Assessment of Melting Kinetics of Sugar-Reduced Silver Ear Mushroom Ice Cream under Various Additive Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Ice Cream Mixture Formulation and Processing
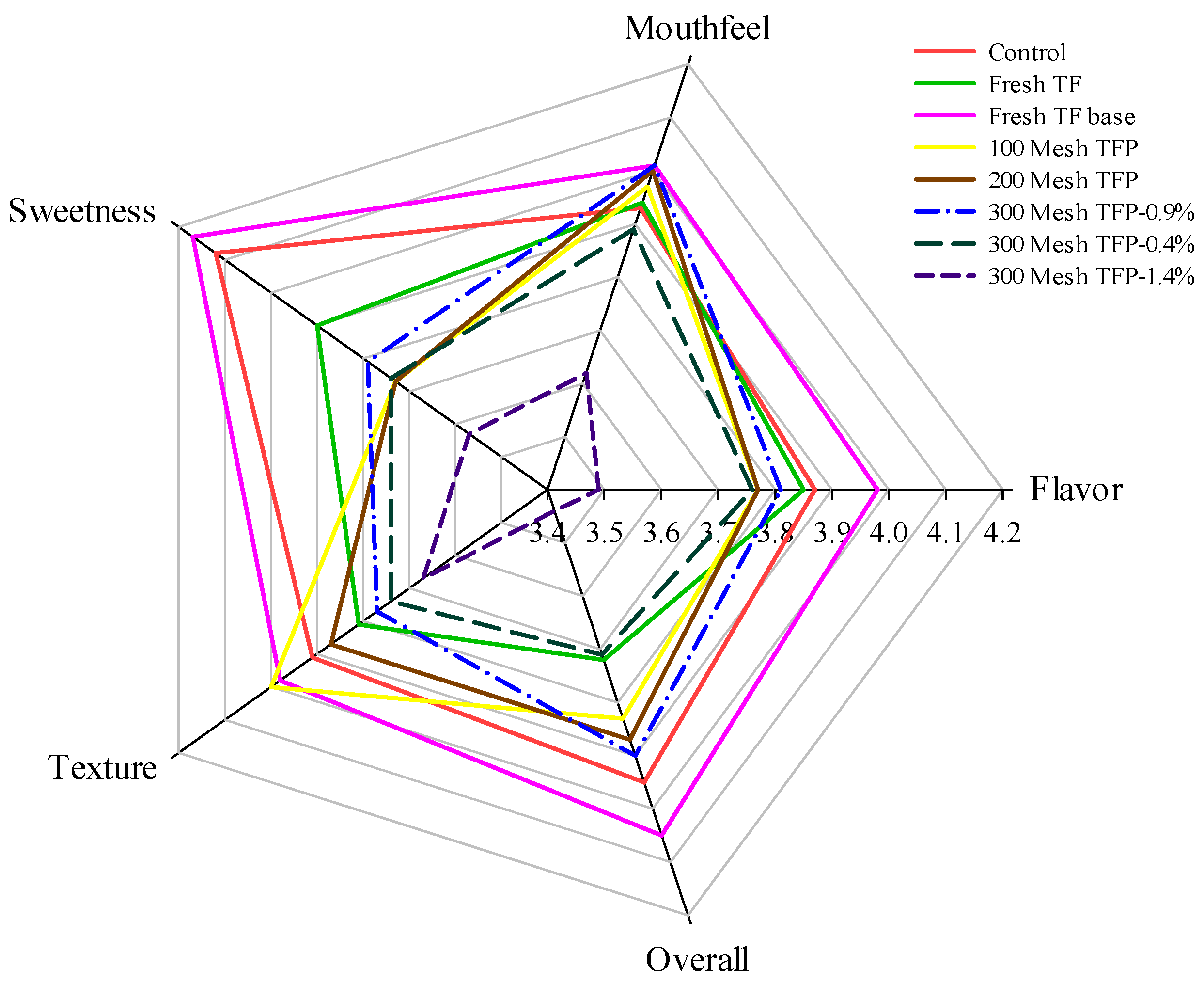
2.2. Consumer Sensory Evaluation
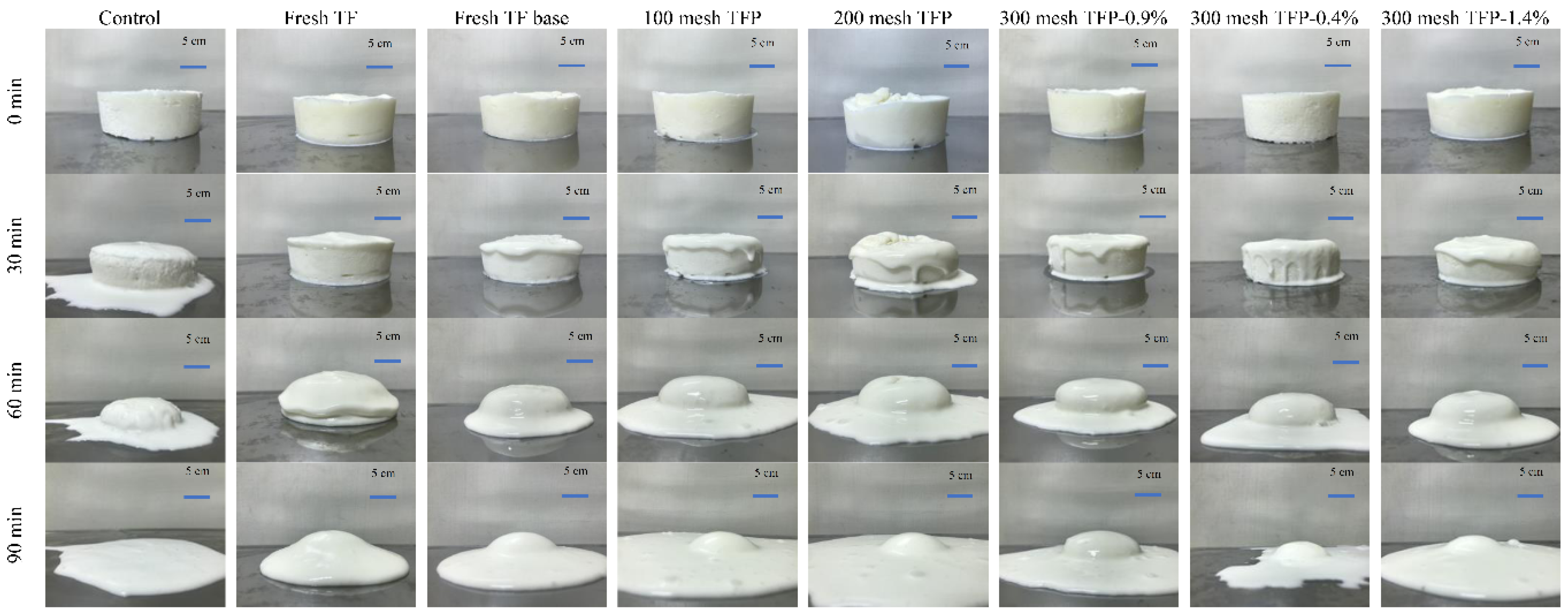
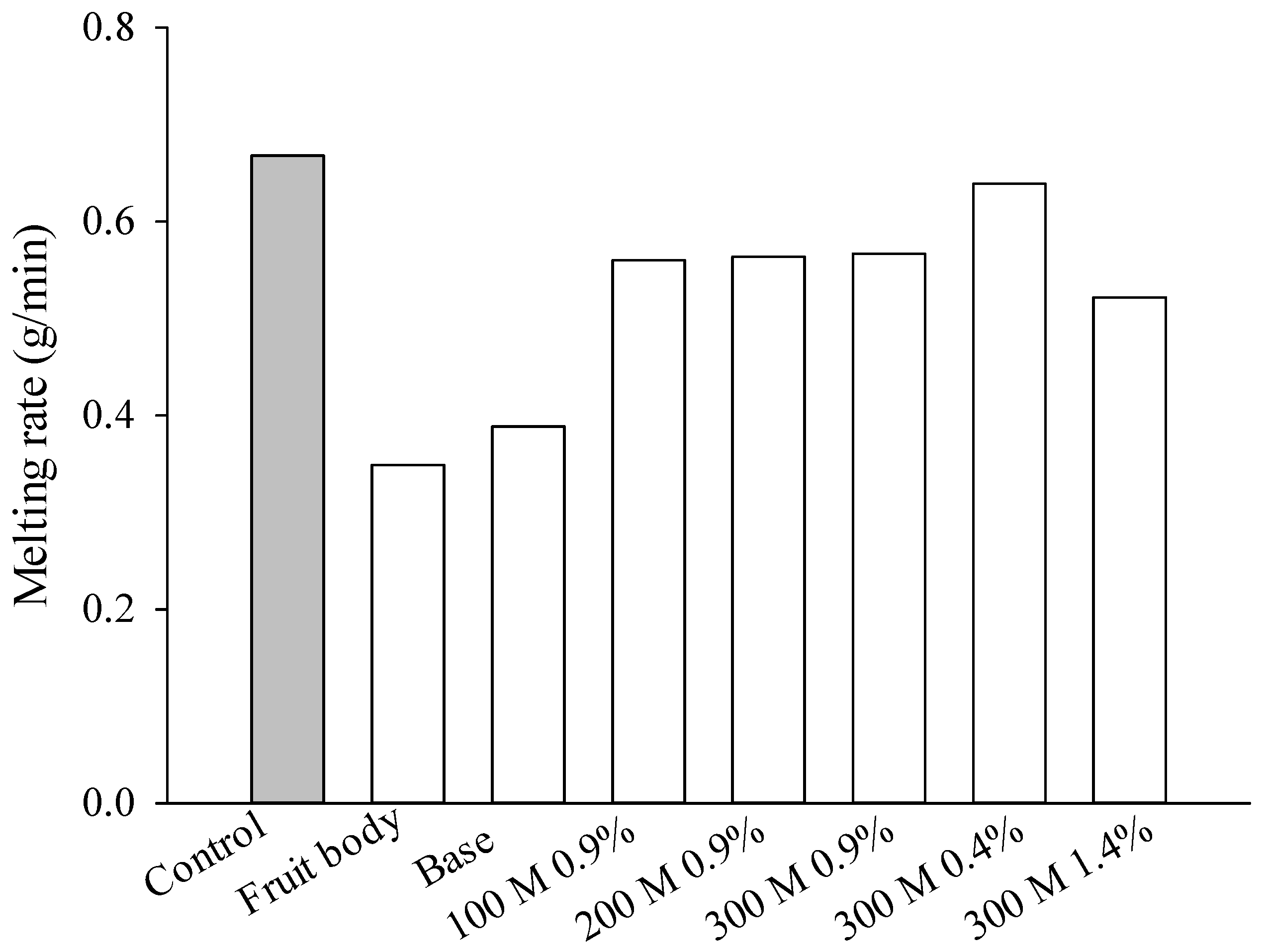
2.3. Melting Behavior and Melting Rate Measurements
2.4. DSC Measurements
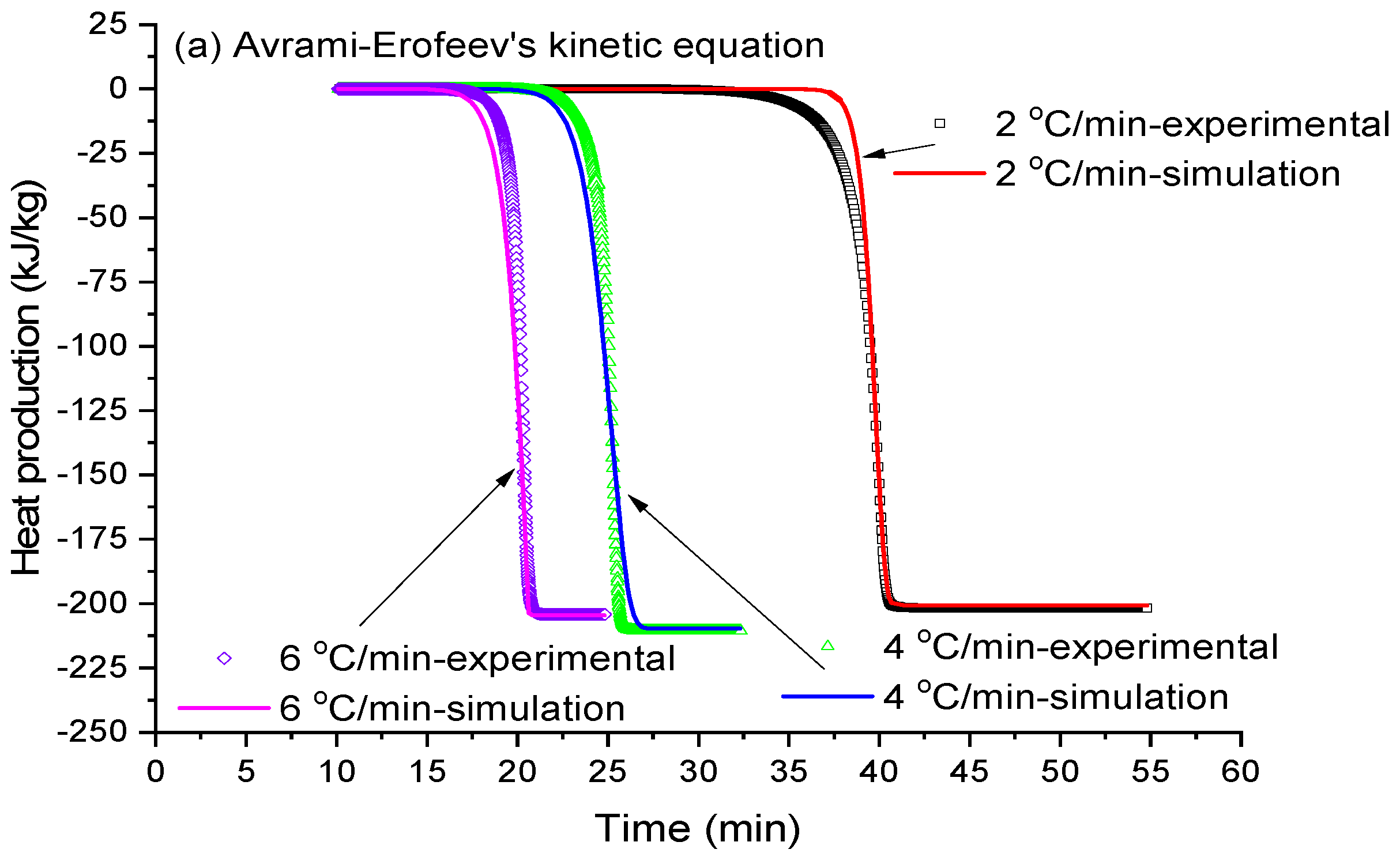
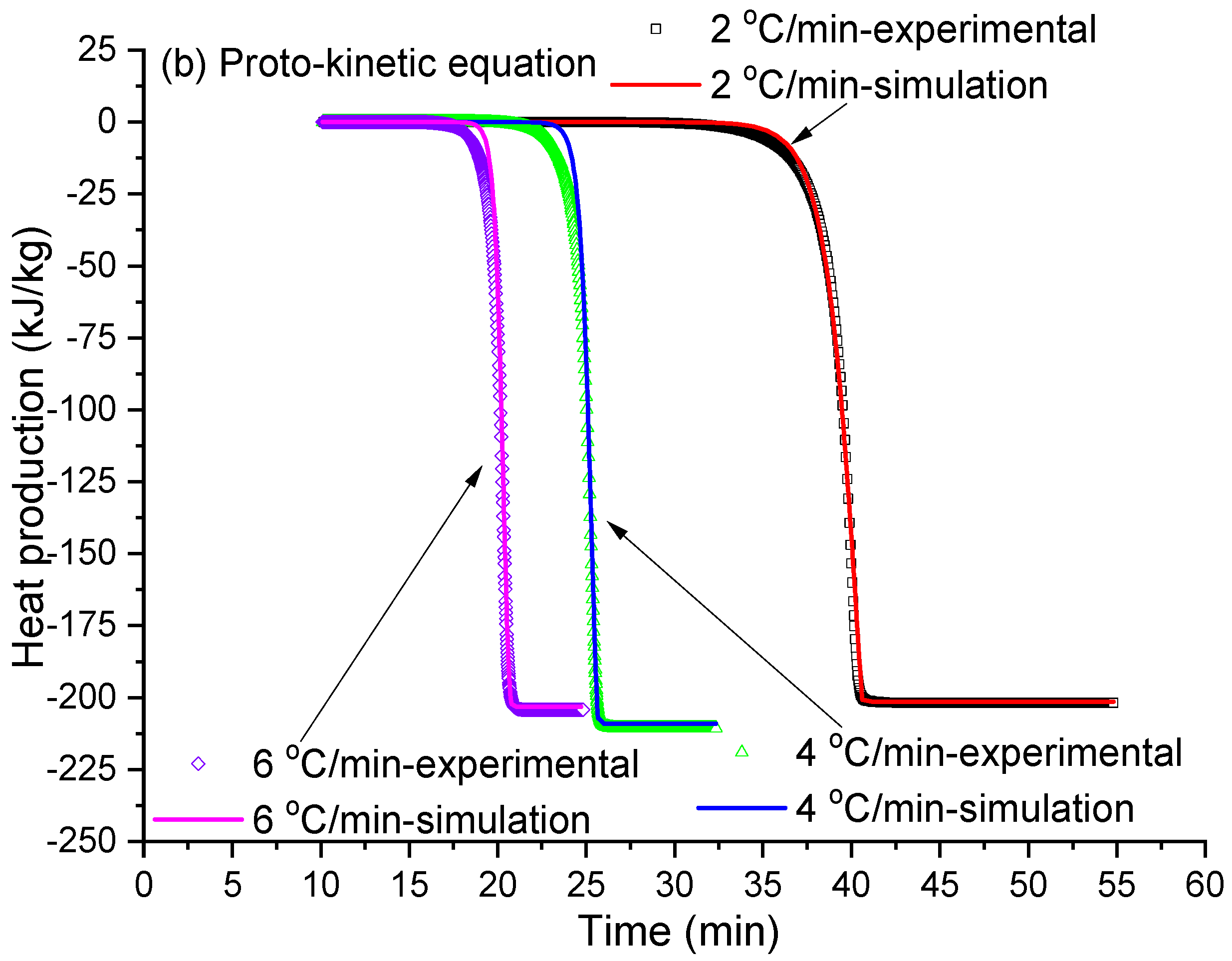
2.5. Melting Kinetic Simulation
2.6. Determination of Physical Characteristic
2.7. Determination of Chemical Characteristics
2.8. Statistical Analyses
3. Results and Discussion
3.1. Sensory Quality Characteristics of T. fuciformis Ice Cream
3.2. Melting Behavior and Melting Rate of T. fuciformis Ice Cream
3.3. Melting Properties of T. Fuciformis Ice Cream by DSC Analysis
3.4. Ice Cream Melting Kinetic Simulation
3.5. Physicochemical Qualities of T. Fuciformis Ice Cream
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurt, A.; Atalar, I. Effects of quince seed on the rheological, structural and sensory characteristics of ice cream. Food Hydrocoll. 2018, 82, 186–195. [Google Scholar] [CrossRef]
- Calligaris, S.; Marino, M.; Maifreni, M.; Innocente, N. Potential application of monoglyceride structured emulsions as delivery systems of probiotic bacteria in reduced saturated fat ice cream. LWT-Food Sci Technol. 2018, 96, 329–334. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Demirci, T.; Akın, N. Production of functional probiotic ice creams with white and dark blue fruits of Myrtus communis: The comparison of the prebiotic potentials on Lactobacillus casei 431 and functional characteristics. LWT-Food Sci. Technol. 2018, 90, 339–345. [Google Scholar]
