Microalgae Water Bioremediation: Trends and Hot Topics
Abstract
1. Introduction
2. Methods
2.1. Document Source Selection
2.2. Bibliometric Analysis
3. Bibliometric Performance Analysis
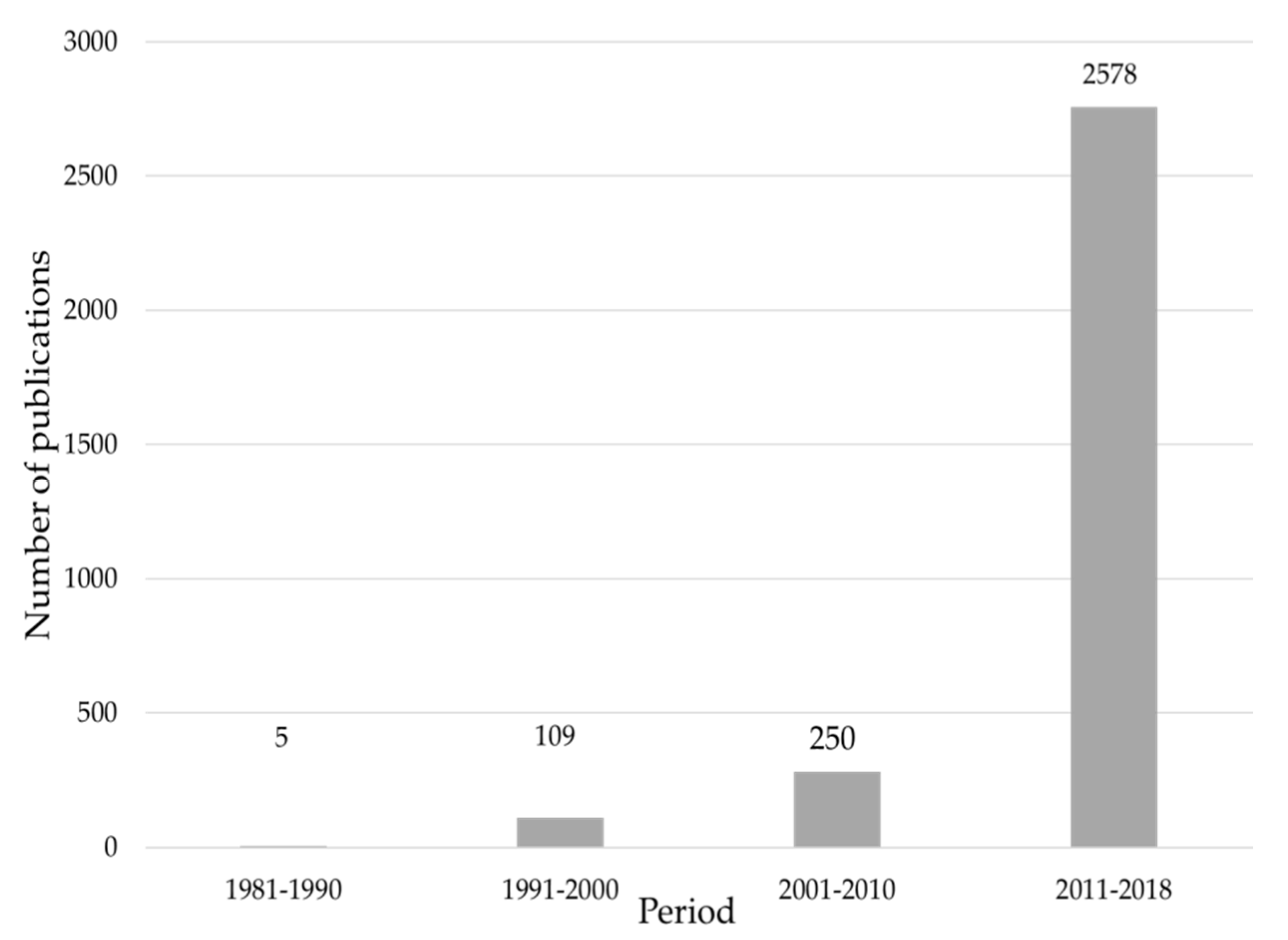
3.1. Publication Production
