Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Supercritical Fluid Extraction
2.3. Experimental Design
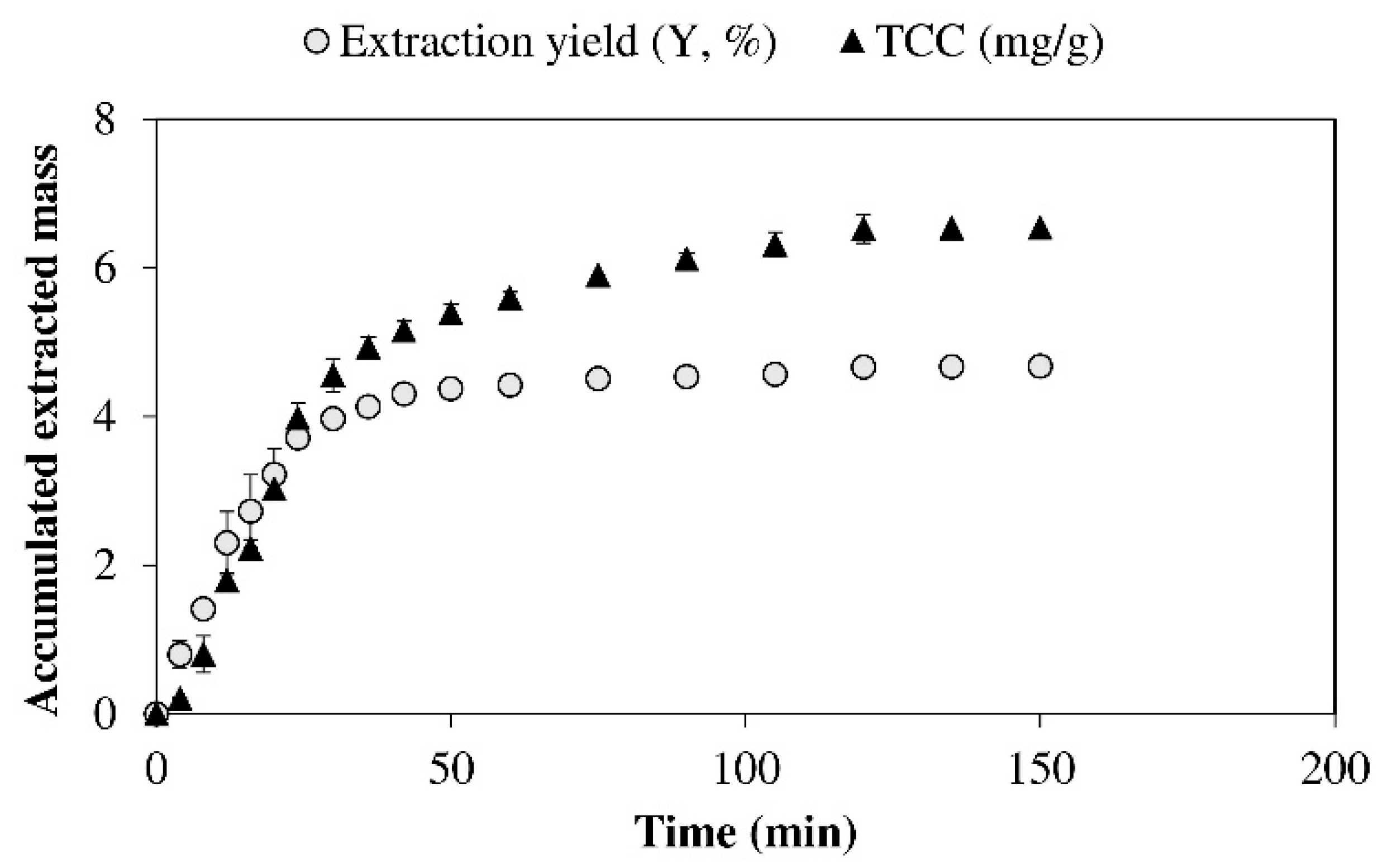
2.4. Kinetic Study
2.5. Extracts Analysis
2.5.1. Total Carotenoids Content (TCC)
2.5.2. Total Carotenoids Recovery
2.5.3. Total Phenol Content (TPC)
2.5.4. Determination of Antioxidant Activity
2.5.5. Extraction of Fatty Acid
2.5.6. Analysis of Fatty Acids
3. Results and Discussion
3.1. Specific Kinetics and Selection of Box-Behnken Design of Supercritical Fluid Extraction from Isochrysis galbana
3.2. Effects of Different Parameters on the Extraction Yield and Total Carotenoids Content and Recovery
+ 0.045·T·Co-solvent - 0.025·Co-solvent2
solvent2
0.14·Co-solvent2
3.3. Total Phenolic Content and Antioxidant Response in Isochrysis galbana by Supercritical Fluid Extraction
0.000012·P·Co-solvent − 0.0014·T2 + 0.00069·T·Co-solvent − 0.0068·Co-solvent2
3.4. Measurement of Fatty Acid Composition
0.10·Cosolvent2
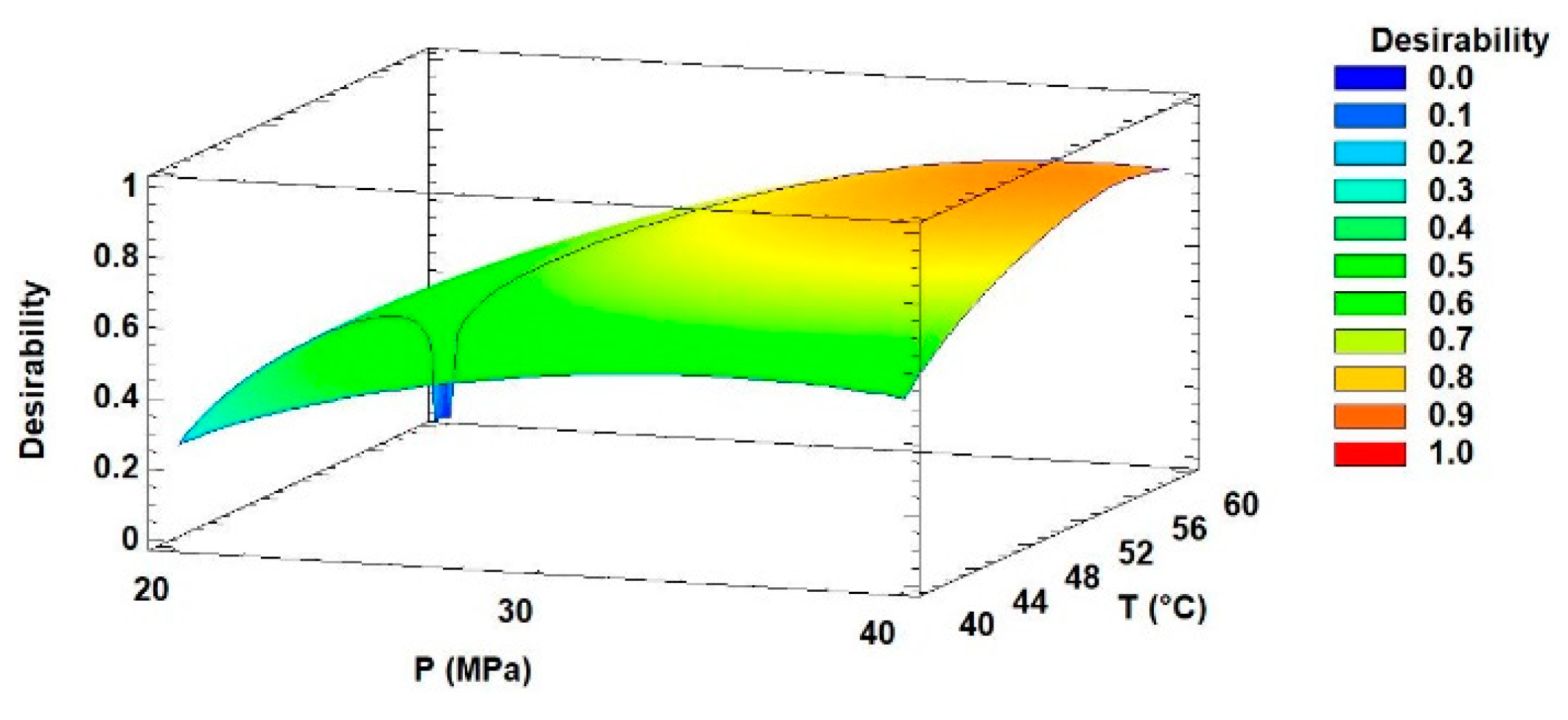
3.5. Desirability Function
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicoletti, M. Microalgae nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Thompkinson, D.K. Multiple Functional Ingredient Approach in Formulating Dietary Supplement for Management of Diabetes: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Svirčev, Z. Microalgae and Cyanobacteria: Food for thought (1). J. Phycol. 2008, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Lee, T.; González-Mariño, G.E. Microalgae for “healthy” foods—Possibilities and challenges. Compr. Rev. Food. Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Gouveia, L.; Coutinho, C.; Mendonça, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional biscuits with PUFA-ω3 from Isochrysis galbana. J. Sci. Food Agric. 2008, 88, 891–896. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R.; Demers, E.; Roussy, M. Variation of lipid class and fatty acid composition of Chaetoceros muelleri and Isochrysis sp. grown in a semicontinuous system. Aquaculture 2003, 221, 393–406. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trac-Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Mulders, K.J.; Weesepoel, Y.; Lamers, P.P.; Vincken, J.-P.; Martens, D.E.; Wijffels, R.H. Growth and pigment accumulation in nutrient-depleted Isochrysis aff. galbana T-ISO. J. Appl. Phycol. 2013, 25, 1421–1430. [Google Scholar] [CrossRef]