- Balthazar, C.F.; Silva, H.L.A.; Esmerino, E.A.; Rocha, R.S.; Moraes, J.; Carmo, M.A.V.; Azevedo, L.; Camps, I.K.D.; Abud, Y.; Sant’Anna, C.; et al. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Pintor-Jardines, A.; Arjona-Román, J.L.; Totosaus-Sánchez, A.; Severiano-Pérez, P.; González-González, L.R.; Escalona-Buendia, H.B. The influence of agave fructans on thermal properties of low-fat, and low-fat and sugar ice cream. LWT-Food Sci. Technol. 2018, 93, 679–685. [Google Scholar] [CrossRef]
- Kataria, A.; Achi, S.C.; Halami, P.M. Effect of encapsulation on viability of Bifidobacterium longum CFR815j and physiochemical properties of ice cream. Indian J. Microbiol. 2018, 58, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cui, J.; Ma, Y.; Yan, T.; Wang, L.; Li, H.; Li, X. Effects of soy-to-milk protein ratio and sucrose fatty acid ester addition on the stability of ice cream emulsions. Food Hydrocoll. 2016, 60, 425–436. [Google Scholar] [CrossRef]
- Moriano, M.E.; Alamprese, C. Organogels as novel ingredients for low saturated fat ice creams. LWT-Food Sci. Technol. 2017, 86, 371–376. [Google Scholar] [CrossRef]
- Moriano, M.E.; Alamprese, C. Honey, trehalose and erythritol as sucrose-alternative sweeteners for artisanal ice cream. A pilot study. LWT-Food Sci. Technol. 2017, 75, 329–334. [Google Scholar] [CrossRef]
- Hiranrangsee, L.; Kumaree, K.K.; Sadiq, M.B.; Anal, A.K. Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. J. Food Sci. Technol. 2016, 53, 3806–3813. [Google Scholar] [CrossRef]
