Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Sample Description
2.3. Extraction of Lipids
2.4. Preparation of Fatty Acid Methyl Esters (FAMEs)
2.5. Free Fatty Acid (FFA) Content from Extracted Oils
2.6. GC Analysis
2.7. Mineral Determination in Seeds Samples
2.8. Protein Determination
2.9. Determination of the Antioxidant Activity
2.10. Statistics
3. Results and Discussion
3.1. Lipid Extraction Yield
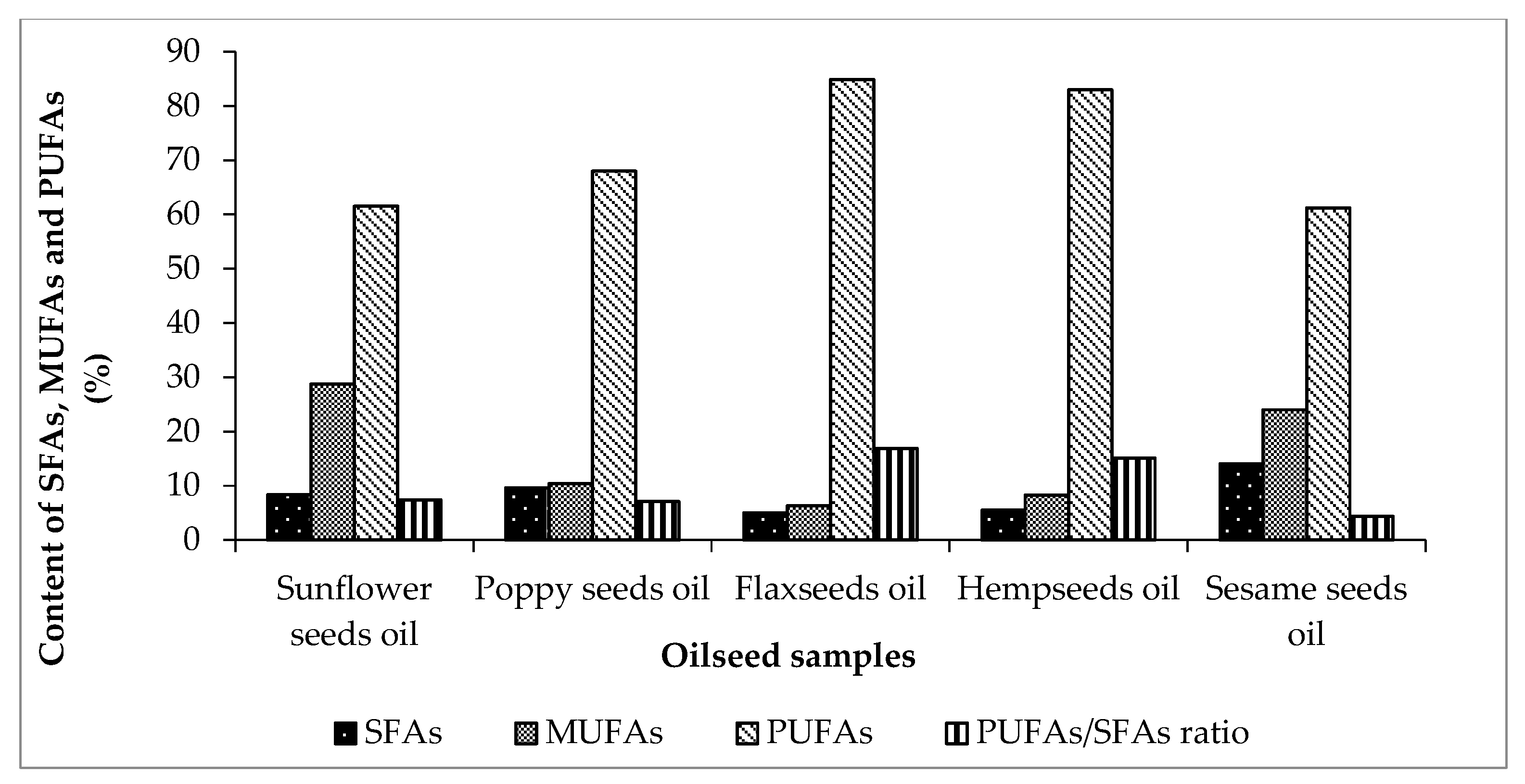
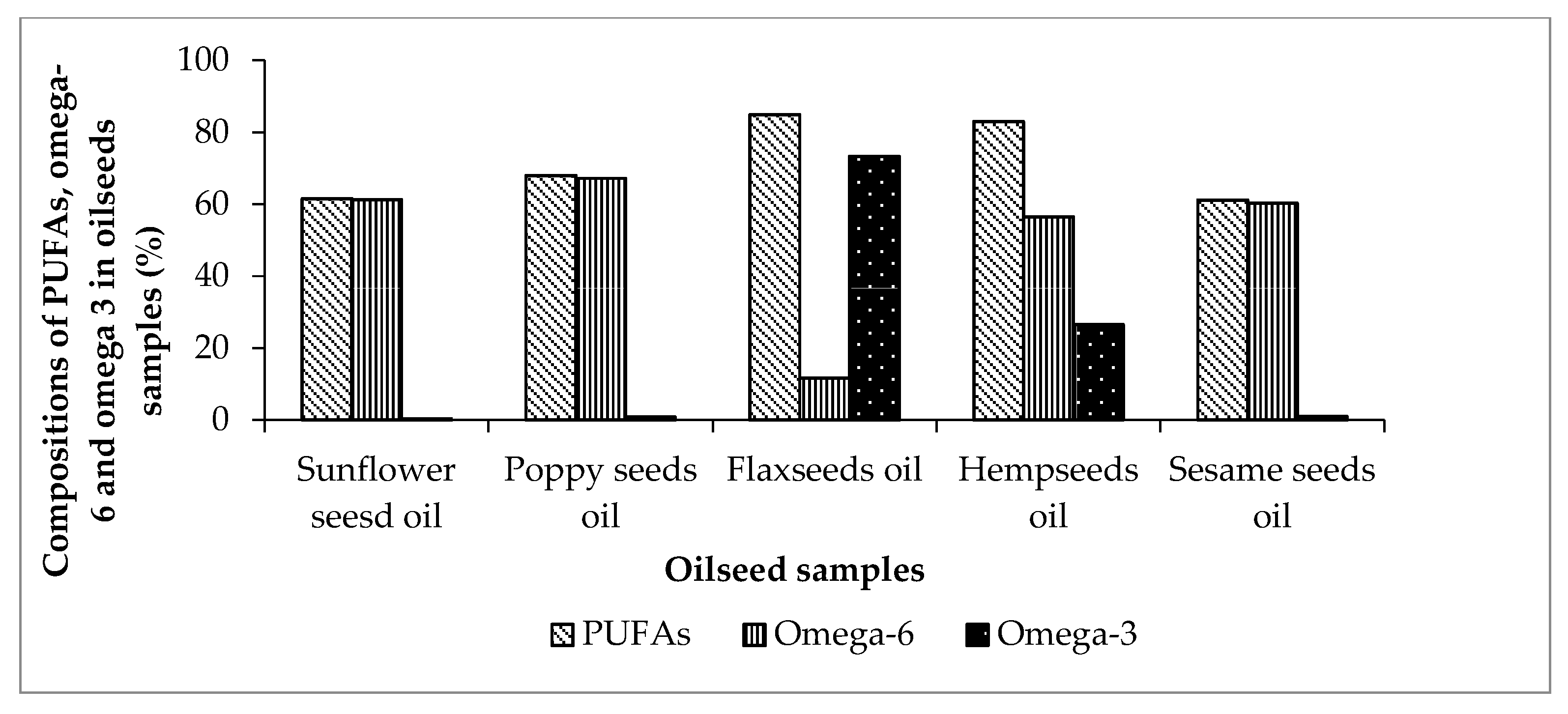
3.2. FAs Composition
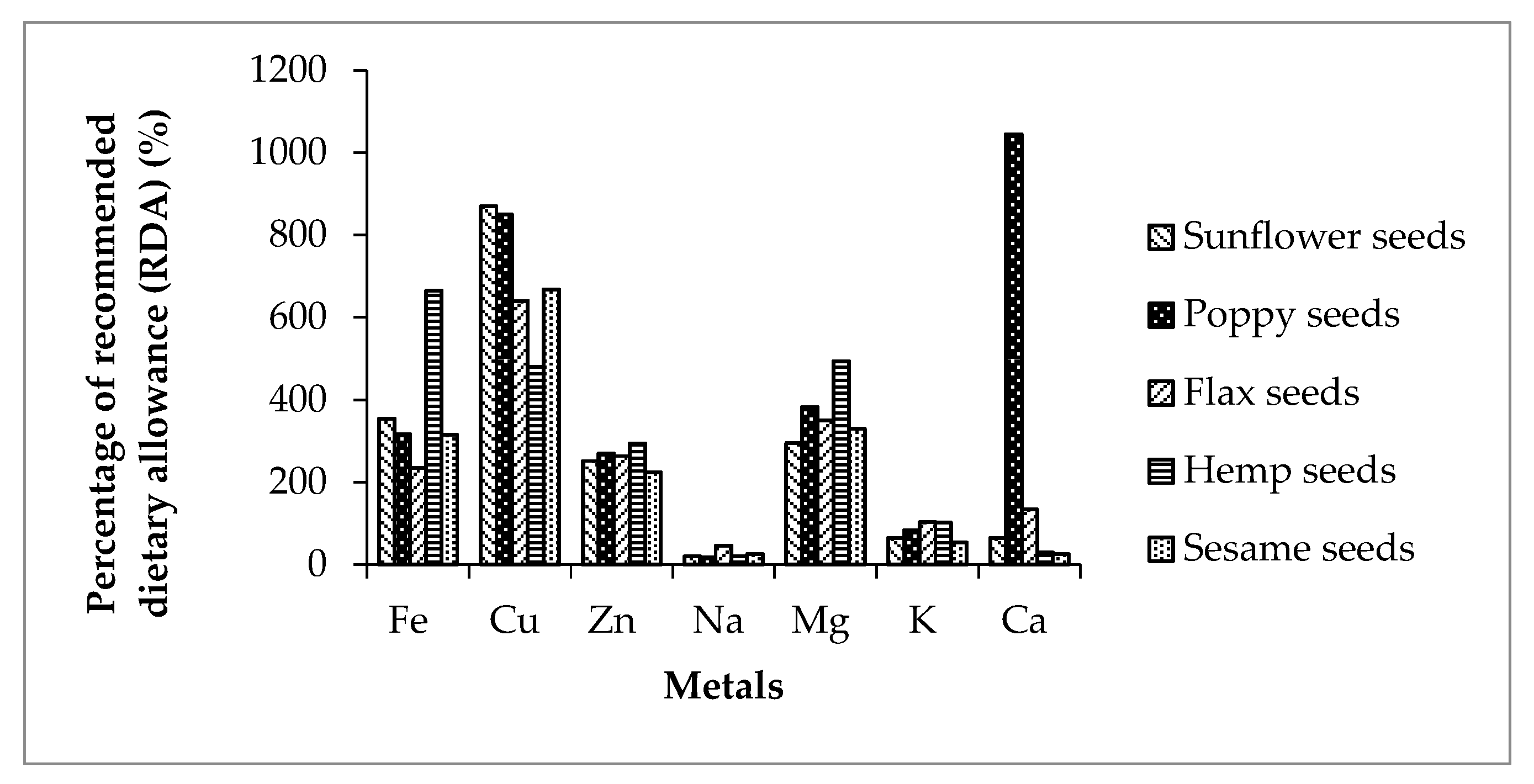
3.3. Minerals, Protein Content and Antioxidant Capacity
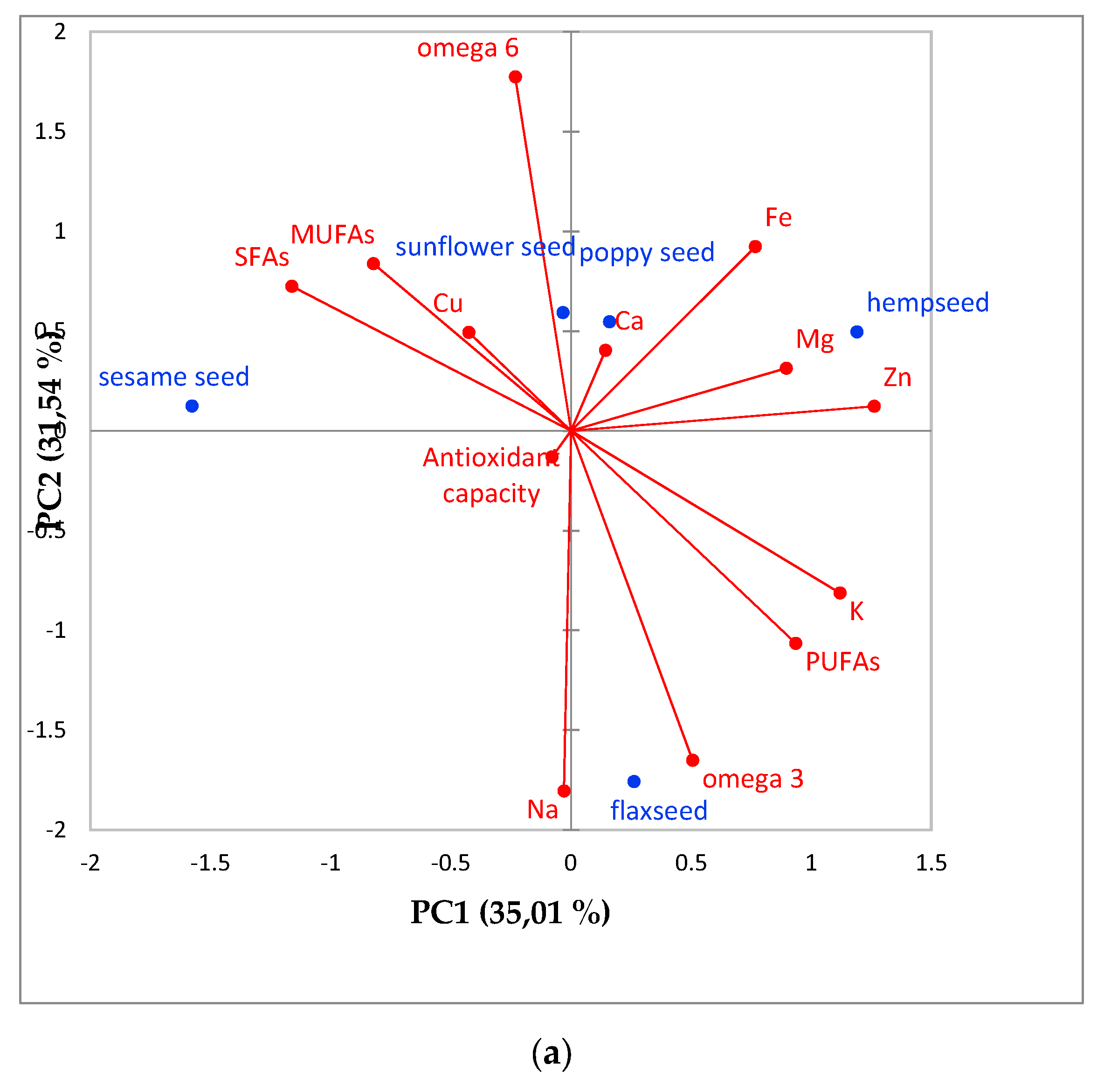
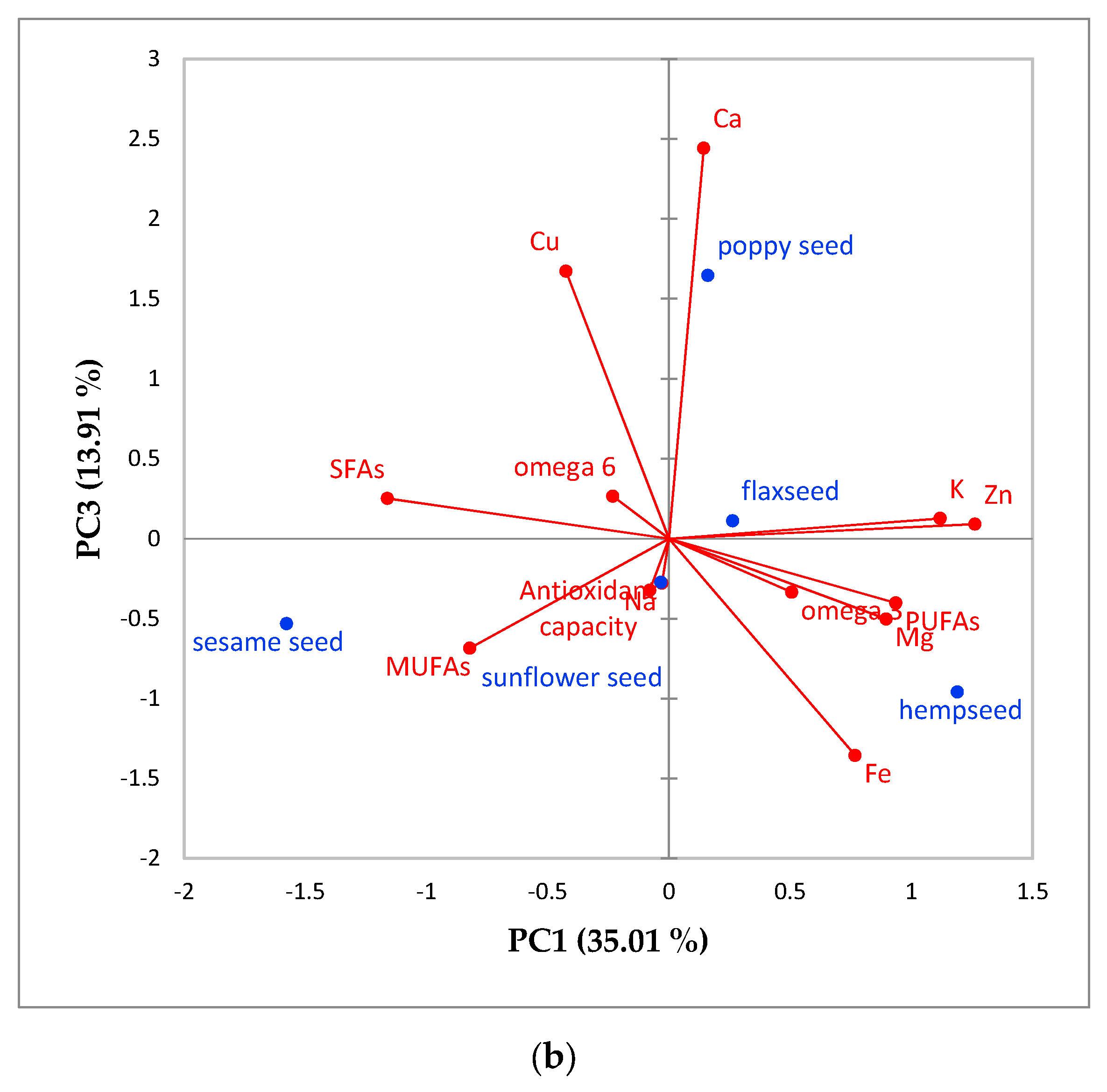
3.4. Results Characterization Using Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrović, M.; Kezić, N.; Bolanća, V. Optimization of the GC method for routine analysis of the fatty acid profile in several food samples. Food Chem. 2010, 122, 285–291. [Google Scholar] [CrossRef]
- Labdeli, A.; Zemour, K.; Simon, V.; Adda, A.; Merah, O. Pistacoa Atlantica Desf., a source of healthy vegetable oil. Appl. Sci. 2019, 9, 2552. [Google Scholar] [CrossRef]
- McClements, D.J. The biophysics of digestion: Lipids. Curr. Opin. Food Sci. 2018, 21, 1–6. [Google Scholar] [CrossRef]
- Béligon, V.; Christophe, G.; Fontanille, P.; Larroche, C. Microbial lipids as potential source to food supplements. Curr. Opin. Food Sci. 2016, 7, 35–42. [Google Scholar] [CrossRef]
- Kozlowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seed. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Mohammed, F.; Abdulwali, G.D.; Tenyang, N.; Ponka, R.; Al-Gadabi, K.; Bchitou, R.; Abdullah, A.H.; Naji, K.M. Chemical composition and mineralogical residence of sesame oil from plants grown in different Yemeni environments. Microchem. J. 2018, 140, 269–277. [Google Scholar] [CrossRef]
