Abstract
The review covers achievements and developments in the field of probiosis and prebiosis originating from sources other than dairy sources, mainly from plant material like cereals. The actual definitions of probiotic microorganisms, prebiotic, and postbiotic compounds and functional food are discussed. The presentation takes into account the relations between selected food components and their effect on probiotic bacteria, as well as effects on some health issues in humans. The review also focuses on the preservation of cereals using probiotic bacteria, adverse effects of probiotics and prebiotics, and novel possibilities for using probiotic bacteria in the food industry.
1. Introduction
Food that improves human’s life and cures diseases is a concept that has many faces. One of the most complicated loops is: nutrient (prebiotic), which is treated by specific bacteria (probiotic) and products (postbiotic) that are biologically active. The probiotics, their nutrients–prebiotics and derived from the latter postbiotics attract considerable attention of clinicians, microbiologists, dieticians and nutritionists as well as food technologists and agriculture. A number of recent contributions covers problems of probiotics and prebiotics in general [1,2], functional, healthy foods and beverages [3,4,5,6,7,8], effects of processing upon some sources [9], consumer preferences [10,11], mathematical models for probiotic fermentation [12] and curing infectious diarrhoea [13]. They perfectly underline the significance of the problem. This review presents novelties in the field of probiotics and then focuses on cereals—a relatively new type of prebiotics. As a main food component, cereals contain a considerable amount of insoluble and soluble fibre utilised as nutrients by probiotics and deliver several interesting postbiotics. Cereal prebiotics are also a healthy alternative for humans who do not tolerate dairy products. However, there is also a group of consumers who do not tolerate cereals. Therefore, in this review, prebiotics originating from other plants are briefly mentioned.
2. Probiotics
In 2013, a panel of experts slightly modified the World Health Organisation/Food and Agriculture Organisation (WHO/FAO) definition of probiotics into “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [14]. Currently, the research in this field focuses mainly on the gastrointestinal (GI) tract. However, bacteria also colonise the skin, urogenital tract, and airways. New microbial niches have been discovered in, thus, far considered sterile organs and tissues, such as the placenta, amniotic fluid, mammary gland and human milk. Very sensitive genetic techniques have confirmed the presence of microbes in the blood. They are Proteobacteria phylum (more than 80%) but also from Actinobacteria, Firmicutes, and Bacteroidetes [15,16]. These findings changed the frontiers of in vivo probiotics research [16,17,18,19,20].
Lactobacillus species are the most commonly utilised fermentative bacteria [21,22]. Although suitability of lactic acid bacteria for fermentation of maize and amaranth has been reported [23], some Lactobacilli, for instance, Lactobacillus acidophilus, poorly grow in cereal products. Therefore, in such applications, it is combined with Rhizopus oryzae [24]. Among fermentative bacteria, these assigned to the genus Weissella of the group of lactic acid bacteria, are particularly interesting. Weissella spp. occur in a wide range of habitats, e.g., on the skin and in the milk and faeces of animals, saliva, breast milk, human faeces and vaginas, plants and vegetables as well as a variety of fermented foods, such as European sourdoughs, Asian and African traditional fermented foods. These microorganisms are receiving attention as potential probiotics delivering novel non-digestible oligosaccharides and extracellular polysaccharides, mainly dextran. Potentially, as bioactive compounds, they might be useful in a wide range of industrial applications, predominantly for bakeries and production of cereal-based fermented functional beverages [25].
Generally, microbes which are recognised as beneficial are called probiotic bacteria. The positive effect of those bacteria is local and distant, involving the whole body.
3. Modern Probiosis
A considerable number of clinical studies confirm the positive effect of probiotics and prebiotics in maintaining health and therapy. They cure diseases of some organs or alleviate their symptoms, for instance irritable bowel syndrome, Helicobacter pylori gastritis, inflammatory bowel disease, diarrhoea, non-alcoholic fatty liver disease, and atopic dermatitis. Clinical studies suggest the effectiveness of probiotics in the treatment of diseases involving the whole body, such as obesity, insulin resistance syndrome, and type 2 diabetes [26,27]. Probiotics also increase body immunity (immunomodulation). Benefits of the prophylactic use of probiotics in different types of cancer and cancer-associated side effects have also been reported [28]. The influence of probiotics on allergy remains unclear. Long-time follow-up of infants fed with cereals containing probiotic L. paracasei spp. paracasei F19 and a control group fed with probiotic free cereals showed no difference in the allergy risk in both groups [29,30]. A supplementation of cereals with probiotics had no effect on caries in children [31].
Maintaining the skin in good condition with relevant probiotic bacteria appears very promising. Skin bacteria prevent skin irritation, including sun irritation, topical allergy, inflammation, acne, dandruff, alopecia, psoriasis and likely skin cancer [32,33,34]. Following these results, the cosmetic industry introduced a wide range of topical products enriched with probiotics and more commonly with prebiotics specific for beneficial skin bacteria. Such an approach to curing vagina, urinary tract, and respiratory tree disorders with their bacterial microflora is less common.
A personalised microbiome is a novel emerging concept [35]. Such an approach is based on personalised diets, individual-specifically composed. Cereals as common components of a diet, are stable in commercial and home-made processing and are very promising.