- Azari-Anpar, M.; Khomeiri, M.; Ghafouri-Oskuei, H.; Aghajani, N. Response surface optimization of low-fat ice cream production by using resistant starch and maltodextrin as a fat replacing agent. J. Food Sci. Technol. 2017, 54, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Azari-Anpar, M.; Tehrani, N.S.; Aghajani, N.; Khomeiri, M. Optimization of the new formulation of ice cream with native Iranian seed gums (Lepidium perfoliatum and Lepidium sativum) using response surface methodology (RSM). J. Food Sci. Technol. 2017, 54, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Cengiz, A.; Kahyaoglu, T. The effect of gum tragacanth on the rheological properties of salep based ice cream mix. Carbohydr. Polym. 2016, 143, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Huang, F.K.; Juan, H.W.; Lin, C.P. Evaluation of food-processing conditions of various particle sizes of Tremella fuciformis powder via DSC and TG analyses. J. Therm. Anal. Calorim. 2018, 134, 857–864. [Google Scholar] [CrossRef]
- Cheung, P.C. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci. Hum. Wellness 2013, 2, 162–166. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Zhang, Y.; Xing, L.; Li, S.; Wang, X.; Sun, Z. Chemical composition and antioxidant properties of five edible Hymenomycetes mushrooms. Int. J. Food Sci. Technol. 2015, 50, 465–471. [Google Scholar] [CrossRef]
- Li, H.; Lee, H.S.; Kim, S.H.; Moon, B.; Lee, C. Antioxidant and antiinflamatory activities of methanol extracts of Tremella fuciformis and its major phenolic acids. J. Food Sci. 2014, 79, 460–468. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wei, Z.X.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L.; Zhang, A.Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef]
- Han, C.K.; Chiang, H.C.; Lin, C.Y.; Tang, C.H.; Lee, H.; Huang, D.D.; Zeng, Y.R.; Chuang, T.N.; Huang, Y.L. Comparison of immunomodulatory and anticancer activities in different strains of Tremella fuciformis Berk. Am. J. Chin. Med. 2015, 43, 1637–1655. [Google Scholar] [CrossRef]