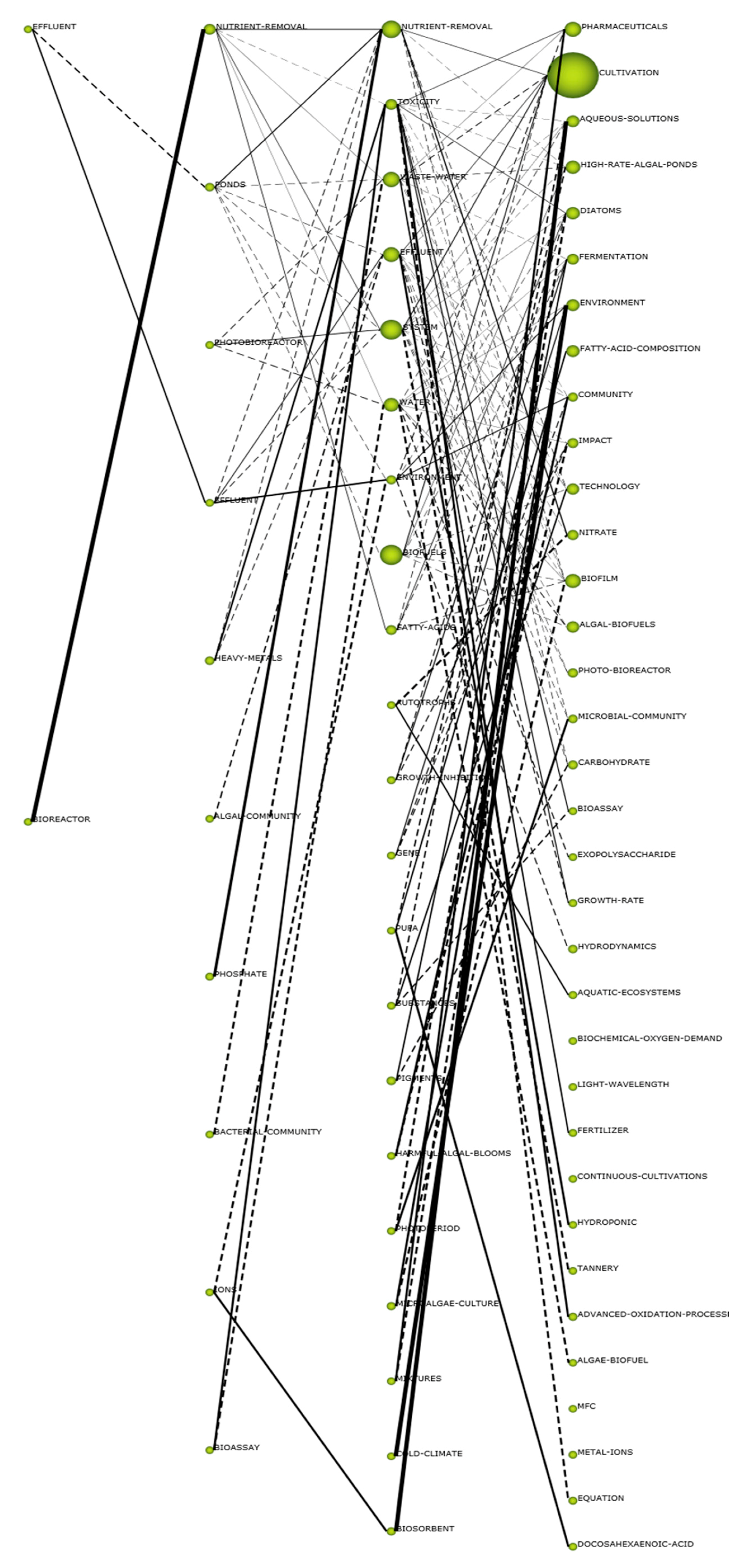
3.2. Content Analysis
- Quadrant Q1: considered motor themes, important for the development of the research field.
- Quadrant Q2: themes well developed.
- Quadrant Q3: represents emergent or declining themes.