4. Prebiotics, Functional Food, Bioactive Ingredients and Nutraceuticals
The old but still valid FAO definition of prebiotics states that ‘a prebiotic is a non-viable (in opposition to probiotics) food component that confers a health benefit on the host associated with modulation of the microbiota’ [36]. However, in December 2016, experts of the International Scientific Association for Probiotics and Prebiotics (ISAPP) recommended the new definition of a prebiotic, ‘a substrate that is selectively utilised by host microorganisms conferring a health benefit’. Thus, a prebiotic may not be a food component. This new definition expands to a wide range of inorganic and organic substances stimulating microorganisms in all niches of the body and not only in the GI tract. It has an essential implication for microbiomes outside of the gastrointestinal tract, especially in the skin, urinary tract, vagina, and so on [37]. In addition, the definition requires documented beneficial health effects of potential prebiotics.
However, that definition evokes some doubts as a number of medications clearly fit this definition. Some antibiotics are utilised by pathogenic bacteria, and the expected effect of those drugs is beneficial for health. Should they be recognised as prebiotics?
These definitions leave apart biological processing involving probiotic bacteria outside the human/animal body (in vitro). Likely, compounds processed outside living organisms using probiotic bacteria, and ingested/used by humans/animals with beneficial effect, should also be accounted for prebiotics.
There is neither a regulation nor any obligatory definition for healthy food, functional food, nutraceuticals, or nutraceutical supplements. Depending on countries, companies, and scientific and consumer societies, various definitions are followed. For instance, functional food is most commonly defined as a food improving health, and nutraceuticals are described as special, pharmaceutical-grade, and standardised nutrients. Another definition states that functional food contains bioactive ingredients, which can be chemical compounds, and the others include also probiotic bacteria. The latter approach implies four main active compounds of functional food: (i) probiotic bacteria, (ii) substrates for their growth (clear prebiotics), (iii) substrates for bacteria metabolism, resulting in biologically active compounds beneficial for the host (could also be called prebiotics), and (iv) biologically active chemical compounds, called recently postbiotics.
4.1. Prebiotic Compounds
Formerly, prebiotics were considered as non-digestible dietary fibre that exert some biological effects involving selective stimulation of growth and bioactivity of beneficial microorganisms either present or therapeutically introduced to the intestine. Probiotics in functional food reside mostly in dairy products. Nevertheless, there is a need for the development of non-dairy probiotics and non-fibre prebiotics.
According to the new definition of prebiotics, polyphenols and fatty acids could be included in this group together with some peptides catabolised by bacteria into active ingredients. Even inorganic materials (i.e., micronutrients necessary for the development of bacteria) used externally and internally could be considered as prebiotics.
4.2. Cereals and Their Derivatives as Prebiotics
Cereals constitute a relatively new group of prebiotics. The most common prebiotics are identified as vegetables and fruit fibres, and dairy products act as probiotic carriers [38]. Grain-based prebiotics, which are a part of a natural everyday diet for most of the human population, are very promising. Cereals are rich in insoluble fibre, soluble fibre (β-glucans and arabinoxylan), galactooligosaccharides (GOSs), fructooligosaccharides (FOSs) and resistant starch [39]. Whole grains are also sources of many phytochemicals, including phytoestrogens, phenolic compounds, antioxidants, phytic acid, and sterols [21,40]. It was shown [41,42,43] that dietary fibre prepared from maize starch are well tolerated by the human organism. They activate the growth of Bacterioidetes and Actinobacteria inhibiting simultaneously the growth of Firmicutes responsible for obesity, similarly, they act also as potato dextrins [44,45].
Products of the lactic acid fermentation of cereals and their derivatives are safe, they enhance the nutritional value of cereals, either improve or change their taste and help to preserve the food. This kind of processing, very common in Asia and Africa is employed in manufacturing beverages, gruels, pancakes, and porridge from fermented rice, sorghum, maize, millet, non-cereal cassava, and wild legume seeds [46]. That food has relatively long shelf life under ambient temperature, and is widely accepted by consumers including expectant/breastfeeding mothers, and sick and recovering persons [47]. Also, in the Western dietary culture, cereals either fermented in vitro or treated by bacteria in vivo used to be added to a variety of functional foods. Frequently, it is a rediscovery of procedures known for centuries, for instance, kvass (a soft drink) containing living bacteria.
Below, the novel impact of prebiotics, especially cereals and their modifications, on probiosis and health of the main body systems will be briefly discussed.
4.2.1. Gastrointestinal Tract
Numerous studies indicated that in contrast to conventional dairy products, cereal products may offer healthier options for the delivery of probiotics. Probiotics are common in dairy products, but cereals are more commonly accepted and consumed [48]. Required cold storage and transportation limit the use of dairy products. A dry food matrix is also a good carrier for probiotic bacteria. The intake of the probiotic Bifidobacterium lactis Bb-12 in an oat-based cereal bar resulted in colonisation in five of the nine subjects, lasting one week after cessation of B. lactis Bb-12 feeding [49]. Fermented oat milk-like beverages could be a compromise between cereal and milk diets. It is rich in probiotic bacteria and has a reasonable ratio of oat β-glucans [50]. Cereal β-glucans improve probiotic-enterocyte adhesion. It could inhibit intestinal colonisation by unfavourable bacteria and, therefore, promote the settlement of probiotic strain [51].
The feeding of a cereal containing Streptococcus thermophilus, B. lactis, L. acidophilus, and zinc reduced the severity and duration of acute gastroenteritis in young children. However, whether this combination is better than either the addition of probiotics or zinc alone is yet to be determined [52].