- Ban, S.; Lee, S.L.; Jeong, H.S.; Lim, S.M.; Park, S.; Hong, Y.S.; Kim, J.E. Efficacy and safety of Tremella fuciformis in individuals with subjective cognitive impairment: A randomized controlled trial. J. Med. Food. 2018, 21, 400–407. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agric. 2018, 98, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Yang, J.H.; Tseng, Y.H.; Lee, C.E.; Mau, J.L. Quality of silver ear steamed bun. J. Food Process. Preserv. 2010, 34, 649–663. [Google Scholar] [CrossRef]
- Cha, M.H.; Heo, J.Y.; Lee, C.; Lo, Y.M.; Moon, B. Quality and sensory characterization of white jelly mushroom (Tremella fuciformis) as a meat substitute in pork patty formulation. J. Food Process. Preserv. 2014, 38, 2014–2019. [Google Scholar] [CrossRef]
- Lin, C.P.; Tsai, S.Y. Differences in the moisture capacity and thermal stability of Tremella fuciformis polysaccharides obtained by various drying processes. Molecules 2019, 24, 2856. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Lin, H.Y.; Hong, W.P.; Lin, C.P. Evaluation of preliminary causes for vitamin D series degradation via DSC and HPLC analyses. J. Therm. Anal. Calorim. 2017, 130, 1357–1369. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Hwang, B.F.; Wang, Y.H.; Lin, C.P. Moisture desorption and thermal properties of polysaccharide from pulsed light irradiated Flammulina velutipes. J. Therm. Anal. Calorim. 2017, 127, 469–481. [Google Scholar] [CrossRef]
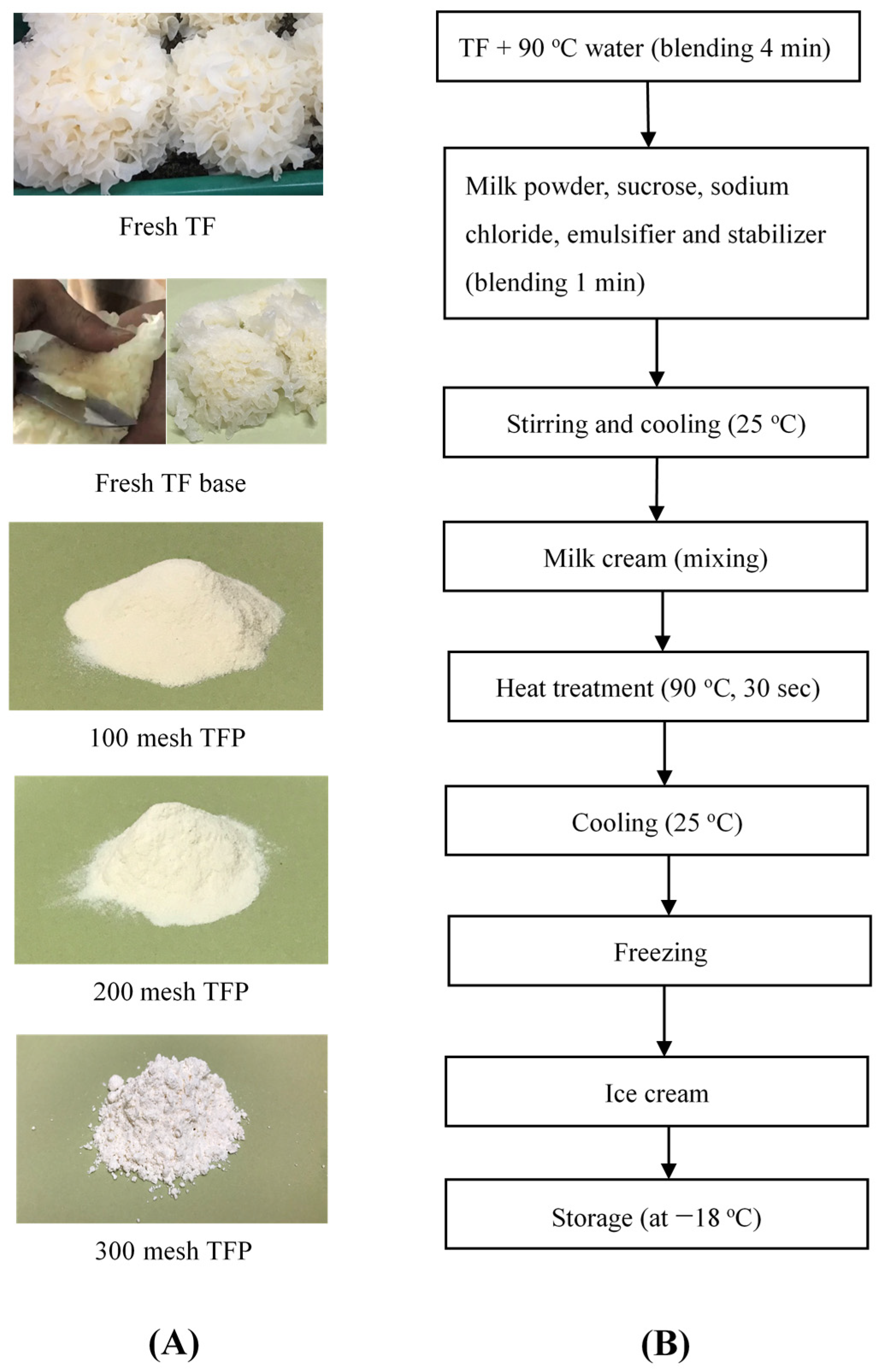
| Ingredients/g | Control | Fresh TF | Fresh TF Base | 100 Mesh TFP | 200 Mesh TFP | 300 Mesh TFP-0.4% | 300 Mesh TFP-0.9% | 300 Mesh TFP-1.4% |
|---|---|---|---|---|---|---|---|---|
| Milk cream | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 |
