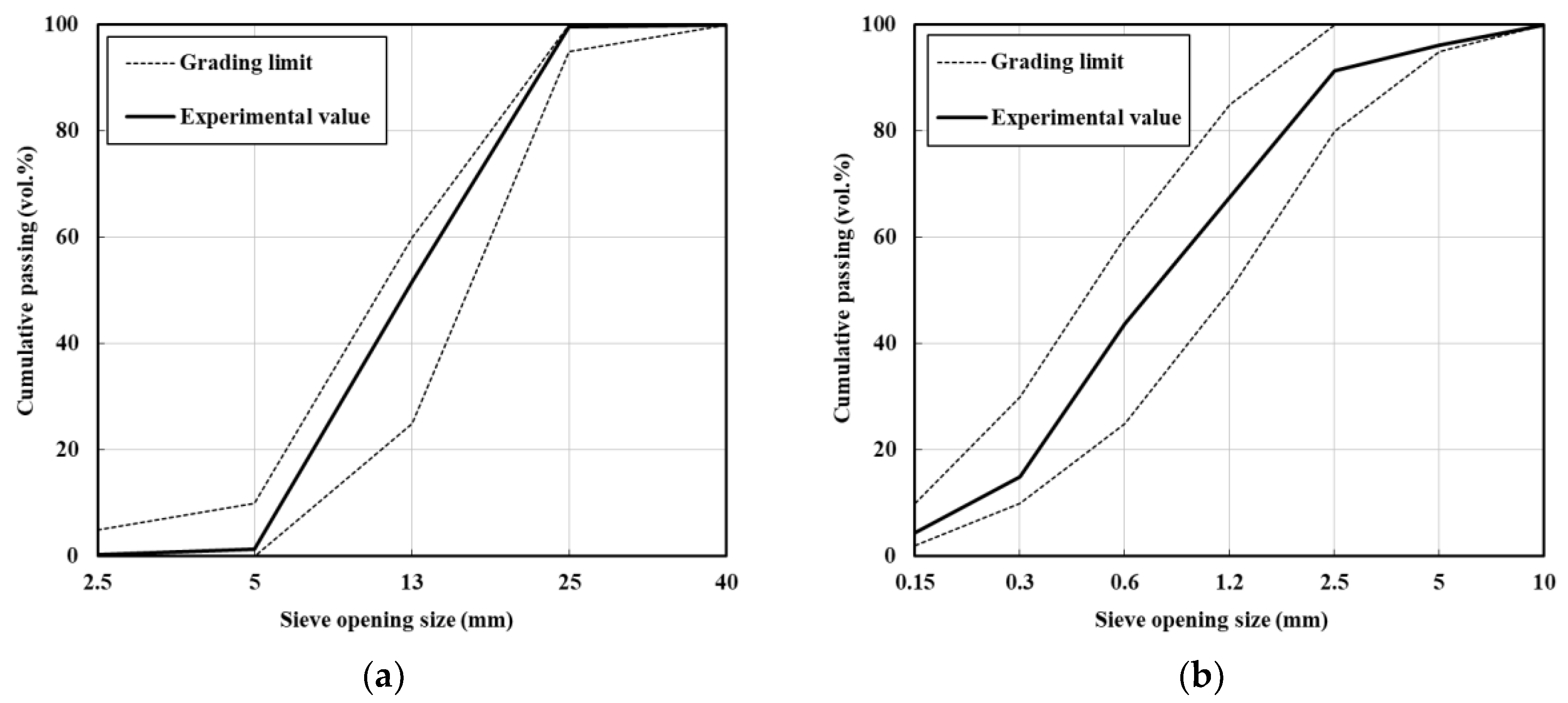
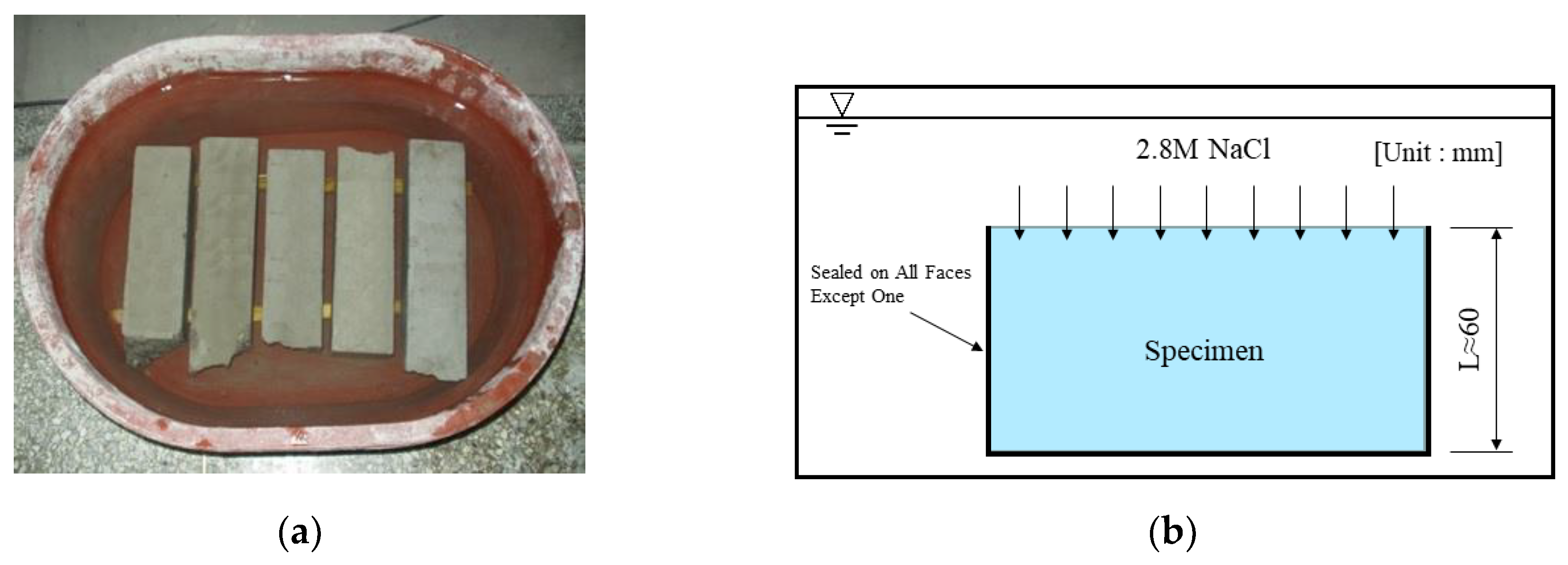
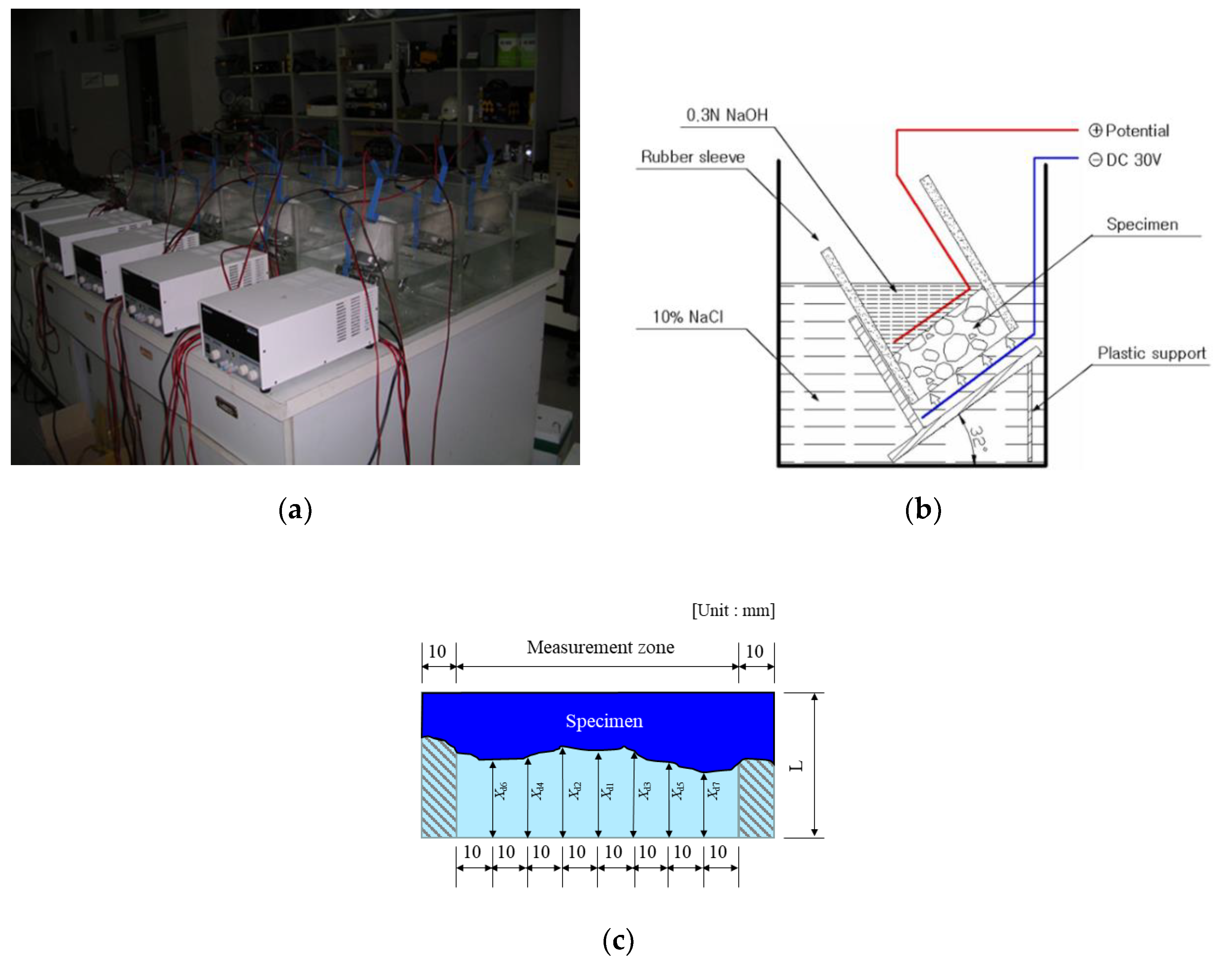
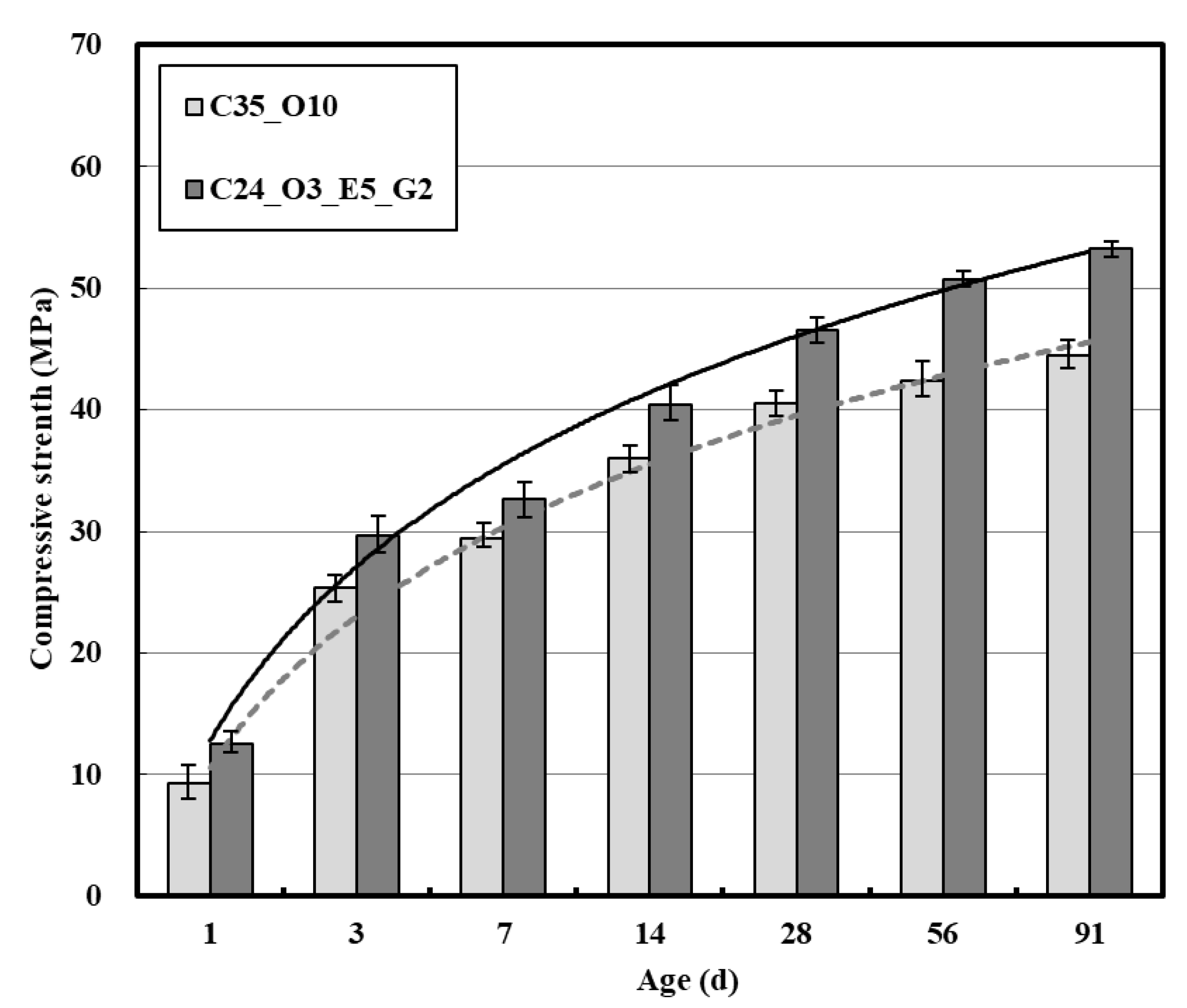

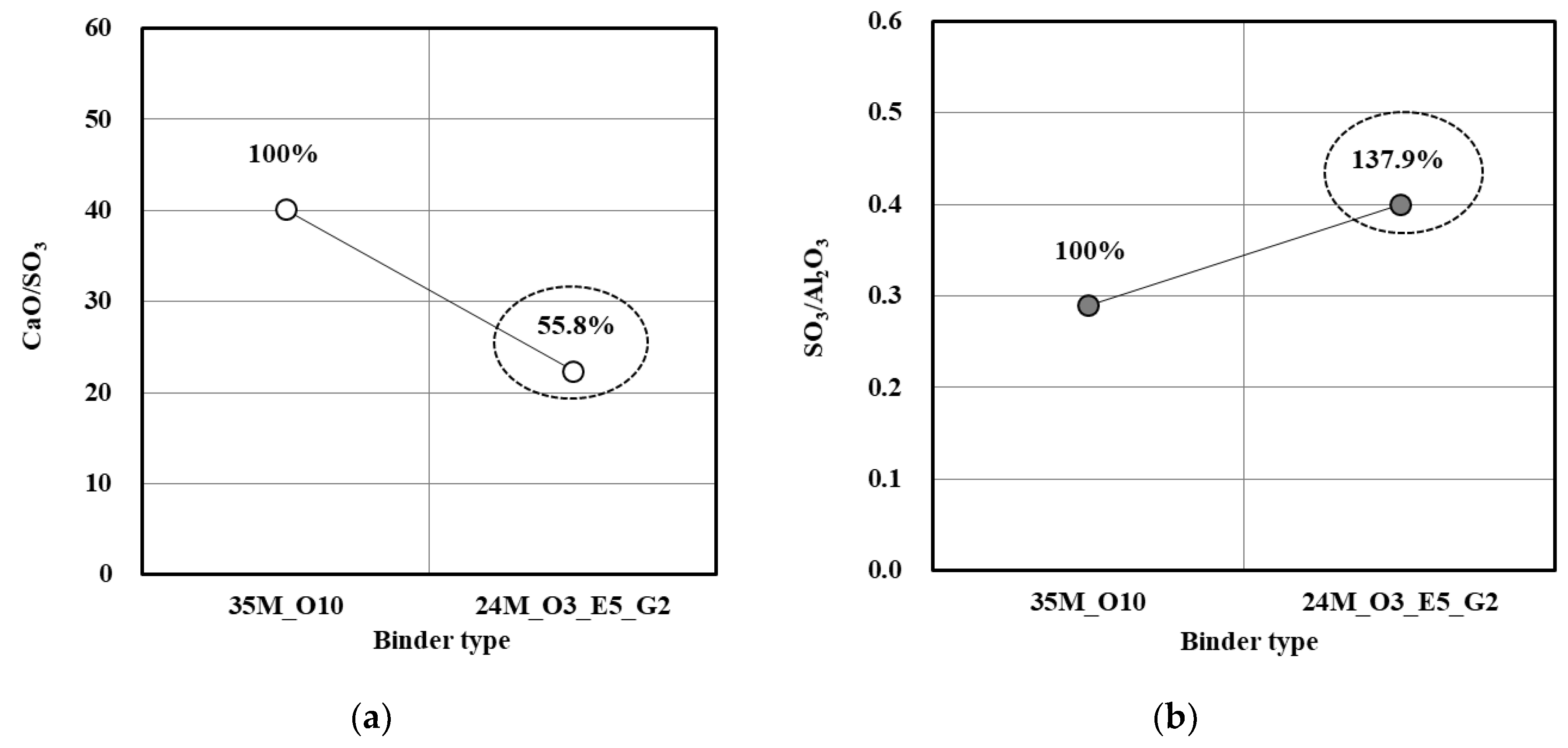
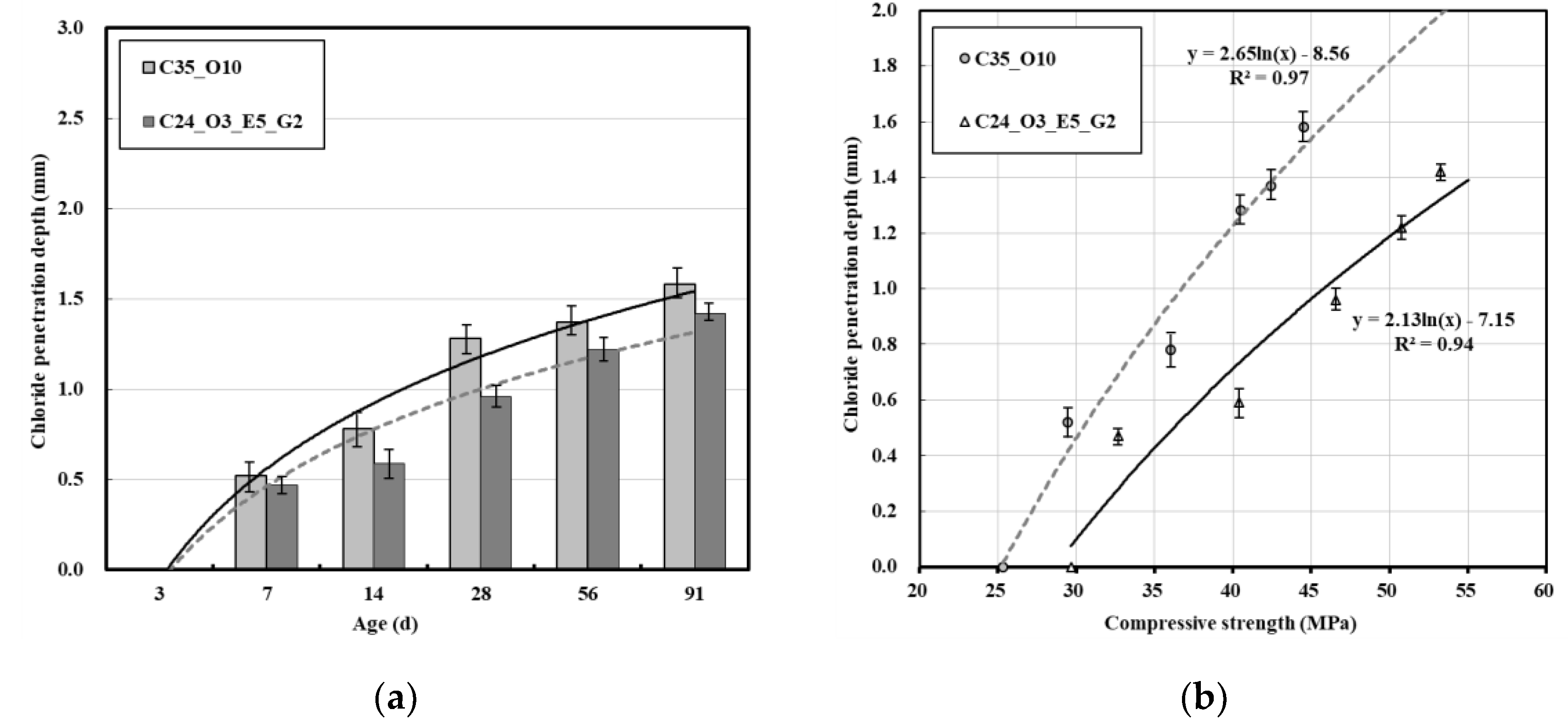

| Material | Property | |
|---|---|---|
| Cement | OPC | ASTM Type I ordinary Portland cement Density: 3150 kg/m3, fineness: 330 m2/kg, mean particle size: 19.46 µm |
| EPC | ASTM Type III early Portland cement Density: 3160 kg/m3, fineness: 488 m2/kg, mean particle size: 14.01 µm | |