- Youzbachi, N.; Elfalleh, W.; Khaldi, A.; Nasri, N.; Tlili, N. Fatty acids and triacylglycerols composition from Tunisian Acacia species seeds oil. Arab. J. Chem. 2019, 12, 3302–3308. [Google Scholar] [CrossRef]
- Bystrická, Z.; Ďuračková, Z. Gas chromatography determination of fatty acids in the human erythrocyte membranes—A review. Prostaglandins Leukotr. Essent. Fatty Acids 2016, 115, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A. Marine omega-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hejtmánková, A.; Kotíková, Z.; Pivec, V.; Střalková, R.; Vollmannová, A.; Bojňanská, T.; Dědina, M. Evaluation of oil content and fatty acid composition in the seeds of grapevine varieties. LWT Food Sci. Technol. 2015, 63, 620–625. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; Gimenez, D.G.; Fernandez-Arche, M.A. Hemp (Cannabis sativa L.) seeds oil: Analytical and phytochemical characterization of unsaponifiable fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Rutusz, K.; Symoniuk, E.; Wroniak, M.; Rudzinska, M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed Camelina (Camelina sativa L.) oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Li, P.; Xu, B.; Ma, F.; Zhang, W. Fatty acid profiles based adulteration detection for flaxseed oil by gas chromatography mass spectrometry. Food Sci. Technol. 2015, 63, 430–436. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim Biophys. Acta 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Ramos, P.A.B.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Chemical composition of lipophilic bark extracts from Pinus pinaster and Pinus pinea cultivated Portugal. Appl. Sci. 2018, 8, 2575. [Google Scholar] [CrossRef]