| Milk powder | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| Sucrose | 40 | 50 | 50 | 40 | 40 | 40 | 40 | 40 |
| Sodium chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Emulsifier | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Sodium carboxymethyl cellulose | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Maltodextrin | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Water | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
| Silver ear | - | 54 | 54 | 4.83 | 4.83 | 2.13 | 4.85 | 7.6 |
| Total | 532.4 | 596.4 | 596.4 | 537.25 | 537.25 | 534.55 | 537.25 | 540.0 |
| Sample | Tg (°C) | mTo (°C) | mTp (°C) | mΔH (kJ/kg) |
|---|---|---|---|---|
| 7.5% sucrose control ice cream | N/A | −2.88 | −0.37 | 196.20 |
| 18% sucrose commercial ice cream | −33.65 | −5.84 | −2.15 | 137.50 |
| 10% sucrose solution | −32.93 | −4.72 | −0.59 | 176.90 |
| 30% sucrose solution | −32.50 | −8.66 | −3.29 | 122.80 |
| 50% sucrose solution | −34.79 | −13.92 | −6.95 | 75.65 |
| Heating Rate (°C/min) | mTo (°C) | mTp (°C) | mΔH (kJ/kg) | |
|---|---|---|---|---|
| Control | 2 | −2.91 | −0.96 | 202.50 |
| 4 | −3.20 | −0.70 | 199.30 | |
| 6 | −2.88 | −0.37 | 196.20 | |
| Fresh TF | 2 | −3.11 | −0.96 | 202.00 |
| 4 | −3.09 | −0.47 | 209.10 | |
| 6 | −3.34 | −0.26 | 206.60 | |
| Fresh TF base | 2 | −3.36 | −1.11 | 198.30 |
| 4 | −3.26 | −0.63 | 201.70 | |
| 6 | −3.18 | −0.25 | 203.50 | |
| 100 mesh TFP | 2 | −3.03 | −0.90 | 203.10 |
| 4 | −3.25 | −0.70 | 203.90 | |
| 6 | −3.30 | −0.43 | 203.40 | |
| 200 mesh TFP | 2 | −2.89 | −0.86 | 197.60 |
| 4 | −3.09 | −0.62 | 201.40 | |
| 6 | −3.16 | −0.32 | 201.40 | |
| 300 mesh TFP-0.9% | 2 | −2.97 | −0.95 | 201.00 |
| 4 | −3.03 | −0.64 | 200.10 | |
| 6 | −3.11 | −0.26 | 211.90 | |