| Mineral admixture | GGBS | Ground granulated blast-furnace slag Density: 2860 kg/m3, fineness: 430 m2/kg, mean particle size: 22.47 µm |
| Fine aggregate | S1 | Washed sea sand, size: 5 mm Fineness modulus: 2.01, density: 2600 kg/m3, absorption: 0.79% |
| S2 | Crushed sand, size: 5 mm Fineness modulus: 3.29, density: 2570 kg/m3, absorption: 0.87% | |
| Coarse aggregate | Crushed granitic aggregate Size: 25 mm, density: 2600 kg/m3, absorption: 0.76% | |
| Chemical admixture | NP | Naphthalene group, density: 1220 kg/m3 |
| PC | Polycarboxylic acid group, density: 1260 kg/m3 | |
| Materials | Chemical Compositions (%) | L.O.I. 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | SO3 | Others | ||
| OPC 1 | 60.34 | 19.82 | 4.85 | 3.30 | 3.83 | 1.08 | 2.90 | 0.86 | 3.02 |
| EPC 2 | 61.44 | 20.33 | 4.72 | 3.42 | 2.95 | 0.95 | 3.73 | 0.79 | 1.67 |
| GGBS 3 | 44.90 | 35.4 | 13.00 | 0.47 | 5.01 | 0.37 | 1.31 | - | 0.69 |
| Mix No. | OPC mix | W/B 1 | Total Unit Weight of Binder (kg/m3) | Proportion of Binder (%) | Chemical Admixture | ||
|---|---|---|---|---|---|---|---|
| OPC | EPC | GGBS | |||||
| C35_O10 | C35 2 | 0.42 | 444 | 100 | - | - | NP |
| C24_O3_E5_G2 | C24 3 | 0.50 | 340 | 30 | 50 | 20 | PC |
