Antiphotoaging Effect of (2′S)-Columbianetin from Corydalis heterocarpa in UVA-Irradiated Human Dermal Fibroblasts
Abstract
1. Introduction
2. Results
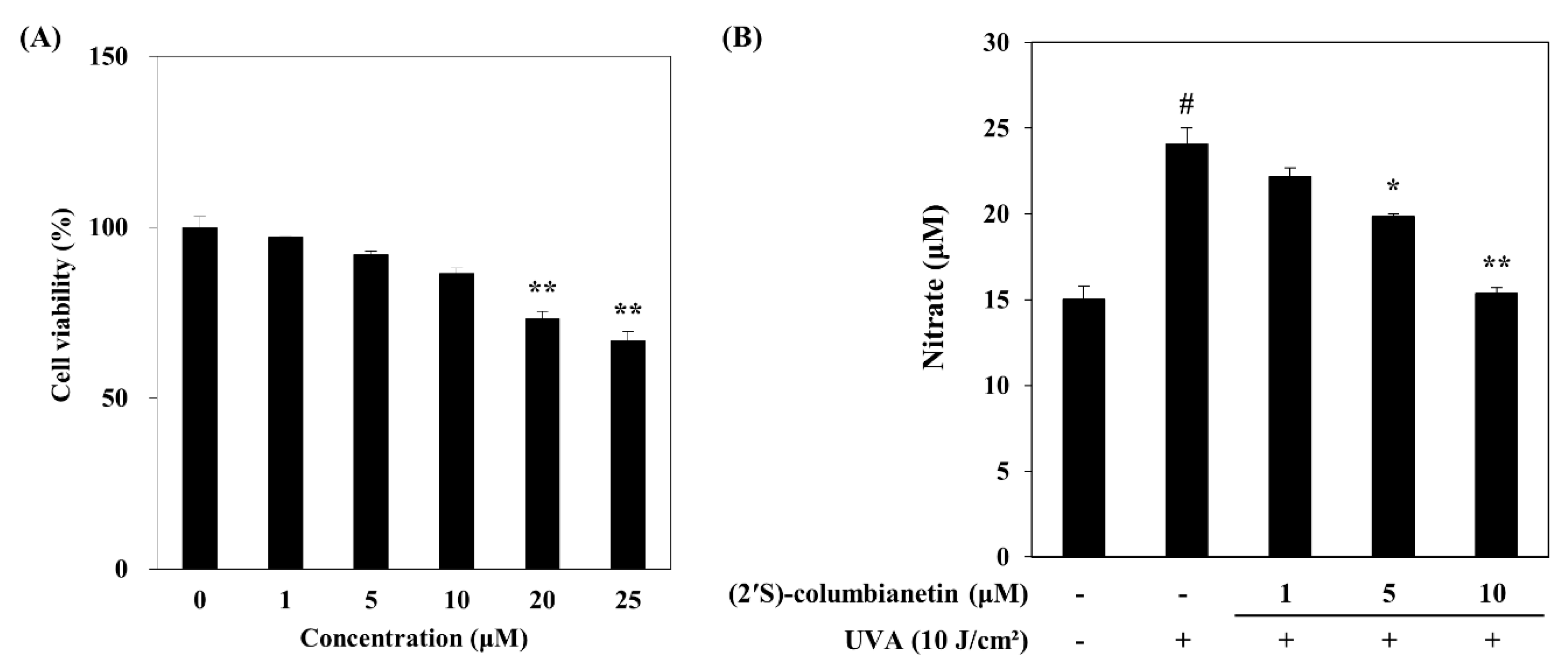
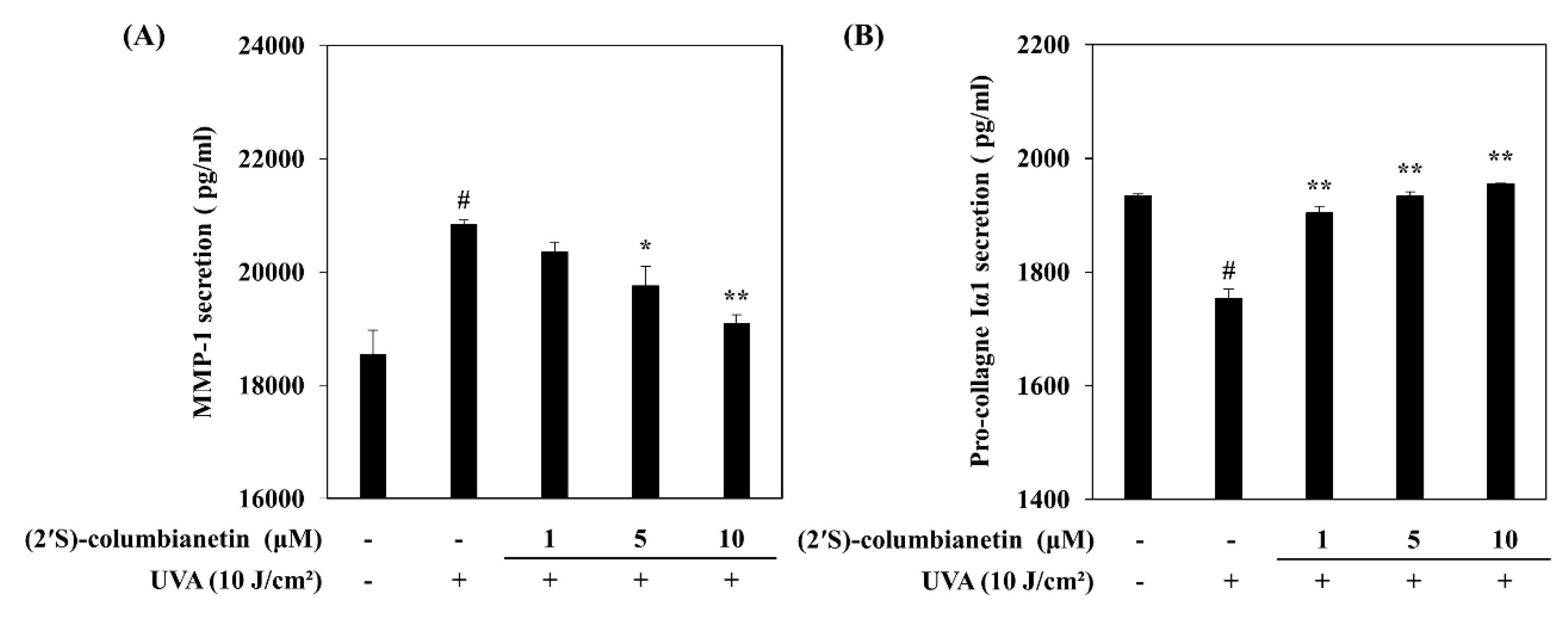
2.1. Effect of (2′S)-Columbianetin on the UVA-Mediated Release of MMP-1 and Type Iα1 Pro-Collagen
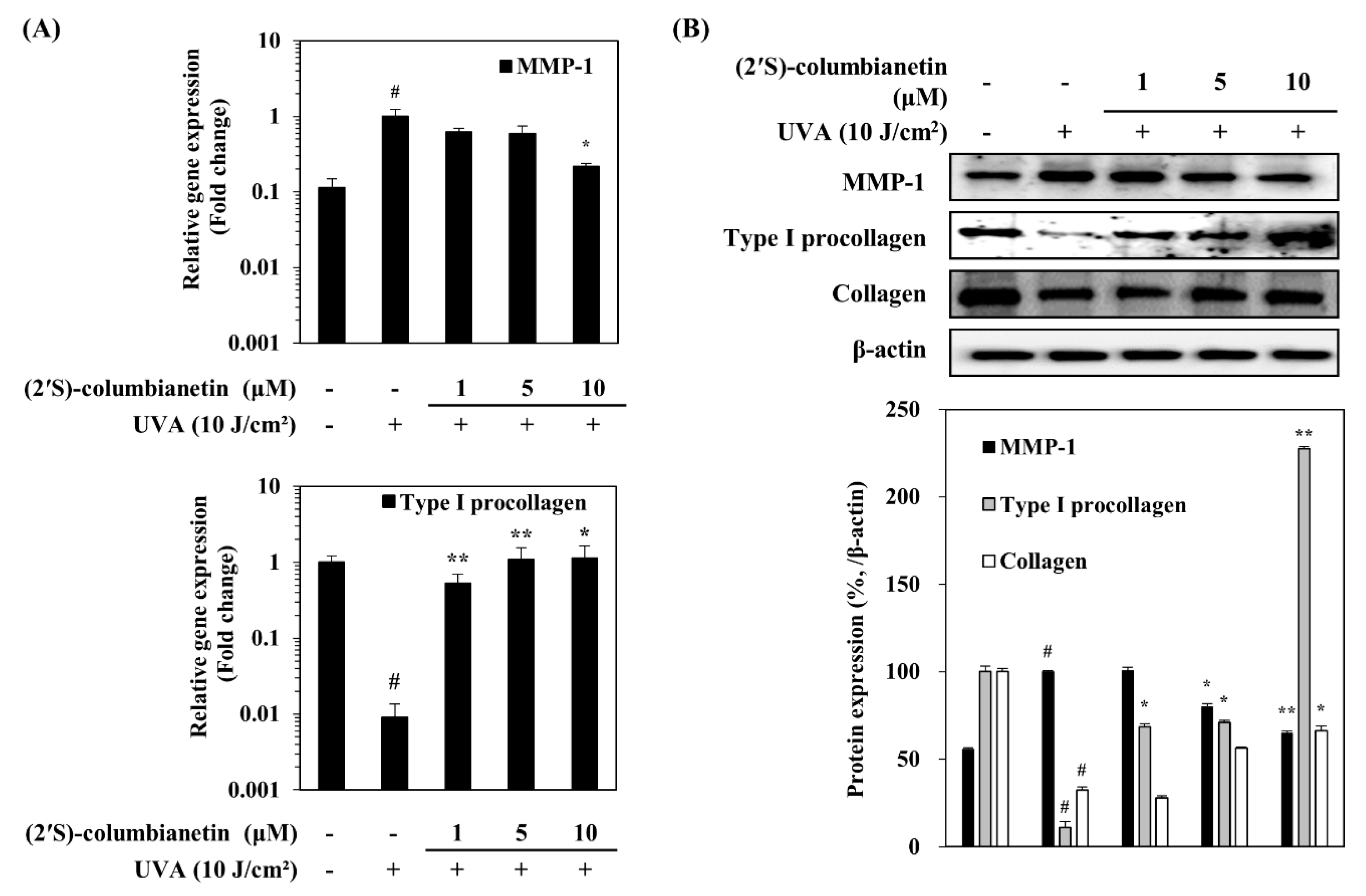
2.2. Effect of (2′S)-Columbianetin on the UVA-Mediated Expression of MMP-1 and Collagen
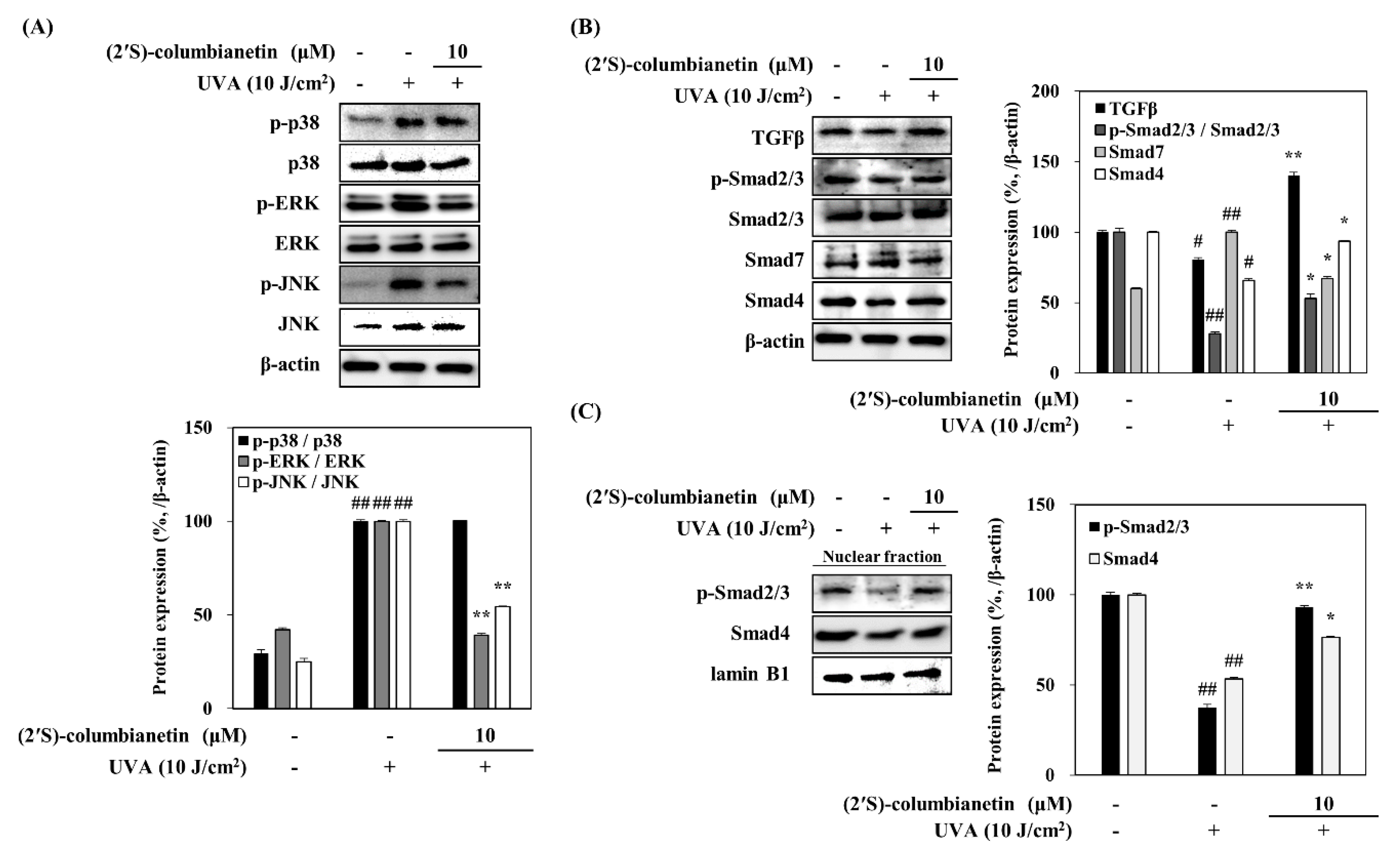
2.3. Effect of (2′S)-Columbianetin on the UVA-Induced MAPK Activation
2.4. Effect of (2′S)-Columbianetin on the UVA-Induced Suppression of TGFβ Pathway
3. Discussion
4. Materials and Methods
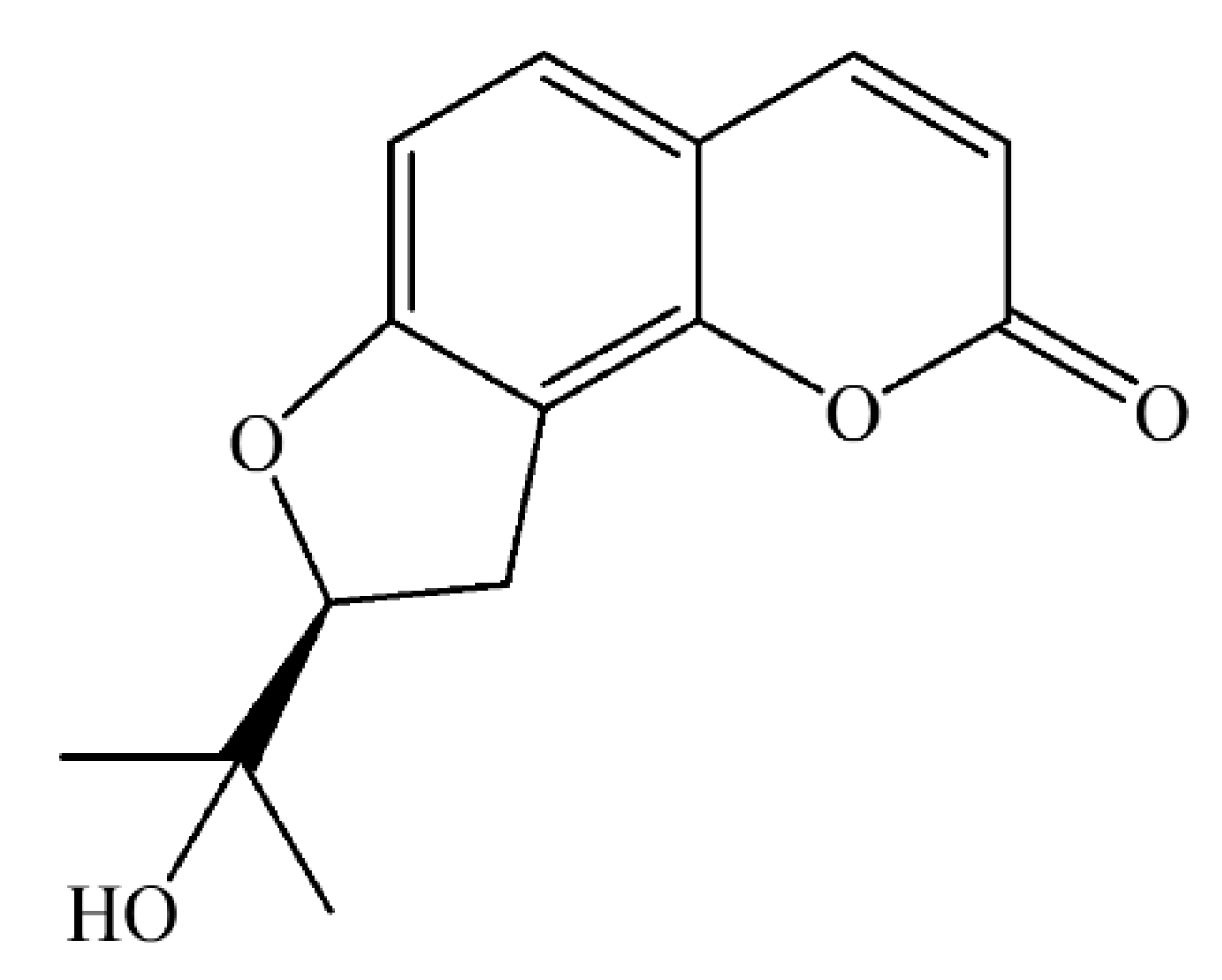
4.1. Isolation of (2′S)-Columbianetin
4.2. Human Dermal Fibroblast (HDF) Culture and Cytotoxicity Assay
4.3. UVA Irradiation