- Kalam, S.; Gul, M.Z.; Singh, R.; Ankati, S. Free radicals: Implications in etiology of chronic diseases and their amelioration through nutraceuticals. Pharmacologia 2015, 6, 11–20. [Google Scholar]
- Miyashita, K. Function of marine carotenoids. In Food Factors for Health Promotion; Karger Publishers: Kyoto, Japan, 2009; Volume 61, pp. 136–146. [Google Scholar]
- Raposo, M.; de Morais, A.; de Morais, R. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef]
- Sousa, I.; Gouveia, L.; Batista, A.P.; Raymundo, A.; Bandarra, N.M. Microalgae in Novel Food Products; Food Chemistry Research Developments: New York, NY, USA, 2008; pp. 75–112. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the significance mnemonic of green analytical practices. Trac-Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Del Pilar Sánchez-Camargo, A.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; van de Laak, C.C.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Moure, A.; Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. J. Appl. Phycol. 2015, 27, 957–964. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K. Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. Afr. J. Biotechnol. 2011, 10, 18855–18862. [Google Scholar]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminalia japonica. Food Sci. Technol. Res. 2008, 14, 573. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Fernández, F.A.; Grima, E.M. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Widowati, I.; Zainuri, M.; Kusumaningrum, H.P.; Susilowati, R.; Hardivillier, Y.; Leignel, V.; Bourgougnon, N.; Mouget, J.-L. Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bali, Indonesia, 25–27 October 2016; p. 012067. [Google Scholar]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Michalak, I.; Dmytryk, A.; Wieczorek, P.P.; Rój, E.; Łęska, B.; Górka, B.; Messyasz, B.; Lipok, J.; Mikulewicz, M.; Wilk, R.; et al. Supercritical algal extracts: A source of biologically active compounds from nature. J. Chem. 2015, 14, 37. [Google Scholar]
