1. Introduction
Microalgae can be a source of high value compounds, such as polyunsaturated fatty acids, pigments (e.g., chlorophylls and carotenoids), polysaccharides, and proteins, with applications in the food, cosmetic, and pharmaceutical industries, among others [
1]. In recent years, the food industry has seen an increased demand for natural, healthy, high quality raw materials, even if the consumer prices of the final product become higher.
In scientific terms, “organic” usually refers to living organisms and compounds with the presence of carbon, but for consumers this word is often linked to food. Food and Agriculture Organization of the United Nations (FAO) reported that the term “organic” should be viewed as a management claim concerning a process having natural inputs rather than a product claim. Inter-governmentally organizations through partnerships with International Federation of Organic Agriculture Movements (IFAOM) set guidelines in 2002 for organic farming production systems, but in legal terms, labelling and certification is set by different global standards depending on where the food is produced.
Food products are an important niche market due to the increasing awareness of customers regarding the nutritional quality and safety of the food they eat.
As a result, the worldwide market volume of food products labelled as “organic” has quadrupled over the last 15 years [
2], a fact that needs to be addressed by the food supply chain.
The concept of quality in food has expanded beyond the chemical and organoleptic characteristics of products and embraced the ethical values and the environmental impact of production mode [
3]. For consumers, good nutrition is not only the determinant for health maintenance but also for improved quality of life from a cultural, social, and psychological holistic perspective. An ever-growing number of consumers are a conscious and informed elite, who are prepared to pay more for certified organic food products because of the credibility of their origin and their label reliability [
4]. Because of this trend, Europe Regulation (EU) 2018/848 has recently been approved, repealing Council Regulation (EC) No 834/2007 on organic production and labelling as organic food products. Euroleaf is the EU logo that identifies packaged organic food products. For consumers, certification is important for two reasons: i) the perception of food safety is enhanced upon purchase by the consumer of the product labelled as “organic”; and ii) companies are able to show that the product meets the necessary requirements, providing a competitive advantage as compared to their direct competitors.
Certain microalgal species, such as
Chlorella vulgaris, Arthrospira platensis (often called “Spirulina”), and
Tetraselmis chui, are commonly used in the food sector as novel foods, either as a whole or as food supplements, approved by FDA (Food and Drug Administration), EFSA (European Food Safety Authority), and Regulation (EC) 2017/2470 Directive 2002/46/CE [
5]. Functional food, as
C. vulgaris biomass, can be directly consumed or incorporated into conventional foods in order to enhance their nutritional content [
6]. This freshwater microalga is known to be a rich source of protein, pigments, vitamins, and
β-glucans that can help in the regulation of lipid concentration in human blood [
6].
One of the most important steps for successful autotrophic microalgal growth is the selection of an adequate culture medium able to supply suitable amounts of nitrogen, phosphorus, iron, and trace minerals that ensure the effective growth of cultures [
7]. Similarly, the abiotic growth conditions, including temperature, pH, and light irradiance play a key role in microalgal growth and are known to significantly affect the final biochemical composition of the biomass. The culture medium and the cultivation conditions are intended to mimic, as much as possible, the natural environment from which the strains are derived, but they can vary according to strain, trophic route, and the purpose of cultivation.
In this context, the present work aimed at establishing and optimizing an effective culture medium for autotrophic production that in the future can label C. vulgaris as “organic biomass”, which will be used as a term of convenience throughout the manuscript. Indeed, all growth media tested and the methods used follow the EU legislation, allowing the authors to label the biomass as such. To reach this goal, various certified organic label substrates were screened for their macronutrient and micronutrient composition as well as for their effect on C. vulgaris growth performance at laboratory scale. Thereafter, the most promising formulation of organic culture medium was compared to a standard synthetic medium in different outdoor photobioreactors and the biochemical composition of the biomass produced was evaluated. To the best of the authors’ knowledge, this is the first study addressing the establishment of an organic culture medium for microalgal production.
2. Materials and Methods
2.1. Microalgae Strain and Inoculum Production
The experiments described in the present work were performed at the facilities of Allmicroalgae Natural Products, between 15 March and 15 August 2017.