- Xiao, L.; Mjøs, S.A.; Haugsgjerd, B.O. Efficiencies of three common lipid extraction methods evaluated by calculating mass balances of the fatty acids. J. Food Compos. Anal. 2012, 25, 198–207. [Google Scholar] [CrossRef]
- Nielsen, S. Food Analysis, 3rd ed.; Springer Science & Business Media, Kluwer Academic: New York, NY, USA, 2003; Volume 10, ISBN 978-0306481758. [Google Scholar]
- Teymouri, A.; Adams, K.J.; Dong, T.; Kumar, S. Evaluation of lipid extractability after flash hydrolysis of algae. Fuel 2018, 224, 23–31. [Google Scholar] [CrossRef]
- Cadar, O.; Miclean, M.; Cadar, S.; Tanaselia, C.; Senila, L.; Senila, M. Assessment of heavy metals in cows milk in Rodnei mountains area, Romania. Environ. Eng. Manag. J. 2015, 14, 2523–2528. [Google Scholar] [CrossRef]
- Adjepong, M.; Valentini, K.; Pickens, C.A.; Li, W.; Appaw, W.; Fenton, J. Quantification of fatty acid and mineral levels of selected seeds, nuts, and oils in Ghana. J. Food Compos. Anal. 2017, 59, 43–49. [Google Scholar] [CrossRef]
- Boffito, D.C.; Pirola, C.; Galli, F.; Michele, A.D.; Bianchi, C.L. Free fatty acids esterification of waste cooking oil and its mixtures with rapeseed oil and diesel. Fuel 2013, 108, 612–619. [Google Scholar] [CrossRef]
- Naozuka, J.; Vieira, E.C.; Nascimento, A.N.; Oliveira, P.V. Elemental analysis of nuts and seeds by axially viewed ICP OES. Food Chem. 2011, 124, 1667–1672. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterization of fatty acids, carotenoid, tocopherol/tocotrienol composition and antioxidant activities in seeds of three Chenopodium quinoa Willd. Genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oil extracted by different methods. Ind. Crop Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by h-hexane. Ind. Crop Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Bratfalean, D.; Cristea, V.M.; Agachi, P.S.; Irimie, D.F. Improvement of sunflower oil extraction by modelling and simulation. Rev. Roum. Chim. 2008, 53, 881–888. [Google Scholar]