| 300 mesh TFP-0.4% | 2 | −2.72 | −0.79 | 209.00 |
| 4 | −2.85 | −0.45 | 212.90 | |
| 6 | −2.79 | −0.19 | 215.30 | |
| 300 mesh TFP-1.4% | 2 | −2.99 | −0.95 | 196.40 |
| 4 | −3.33 | −0.66 | 202.50 | |
| 6 | −3.24 | −0.19 | 209.60 |
| Ice Cream | Heating Rate | 2 | 4 | 6 | |||
|---|---|---|---|---|---|---|---|
| Parameter | Avrami Erofeev’s | Proto | Avrami Erofeev’s | Proto | Avrami Erofeev’s | Proto | |
| Control | ln(k0) | 1.000E-06 | 3.0478 | 30.4182 | 3.3370 | 12.2110 | 3.6327 |
| Ea | 18.2713 | 16.3207 | 76.0868 | 15.8026 | 36.0000 | 15.8015 | |
| n/n1 | 0.3944 | 1.0082 | 0.8008 | 0.9998 | 0.7618 | 0.9987 | |
| n2 | N/A | 0.0909 | N/A | 0.3000 | N/A | 0.3278 | |
| ΔH | 205.8837 | 204.2369 | 200.0942 | 201.9552 | 200.2802 | 200.4360 | |
| Fresh TF | ln(k0) | 43.5299 | 23.3498 | 19.8606 | 24.3940 | 47.0421 | 24.1891 |
| Ea | 106.2144 | 62.6209 | 54.2131 | 62.4601 | 116.3713 | 61.3940 | |
| n/n1 | 0.8283 | 0.7573 | 0.6491 | 0.8687 | 0.1507 | 0.8505 | |
| n2 | N/A | 0.2359 | N/A | 0.4905 | N/A | 0.6690 | |
| ΔH | 200.7589 | 201.4170 | 209.7087 | 209.0389 | 204.5348 | 203.0871 | |
| Fresh TF base | ln(k0) | 8.3507 | 23.9527 | 23.8063 | 24.4070 | 47.4876 | 23.9588 |
| Ea | 27.0063 | 62.5884 | 61.0614 | 62.7213 | 114.0540 | 62.3057 | |
| n/n1 | 0.9569 | 0.8875 | 0.8451 | 0.8391 | 0.7214 | 0.6975 | |
| n2 | N/A | 0.3678 | N/A | 0.5185 | N/A | 0.4523 | |
| ΔH | 194.3651 | 196.5890 | 199.2232 | 199.6637 | 201.3932 | 204.5091 | |
| 100 mesh TFP | ln(k0) | 10.2859 | 10.4017 | 7.9790 | 10.6846 | 32.4467 | 11.0755 |
| Ea | 31.3031 | 31.9875 | 28.2942 | 31.9423 | 79.8970 | 31.3979 | |
| n/n1 | 0.9525 | 0.9872 | 0.7214 | 0.9323 | 0.7972 | 0.9828 | |
| n2 | N/A | 0.4203 | N/A | 0.4366 | N/A | 0.5828 | |
| ΔH | 204.9092 | 203.7050 | 204.6270 | 204.6387 | 203.7039 | 203.3011 | |