Chlorella vulgaris 0002CA was obtained from the Allmicroalgae culture collection. All growth trials were performed using a two-stage growth process, where a heterotrophic culture was used to inoculate the autotrophic production systems, following the approach described in Reference [
8]. Accordingly, the heterotrophic inoculum of
C. vulgaris was kept cryopreserved in liquid nitrogen in a working cell bank and transferred to 50 mL Erlenmeyer flasks when necessary. The cultures grown in Erlenmeyer flasks were scaled up to inoculate a 7 L bench-top fermenter (New Brunswick BioFlo
®/CelliGen
®115; Eppendorf AG, Hamburg, Germany). The fermenter was maintained at 28 °C and the pH at 6.5 by the addition of ammonium (24% in weight). Cultures obtained from this fermenter were used to inoculate the autotrophic production systems, namely, 2 L bubble columns, flat panels (FP), and tubular photobioreactors used in the present work.
2.2. Preparation of Organic Medium
The commercial organic substrates (OS) used during this study consisted of biodegradable compounds and were all certified for biological production: i) Bioscape Humix (Atlanlusi, Leiria, Portugal); ii) Nutrimais (Lipor, Porto, Portugal); iii) Nutriverde (Algar, Faro, Portugal); and iv) EcoMix4 (DCM, Grobbendonk, Belgium).
To prepare the liquid culture medium, the granular OS were dissolved by sequentially increasing their concentrations, until a maximum of 300 g L−1 at 60 °C for 2 h, under constant agitation (magnetic stirrer). After the solubilization, the non-soluble compounds and residues were centrifuged (model z400k, Hermle, Wehingen, Germany) at 4500× g for 15 min, and the supernatant was further filtered (0.2 μm) and stored for the elemental analysis and laboratory trials.
Taking into account the N concentration obtained, the quantities of each substrate and the control were added in the following percentages (% v/v): Bioscape Humix (0.18 %); ii) Nutrimais (17.39 %); iii) Nutriverde (55.71 %); and iv) Eco-Mix 4 (3.13 %); synthetic medium (0.20 %).
To prepare the optimized organic culture medium for the outdoor trials, 25 kg of Bioscape Humix and 30 kg of EcoMix4 were solubilized in 200 L of water at 18 °C for 2 h, in a tank with constant aeration to facilitate mass transfer. After the solubilization, the mixture was filtered using cartridges of different pore sizes, namely, 2000, 50, 20, 10, 1, and 0.2 μm (CEPEX, Barcelona, Spain). The liquid culture medium was stored in 20 L bottles at 4 °C until further use.
2.3. Laboratory Trials
Laboratory trials were conducted in 2 L bubble column photobioreactors under constant aeration (Midisart 2000 Filter, 0.2 µm PTFE, Odivelas, Portugal). Experiments were carried out at room temperature (25 ± 1 °C), using a constant (24 h) photon flux density of 100 μmol photons m
−2 s
−1 and an initial pH of 6.8–7.1. The four organic culture media were diluted to obtain a final concentration of 2 mmol L
−1 of N (nitrates and ammonium), and a synthetic culture medium based on Guillard’s F2, adapted to the local water supply [
9] was used as control, at a final concentration of 2 mmol L
−1 of N. The assays started with an initial
C. vulgaris biomass concentration of 0.17 g L
−1. All experiments were performed in triplicate, and sampling was carried out every two days.
2.4. Outdoor Trials in Photobioreactors
The first outdoor trial was performed in 125 L FP, under constant aeration (filtered at 0.2 µm). The pH in the culture was kept below 8 by the introduction of CO2 in the culture system using a pulse system, while the temperature was kept below 30 °C using a water-sprinkling system. The developed organic medium, a mixture of Bioscape and EcoMix4 (1:1.2, m/m), was supplied around 0.91 % v/v to achieve a final concentration of 2 mmol L−1 N. The synthetic medium at the same N concentration was used as control.