- Quadrant Q4: characterized by basic and transversal themes, nevertheless not well developed.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations Educational, Scientific and Cultural Organization. International Initiative on Water Quality; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2015. [Google Scholar]
- Mohamad, S.; Fares, A.; Judd, S.; Bhosale, R.; Kumar, A.; Gosh, U.; Khreisheh, M. Advanced wastewater treatment using microalgae: Effect of temperature on removal of nutrients and organic carbon. In Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2017; Volume 67. [Google Scholar] [CrossRef]
- Menger-Krug, E.; Niederste-Hollenberg, J.; Hillenbrand, T.; Hiessl, H. Integration of microalgae systems at municipal wastewater treatment plants: Implications for energy and emission balances. Environ. Sci. Technol. 2012, 46, 11505–11514. [Google Scholar] [CrossRef] [PubMed]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 860–871. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Emparan, Q.; Harun, R.; Danquah, M.K. Role of phycoremediation for nutrient removal from wastewaters: A review. Appl. Ecol. Environ. Res. 2019, 17, 889–915. [Google Scholar] [CrossRef]
- Menció, A.; Mas-Pla, J.; Otero, N.; Regàs, O.; Boy-Roura, M.; Puig, R.; Bach, J.; Domènech, C.; Zamorano, M.; Brusi, D.; et al. Nitrate pollution of groundwater; all right…, but nothing else? Sci. Total Environ. 2016, 539, 241–251. [Google Scholar] [CrossRef]
- Schaum, C. Phosphorus: Polluter and Resource of the Future-Removal and Recovery from Wastewater. Water Intell. Online 2018, 17, 450–457. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Leslie, H.A.; Quinn, B. Microplastics in drinking water: A review and assessment. Curr. Opin. Environ. Sci. Heal. 2019, 7, 69–75. [Google Scholar] [CrossRef]
- Muñoz, I.; Gómez-Ramos, M.J.; Agüera, A.; Fernández-Alba, A.R.; García-Reyes, J.F.; Molina-Díaz, A. Chemical evaluation of contaminants in wastewater effluents and the environmental risk of reusing effluents in agriculture. TrAC Trends Anal. Chem. 2009, 28, 676–694. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants-Opportunities and challenges. Water Res. 2019, 164. [Google Scholar] [CrossRef]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.H.; Cheng, C.L.; Guo, W.Q.; Nagarajan, D.; Ren, N.Q.; Lee, D.J.; Chang, J.S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Xu, X.Q.; Wang, X.X.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.G.; Fick, J. Algal cultivation in urban wastewater: An efficient way to reduce pharmaceutical pollutants. J. Appl. Phycol. 2017, 29, 255–262. [Google Scholar] [CrossRef]
- Varjani, S.; Agarwal, A.K.; Gnansounou, E.; Gurunathan, B. Bioremediation: Applications for Environmental Protection and Management; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Marchello, A.E.; Lombardi, A.T.; Dellamano-Oliveira, M.J.; De Souza, C.W.O. Microalgae population dynamics in photobioreactors with secondary sewage effluent as culture medium. Brazilian J. Microbiol. 2015, 46, 75–84. [Google Scholar] [CrossRef]
- Zhu, J.; Rong, J.; Zong, B. Factors in mass cultivation of microalgae for biodiesel. Cuihua Xuebao Chin. J. Catal. 2013, 34, 80–100. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry and Biotechnology. In Climate Change 2013-The Physical Science Basis; CRC Press, Taylor & Francis Group: Pisa, Italy, 2014; pp. 1–30. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, F. Growing Phototrophic Cells without Light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Pal, P.; Chew, K.W.; Yen, H.-W.; Lim, J.W.; Lam, M.K.; Show, P.L. Cultivation of Oily Microalgae for the Production of Third-Generation Biofuels. Sustainability 2019, 11, 5424. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: A review. Algal Res. 2017, 24, 425–437. [Google Scholar] [CrossRef]