- Boonchum, W.; Peerapornpisal, Y.; Kanjanapothi, D.; Pekkoh, J.; Pumas, C.; Jamjai, U.; Amornlerdpison, D.; Noiraksar, T.; Vacharapiyasophon, P. Antioxidant activity of some seaweed from the Gulf of Thailand. Int. J. Agric. Biol. 2011, 13, 95–99. [Google Scholar]
- Roh, M.-K.; Uddin, M.S.; Chun, B.-S. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Büyükokuroğlu, M.; Gülçin, I.; Oktay, M.; Küfrevioğlu, O. In vitro antioxidant properties of dantrolene sodium. Pharmacol. Res. 2001, 44, 491–494. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibanez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercri. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef]
- Kühn, S.; Temelli, F. Recovery of bioactive compounds from cranberry pomace using ternary mixtures of CO2+ ethanol+ water. J. Supercrit. Fluids 2017, 130, 147–155. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. Trac-Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Di Mascio, P.; Murphy, M.E.; Sies, H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991, 53, 194S–200S. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Marino, T.; Karatza, D.; Musmarra, D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules 2019, 24, 782. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Mouahid, A.; Toudji, S.-A.A.; Lépine, O.; Badens, E. Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J. Supercrit. Fluids 2013, 79, 337–344. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Supercritical CO2 extraction of rosehip seed oil: Fatty acids composition and process optimization. J. Supercrit. Fluids 2007, 41, 421–428. [Google Scholar] [CrossRef]
- Cheung, P.C. Temperature and pressure effects on supercritical carbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem. 1999, 65, 399–403. [Google Scholar] [CrossRef]




| Terms of the Model | Y | TCC | TC recovery | TPC | TEAC | FAMEs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated | p-value | Estimated | p-value | Estimated | p-value | Estimated | p-value | Estimated | p-value | Estimated | p-value | |
| constant | 51.47 | 35.73 | 146.47 | –619.40 | –2.85 | –17.72 | ||||||
| A:P | –0.09 | 0.05 * | 0.11 | 0.18 | 0.44 | 0.18 | 2.38 | 0.32 | –0.0007 | 0.02* | 0.07 | 0.15 |