Thereafter, a proof of concept assay was performed in 10 m3 horizontal tubular photobioreactors composed of polymethyl methacrylate (PMMA) tubes (Φi = 56 mm). Trials were conducted with a culture velocity of 1 m s−1 and an automatic CO2 injection system using a pH set point of 8.0. The turbidity, pH, and temperature were registered in real-time using an in-house automated system. After the biomass production was finalized, the culture was harvested using an ultrafiltration system (PALL WUSP-6443, Port Washington, NY, USA) and dried using an MDR-150 high-speed centrifugal spray drier. Similarly, a mixture of Bioscape and EcoMix4 (1:1.2, m/m) was supplied in order to achieve a final concentration of 2 mmol L−1 N.
2.5. Nitrates and Ammonium Determination
Culture media and microalgal samples collected were centrifuged for 15 min at 4500×
g. Nitrates were determined using a Genesys 10S UV-VIS spectrophotometer (Thermo Scientific, Waltham, MA, USA). The blank solution was made with 0.1 mol L
−1 of HCl. The OD of each sample was calculated from the difference between the value at 220 nm and twice the value at 275 nm, and compared with a calibration curve constructed with sodium nitrate [
10].
Ammonium levels were determined using an Ammonium–Ammonia Sera test (Sera, Heinsberg, Germany) by comparison to a calibration curve plotted against known ammonium nitrate concentrations.
2.6. Growth Assessment
Optical density (OD) was determined at wavelengths of 600 and 740 nm, as an indirect measurement of the growth of the culture. Dry weight (DW) was determined by filtering a known volume of culture in glass microfibre filters (0.7 µm, VWR, Amadora, Portugal) that were later dried using a moisture analyser (Kern DBS, Balingen-Frommern, Germany). Calibration curves were established relating the OD and DW as shown below in Equation (1):
Specific growth rate (
µ) was calculated according to Equation (2):
where
N1 and
N2 refer to biomass concentrations (g L
−1) at the time points (days)
and
, respectively.
The volumetric productivity in biomass (
Pmax) during the culture period was calculated according to Equation (3), expressed in g L
−1 day
−1, from initial time
t0 to
tf with
Xf and
X0 as final and initial concentrations, respectively.
2.7. Biochemical Composition
2.7.1. Proximate Composition
The moisture content was determined by gravimetry, by heating the samples at 100 °C for 2 h, until a constant weight measurement was obtained.
The protein content was determined by elemental analysis of C, H, and N using a Vario el III (Vario EL, Elementar Analyser GmbH, Hanau, Germany), according to the procedure provided by the manufacturer. Total protein was estimated by multiplying the N content by 6.25 [
11].
The total lipid content was determined following the Bligh and Dyer method [
12] with a few modifications [
13]. Briefly, the lipids were extracted using a mixture of chloroform, methanol, and water (2:2:1), and cell lysis was ensured by an Ultra-Turrax (IKA, Staufen, Germany) disperser for 2 min. Thereafter, the organic phase containing the lipids was separated by centrifugation (10 min at 3500×
g) and transferred to a glass vial. A known volume of the organic phase was evaporated and weighted, and the total lipids were determined gravimetrically.
The total ash content was determined by weighing the biomass, placing it in ceramic cups, and incinerating it for 5 h at 550 °C using a furnace (J. P. Selecta, Sel horn R9-L, Barcelona, Spain).
Dietary fibres were determined by Silliker (Vila Nova de Gaia, Portugal) using the PAFQ 230.2 method.
Digestible carbohydrates were calculated by difference of the total and other macronutrients, and the calorific value (energy) was calculated using standard equations (Reg. EU Nº 1169/2011).
2.7.2. Determination of Heavy Metals
Heavy metals were determined by Silliker with a Varian 730-ES atomic emission spectrometry with inductively coupled plasma (ICP-OES). Cadmium, arsenic, tin, and lead were determined as per the EN 14084:03, PAFQ 364N, PAFQ 202.1, and F023730.1 methods, respectively.
2.7.3. Pigment Analysis
Pigments were determined using the Ritchie method [
14], with minor modifications. Briefly, samples were centrifuged at 2547×
g for 15 min using a Hermle centrifuge (HERMLE Labortechnik GmbH, Wehingen, Germany). Thereafter, the supernatant was discarded, pigments were extracted by bead milling in acetone and the full absorbance spectrum of the extract was obtained with a Genesys 10S UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA).