- Rashid, U.; Ibrahim, M.; Nehdi, A.I.; IbrahimAl-Resayes, S.; Ullah, S.; Mehmood, M.A.; Shahzadi, S. Synthesis and characterization of poppy seeds oil methyl esters. Chin. J. Chem. Eng. 2016, 24, 1087–1096. [Google Scholar] [CrossRef]
- Zanqui, A.B.; Rodrigues de Morais, D.; Marques da Silva, C.; Santos, J.M.; Gomes, S.T.; Visentainer, J.V.; Eberlin, M.N.; Cardozo-Filho, L.; Matsushita, M. Subcritical extraction of flaxseed oil with n-propane: Composition and purity. Food Chem. 2015, 188, 452–458. [Google Scholar] [CrossRef]
- Junpeng, Y.; Qi, Z.; Xin, L.; Xinsheng, W.; Bing, L.; Wenxue, Z. Steam explosion technology based for oil extraction from sesame (Sesamum indicum L.) seeds. J Saudi Soc. Agric. Sci. 2016. [Google Scholar] [CrossRef]
- Chung, I.; Kim, J.-K.; Park, I.; Oh, J.-Y.; Kim, S.-H. Effects of milk type, production month, and brand on fatty acid composition: A case study in Korea. Food Chem. 2016, 196, 138–147. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Salimon, J.; Omar, T.A.; Salih, N. An accurate and reliable method for identification and quantification of fatty acids and trans fatty acids in food fats samples using gas chromatography. Arab. J. Chem. 2017, 10, S1875–S1882. [Google Scholar] [CrossRef]
- Lu, J.; Wang, P.; Ke, Z.; Liu, X.; Kang, Q.; Hao, L. Effect of metal ions on the enzymatic hydrolysis of hemp seeds oil by lipase Candida sp. Int. J. Biol. Macromol. 2018, 114, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Siroho, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits—A review. Pharma Nutr. 2019, 10, 100162. [Google Scholar] [CrossRef]