| B:T | –1.44 | 0.14 | –1.73 | 0.67 | –7.10 | 0.67 | 17.03 | 0.19 | 0.124 | 0.81 | 0.44 | 0.43 |
| C:Co-solvent | –1.23 | 0.0012 * | –2.27 | 0.009* | –9.32 | 0.009 * | –21.94 | 0.07 | 0.0364 | 0.05 * | –0.63 | 0.01 * |
| AA | 0.00002 | 0.83 | –0.00021 | 0.31 | –0.00086 | 0.31 | –0.0045 | 0.05 * | –3.33 E–7 | 0.91 | –0.0001 | 0.06 |
| AB | 0.0019 | 0.051 | 0.0004 | 0.83 | 0.0016 | 0.84 | 0.0030 | 0.87 | 0.00003 | 0.29 | 0.0004 | 0.64 |
| AC | 0.0001 | 0.96 | 0.0049 | 0.33 | 0.020 | 0.33 | 0.0775 | 0.12 | –0.00001 | 0.86 | 0.0002 | 0.91 |
| BB | 0.0078 | 0.37 | 0.014 | 0.48 | 0.058 | 0.48 | –0.2061 | 0.29 | –0.00136 | 0.01 * | –0.0064 | 0.17 |
| BC | 0.045 | 0.06 | 0.037 | 0.45 | 0.151 | 0.45 | 0.2357 | 0.59 | 0.00069 | 0.36 | 0.032 | 0.16 |
| CC | –0.025 | 0.63 | 0.033 | 0.79 | 0.136 | 0.79 | –0.7877 | 0.50 | –0.00677 | 0.01 * | –0.061 | 0.10 |
| Lack-of-Fit | 0.13 | 0.29 | 0.30 | 0.10 | 0.05 | 0.04 | ||||||
| Statistics for the goodness of fit of the model | ||||||||||||
| R2 | 0.930 | 0.825 | 0.825 | 0.806 | 0.918 | 0.874 | ||||||
| Adjusted R2 | 0.804 | 0.510 | 0.510 | 0.457 | 0.770 | 0.647 | ||||||
| RSD | 1.506 | 3.565 | 14.616 | 33.068 | 0.054 | 1,567 | ||||||
| P | 0.336 | 0.905 | 0.905 | 0.356 | 0.871 | 0.251 | ||||||
| C.V. | 0.639 | 0.647 | 0.647 | 0.676 | 0.529 | 0.558 | ||||||
| Run | P | T | Co-solvent | Y | TCC | TC Recovery | TPC | TEAC | FAMEs |
|---|---|---|---|---|---|---|---|---|---|
| (MPa) | ( °C) | (%) | (%, w/w) | (mg/g) | (%, w/w) | (mg GAE/g) | (mmol TE/g) | (mg/g) | |
| 1 | 30 | 40 | 8 | 5.71 ± 0.24 | 14.09 ± 0.55 | 57.75 ± 2.30 | 93.33 ± 3.52 | 0.11 ± 5.3 × 10−3 | 3.41 ± 0.13 |
| 2 | 40 | 40 | 4 | 6.16 ± 0.16 | 9.66 ± 0.32 | 39.61 ± 1.45 | 50.93 ± 2.01 | 0.28 ± 1.3 × 10−2 | 5.56 ± 0.16 |
| 3 | 40 | 60 | 4 | 10.25 ± 0.49 | 6.22 ± 0.29 | 25.49 ± 1.10 | 22.89 ± 1.02 | 0.33 ± 1.4 × 10−2 | 5.41 ± 0.21 |
| 4 | 40 | 50 | 0 | 2.28 ± 0.11 | 4.05 ± 0.16 | 16.61 ± 0.74 | 5.98 ± 0.23 | 0.22 ± 1.1 × 10−2 | 1.18 ± 0.04 |
| 5 | 20 | 50 | 0 | 1.09 ± 0.03 | 1.34 ± 0.06 | 5.50 ± 0.21 | 5.71 ± 0.20 | 0.15 ± 7.5 × 10−3 | 0.47 ± 0.02 |
| 6 | 30 | 50 | 4 | 5.79 ± 0.21 | 7.02 ± 0.33 | 28.78 ± 1.35 | 109.31 ± 3.56 | 0.31 ± 1.4 × 10−2 | 7.57 ± 0.26 |
| 7 | 40 | 50 | 8 | 8.78 ± 0.32 | 15.33 ± 0.72 | 62.85 ± 2.68 | 157.16 ± 3.66 | 0.31 ± 1.5 × 10−2 | 7.19 ± 0.33 |