2.8. Elemental Analysis of Organic Substrates
The different chemical elements were determined using an Agilent Technologies 4200 microwave plasma-atomic emission spectroscopic (MP-AES) analyser (Santa Clara, CA, USA), according to Agilent’s technical note (5990-9005EN).
2.9. Statistical Analysis
Statistical analysis was carried out using a one-way ANOVA to compare the different OS, while a t-student test was performed to compare two groups (organic production versus synthetic medium). All tests were performed with a 95% confidence level using R software (version 3.6.2).
3. Results and Discussion
3.1. Characterization of The Organic Substrates
Considering that the OS studied are complex media, it is essential to know the main elements of which they are composed, in order to meet the nutritional requirements of microalgae. Accordingly, the elements that are known to be most important for microalgae growth were determined by elemental analysis (
Table 1).
From
Table 1, it is possible to compare the nutrients concentrations of each OS stock solution, so their usage could be adjusted the best way possible with that of the non-organic medium, in order to achieve an equivalent final concentration. All OS displayed very different concentrations for all elements analysed.
The results obtained revealed that Bioscape clearly presented the highest N and P values (1133.9 mmol L
−1 and 864.2 µmol L
−1, respectively), followed by EcoMix4 (63.8 mmol L
−1 and 566.4 µmol L
−1, respectively), while Nutrimais (11.5 mmol L
−1 and 167.3 µmol L
−1, respectively) and Nutriverde (3.6 mmol L
−1 and 135.4 µmol L
−1, respectively) showed the lowest values. The main macronutrients, N and P, used for microalgal growth, since N is crucial for the biosynthesis of protein and amino acids thereof, while P is essentially used for energy transfer, membrane synthesis (phospholipids), and nucleic acid (DNA and RNA) biosynthesis [
15]. However, the optimum N:P ratio must be taken into account, which is within the range of 6.8–10 for the growth of freshwater algae [
16]. In this context, Bioscape showed the least balanced N:P ratio (1,289), followed by EcoMix4 (113), Nutrimais (69), and Nutriverde (27). These results show that solubilization of P in the different culture media is low, and that all solutions present an unfavourable N:P ratio.
Another key element for microalgal growth is Fe, essential for different metabolic pathways and for chlorophyll biosynthesis [
17,
18]. Among the OS studied, Bioscape was the one that showed the highest Fe concentration, 101.4 µmol L
−1, while in the remaining OS it ranged from 34.6 to 66.8 µmol L
−1. Even though none of the OS displayed ideal concentrations of Fe, the organic Regulation (EU) 2018/848 allows the use of 2% Fe chelated with EDDHSA. Those rules comply with the principles set in the regulation mutatis mutandis concerning phytoplankton production [
19].
Regarding the other micronutrients essential for microalgae growth, Bioscape was once again the OS that presented the highest concentrations, with particularly high contents of Mg (2950 µmol L−1) and Mn (28.9 µmol L−1). However, no OS contained toxic heavy metals, such as Hg, Pb, Ni, Cu, Zn, Cd, and Cr (data not shown) in concentrations higher than those allowed by law (Regulation (EC) N.o 629/2008).
3.2. Screening of Organic Substrates for Autotrophic Production of C. vulgaris
A preliminary trial was carried out at laboratory scale (2 L bubble column reactors) using the four OS and the synthetic culture medium at a normalized N concentration of 2 mmol L
−1 as control (
Figure 1). The results obtained revealed that
C. vulgaris grew best in Bioscape and EcoMix4, reaching a final biomass concentration of ~0.6 g L
−1, similar to that obtained with the synthetic growth medium (control;
p ≥ 0.05). On the other hand, significantly slower growth performance was observed in cultures grown with Nutrimais and Nutriverde (
p < 0.05), which reached about half of the final biomass concentration (~ 0.31 g L
−1) obtained with the other OS. Although Nutriverde and Nutrimais displayed an N:P ratio closer to the optimum, the higher volume of both culture media needed to reach an N content of 2 mmol L
−1 augmented its colour and, therefore, the light attenuation of the final medium. As a result, the photosynthetic capacity of cultures in this specific medium might have been compromised. Alternatively, these culture media might lack some micronutrients essential for microalgal growth or contained compounds that inhibited cell division.