- Gao, F.; Cui, W.; Xu, J.P.; Li, C.; Jin, W.H.; Yang, H.L. Lipid accumulation properties of Chlorella vulgaris and Scenedesmus obliquus in membrane photobioreactor (MPBR) fed with secondary effluent from municipal wastewater treatment plant. Renew. Energy 2019, 136, 671–676. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, S.M.; Tahoon, M.A.; Ben Rebah, F. Agro-industrial waste materials and wastewater as growth media for microbial bioflocculants production: A review. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Lavrinovičs, A.; Juhna, T. Review on Challenges and Limitations for Algae-Based Wastewater Treatment. Constr. Sci. 2018, 20, 17–25. [Google Scholar] [CrossRef]
- Rawat, I.; Gupta, S.K.; Shriwastav, A.; Singh, P.; Kumari, S.; Bux, F. Microalgae Applications in Wastewater Treatment. Algae Biotechnol. 2016, 249–268. [Google Scholar] [CrossRef]
- Herrador, M. The Microalgae/Biomass Industry in Japan -An Assessment of Cooperation and Business Potential With European Companies; EU Japan Centre Industrial Cooperation: Tokyo, Japan, 2016. [Google Scholar]
- Nur, M.M.A.; Buma, A.G.J. Opportunities and Challenges of Microalgal Cultivation on Wastewater, with Special Focus on Palm Oil Mill Effluent and the Production of High Value Compounds. Waste Biomass Valorization 2019, 10, 2079–2097. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.P.; Liu, Z.H.; Martin, G.; Sun, Z. Integration of Waste Valorization for Sustainable Production of Chemicals and Materials via Algal Cultivation. Top. Curr. Chem. 2017, 375. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Mariano, A.B.; Vargas, J.V.C. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Energy Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Fazal, T.; Mushtaq, A.; Rehman, F.; Ullah Khan, A.; Rashid, N.; Farooq, W.; Rehman, M.S.U.; Xu, J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew. Sustain. Energy Rev. 2018, 82, 3107–3126. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ayre, J.M.; Moheimani, N.R.; Ubi, B.E.; Ogbonna, J.C. Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. ALGAL 2016, 17, 268–276. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Patel, A.; Mehtani, J.; Pruthi, P.A.; Pruthi, V.; Poluri, K.M. Co-culturing of oleaginous microalgae and yeast: Paradigm shift towards enhanced lipid productivity. Environ. Sci. Pollut. Res. 2019, 26, 16952–16973. [Google Scholar] [CrossRef] [PubMed]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Co-culture for lipid production: Advances and challenges. Biomass Bioenergy 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Eroglu, E.; Agarwal, V.; Bradshaw, M.; Chen, X.; Smith, S.M.; Raston, C.L.; Swaminathan Iyer, K. Nitrate removal from liquid effluents using microalgae immobilized on chitosan nanofiber mats. Green Chem. 2012, 14, 2682. [Google Scholar] [CrossRef]
- Mallick, N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: A review. BioMetals 2002, 15, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T.; Geest, T.; Iversen, J.J.L. Phototrophic growth in the lumostat: A photo-bioreactor with on-line optimization of light intensity. J. Appl. Phycol. 1996, 8, 345–352. [Google Scholar] [CrossRef]
- Zohri, A.-N.A.; Ragab, S.W.; Mekawi, M.I.; Mostafa, O.A.A. Comparison between batch, fed-batch, semi-continuous and continuous techniques for bio-ethanol production from a mixture of egyptian cane and beet molasses. Egypt. Sugar J. 2017, 9, 89–111. [Google Scholar]
- Ho, S.-H.; Ye, X.; Hasunuma, T.; Chang, J.-S.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef]
- Sforza, E.; Enzo, M.; Bertucco, A. Design of microalgal biomass production in a continuous photobioreactor: An integrated experimental and modeling approach. Chem. Eng. Res. Des. 2014, 92, 1153–1162. [Google Scholar] [CrossRef]
- Ravindran, B.; Gupta, S.K.; Cho, W.M.; Kim, J.K.; Lee, S.R.; Jeong, K.H.; Lee, D.J.; Choi, H.C. Microalgae potential and multiple roles-current progress and future prospects-an overview. Sustainability 2016, 8, 1215. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae-A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Koreiviene, J.; Paskauskas, R. EU Project of LIFE Programme ‘Algae Service for LIFE’ Develops Ecologicaly Sustainable Bioproducts from Freshwater Cyanobacteria and Macroalgae Biomass. Botanica 2019, 25, 176–185. [Google Scholar] [CrossRef]