| 200 mesh TFP | ln(k0) | 28.3391 | 19.6084 | 36.7518 | 19.2370 | 11.6076 | 19.8417 |
| Ea | 71.8479 | 52.5000 | 90.7832 | 52.3321 | 35.2615 | 51.1932 | |
| n/n1 | 0.8875 | 0.9407 | 0.7486 | 0.7454 | 0.6929 | 0.9170 | |
| n2 | N/A | 0.3689 | N/A | 0.1915 | N/A | 0.6586 | |
| ΔH | 195.3120 | 195.6301 | 201.8613 | 203.1085 | 204.6257 | 203.7534 | |
| 300 mesh TFP-0.9% | ln(k0) | 40.9786 | 25.0972 | 14.8281 | 25.0105 | 15.8715 | 27.8490 |
| Ea | 100.4681 | 65.4591 | 42.6276 | 64.1818 | 43.3537 | 69.4753 | |
| n/n1 | 0.8353 | 0.8577 | 0.7291 | 0.8086 | 0.8278 | 0.8497 | |
| n2 | N/A | 0.3644 | N/A | 0.8310 | N/A | 0.6155 | |
| ΔH | 200.5455 | 200.7974 | 199.3297 | 200.1815 | 212.2757 | 210.7239 | |
| 300 mesh TFP-0.4% | ln(k0) | 29.5765 | 14.7524 | 17.2494 | 14.8923 | 29.9857 | 14.3501 |
| Ea | 74.6918 | 41.1389 | 48.1955 | 41.2655 | 74.5872 | 38.7238 | |
| n/n1 | 0.8821 | 1.0046 | 0.6937 | 0.9120 | 0.8006 | 0.9589 | |
| n2 | N/A | 0.4052 | N/A | 0.6784 | N/A | 0.6391 | |
| ΔH | 208.6359 | 208.4884 | 216.9507 | 211.5991 | 207.9163 | 214.1946 | |
| 300 mesh TFP-1.4% | ln(k0) | 12.2034 | 25.2985 | 11.8881 | 26.1150 | 29.1130 | 26.3340 |
| Ea | 38.4980 | 66.4692 | 36.1361 | 66.3921 | 72.7000 | 66.3508 | |
| n/n1 | 0.7198 | 0.7998 | 0.7568 | 0.8430 | 0.7894 | 0.8206 | |
| n2 | N/A | 0.2044 | N/A | 0.5349 | N/A | 0.6323 | |
| ΔH | 197.3906 | 196.7799 | 200.5685 | 204.5810 | 208.5698 | 208.6545 | |
| Control | Fresh TF Base | 300 Mesh TFP-0.9% | |
|---|---|---|---|
| Physical characteristics | |||
| pH | 6.91 ± 0.03a | 6.69 ± 0.05b | 6.80 ± 0.12ab |
| Color coordinate L | 82.36 ± 2.77a | 81.98 ± 0.76a | 79.54 ± 0.42a |
| a | 3.01 ± 0.29a | 3.38 ± 0.18a | 3.37 ± 0.02a |
| b | 8.66 ± 0.48b | 10.89 ± 1.08a | 10.38 ± 0.91ab |