Higher biomass productivities were obtained using EcoMix4 (0.038 ± 0.003 g L
−1 d
−1), Bioscape (0.033 ± 0.005 g L
−1 d
−1), and synthetic (0.037 ± 0.001 g L
−1 d
−1) culture media without significant differences among them (
p ≥ 0.05;
Table 2).
C. vulgaris grown with Nutrimais displayed a productivity of 0.017 ± 0.002 g L
−1 d
−1, significantly lower than that achieved in the aforementioned media, but significantly higher than those obtained with Nutriverde (0.009 ± 0.002 g L
−1 d
−1;
p < 0.05). Similarly, cultures grown with EcoMix4 (0.122 ± 0.009 d
−1), Bioscape (0.111 ± 0.011 d
−1), and synthetic (0.123 ± 0.001 d
−1) culture media displayed higher specific growth rates (
µ), but identical among them (
p ≥ 0.05), followed by Nutrimais (0.075 ± 0.004 d
−1), and Nutriverde with the lowest
µ (0.036 ± 0.008 d
−1;
p < 0.05).
Regarding reagents’ cost, synthetic and Bioscape were the most competitive media with 1.32 and 1.56 €/kg of biomass produced. Nutrimais and EcoMix4 followed with a cost of 14.23 and 21.24 €/kg of biomass produced. The most expensive media was Nutriverde with 58.13 €/kg of biomass. Recently, there has been a demand for products labelled as organic by the consumers who refuse to include “non-organic” products in their diet, independently of the price. Hence, the low supply and high demand in this field, enables higher consumer prices and gives the companies the opportunity to increase the sales volume.
Results show that all cultures grown using the different OS consumed preferentially ammonia over nitrates, being ammonia almost fully consumed (except for Nutriverde), while nitrates were consumed at a much slower rate. Perez-Garcia
et al. [
20] reported that N is preferably assimilated in the following order: NH
4+ > NO
3− > NO
2− > CH
4N
2O. The assimilation of nitrates requires transport through the cell membrane and reduction to ammonia, which demands energy, via consumption of carbon and protons. The assimilation of ammonia at high pH causes an imbalanced reaction that lowers the pH, according Reaction (4) [
21,
22]. For this reason, it is important to avoid the addition of excess ammonia.
3.3. Synthetic Culture Medium versus Organic Growth Medium in 125 L Flat Panels
Considering the results obtained at laboratory scale and from the elemental analysis, a combination of two OS was considered, and a mixture of Bioscape and EcoMix4 (1:1.2, m/m) selected, and later was tested in 125 L FP. The blend was compared to the synthetic medium as control (
Figure 2).
The results obtained revealed that both the formulated organic medium and the non-organic medium led to similar microalgal growth performance and biomass concentrations, 0.77 ± 0.07 and 0.82 ± 0.06 g L−1, respectively, showing no significant differences throughout the culture growth period (p ≥ 0.05). Similarly, there were no significant differences in the volumetric biomass productivities and µ of the organic (0.04 ± 0.02 g L−1 d−1; 0.067 ± 0.017 d−1, respectively) and synthetic (0.04 ± 0.03 g L−1 d−1; 0.074 ± 0.003 d−1, respectively) culture media.
Producing this industrial medium cost 3.86 €/kg of biomass, given the operational expenditures of mixing both reagents and their solubility at this scale. The chosen substrates were not based only on price. Nutrimais was cheaper than EcoMix4 but the mass needed was 5.5 times higher than EcoMix4. As a result, it took longer to solubilize, and the separation solid/liquid was more expensive.