- Börner, K.; Chen, C.; Boyack, K.W. Visualizing knowledge domains. Annu. Rev. Inf. Sci. Technol. 2005, 37, 179–255. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. SciMAT: A new science mapping analysis software tool. J. Am. Soc. Inf. Sci. Technol. 2012, 63, 1609–1630. [Google Scholar] [CrossRef]
- Herrera-Viedma, E.; Martinez, M.A.; Herrera, M. Bibliometric Tools for Discovering Information in Database. In Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer Verlag: Berlin/Heidelberg, Germany, 2016; Volume 9799, pp. 193–203. [Google Scholar] [CrossRef]
- López-Robles, J.R.; Otegi-Olaso, J.R.; Porto Gómez, I.; Cobo, M.J. 30 years of intelligence models in management and business: A bibliometric review. Int. J. Inf. Manag. 2019, 48, 22–38. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Martínez, M.E.; Sánchez, S.; Jiménez, J.M.; El Yousfi, F.; Muñoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Klekner, V.; Kosaric, N. Degradation of phenols by algae. Environ. Technol. (UK) 1992, 13, 493–501. [Google Scholar] [CrossRef]
- Ellis, B.E. Degradation of phenolic compounds by fresh-water algae. Plant. Sci. Lett. 1977, 8, 213–216. [Google Scholar] [CrossRef]
- Scragg, A.H. The effect of phenol on the growth of Chlorella vulgaris and Chlorella VT-1. Enzyme Microb. Technol. 2006, 39, 796–799. [Google Scholar] [CrossRef]
- Mahan, C.A.; Majidi, V.; Holcombe, J.A. Evaluation of the metal uptake of several algae strains in a multicomponent matrix utilizing inductively coupled plasma emission spectrometry. Anal. Chem. 1989, 61, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae-A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.H.; Ramanan, R.; Kim, B.H.; Oh, H.M.; Kim, H.S. Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour. Technol. 2013, 131, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Heydarizadeh, P.; Boureba, W.; Zahedi, M.; Huang, B.; Moreau, B.; Lukomska, E.; Couzinet-Mossion, A.; Wielgosz-Collin, G.; Martin-Jézéquel, V.; Bougaran, G. Response of CO2-starved diatom Phaeodactylum tricornutum to light intensity transition. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Salim, S.; Bosma, R.; Vermuë, M.H.; Wijffels, R.H. Harvesting of microalgae by bio-flocculation. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Santos, C.E.; de Coimbra, R.N.; Bermejo, S.P.; Pérez, A.I.G.; Cabero, M.O. Comparative Assessment of Pharmaceutical Removal from Wastewater by the Microalgae Chlorella sorokiniana, Chlorella vulgaris and Scenedesmus obliquus. In Biological Wastewater Treatment and Resource Recovery; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhuang, L.L.; Xu, X.Q.; Deantes-Espinosa, V.M.; Wang, X.X.; Hu, H.Y. Microalgal attachment and attached systems for biomass production and wastewater treatment. Renew. Sustain. Energy Rev. 2018, 92, 331–342. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: An experimental study. J. Appl. Phycol. 2007, 19, 417–423. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ehrampoush, M.H.; Mahvi, A.H.; Dehghani, M.; Faramarzian, M.; Eslami, H. A comparative study of hybrid membrane photobioreactor and membrane photobioreactor for simultaneous biological removal of atrazine and CNP from wastewater: A performance analysis and modeling. Chem. Eng. J. 2019, 355, 428–438. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. An approach for detecting, quantifying, and visualizing the evolution of a research field: A practical application to the Fuzzy Sets Theory field. J. Informetr. 2011, 5, 146–166. [Google Scholar] [CrossRef]
- Edwards, P.; Sinchumpasak, O.A.; Tabucanon, M. The harvest of microalgae from the effluent of a sewage fed high rate stabilization pond by Tilapia nilotica. Part 2: Studies of the fish ponds. Aquaculture 1981, 23, 107–147. [Google Scholar] [CrossRef]
- Edwards, P.; Sinchumpasak, O.A. The harvest of microalgae from the effluent of a sewage fed high rate stabilization pond by Tilapia nilotica. Part 1: Description of the system and the study of the high rate pond. Aquaculture 1981, 23, 83–105. [Google Scholar] [CrossRef]
- Edwards, P.; Sinchumpasak, O.A.; Labhsetwar, V.K.; Tabucanon, M. The harvest of microalgae from the effluent of a sewagefed high rate stabilization pond by Tilapia nilotica. Part 3: Maize cultivation experiment, bacteriological studies, and economic assessment. Aquaculture 1981, 23, 149–170. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar] [CrossRef]