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Lee, J.H.; An, H.T.; Chung, J.H.; Kim, K.H.; Eun, H.C.; Cho, K.H. Acute effects of UVB radiation on the proliferation and differentiation of keratinocytes. Photodermatol. Photoimmunol. Photomed. 2002, 18, 253–261. [Google Scholar] [CrossRef]
- Krutmann, J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000, 23, S22–S26. [Google Scholar] [CrossRef]
- Vile, G.F.; Tyrrell, R.M. Uva radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radic. Biol. Med. 1995, 18, 721–730. [Google Scholar] [CrossRef]
- Xu, Y.; Fisher, G.J. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J. Dermatol. Sci. Suppl. 2005, 1, S1–S8. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, 167, re2:1–re2:15. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, N.; Li, S.P.; Ahonen, M.; Foschi, M.; Han, J.; Kähäri, V.M. Activation of p38α MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J. Biol. Chem. 2002, 277, 32360–32368. [Google Scholar] [CrossRef]
- Panich, U.; Chaiprasongsuk, A.; Lohakul, J.; Soontrapa, K.; Sampattavanich, S. Photoprotective role of Nrf2 in UVA-mediated MMP-1 via MAPK/AP-1 signaling in keratinocyte HaCaT cells and mouse skin: The photoprotective effects of sulforaphane. Free Radic. Biol. Med. 2016, 100, S44. [Google Scholar] [CrossRef]
- Gambichler, T.; Skrygan, M.; Tomi, N.S.; Breuksch, S.; Altmeyer, P.; Kreuter, A. Significant downregulation of transforming growth factor-β signal transducers in human skin following ultraviolet-A1 irradiation. Br. J. Dermatol. 2007, 156, 951–956. [Google Scholar] [CrossRef]
- Cathcart, J.M.; Cao, J. MMP inhibitors: Past, present and future. Front. Biosci. 2015, 20, 1164–1178. [Google Scholar]
- Mannello, F. Natural bio-drugs as matrix metalloproteinase inhibitors: New perspectives on the horizon? Recent Pat. Anticancer Drug Discov. 2008, 1, 91–103. [Google Scholar] [CrossRef]
- Shu, L.; Cheung, K.L.; Khor, T.O.; Chen, C.; Kong, A.N. Phytochemicals: Cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010, 29, 483–502. [Google Scholar] [CrossRef]