| 8 | 30 | 50 | 4 | 5.19 ± 0.24 | 10.83 ± 0.51 | 44.38 ± 1.89 | 94.40 ± 4.02 | 0.40 ± 1.8 × 10−2 | 7.63 ± 0.29 |
| 9 | 30 | 50 | 4 | 4.36 ± 0.20 | 6.00 ± 0.28 | 24.57 ± 1.02 | 120.77 ± 4.02 | 0.33 ± 1.5 × 10−2 | 6.87 ± 0.32 |
| 10 | 30 | 60 | 8 | 12.82 ± 0.06 | 19.01 ± 0.92 | 77.93 ± 2.87 | 76.06 ± 3.52 | 0.20 ± 9.0 × 10−3 | 8.82 ± 0.38 |
| 11 | 30 | 60 | 0 | 1.64 ± 0.08 | 2.74 ± 0.18 | 11.24 ± 0.42 | 37.71 ± 1.44 | 0.04 ± 1.0 × 10−3 | 2.86 ± 0.12 |
| 12 | 20 | 40 | 4 | 5.73 ± 0.12 | 9.11 ± 0.45 | 37.33 ± 1.63 | 67.87 ± 3.21 | 0.15 ± 6.2 × 10−3 | 3.50 ± 0.13 |
| 13 | 20 | 50 | 8 | 7.43 ± 0.35 | 4.85 ± 0.23 | 19.87 ± 0.75 | 32.90 ± 1.20 | 0.26 ± 1.2 × 10−2 | 6.09 ± 0.28 |
| 14 | 30 | 40 | 0 | 1.80 ± 0.08 | 3.72 ± 0.17 | 15.25 ± 0.65 | 92.69 ± 3.54 | 0.06 ± 2.1 × 10−3 | 2.63 ± 0.11 |
| 15 | 20 | 60 | 4 | 2.15 ± 0.10 | 4.10 ± 0.20 | 16.80 ± 0.82 | 28.04 ± 1.32 | 0.07 ± 2.9 × 10−3 | 1.78 ± 0.07 |
| Fatty Acid | Common Name | Run | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| C14:0 | Myristic acid | 20.0 ± 0.5 | n.d. | n.d. | 22.8 ± 1.02 | 4.9 ± 0.21 | n.d. | n.d. | 3.7 ±0.2 | n.d. | 24.2 ±1.2 | n.d. | 0.2 ± 0.01 | n.d. | n.d. | n.d. |
| C16:0 | Palmitic acid | n.d. | 4.9 ± 0.2 | 13.8 ± 0.5 | 9.1 ± 0.40 | 6.6 ± 0.28 | 5.6 ± 0.1 | n.d. | 7.1 ±0.3 | n.d. | n.d. | n.d. | 7.8 ± 0.3 | 8.5 ±0.38 | 11.0 ±0.4 | 9.7 ± 0.4 |
| C16:1 | Palmitoleic acid | 9.8 ± 0.3 | n.d. | n.d. | n.d. | n.d. | 9.0 ± 0.3 | 8.9 ±0.3 | 14.9 ±0.5 | n.d. | 30.1 ±1.3 | 3.0 ±0.1 | n.d. | n.d. | 7.4 ±0.3 | n.d. |
| C18:0 | Stearic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.4 ±0.06 | n.d. | 2.4 ±0.05 | 3.1 ±0.1 | 1.7 ± 0.08 | 1.4 ±0.06 | 1.3 ±0.04 | 1.4 ± 0.07 |
| C18:1 | Oleic acid | n.d. | n.d. | n.d. | n.d. | 2.2 ± 0.09 | 0.6 ± 0.02 | n.d. | 2.3 ±0.10 | n.d. | n.d. | n.d. | 47.7 ±1.7 | 36.7 ±1.5 | 37.9 ±1.7 | 36.2 ± 1.5 |
| C18:2 | Linoleic acid | 39.1 ± 1.7 | 43.5 ± 1.9 | 6.0 ± 0.3 | 2.7 ± 0.11 | 35.0 ± 1.42 | 48.1 ± 1.7 | 41.9 ± 1.8 | 40.7 ±1.8 | 53.6±1.68 | 43.3 ±1.8 | 88.1 ±3.5 | 26.1 ±1.2 | 17.9 ±0.7 | 18.9 ±0.7 | 18.2 ± 0.8 |
| C18:3 | Linolenic acid | 26.2 ± 1.2 | 36.5 ± 1.7 | 54.1 ± 2.5 | 48.4 ± 1.89 | 28.7 ± 1.25 | 25.5 ± 1.2 | 24.9± 1.0 | n.d. | 42.0 ±1.89 | n.d. | 0.9 ±0.04 | 4.0 ±0.1 | 19.6 ±0.8 | 2.9 ±0.1 | n.d. |