| Mix No. 1 | W/B 2 | S/A 3 (%) | Unit Weight (kg/m3) | NP (B×%) | PC (B×%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| W 4 | OPC | EPC | GGBS | S 5 | G 6 | |||||
| C35_O10 | 0.42 | 48.5 | 185 | 444 | 908 | 919 | 0.7 | |||
| C24_O3_E5_G2 | 0.50 | 48.5 | 165 | 102 | 170 | 68 | 910 | 921 | 0.7 | |
| Evaluation Item | Test Method |
|---|---|
| Slump (mm) | ASTM C143 |
| Air contents (%) | ASTM C231 |
| Compressive strength (MPa) | ASTM C873; ASTM C39 |
| Chloride penetration depth (mm) | NT Build 443 |
| Chloride ion diffusion coefficient (m2/s) | NT Build 492 |
| Porosity (%) | ASTM D 4404 |
| Mix No. | Slump (mm) | Air Contents (%) | ||
|---|---|---|---|---|
| Initial | After 60 min | Initial | After 60 min | |
| C35_O10 | 195 | 160 | 4.6 | 4.6 |
| C24_O3_E5_G2 | 190 | 180 | 4.6 | 4.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Lee, J. Evaluation of Chloride Resistance of Early-Strength Concrete Using Blended Binder and Polycarboxylate-Based Chemical Admixture. Appl. Sci. 2020, 10, 2972. https://doi.org/10.3390/app10082972
Lee T, Lee J. Evaluation of Chloride Resistance of Early-Strength Concrete Using Blended Binder and Polycarboxylate-Based Chemical Admixture. Applied Sciences. 2020; 10(8):2972. https://doi.org/10.3390/app10082972
Chicago/Turabian StyleLee, Taegyu, and Jaehyun Lee. 2020. "Evaluation of Chloride Resistance of Early-Strength Concrete Using Blended Binder and Polycarboxylate-Based Chemical Admixture" Applied Sciences 10, no. 8: 2972. https://doi.org/10.3390/app10082972
APA StyleLee, T., & Lee, J. (2020). Evaluation of Chloride Resistance of Early-Strength Concrete Using Blended Binder and Polycarboxylate-Based Chemical Admixture. Applied Sciences, 10(8), 2972. https://doi.org/10.3390/app10082972