Cereal-based fermented food products should be taken into account as a promising source of new probiotics. In Nigerian traditional fermented food, Pichia kluyveri LKC17, Issatchenkia orientalis OSL11, Pichia kudriavzevii OG32, Pichia kudriavzevii ROM11, and Candida tropicalis BOM21 were found. They are potentially promising new probiotic strains [53].
4.2.2. Skin
In cosmetology, prebiotics, such as FOSs (mainly extracted from fruits and vegetables), mannooligosacharides, synthetic or lactose-derived lactulose and mainly cereal β-glucans are widely used [54,55]. These prebiotics restore and stimulate the skin beneficial probiotic bacteria and fungi. Moreover, balancing skin pH with prebiotics in cosmetics improves the hydration of surface layers of the skin and normalises its keratinisation and exfoliation. Oligosaccharide probiotics of hairy skin limit the growth of Malassezia furfur responsible for dandruff and maintain the production of sebum. Possibly a stimulation of dermatophytes and blastomycetes is involved. “Fermented” cosmetics, naturally processed synbiotics, mainly from Asia, design new trends in cosmetology. The technology of their production is different, but the final effect resembles that of the use of extracted compounds.
4.2.3. Urogenital Tract
Little is known about the role of prebiotics in maintaining vaginal microflora although vaginal and women lower urinary tract infections are extremely common. Only a few older studies about the positive effects of FOSs and GOSs in vaginal treatment are available [56]. The urogenital tract could be colonised by bacteria administered orally with or without prebiotics [57]. Gut microflora interacts with the urogenital tract, the so-called gut-urogenital axis. It is known that proper gut microbiome stimulated by oral delivery of prebiotics reduces the level of some uremic nephrovascular toxins in patients with chronic kidney diseases. Gut dysbiosis is responsible for the progression of kidney disease [58]. It leads to new therapeutic opportunities for prebiotic and probiotic supplementation preventing kidney diseases and limiting their progress.
Prebiotics and probiotics could also be helpful in men during reproduction. Half-year treatment with orally delivered synbiotic composition of lactic acid bacteria (LAB) and arabinogalactan, FOS and L-glutamine increases the volume of the ejaculate and the quality/quantity of spermatozoa in patients suffering from idiopathic oligoasthenoteratospermia [59]. Likely, such an effect could also be seen in healthy men.
4.2.4. Metabolic Diseases
The dysbiotic gut is linked to obesity, type 1 and type 2 diabetes, and non-alcoholic fatty liver disease. The transfer of gut microflora from obese animals induces their metabolic disease and obesity in followed animals. Conversely, the transfer of pathogen-free microbiota from lean healthy human donors to patients with metabolic disease can increase the sensitivity to insulin [60].
A diet based on rich in prebiotic-fermentable fibre, β-glucans, and plant polyphenols whole-grain cereals, vegetables, and legumes significantly reduces glycaemia, HbA1c, and insulinaemia. It also lowers blood cholesterol and low-density lipoprotein cholesterol. The ratio of low-density lipoprotein cholesterol/high-density lipoprotein cholesterol, triglycerides, blood urea, microalbuminuria, body weight, and body fat in type 2 diabetes patients also decrease [61].
Excessive consumption of red meat increases the risk of cardiovascular diseases. Intestinal microbiota metabolise metabolites of choline, phosphatidylcholine, and L-carnitine into proatherogenic trimethylamine-N-oxide (TMAO) [62].
5. New-Quality—Processing of Foodstuff by Probiotic Bacteria In Vitro
Malting of cereal grains and probiotic lactic acid fermentation of plant-based media increase the nutritional quality of final products. Fermentation is one of the oldest technologies in food processing. LAB are commonly used for the fermentation of a large variety of foods. Most of the commercialised lactic acid fermented products originate from dairy sources. Although they are good matrices for probiotics, their consumption is limited because of growing both veganism and the number of lactose-intolerant individuals and propagation of various restricted diets. Thus, the development of non-dairy probiotic products, such as fruits, vegetables, and cereals as food matrices, is very promising [63]. Particularly, products from cereals fermented with probiotic bacteria pay considerable attention as a cheap way to nutritionally rich food either with or without probiotic bacteria. The most common fermented products of grain origin are presented in Table 1. In many cultures, including Western countries, many fermented products are heated and cooked prior to consumption. These procedures kill probiotic microorganisms [9].

Table 1.
Products from fermented cereals.
Fermentation of cereals provides products of longer shelf life, less allergy and higher nutritional value (vitamins and essential amino acids), and appreciated organoleptic properties.
6. Postbiotics
Recently a new term “postbiotic” was introduced [64]. It is defined as a combination of all bioactive components generated by bacteria, for instance, on fermentation, which acts beneficially when introduced into the human organism (Figure 1). Postbiotics can include bacterial lysates with cell surface proteins, bacterial enzymes and peptides, metabolites produced by bacteria such as teichoic acids, peptidoglycan-derived neuropeptides, polysaccharides, and lower organic acids, for instance, lactic acid. Postbiotics can stimulate the immunological system, likely involving bowel and intestine developing anti-inflammatory, immunomodulatory, anti-obesogenic, antihypertensive, anti-proliferative, antioxidative, and hypocholesterolemic activity.
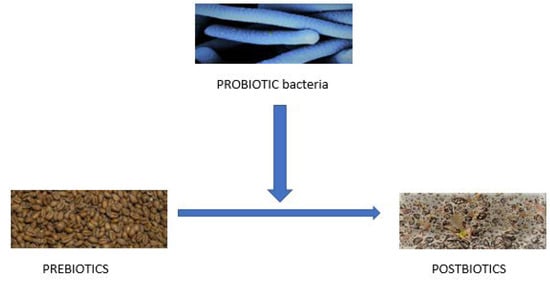
Figure 1.