| WI | 82.84 ± 1.27a | 82.77 ± 2.74a | 80.29 ± 0.88a |
| Hardness (N/mm2) | 3.44 ± 0.53a | 1.67 ± 0.49b | 2.33 ± 0.48b |
| Viscosity (cp) | 35.40 ± 0.11c | 558.05 ± 10.25a | 410.10 ± 17.98b |
| Chemical composition | |||
| Dry matter (%) | 26.08 ± 1.01c | 43.88 ± 1.87a | 39.95 ± 1.68b |
| Ash (DM, %) | 0.51 ± 0.01a | 0.50 ± 0.01a | 0.50 ± 0.02a |
| Fat (DM, %) | 55.02 ± 3.31a | 53.67 ± 2.66a | 54.99 ± 3.03a |
| Protein (DM, %) | 16.13 ± 0.38c | 17.84 ± 0.15a | 17.24 ± 0.25b |
| Water-soluble dietary fiber (DM, %) | 0.09 ± 0.02b | 0.19 ± 0.02a | 0.16 ± 0.01a |
| Water-insoluble dietary fiber (DM, %) | 0.23 ± 0.06a | 0.27 ± 0.04a | 0.24 ± 0.04a |
| Polysaccharide (DM, mg/g) | 113.40 ± 2.38c | 200.50 ± 1.31b | 281.01 ± 5.48a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-Y.; Tsay, G.J.; Li, C.-Y.; Hung, Y.-T.; Lin, C.-P. Assessment of Melting Kinetics of Sugar-Reduced Silver Ear Mushroom Ice Cream under Various Additive Models. Appl. Sci. 2020, 10, 2664. https://doi.org/10.3390/app10082664
Tsai S-Y, Tsay GJ, Li C-Y, Hung Y-T, Lin C-P. Assessment of Melting Kinetics of Sugar-Reduced Silver Ear Mushroom Ice Cream under Various Additive Models. Applied Sciences. 2020; 10(8):2664. https://doi.org/10.3390/app10082664
Chicago/Turabian StyleTsai, Shu-Yao, Gregory J. Tsay, Chien-Yu Li, Yu-Tzu Hung, and Chun-Ping Lin. 2020. "Assessment of Melting Kinetics of Sugar-Reduced Silver Ear Mushroom Ice Cream under Various Additive Models" Applied Sciences 10, no. 8: 2664. https://doi.org/10.3390/app10082664
APA StyleTsai, S.-Y., Tsay, G. J., Li, C.-Y., Hung, Y.-T., & Lin, C.-P. (2020). Assessment of Melting Kinetics of Sugar-Reduced Silver Ear Mushroom Ice Cream under Various Additive Models. Applied Sciences, 10(8), 2664. https://doi.org/10.3390/app10082664