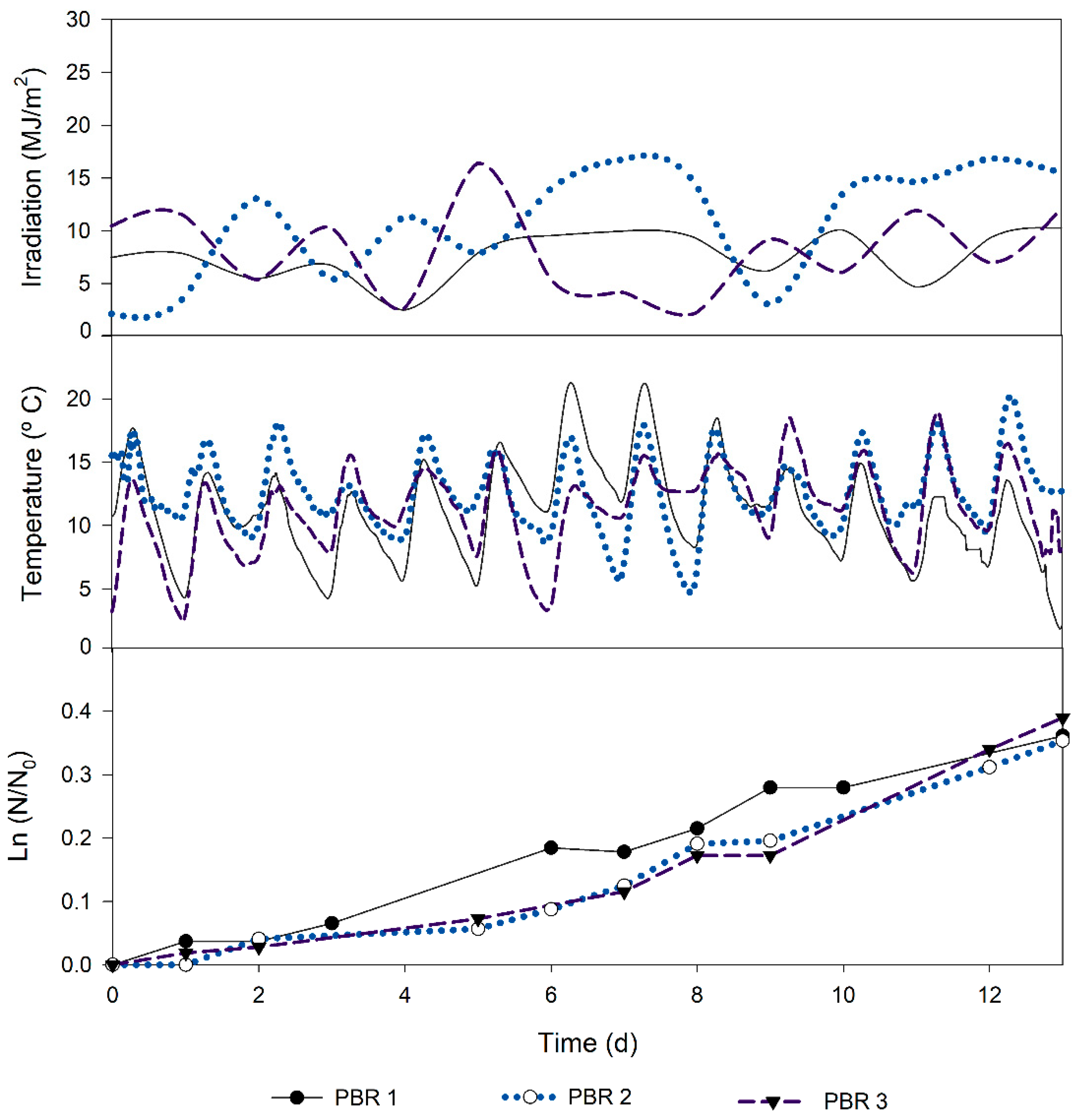
3.4. Growth Using the Optimized Organic Growth Medium in 10 m3 Tubular Photobioreactors
Finally, a proof of concept was performed in 10 m
3 tubular photobioreactors aiming at assessing the performance in a significantly higher culture volume (
Figure 3). The average daily radiation in the three growth periods was 8.89 ± 4.26 MJ m
−2. The average cultivation temperature was 11.84 ± 0.73 °C, which is under the optimal growth condition of 30 °C known for this strain [
23]. The logarithmic growth curves obtained for
C. vulgaris cultivated using the optimized organic culture medium show that cultures grew steadily during the 13 days of cultivation, displaying a similar growth performance among the three photobioreactors. These results are highlighted by the consistency of the values obtained for each experimental replicate in terms of biomass productivity (0.023 ± 0.001 g L
−1 d
−1), specific growth rate (0.026 ± 0.002 d
−1), and final dry weight (1.04 ± 0.09 g L
−1,
Table 3).