Relation between prebiotics (grains), probiotics bacteria, and postbiotics (selected fermented products and selected bacteria residue).
In the food, as well medical technology, fermentation is the most common source of postbiotics.
An increase in the B vitamins content in cereal grains presents another spectacular example of postbiotic fermentation. Grains contain considerable levels of the B group vitamins. Frequently, these vitamins are removed upon milling or destroyed on thermal processing. Fermentation of cereals, as well as their pre-treatment with LAB, supports bacterial synthesis elevating the content of vitamins B1, B2, B3, B9, B11, and B12. Potentially it is a strategy for increasing the B vitamin content in cereal-based products [65,66].
LAB fermentation of grains significantly enhanced levels of total lysine, protein fractions, sugars, soluble dietary fibre in vitro, and bioavailability of Ca, Fe and Zn. Also, LAB fermentation of wheat could provide antihypertensive angiotensin I converting enzyme (ACE)-inhibitory peptides and α-aminobutyric acid (GABA) and antioxidant peptides [67,68]. There are attempts to use enzymes instead of probiotic bacteria to get more specific effects. Purified phytases from Bifidobacterium longum spp. infantis and Bifdobacterium pseudocatenulatum reduced the content of phytates in cereal mixtures and led to increased levels of myo-inositol triphosphate. They also increased the solubility of zinc [69].
The new approach is not only connected with enriching food in postbiotics, but also with the deletion of some potentially harmful components of the food during probiotic-induced fermentation. It was found that fermentation with probiotic bacteria L. paracasei CBA L74 decreases the concentration of harmful gliadin peptides in celiac patients [70]. The mechanism involves LAB proline-specific peptidase systems. As α-gliadins in gluten are rich in proline, they are hindered from hydrolysis by enzymes of the GI tract. A joint action of peptidase from LAB strains and GI enzymes could offer a solution for celiac patients. A recently exhausted review on the potential application of postbiotics in early life nutrition and beyond was published [71]. That review considers postbiotics as an efficient and safe way of improving health conditions, is also suitable as a more safe alternative in case of immunocompromised or severely ill new-borns.
6.1. Better Taste—An Extension to the “Beneficial” Action of Probiotics
Flavour is one of the most important organoleptic property of food. Sourdough fermentations employ complex and diverse microflora consisting of yeasts and LAB, mainly Lactobacillus. These complex fermentations add characteristic sensory attributes to the baked bread. Because probiotic microorganisms are killed upon baking, bread is not symbiotic [4]. Therefore, the fermented unbaked/uncooked cereals are the most interesting for their unique sour with mild sweet and malty taste.
The industry seems to be focused on cereal beverages. Fermentation of malt, barley, and barley-malt media by potentially probiotic L. paracasei and L. delbrueckii resulted in better conservation of processed beverages [72]. In Eastern Europe, soft drinks and sour rye soups based on fermented bread, wheat or cereals are common for centuries. Japanese cuisine is particularly rich in fermented foods. It includes fermented fruits and vegetables, fish, soy beans, and other sources. The common Japanese soup, miso is based on soy beans fermented with koji malt rich in Aspergillus orizaee. The same microorganism is utilised in the production of traditional soy sauce (shoyu). Soybean sprouts are fermented with Bacillus subtilis into traditional Japanese appetizer – natto. A traditional fermented dish involves pickles, carrots, daikon, turnip, eggplant, and rice bran all pickled together in vinegar. Umeboshi is prepared from Japanese plums through lactic acid fermentation [73,74]. In China, particularly in Taiwan, stinky tofu made by fermentation chiefly involves LAB, although stinky tofu fermentation does not have a fixed formula and wide regional and individual variations exist in its manufacture and preparation [75]. Lactobacillus spp. is specially blended with vegetables, chiefly a Peking cabbage, into a traditional Korean dish called kimchi. Also, Indian regional cuisines are rich in fermented foods [39].
These new tastes are based chiefly on well-known fermentative bacteria strains such as Bifidobacterium breve, although new or unusual probiotic microorganisms like yeast are used [76]. The probiotic yeast Pichia kudriavzevii OG32 improved the sensory and some functional properties, such as an increase in viscosity [77].
6.2. Probiotics in the Grain Preservation
In the agricultural industry, probiotic microorganisms hinder the colonisation of sorghum grains and peanuts [78]. For instance, LAB reduces the content of phytate, an anti-nutrient in cereals and inhibits moulding [79,80]. Also, Saccharomyces cerevisiae var. boulardii, S. cerevisiae UFMG 905, and L. delbrueckii UFV H2b20 reduce aflatoxin and spore production by Aspergillus parasiticus IMI 242695 in peanuts [81]. What is more, fermented food contains some antimicrobial agents, mostly bacteriocins. The subject is still explored as interesting for the agricultural industry.
7. The Recognition and Uptake of Prebiotics among Consumers
Modern consumers pay considerable attention to their lifestyle. It generates increased demand for food promoting health and wellness through busting immune system providing a reduced risk of certain cancers and cardiovascular diseases, balanced metabolism and proper weight, improved eyesight, memory and physical efficiency [82]. Motivations depend on age and gender. Young men, as opposed to women and older men, pay less attention to the consequences of consuming functional food [83]. Other study indicates that only 20% and 7% of patients could correctly define probiotics and prebiotics, respectively. More patients consumed probiotics (53%) than prebiotics (38%). Among the most frequently consumed probiotic and prebiotic products were yogurts (72%) and cereals/granola bars (55%). Patients considered probiotics and prebiotics most beneficial for ‘digestion or gut health’, but the most common reason to consume these products was ‘to taste or try’ (36% and 43%, respectively) [84].