- James, C.M.; Al-Khars, A.M. An intensive continuous culture system using tubular photobioreactors for producing microalgae. Aquaculture 1990, 87, 381–393. [Google Scholar] [CrossRef]
- Familletti, P.C. Air Lift Bioreactor. U. S. Patent 4,649,117, 10 March 1987. [Google Scholar]
- Armstrong, D.W.; Ottawa, L.; Fleming, P.; Grenzowski, D. Cell Culture Bioreactor. U.S. Patent 4,906,577, 6 March 1990. [Google Scholar]
- Raymond, L.P. Mass Algal Culture System. U. S. Patent 4,253,271, 3 March 1981. [Google Scholar]
- Jais, N.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Hashim, M.K.A. The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol. Environ. Policy. 2017, 19, 37–52. [Google Scholar] [CrossRef]
- AlMomani, F.A.; Örmeci, B. Performance Of Chlorella Vulgaris, Neochloris Oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent and centrate. Ecol. Eng. 2016, 95, 280–289. [Google Scholar] [CrossRef]
- Goncalves, E.C.; Wilkie, A.C.; Kirst, M.; Rathinasabapathi, B. Metabolic regulation of triacylglycerol accumulation in the green algae: Identification of potential targets for engineering to improve oil yield. Plant. Biotechnol. J. 2016, 14, 1649–1660. [Google Scholar] [CrossRef]
- Çetinkaya Dönmez, G.; Aksu, Z.; Öztürk, A.; Kutsal, T. A comparative study on heavy metal biosorption characteristics of some algae. Process. Biochem. 1999, 34, 885–892. [Google Scholar] [CrossRef]
- Matsunaga, T.; Takeyama, H.; Nakao, T.; Yamazawa, A. Screening of marine microalgae for bioremediation of cadmium-polluted seawater. J. Biotechnol. 1999, 70, 33–38. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients From Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Chen, F.; Johns, M.R. Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process. Biochem. 1996, 31, 601–604. [Google Scholar] [CrossRef]
- Ruxton, C.; Reed, S.; Simpson, M.; Millington, K. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. Discovery Service for Endeavour College of Natural Health Library. J. Hum. Nutr. Diet. 2004, 20, 275–285. [Google Scholar] [CrossRef]
- Giger, W. Hydrophilic and amphiphilic water pollutants: Using advanced analytical methods for classic and emerging contaminants. Anal. Bioanal. Chem. 2009, 393, 37–44. [Google Scholar] [CrossRef]
- Boxall, A.B.A. New and Emerging Water Pollutants arising from Agriculture; OECD: Paris, France, 2012. [Google Scholar]
- Dulio, V.; Assoumani, A. Contaminants of Emerging Concern in Urban Wastewater; Position Paper to the Workshop on Prioritisation of Emerging Contaminants in Urban Wastewater; Norman: Paris, France, 2019. [Google Scholar]
- Pereira, S.A.; Araújo, V.Q.; Reboucas, M.V.; Vieira, F.S.V.; De Almeida, M.V.A.; Chinalia, F.A.; Nascimento, I.A. Toxicity of biodiesel, diesel and biodiesel/diesel blends: Comparative sub-lethal effects of water-soluble fractions to microalgae species. Bull. Environ. Contam. Toxicol. 2012, 88, 234–238. [Google Scholar] [CrossRef]
- Asselborn, V.; Fernández, C.; Zalocar, Y.; Parodi, E.R. Effects of chlorpyrifos on the growth and ultrastructure of green algae, Ankistrodesmus gracilis. Ecotoxicol. Environ. Saf. 2015, 120, 334–341. [Google Scholar] [CrossRef]
- Chung, M.K.; Hu, R.; Wong, M.H.; Cheung, K.C. Comparative toxicity of hydrophobic contaminants to microalgae and higher plants. Ecotoxicology 2007, 16, 393–402. [Google Scholar] [CrossRef]
- Posadas, E.; Serejo, M.L.; Blanco, S.; Pérez, R.; García-Encina, P.A.; Muñoz, R. Minimization of biomethane oxygen concentration during biogas upgrading in algal-bacterial photobioreactors. Algal Res. 2015, 12, 221–229. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Biological Processing of Agricultural and Food Wastes to Recover Energy, Nutrients and Water for Sustainable Agricultural Production-North Carolina A&T State University. Available online: https://reeis.usda.gov/web/crisprojectpages/1003263-biological-processing-of-agricultural-and-food-wastes-to-recover-energy-nutrients-and-water-for-sustainable-agricultural-production.html (accessed on 13 January 2020).