| C20:0 | Eicosanoic acid | n.d. | n.d. | 5.8 ± 0.2 | 3.5 ± 0.17 | 3.9 ± 0.15 | 1.6 ± 0.04 | 2.9±0.05 | 1.5 ±0.06 | n.d. | n.d. | 4.1 ±0.2 | 10.2 ±0.5 | 12.6 ±0.5 | 15.3 ±0.7 | 22.6 ± 1.1 |
| C21:0 | Methyl heneicosanoate | n.d. | n.d. | n.d. | n.d. | 10.8 ± 0.42 | n.d. | 9.2±0.3 | 8.8 ±0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C22:2 | Cis-13,16- docosadienoic acid methyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.2 ±0.1 | 3.4 ±0.1 | 3.6 ± 0.1 | 8.2 ± 0.3 |
| C20:3 | Cis-11-14-17-eicosatrienoic acid methyl ester | 5.1 ± 0.2 | 8.5 ± 0.3 | 11.5 ± 0.5 | 7.8 ± 0.3 | 6.0 ± 0.3 | 6.2 ± 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Others | n.d. | 6.5 ± 0.3 | 8.8 ± 0.4 | 5.8 ± 0.2 | 1.9 ± 0.08 | 3.4 ± 0.1 | 12.2± 0.6 | 18.6 ±0.8 | 4.4 ±0.1 | n.d. | n.d. | n.d. | n.d. | 1.8 ±0.0 | 3.8 ± 0.1 | |
| Ʃ SFAs | Total saturated fatty acids | 20.0 | 6.7 | 23.3 | 35.4 | 26.2 | 7.2 | 24.3 | 42.1 | 4.4 | 26.6 | 7.1 | 20.0 | 22.5 | 29.3 | 37.5 |
| Ʃ MUFAs | Total monounsaturated fatty acids | 9.8 | 4.8 | 5.2 | 5.8 | 4.1 | 13.0 | 8.9 | 17.2 | n.d. | 30.1 | 3.0 | 47.7 | 36.7 | 45.3 | 36.2 |
| Ʃ PUFAs | Total polyunsaturated fatty acids | 70.3 | 88.5 | 71.6 | 58.8 | 69.7 | 79.8 | 66.8 | 40.7 | 95.6 | 43.3 | 89.9 | 32.4 | 40.9 | 25.4 | 26.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Domínguez, M.C.; Cerezal, P.; Salinas, F.; Medina, E.; Renato-Castro, G. Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana . Appl. Sci. 2020, 10, 2789. https://doi.org/10.3390/app10082789
Ruiz-Domínguez MC, Cerezal P, Salinas F, Medina E, Renato-Castro G. Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana . Applied Sciences. 2020; 10(8):2789. https://doi.org/10.3390/app10082789
Chicago/Turabian StyleRuiz-Domínguez, Mari Carmen, Pedro Cerezal, Francisca Salinas, Elena Medina, and Gabriel Renato-Castro. 2020. "Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana " Applied Sciences 10, no. 8: 2789. https://doi.org/10.3390/app10082789
APA StyleRuiz-Domínguez, M. C., Cerezal, P., Salinas, F., Medina, E., & Renato-Castro, G. (2020). Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana . Applied Sciences, 10(8), 2789. https://doi.org/10.3390/app10082789