In Europe, a high percentage of the population consumes functional food. Women, older people (35–60 years), and those with university educations pay attention to natural and eco products, nutritional value, freshness, food safety, and quality guarantees. Fibre-rich breads/cookies were consumed, first of all, by individuals with medium education. Females more often consumed soy milk, fibre-rich bread/cookies and teas; males and females with a medium income preferred breakfast cereal. Obese individuals less likely consumed breakfast cereals and fibre-rich bread/cookies [85]. Older persons commonly consumed functional foods such as yogurts with probiotics (56.0%), eggs with omega-3 fatty acids (37.0%), and bread with fibre (35.5%). Young consumers are more open to high-technology food processing.
8. Limitations of Functional Food
Several papers document benefits from the use of probiotics and prebiotics and their generally positive role. However, recent, extended metagenomic study of the human GI microbiome indicates that person-, region-, and strain-specific mucosal colonisation by supplemented bacteria is not obvious. Probiotics have a transient and individualised impact on the mucosal microbiome [86]. After antibiotic therapy, the colonisation of the GI tract by exogenous probiotics is markedly delayed and persistently incomplete. It appears that the best way to promote the long-term improvement of GI microflora is the opposite way of supplementation, that is, faecal microbiome transplantation [87]. However, this kind of therapy seems to be much less acceptable than oral supplementation. Enhancing the number of supplemented bacteria is not the best solution. An excess of probiotic supplementation is unfavourable. An excess of LAB can result in some local problems such as belching, bloating, discomfort or even stomach ache as well as rectal sensations, including gases and loose stools [88]. Likely, such effects could also be distant and generally associated with lactic acidosis. This metabolic disturbance is connected with brain fogginess, a complex of cognitive dysfunction involving memory problems, lack of mental clarity, poor concentration, and mental fatigue [89]. Recent data indicate that Bifidobacterium might also have an adverse effect on glucose metabolism by reducing butyrate-producing microbes [90].
It should be taken into account that probiotics are sold as medications and/or food supplements, and they are usually well defined and their dosage is known. Prebiotics are a common component of food and rarely a partially purified supplements. So, the knowledge of the adverse effects of overdosing of prebiotics is very limited. This subject is ignored even in serious well-composed scientific studies, such as the effects of formulae supplemented with prebiotics upon the health of infants [91]. However, some reports demonstrate an adverse effect. It could be related to an overgrowth of probiotic bacteria induced by excessive supplementation of prebiotics [92]. Moreover, also straight effects can be observed. For instance, an excess of inulin aggravates atherosclerosis in hypercholesterolemic mice and supplementation with fibre could limit access to microelements [93,94] should be taken under consideration.
In Summary
In modern pre- and pro-biosis, non-dairy products did not meet yet sufficient attention. Particularly, cereals offer new intriguing prospects. They are commonly consumed all over the World for their availability and nutritional valour. Recent research points to them as prebiotics. Among others, prebiotic of non-dairy origin are efficient in preventing atherosclerotic disease and cardiac incidences, prevents intestinal disorders, including cancer, reduces high blood sugar, and improves the course of some metabolic diseases. A variety of cereals, specific bacteria used for fermentation, and finally thousands of mid- and final biologically active products called postbiotics offer a huge potential of this part of nutrition and stimulate future scientific research in this area.
Author Contributions
Contribution of both authors is equal. P.T. (Przemysław Tomasik) & P.T. (Piotr Tomasik) conceived the original idea, searched the published papers, discussed and analysed collected data, wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Tomasik, P.J.; Tomasik, P. Prebiotics and probiotics. Cereal Chem. 2003, 80, 113–117. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ewe, J.-A.; Sau-Chan Tham, C.; Liong, M.-Z. Carriers of probiotic organisms. In Probiotics: Biology, Genetics, and Health Aspects; Book Series: Microbiology Monographs; Springer: Berlin, Germany, 2011; Volume 21, pp. 191–220. [Google Scholar]
- Saxelin, M. Probiotic formulations and applications, the current probiotics market and changes in the marketplace: A European perspectives. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S76–S79. [Google Scholar] [CrossRef]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy based probiotics: A healthy treat for intestine. Crit. Rev. Food Sci. Nutr. 2015, 56, 1856–1867. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Enujiugha, V.N.; Badejo, A.A. Probiotic potentials of cereal-based beverages. Crit. Rev. Food Sci. Nutr. 2017, 57, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Greppi, A.; Saubade, F.; Botta, C.; Humblot, C.; Guyot, J.P.; Cocolin, L. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 2017, 62, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, I. Fermented cereal beverages: From probiotic, prebiotic and symbiotic towards nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Procopio, S.; Becker, T. Influence of malting and lactic acid fermentation on functional bioactive components in cereal-based raw materials: A review paper. Int. J. Food Sci. Technol. 2016, 51, 14–22. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Faubion, J.M. The beneficial use of cereal and cereal compounds in probiotic foods. Food Rev. Int. 2009, 25, 103–114. [Google Scholar] [CrossRef]