| Microalgae Cultivation | Description |
|---|---|
| System | |
| Open Culture System | Ponds, tanks, lakes and raceway ponds are examples of open culture systems. These outdoor systems were the first and are the most used for large-scale production of microalgae biomass due to their simple construction, easy operation and low energy demands [32]. However, open culture systems not only are affected by climate conditions and exterior contamination as they also show low productivity and loss of nutrients by evaporation [33]. |
| Closed Culture System | Closed systems are usually denominated by photobioreactors and are characterized by not allowing exchanges between the microalgae culture and the external environment [20], presenting different designs, such as tubular, plastic bags, flat-plate and bubble-column [34]. Closed-culture systems overcome some of the challenges faced by open systems, such as higher productivity, less nutrient evaporation and contamination [35]. Nevertheless, these systems present a high energy demand, being their implementation expensive [34]. |
| Method | |
| Co-Culture Method | Microalgae exist in nature as a part of a community, benefiting from the interaction among microorganisms [36]. In a co-culture method, more than one species is grown in the same medium, so it should be taken into account that the species selected have similar growth requirements [37]. Nowadays, co-culture of microalgae with yeast or bacteria has shown potential to enhance the phycoremediation and biomass yield [26]. In this cultivation method, microalgae synthesize higher contents of exopolysaccharides to support the growth in hostile conditions [38]. |
| Immobilized Method | When compared with microalgae free-cell cultivation, the immobilization process can overcome the biomass-harvesting challenge, ensuring that metabolic substances can diffuse through the polymer gel matrix [39]. Nevertheless, the costs of the polymer and the immobilization process, accumulation of metabolic products and the efficiency of the bioremediation are some of the drawbacks of this cultivation method [6]. Still, previous studies show the potential of immobilized microalgae in wastewater bioremediation and metal recovery [40]. |
| Strategy | |
| Batch | This is a simple closed system and low-cost strategy, since it does not require much control. This strategy is characterized by no renewal of culture medium, so the microalgae culture grows until it reaches the decline phase [20]. In practice, the culture could crash for several reasons such as nutrient or oxygen depletion, self-shading, pH variations or contamination [41]. |
| Semi-Continuous | This strategy is like a batch system but, in this case, the culture medium is renewed periodically, while effluent is removed at the same time [20]. Nevertheless, this strategy could not be appropriate for microalgae cultivation because light is a limiting-factor that affects biomass productivity [42]. |
| Continuous | A continuous strategy that consists in the constant renewal of growth medium, in which the volume of culture medium that is supplemented is the same as the volume of culture that is removed [20]. The advantage of continuous renewal of medium is the achievement of high biomass productivity [43]. Also, it is easy to scale-up for industrial microalgae production due to the simple operation of the cultivation system [44]. |
| Author | Number of Publications | h-Index | Affiliation | Country |
|---|---|---|---|---|
| Raul Muñoz | 44 | 53 | Valladolid University | Spain |
| Joan García | 34 | 58 | Valencia University | Spain |
| Roger Ruan | 32 | 60 | Minnesota University | USA |
| Paul Chen | 27 | 53 | Minnesota University | USA |
| Ivet Ferrer | 27 | 36 | Polytechnic University of Catalunya | Spain |
| Jo-Shuo Chang | 26 | 89 | National Cheng Kung University | Taiwan |
| S. Venkata Mohan | 26 | 71 | CSIR-Indian Institute of Chemical Technology | India |
| Enrica Uggetti | 25 | 17 | Polytechnic University of Catalunya | Spain |
| Byong-Hun Jeon | 24 | 44 | Hanyang University | South Korea |
| Wenguang Zhou | 24 | 26 | Nanchang University | China |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, D.; Rocha, A.C.; Pereira, L.; Verdelhos, T. Microalgae Water Bioremediation: Trends and Hot Topics. Appl. Sci. 2020, 10, 1886. https://doi.org/10.3390/app10051886
Pacheco D, Rocha AC, Pereira L, Verdelhos T. Microalgae Water Bioremediation: Trends and Hot Topics. Applied Sciences. 2020; 10(5):1886. https://doi.org/10.3390/app10051886
Chicago/Turabian StylePacheco, Diana, Ana Cristina Rocha, Leonel Pereira, and Tiago Verdelhos. 2020. "Microalgae Water Bioremediation: Trends and Hot Topics" Applied Sciences 10, no. 5: 1886. https://doi.org/10.3390/app10051886
APA StylePacheco, D., Rocha, A. C., Pereira, L., & Verdelhos, T. (2020). Microalgae Water Bioremediation: Trends and Hot Topics. Applied Sciences, 10(5), 1886. https://doi.org/10.3390/app10051886