- Todea, D.; Cadar, O.; Simedru, D.; Roman, C.; Tanaselia, C.; Suatean, I.; Naghiu, A. Determination of major- -to-trace minerals and polyphenols in different apple cultivars. Not. Bot. Horti. Agrobo. 2014, 42, 523–529. [Google Scholar] [CrossRef]
- Danhassan, M.S.; Salihu, A.; Inuwa, H.M. Effect of boiling on protein, mineral, dietary fibre and antinutrient compositions of Nymphaea lotus (Linn) seeds. J. Food Compos. Anal. 2018, 67, 184–190. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rožnowski, J.; Fortuna, T. Phenolic profile and antioxidant activivity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Levei, E.; Ponta, M.; Senila, M.; Miclean, M.; Frentiu, T. Assessment of contamination and origin of metals in mining affected river sediments: A case study of the Aries River catchment, Romania. J. Serb. Chem. Soc. 2014, 79, 1019–1036. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.A.; Senila, L.R.; Oprea, G.M.; Roman, C.M. Mercury in soil and perennial plants in a mining-affected urban area from Northwestern Romania. J. Environ. Sci. Health. Part. A 2012, 47, 614–621. [Google Scholar] [CrossRef]
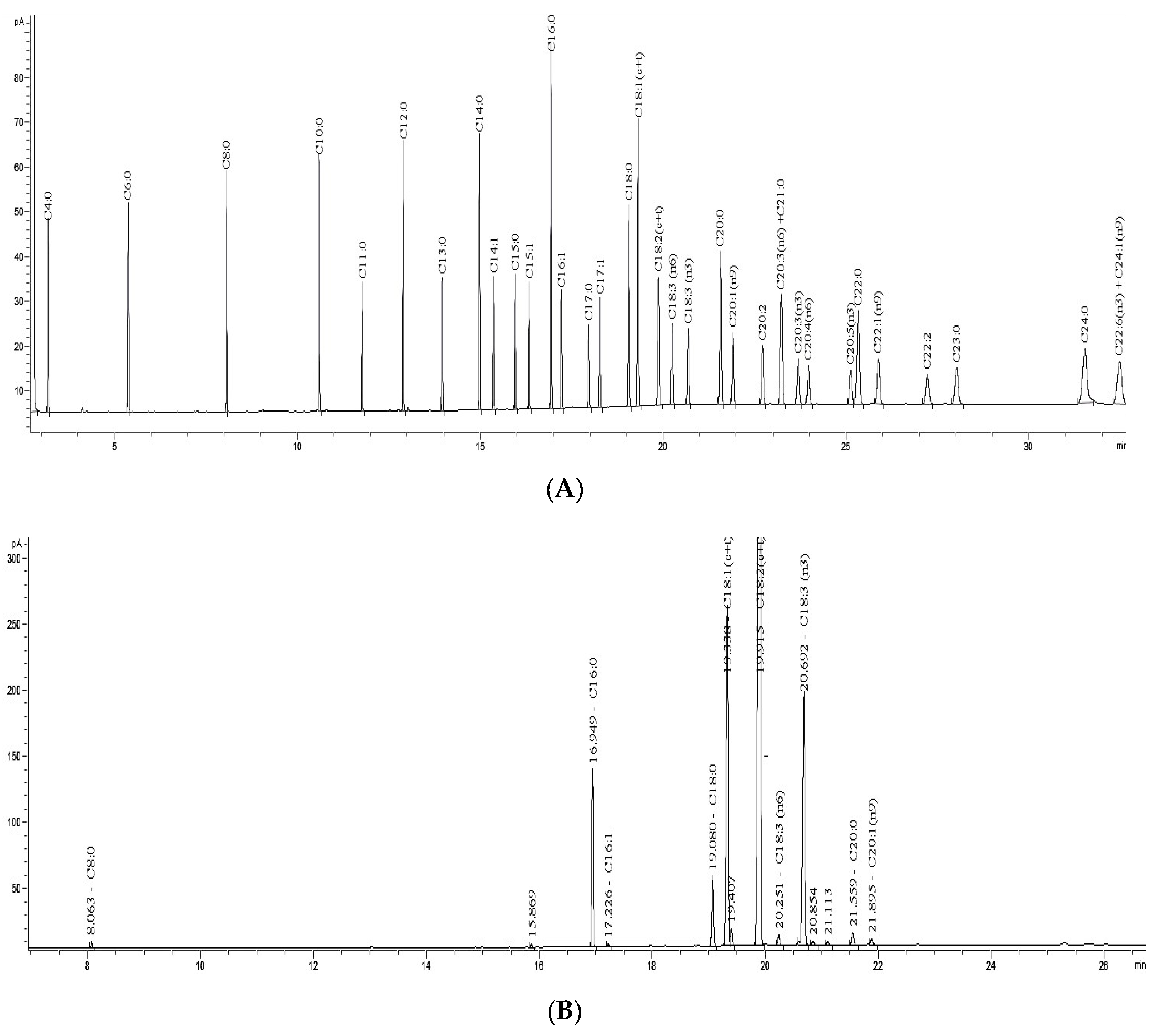
| Sample | Lipid Yield (%) (This Work)* | Lipid Yield (%) (Related Studies) | Extraction Method | Reference |
|---|---|---|---|---|
| Hemp seed | 30.8 ± 0.21 | 29.6 | extraction with n-hexane | [26] |
| Sunflower seed | 54.1 ± 0.50 | 49.1 | extraction with petroleum ether in Soxhlet apparatus | [27] |
| Poppy seed | 38.2 ± 0.31 | 46.8 | cold press extraction | [28] |
| Flax seed | 31.2 ± 0.20 | 35.0 | extraction with choloroform:methanol (2:1(v/v)) | [29] |
| 41.0 | supercritical extraction with n-propane | |||