- Furtado Martins, E.M.; Ramos, A.M.; Lago Vanzela, E.S.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of vegetable origin: A new alternative for the consumption of probiotic bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H. Probiotics and prebiotics in pediatrics: Where are we now? Turk. J. Pediatr. 2007, 49, 231–244. [Google Scholar] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Assadi, H.; Naeimi, B.; Gharibi, S.; Khosravi, A.; Dobaradaran, S.; Taherkhani, R.; Tajbakhsh, S. Detection of Acinetobacter spp. in blood cultures by an improved fluorescent in situ hybridization assay. Pol. J. Microbiol. 2018, 67, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Païssé, S.; Valle, C.; Servant, F.; Courtney, M.; Burcelin, R.; Amar, J.; Lelouvier, B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016, 56, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The bacterial ecosystem of mother’s milk and infant’s mouth and gut. Front. Microbiol. 2017, 8, 1214–1224. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S.; Keelan, J.A. Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit. Revs. Microbiol. 2017, 43, 352–369. [Google Scholar] [CrossRef]
- Gokavi, S.; Zhang, L.W.; Huang, M.-K.; Xin Zhao, X.; Guo, M. Oat-based synbiotic beverage fermented by Lactobacillus plantarum, Lactobacillus paracasei ssp. casei, and Lactobacillus acidophilus. J. Food Sci. 2005, 70, M216–M223. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Pelikanova, J.; Liptakova, D.; Valik, L. Suitability of lactic acid bacteria for fermentation of maize and amaranth. J. Food Nutr. Res. 2015, 54, 354–364. [Google Scholar]
- Gao, F.; Cai, S.; Nout, R.M.J.; Wang, Y.; Xia, Y.; Li, Y.; Ji, B. Production of oat-based synbiotic beverage by two-stage fermentation with Rhizopus oryzae and Lactobacillus acidophilus. J. Food Agric. Environ. 2012, 10, 175–179. [Google Scholar]
- Fusco, V.; Quero, G.M.; Cho, G.-S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.B.M.; Skov, L.; Thyssen, J.P.; Jensen, P. Role of the gut microbiota in atopic dermatitis: A systematic review. Acta Dermato-Venereol. 2019, 99, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Blasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Revs. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Chorell, E.; Karlsson Videhult, F.; Hernell, O.; Antti, H.; West, C.E. Impact of probiotic feeding during weaning on the serum lipid profile and plasma metabolome in infants. Br. J. Nutr. 2013, 110, 116–126. [Google Scholar] [CrossRef]
- West, C.E.; Hammarstrom, M.-L.; Hernell, O. Probiotics in primary prevention of allergic disease – follow-up at 8–9 years of age. Allergy 2013, 68, 1015–1020. [Google Scholar] [CrossRef]
- Hasslöf, P.; West, C.E.; Karlsson Videhult, F.; Brandelius, C.; Stecksén-Blicks, C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. 2013, 47, 559–565. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and microbiota: A systematic review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Deidda, F.; Amoruso, A.; Nicola, S.; Graziano, T.; Pane, M.; Mogna, L. New approach in acne therapy: A specific bacteriocin activity and a targeted anti IL-8 property in Just 1 probiotic strain, the L. salivarius LS03. J. Clin. Gastroenterol. 2018, 52, S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Nwanodi, O. Skin protective nutraceuticals: The current evidence in brief. Healthcare (Basel) 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, M.; Sharma, A.K.; Sharma, A.; Gupta, G.K. Understanding the microbiome: Emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin. Cancer Biol. 2018, 52(pt.1), 1–8. [Google Scholar] [CrossRef]
- FAO. Technical Meeting on Prebiotics; FAO: Rome, Italy, 2008; pp. 1–12. [Google Scholar]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Revs. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Cereal based functional food of Indian subcontinent. A review. J. Food Sci. Technol. Mysore 2012, 49, 665–672. [Google Scholar] [CrossRef]
- Reque, P.M.; Pinilla, C.M.B.; Gautério, G.V.; Kalil, S.J.; Brandelli, A. Xylooligosaccharides production from wheat middlings bioprocessed with Bacillus subtilis. Food Res. Int. 2019, 126, 108673. [Google Scholar] [CrossRef]
- Sharma, M.; Devi, M. Probiotics: A comprehensive approach towards health foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 537–552. [Google Scholar] [CrossRef]
- Barczynska, R.; Slizewska, K.; Litwin, M.; Szalecki, M.; Kapusniak, J. Effect of dietary fiber preparations made from maize starch and the growth and activity of selected bacteria from the Formicutes, Bacteroidetes and Actinobacteria phyla in fecal samples from obese children. Acta Biochim. Polon. 2015, 63, 261–266. [Google Scholar]
- Barczynska, R.; Kapusniak, J.; Litwin, M.; Slizewska, K.; Szalecki, M. Dextrins from maize starch as substances activating the growth of Bacteroidetes and Actinobacteria simultaneously inhibiting the growth of Firmicutes resposnisble for the occurrence of obesity. Plant Foods Hum. Nutr. 2016, 71, 190–196. [Google Scholar] [CrossRef]
- Barczynska, R.; Jurgonski, A.; Slizewska, K.; Juskiewicz, J.; Kapusniak, J. Cornstarch dextrin changes intestinal microbiota and iys metabolic activity in rats fed a basal and high-fat diet. Br. Food J. 2019, 121, 2219–2232. [Google Scholar] [CrossRef]