3.5. Evaluation of Organic C. vulgaris Biomass
In order to have an “organic” certification, the final product needs to meet the requirements of EU Regulation 2018/848 on organic production and labelling of organic products. Furthermore, its nutritional composition should be appealing to buyers in terms of food quality standards when compared with non-organic products, and above all, ensure food safety requirements for the consumer. Therefore, the biochemical composition of biomass produced in the tubular photobioreactors using the optimized organic culture medium and the synthetic medium was analysed (
Table 4).
The mean protein content of
C. vulgaris grown in organic and synthetic media was 57.2 and 58.6 g 100 g
−1, respectively. These values are consistent with the values reported by Ogbonna et al. [
24], but considerably higher than those reported by Tokuşoglu et al. [
25] and Batista et al. [
26] in which
C. vulgaris biomass presented lower protein contents (38.0–47.82 g 100 g
−1). Proteins have a special role in human nutrition due to the presence of essential amino acids, which are building blocks for proteins and, thus, for growth, cell metabolism, and tissue maintenance. This result is highly significant, as the demand for alternatives to animal protein is on the rise, in particular, due to the growing trend of consumers choosing vegan and vegetarian diets.
The mean values of total lipids obtained in the biomass grown in organic medium (9.4 g 100 g
−1) were slightly lower than those obtained with the synthetic culture medium (11.0 g 100 g
−1). The lipid content here reported for biomass grown using both media is similar to the values previously reported in the literature for
C. vulgaris [
24].
Regarding carbohydrates, organically grown
C. vulgaris displayed a higher content, 4.4 g 100 g
−1, as compared to that detected in the biomass produced using synthetic medium, 1.5 g 100 g
−1. This difference can be a sign of stress in the cultures grown with the organic medium, which can be related to the unfavourable N:P ratio. Similarly, a slightly higher content of dietary fibres was detected in the biomass grown using the organic (16.9 g 100 g
−1) than in the synthetic (15.5 g 100 g
−1) culture media. Dietary fibres are present in plant cells and can help increase the feeling of satiety, regulate blood glucose levels, and solve obstipation problems [
27,
28].
Organically grown biomass and non-organically grown biomass had similar contents of ash and moisture, namely 9.2−10.8 and 2.8-3.0 g 100 g
−1, respectively. Low moisture values are important to increase the shelf life of the product once it is kept as a microalgal powder [
29].
The pigment content of organically and non-organically grown biomass revealed to be very similar (10.7–12.6 mg g
−1). The biomass of
C. vulgaris is a rich source of chlorophyll, and the values here reported are in range of the values previously reported by other authors, 8.58–15.4 mg g
−1 [
30,
31]. Photosynthetic pigments have well described antioxidant properties and can be used as natural colourants [
32].
For questions of food safety, it is important to ensure that there are no harmful concentrations of heavy metals such as lead, cadmium, tin, and arsenic present in food. The analysis for these contaminants is regulated by the legislative Commission Regulation (EC) No 629/2008 of 2 July 2008, setting maximum levels for certain contaminants in foodstuffs. Algae are also included since they tend to accumulate cadmium naturally, so for dried products derived from seaweeds the limit established is 3 mg kg
−1 [
33]. Organically grown biomass displayed residual mean values of tin (2.5 mg kg
−1) and lead (0.09 mg kg
−1), both below the values allowed by the European legislation. The non-organically grown biomass presented residual contents of lead (0.12 mg kg
−1) and arsenic (0.21 mg kg
−1), which are also in accordance with the European legislation, and so there was no need to eliminate these compounds from the original medium. All remaining heavy metals were below the limits of quantification of the methods used.