| Sesame seed | 40.0 ± 0.40 | 41.6 | extraction with petroleum ether in Soxhlet apparatus | [30] |
| Fatty Acid | Sunflower Seed Oil | Reference [6] | Poppy Seed Oil | Reference [28] | Flaxseed Oil | Reference [14] | Hempseed Oil | Reference [12] | Sesame Seed Oil | Reference [30] |
|---|---|---|---|---|---|---|---|---|---|---|
| C8:0 | 0.21 ± 0.01 | nd | nd | nd | 0.46 ± 0.04 | nd | nd | nd | nd | nd |
| C10:0 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| C12:0 | nd | 0.02 | nd | nd | nd | 0.0035 | 0.001 ± 0.01 | nd | nd | nd |
| C14:0 | 0.05 ± 0.02 | 0.09 | 0.04 ± 0.02 | nd | 0.02 ± 0.01 | 0.0332 | 0.017 ± 0.01 | nd | 0.01 ± 0.01 | 0.04 |
| C14:1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| C16:0 | 3.94 ± 0.02 | 6.2 | 7.79 ± 0.07 | 8.99 | 2.51 ± 0.07 | 6.12 | 3.17 ± 0.05 | 5.62 | 6.57 ± 0.04 | 14.2 |
| C16:1(n-7) | 0.90 ± 0.01 | 0.12 | 1.47 ± 0.02 | nd | 0.41 ± 0.01 | 0.03 | 0.55 ± 0.02 | 0.31 | 0.91 ± 0.01 | 0.31 |
| C18:0 | 2.17 ± 0.01 | 2.8 | 1.59 ± 0.08 | 2.15 | 1.93 ± 0.06 | 4.11 | 1.49 ± 0.03 | 2.68 | 3.89 ± 0.02 | 7.35 |
| C18:1 (cis+trans) (n-9) | 26.0 ± 0.50 | 28.0 | 8.34 ± 0.09 | 13.7 | 5.28 ± 0.09 | 15.4 | 5.75 ± 0.08 | 11.90 | 21.5 ± 0.60 | 34.4 |
| C18:2(cis+trans) (n-6) | 61.0 ± 0.70 | 62.2 | 67.2 ± 0.80 | 74.1 | 11.6 ± 0.20 | 13.6 | 55.9 ± 0.30 | 55.1 | 60.2 ± 0.50 | 40.8 |
| C18:3 (n-6) | 0.20 ± 0.02 | nd | 0.04 ± 0.01 | nd | 0.005 ± 0.01 | 0.18 | 0.58 ± 0.02 | nd | 0.02 ± 0.01 | nd |
| C18:3 (n-3) | 0.21 ± 0.02 | 0.16 | 0.77 ± 0.02 | 1.0 | 73.2 ± 0.40 | 59.4 | 26.5 ± 0.30 | 16.7 | 0.91 ± 0.02 | nd |
| C20:0 | 0.29 ± 0.03 | 0.21 | 0.17 ± 0.05 | nd | 0.11 ± 0.01 | 0.14 | 0.63 ± 0.03 | 2.50 | 0.71 ± 0.06 | 1.02 |
| C20:1 (n-9) | 1.60 ± 0.04 | 0.18 | 0.45 ± 0.03 | nd | 0.59 ± 0.02 | 0.096 | 1.99 ± 0.02 | 1.44 | 1.45 ± 0.02 | 0.47 |