- Slizewska, K.; Libudzisz, Z.; Barczynska, R.; Kapusniak, J.; Zdunczyk, Z.; Juskiewicz, J. Dietary resistant dextrins positvey modulate fecal and cecal microbiota composition in young rats. Acta Biochim. Polon. 2015, 62, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Barczynska, R.; Jurgonski, A.; Slizewska, K.; Juskiewicz, J.; Kapusniak, J. Effect of potato dextrin on the composition and metabolism of the gut microbiota in rats fed standard and high-fat diets. J. Funct. Food 2017, 34, 398–407. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Hucha, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African fermented foods and probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Kalui, C.M.; Mathara, J.M.; Kutima, P.M. Probiotic potential of spontaneously fermented cereal based foods —A review. Afr. J. Biotechnol. 2010, 9, 2490–2498. [Google Scholar]
- Gupt, M.; Bajaj, B.K. Selection criteria for probiotics and potential of cereal based food products as novel probiotic-carriers. Curr. Nutr. Food Sci. 2016, 12, 157–174. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Kurvinen, T.; Rissanen, P. Use of a probiotic Bifidobacterium in a dry food matrix, an in vivo study. Int. J. Food Microbiol. 2004, 95, 103–106. [Google Scholar] [CrossRef]
- Bernat, N.; Chafer, M.; Gonzalez-Martınez, C.; Rodrıguez-Garcıa, J.; Chiralt, A. Optimization of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Sci. Technol. Int. 2014, 20, 145–157. [Google Scholar]
- Arena, M.P.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozz, V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef]
- Shamir, R.; Makhoul, I.R.; Etzioni, A.; Shehadeh, N. Evaluation of diet containing probiotics and zinc for the treatment of mild diarrheal illness in children younger than one year of age. J. Am. Coll. Nutr. 2005, 24, 370–375. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Sanni, A.I.; Agrawal, R. Probiotic potentials of yeasts isolated from some cereal-based Nigerian traditional fermented food products. J. Appl. Microbiol. 2015, 119, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Khositsuntiwong, N.; Manosroi, A. Biological activities of fructooligosaccharide (FOS)-containing Coix lachryma-jobi Linn. extract. J. Food Sci. Technol. 2014, 51, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.; Al-Ghazzewi, F.H. Intrinsic and extrinsic carbohydrates in the vagina: A short review on vaginal glycogen. Int. J. Biol. Macromol. 2018, 112, 203–206. [Google Scholar] [CrossRef] [PubMed]
- De Alberti, D.; Russo, R.; Terruzzi, F.; Nobile, V.; Ouwehand, A.C. Lactobacilli vaginal colonisation after oral consumption of Respecta(®) complex: A randomised controlled pilot study. Arch. Gynecol. Obstetrics 2015, 29, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics easing renal failure by improving gut microbiology (Synergy): A randomized trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Fava, F.; Viola, R. ‘The way to a man’s heart is through his gut microbiota’—dietary pro- and prebiotics for the management of cardiovascular risk. Proc. Nutr. Soc. 2014, 73, 172–185. [Google Scholar] [CrossRef]
- Fallucca, F.; Porrata, C.; Fallucca, S.; Pianesi, M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabet. Metabol. Res. Revs. 2014, 30 (Suppl. 1), 48–54. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Shaikh Uzma, A.; Deshpande, H.W.; Kulkarni, D.B. A review on probiotic beverages prepared using vegetables. Int. J. Chem. Stud. 2018, 6(5), 61–65. [Google Scholar]
- Aguilar-Toala, J.; Garcia-Valera, R.; Garcia, H.S.; Mata-Haro, V.; Cordova-Gonzalez, A.F.; Hernandez-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Arora, S.; Jood, S.; Khetarpaul, N. Effect of germination and probiotic fermentation on nutrient profile of pearl millet based food blends. Br. Food J. 2011, 113, 470–481. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876–895. [Google Scholar]
- Ayyash, M.; Johnson, S.K.; Liu, S.Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In vitro investigation of bioactivities of solid-state fermented lupin, quinoa and wheat using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef]
- Sanz-Penell, J.M.; Frontela, C.; Ros, G.; Martinez, C.; Monedero, V.; Haros, M. Application of bifidobacterial phytases in infant cereals: Effect on phytate contents and mineral dialyzability. J. Agric. Food Chem. 2012, 60, 11787–11792. [Google Scholar] [CrossRef]
- Sarno, M.; Lania, G.; Cuomo, M.; Passannanti, F.; Budelli, A.; Fasano, F.; Troncone, R.; Auricchio, S.; Barone, M.V.; Nigro, R.; et al. Lactobacillus paracasei CBA L74 interferes with gliadin peptides entrance in Caco-2 cells. Int. J. Food Sci. Nutr. 2014, 65, 953–959. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Salari, M.; Razavi, S.H.; Gharibzahedi, S.M.T. Characterising the synbiotic beverages based on barley and malt flours fermented by Lactobacillus delbrueckii and paracasei strains. Qual. Assur. Saf. Crops Foods 2015, 7, 355–361. [Google Scholar] [CrossRef]
- Bailey, R. Functional Foods in Japan: Foshu (“foods for Specified Health Uses”) and “foods with Nutrient Function Claims”. In Regulation of Functional Foods and Nutraceuticals: A Global Perspective; Hasler, C.M., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 247–261. ISBN 9780813811772. [Google Scholar]
- Saito, M. Role of FOSHU (food for specified health uses) for healthier life. Yakugaku Zasshi 2007, 127, 407–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- China Dictionary.net. Available online: https://nortonsafe.search.ask.com/web?q=china%20dictionary&hp=0&year=2015&installstatus=updated&schemaver=1.0.0.0&os=windows&geo=us&schemacat=sbu_w&ssdcat=321&dsp=0&3in1=0&showuninstallsurvey=1&locale=pl_us&machinelocation=191&version=22.16.2.22&templatecat=sbu_w_ns_cto&sw=0&vendortesteligible=no&tb=0&osvers=6.3&vendor=iac&vendorsrc=firefox&olpchannel=configure_to_order&installsource=nag&source=nag&o=APN12174&prt=ngc&ver=3.3.0.4&tpr=111&chn=3000&guid=f36725de-2708-4191-858d-1f2391ffb9af&doi=2018-11-28 (accessed on 29 July 2018).