- Tan, W.; Lu, J.; Huang, M.; Li, Y.; Chen, M.; Wu, G.; Gong, J.; Zhong, Z.; Xu, Z.; Dang, Y.; et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin. Med. 2011, 6, 27. [Google Scholar] [CrossRef]
- Kimura, Y. Antitumor and antimetastatic actions of various natural products. Stud. Nat. Prod. Chem. 2008, 34, 35–76. [Google Scholar]
- Ahn, B.N.; Kim, J.A.; Kong, C.S.; Seo, Y.; Kim, S.K. Protective effect of (2′S)-columbianetin from corydalis heterocarpa on UVB-induced keratinocyte damage. J. Photochem. Photobiol. B Biol. 2012, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Park, S.Y.; Seo, Y.; Kong, C.S. Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-D-galactopyranoside in UVA-irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules 2020, 25, 1331. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Conlon, G.A.; Murray, G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Min, J.L.; Ahn, J.; Kim, S.; Hyeon, H.K.; Kyu, H.K.; Hee, C.E.; Jin, H.C. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J. Investig. Dermatol. 2004, 123, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.-O.; Pellieux, C.; Briviba, K.; Pierlot, C.; Aubry, J.-M.; Sies, H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur. J. Biochem. 1999, 260, 917–922. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Control of connective tissue gene expression by TGF beta: Role of Smad proteins in fibrosis. Curr. Rheumatol. Rep. 2002, 4, 143–149. [Google Scholar] [CrossRef]
- Yan, X.; Liao, H.; Cheng, M.; Shi, X.; Lin, X.; Feng, X.H.; Chen, Y.G. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/Smad signaling. J. Biol. Chem. 2016, 291, 382–392. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Karadeniz, F.; Lee, J.I.; Kim, H.R.; Seo, Y.; Kong, C.-S. Antiphotoaging Effect of (2′S)-Columbianetin from Corydalis heterocarpa in UVA-Irradiated Human Dermal Fibroblasts. Appl. Sci. 2020, 10, 2568. https://doi.org/10.3390/app10072568
Oh JH, Karadeniz F, Lee JI, Kim HR, Seo Y, Kong C-S. Antiphotoaging Effect of (2′S)-Columbianetin from Corydalis heterocarpa in UVA-Irradiated Human Dermal Fibroblasts. Applied Sciences. 2020; 10(7):2568. https://doi.org/10.3390/app10072568
Chicago/Turabian StyleOh, Jung Hwan, Fatih Karadeniz, Jung Im Lee, Hye Ran Kim, Youngwan Seo, and Chang-Suk Kong. 2020. "Antiphotoaging Effect of (2′S)-Columbianetin from Corydalis heterocarpa in UVA-Irradiated Human Dermal Fibroblasts" Applied Sciences 10, no. 7: 2568. https://doi.org/10.3390/app10072568
APA StyleOh, J. H., Karadeniz, F., Lee, J. I., Kim, H. R., Seo, Y., & Kong, C.-S. (2020). Antiphotoaging Effect of (2′S)-Columbianetin from Corydalis heterocarpa in UVA-Irradiated Human Dermal Fibroblasts. Applied Sciences, 10(7), 2568. https://doi.org/10.3390/app10072568