| C20:4 (n-6) | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| C20:5 (n-3) | 0.09 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | 0.05 ± 0.01 | nd |
| C22:0 | nd | nd | nd | nd | nd | nd | nd | 0.40 | nd | 0.26 |
| C22:1 (n-9) | 0.22 ± 0.02 | nd | nd | nd | nd | 0.026 | nd | nd | 0.14 ± 0.01 | nd |
| C23:0 | 1.67 ± 0.02 | nd | nd | nd | nd | 0.0081 | 0.17 ± 0.02 | nd | 2.86 ± 0.03 | nd |
| C24:0 | nd | nd | 0.003 ± 0.01 | nd | nd | 0.0418 | 0.008 ± 0.02 | nd | nd | 0.11 |
| C24:1 (n-9)+ C22:6 (n-3) | 0.03 ± 0.01 | nd | 0.08 ± 0.01 | nd | nd | nd | nd | nd | nd | nd |
| SFAs | 8.33 ± 0.02 | 9.4 | 9.59 ± 0.04 | 11.14 | 5.03 ± 0.05 | 10.5 | 5.48 ± 0.08 | 11.20 | 14.0 ± 0.07 | 25.7 |
| MUFAs | 28.7 ± 0.08 | 28.3 | 10.3 ± 0.09 | 13.70 | 6.28 ± 0.08 | 15.6 | 8.29 ± 0.07 | 13.34 | 24.0 ± 0.05 | 35.2 |
| PUFAs | 61.5 ± 0.08 | 62.4 | 67.9 ± 0.10 | 75.13 | 84.8 ± 0.08 | 73.2 | 82.9 ± 0.09 | 75.46 | 61.2 ± 0.07 | 40.82 |
| PUFAs/SFAs ratio | 7.4 ± 0.04 | 6.63 | 7.1 ± 0.01 | 6.74 | 16.9 ± 0.02 | 7.00 | 15.1 ± 0.09 | 6.7 | 4.4 ± 0.07 | 1.58 |
| Component | Sunflower Seed | Poppy Seed | Flaxseed | Hempseed | Sesame Seed |
|---|---|---|---|---|---|
| Mg (mg/kg) | 1180.0 ± 1.15 | 1528.0 ± 1.25 | 1403.0 ±1.52 | 1975.0 ± 1.20 | 1320.0 ± 1.20 |
| K (mg/kg) | 2270.0 ± 0.57 | 2833.0 ± 1.05 | 3509.0 ± 1.50 | 3479.0 ± 1.02 | 1823.0 ± 1.31 |
| Ca (mg/kg) | 644.0 ± 1.0 | 10445.0 ± 1.98 | 1337.0 ± 1.0 | 298.0 ± 0.89 | 257.0 ± 0.75 |
| Na (mg/kg) | 485.0 ± 1.00 | 425.0 ± 1.02 | 1090.0 ± 1.21 | 505.0 ± 0.85 | 610.0 ± 0.95 |
| Fe (mg/kg) | 53.1 ± 0.89 | 47.6 ± 0.67 | 35.3 ± 0.20 | 99.7 ± 0.35 | 47.3 ± 0.63 |
| Zn (mg/kg) | 37.7 ± 0.44 | 40.4 ± 0.20 | 39.5 ± 0.30 | 44.2 ± 0.20 | 33.7 ± 0.51 |
| Cu (mg/kg) | 17.4 ± 0.54 | 17.0 ± 0.29 | 12.8 ± 0.12 | 9.60 ± 0.12 | 13.4 ± 0.11 |