- Salmeron, I.; Rozada, R.; Thomas, K.; Ortega-Rivas, E.; Pandiella, S.S. Sensory characteristics and volatile composition of a cereal beverage fermented with Bifidobacterium breve NCIMB 702257. Food Sci. Technol. Int. 2014, 20, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, O.R.; Agrawal, R.; Sanni, A.I. Development of cereal-based functional food using cereal mix substrate fermented with probiotic strain—Pichia kudriavzevii OG32. Food Sci. Nutr. 2015, 3, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Raghavender, C.R.; Reddy, B.N.; Salleh, B. Biological control of Aspergillus flavus growth and subsequent aflatoxin B1 production in sorghum grains. Afr. J. Biotechnol. 2010, 9, 4247–4250. [Google Scholar]
- Quattrini, M.; Bernardi, C.; Stuknytė, M.; Masotti, F.; Passera, A.; Ricci, G.; Vallone, L.; De Noni, I.; Brasca, M.; Fortina, M.G. Functional characterization of Lactobacillus plantarum ITEM 17215: A potential biocontrol agent of fungi with plant growth promoting traits, able to enhance the nutritional value of cereal products. Food Res. Int. 2018, 106, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Wacoo, A.P.; Mukisa, I.M.; Meeme, R.; Byakika, S.; Wendiro, D.; Sybesma, W.; Kort, R. Probiotic enrichment and reduction of aflatoxins in a traditional African maize-based fermented food. Nutrients 2019, 11, 265. [Google Scholar] [CrossRef]
- da Silva, J.F.; Peluzio, J.M.; Prado, G.; Madeira, J.E.; Silva, M.O.; de Morais, P.B.; Rosa, C.A.; Pimenta, R.S.; Nicoli, J.R. Use of probiotics to control aflatoxin production in peanut grains. Sci. World J. 2015, 2015, 959138. [Google Scholar] [CrossRef]
- Kraus, A. Development of functional food with the participation of the consumer. Motivators for consumption of functional products. Int. J. Consum. Stud. 2015, 39, 2–11. [Google Scholar] [CrossRef]
- Kraus, A.; Annunziata, A.; Vecchio, R. Sociodemographic factors differentiating the consumer and the motivations for functional food consumption. J. Am. Coll. Nutr. 2017, 36, 116–126. [Google Scholar] [CrossRef]
- Betz, M.; Uzueta, A.; Rasmussen, H.; Gregoire, M.; Vanderwall, C.; Witowich, G. Knowledge, use and perceptions of probiotics and prebiotics in hospitalised patients. Nutr. Diet. 2015, 72, 261–266. [Google Scholar] [CrossRef]
- Ozen, A.E.; Bibiloni, M.; Pons, A.; Tur, J.A. Sociodemographic and lifestyle determinants of functional food consumption in an adult population of the Balearic Islands. Ann. Nutr. Metabol. 2013, 63, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar]
- Goetze, O.; Fruehauf, H.; Pohl, D.; Giarrè, M.; Rochat, F.; Ornstein, K.; Menne, D.; Fried, M.; Thumshirn, M. Effect of a prebiotic mixture on intestinal comfort and general wellbeing in health. Brit. J. Nutr. 2008, 100, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Rehman, A.; Yu, S.; Andino, N.M. Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clin. Translat. Gastroenterol. 2018, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Repts. 2017, 7, 11789. [Google Scholar] [CrossRef]
- Skorka, A.; Piescik-Lech, M.; Kołodziej, M.; Szajewska, H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Brit. J. Nutr. 2018, 119, 810–825. [Google Scholar] [CrossRef]
- François, I.E.; Lescroart, O.; Veraverbeke, W.S.; Windey, K.; Verbeke, K.; Broekaert, W.F. Tolerance and the effect of high doses of wheat bran extract, containing arabinoxylan-oligosaccharides, and oligofructose on fecal output: A double-blind, randomized, placebo-controlled, cross-over trial. J. Nutr. Sci. 2014, 3, e49. [Google Scholar] [CrossRef]
- Hoving, L.R.; de Vries, M.R.; de Jong, R.C.M.; Katirae, i.S.; Pronk, A.; Quax, P.H.A.; van Harmelen, V.; Willems van Dijk, K. The prebiotic inulin aggravates accelerated atherosclerosis in hypercholesterolemic APOE*3-Leiden mice. Nutrients 2018, 10, 172. [Google Scholar] [CrossRef]
- Lin, F.; Wu, H.; Zeng, M.; Yu, G.; Dong, G.; Yang, H. Probiotic/prebiotic correction for adverse effects of iron fortification on intestinal resistance to Salmonella infection in weaning mice. Food Funct. 2018, 9, 1070–1078. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).