| Al (mg/kg) | 8.30 ± 0.20 | 38.2 ± 0.80 | 8.40 ± 0.15 | 10.1 ± 0.56 | 13.1 ± 0.42 |
| Protein (%) | 22.0 ± 0.40 | 23.7 ± 0.50 | 26.6 ± 0.80 | 36.6 ± 0.26 | 28.2 ± 0.63 |
| Antioxidant capacity (µmol Trolox/mg sample) | 0.45 ± 0.03 | 0.05 ± 0.03 | 0.21 ± 0.04 | 0.03 ± 0.02 | 0.08 ± 0.04 |
| DPPH (µmol Trolox/mg sample) | 0.47 ± 0.02 | 0.06 ± 0.03 | 0.25 ± 0.02 | 0.04 ± 0.03 | 0.09 ± 0.03 |
| Variable | PC1 | PC2 |
|---|---|---|
| Eigenvalue | 6.6 | 3.4 |
| Variability (%) | 51.0 | 26.0 |
| Cumulative | 51.0 | 77.0 |
| Factor loadings after varimax rotation | ||
| SFAs | −0.896 | 0.398 |
| MUFAs | −0.634 | 0.461 |
| PUFAs | 0.721 | −0.586 |
| Antioxidant capacity | −0.061 | −0.072 |
| Mg | 0.691 | 0.172 |
| K | 0.863 | −0.447 |
| Ca | 0.110 | 0.222 |
| Na | −0.023 | −0.994 |
| Fe | 0.591 | 0.508 |
| Zn | 0.973 | 0.067 |
| Cu | −0.328 | 0.271 |
| Omega-6 | −0.179 | 0.976 |
| Omega-3 | 0.390 | −0.909 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, L.; Neag, E.; Cadar, O.; Kovacs, M.H.; Becze, A.; Senila, M. Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds. Appl. Sci. 2020, 10, 1589. https://doi.org/10.3390/app10051589
Senila L, Neag E, Cadar O, Kovacs MH, Becze A, Senila M. Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds. Applied Sciences. 2020; 10(5):1589. https://doi.org/10.3390/app10051589
Chicago/Turabian StyleSenila, Lacrimioara, Emilia Neag, Oana Cadar, Melinda Haydee Kovacs, Anca Becze, and Marin Senila. 2020. "Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds" Applied Sciences 10, no. 5: 1589. https://doi.org/10.3390/app10051589
APA StyleSenila, L., Neag, E., Cadar, O., Kovacs, M. H., Becze, A., & Senila, M. (2020). Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds. Applied Sciences, 10(5), 1589. https://doi.org/10.3390/app10051589








